Hepatitis C virus burden: Treating and educating people without prejudice
Elettra Merola, Elisa Menotti, Giovanna Branz, Andrea Michielan, Sonia Seligmann, Annora Ratti, Flora Agugiaro, Luisa Moser, Giovanni Vettori, Anna Franceschini, William Mantovani, Riccardo Pertile, Giovanni de Pretis, Cecilia Pravadelli
Elettra Merola, Elisa Menotti, Andrea Michielan, Sonia Seligmann, Flora Agugiaro, Luisa Moser, Giovanni Vettori, Giovanni de Pretis, Cecilia Pravadelli, Department of Gastroenterology, Santa Chiara Hospital, Azienda Provinciale Per I Servizi Sanitari (APSS), Trento 38122, Italy
Giovanna Branz, Annora Ratti, Anna Franceschini, Department of SerD, Service for Drug Addiction, Azienda Provinciale Per I Servizi Sanitari (APSS), Trento 38122, Italy
William Mantovani, Department of Prevention, Azienda Provinciale Per I Servizi Sanitari (APSS), Trento 38122, Italy
Riccardo Pertile, Department of Clinical and Evaluative Epidemiology, Azienda Provinciale Per I Servizi Sanitari (APSS), Trento 38122, Italy
Abstract BACKGROUND Hepatitis C virus (HCV) infection has a worldwide incidence of 1.1%. In Italy, 60% of people who inject drugs (PWIDs) and are receiving assistance for substance use disorder are infected with HCV. However, this subset of patients has extremely limited access to care due to multiple factors, including alcohol abuse, psychological comorbidities, and homeless status.AIM To describe the impact of our HCV-dedicated service for substance use disorder (SSUD) service on PWIDs receiving anti-HCV therapy.METHODS A dedicated, multidisciplinary team was set up at the SSUD of Trento in October 2020 to provide antiviral treatment to HCV RiboNucleic Acid-positive patients with an active or previous history of substance abuse. The treatment was followed by a health education program. Patients were treated with Direct-Acting Antivirals (DAAs). Data were retrospectively analyzed to assess the efficacy of our dedicated program in terms of therapy completion, HCV eradication, and compliance (primary endpoint). The rate of HCV reinfection and DAA-related toxicity were also assessed (secondary endpoints).RESULTS A total of 40 patients were enrolled in the study: 28 (70.0%) were treated with Sofosbuvir/Velpatasvir, while 12 (30.0%) received Glecaprevir/Pibrentasvir. At the time of inclusion in the study, 36 patients were receiving opioid agonist maintenance therapy, whilst another 4 had just finished the treatment. 37.5% had a history of alcoholism and 42.5% received concomitant psychiatric treatment. All 40 patients (100.0%) completed the therapy cycle and 92.5% of patients adhered to the program. All patients tested negative for viral load at the end of the treatment. There were no significant drug interactions with common psychiatric treatments and no side effects were observed. The sustained virological response was achieved in 92.5% of cases with good tolerability, although two patients discontinued treatment temporarily. After HCV eradication, one patient died from an overdose, another from complications of cirrhosis, and one reinfection occurred.CONCLUSION Very high adherence to therapy and good tolerability was observed in our series of HCV patients treated at the SSUD, regardless of the substance abuse condition. Further validation in a larger population is required.
Key Words: Hepatitis C virus; Service for substance use disorder; Direct-acting antivirals; Sustained virologic response; Compliance; Tolerability
lNTRODUCTlON
Hepatitis C virus (HCV) infection has an incidence of around 1.1% worldwide, making it one of the main issues in the area of public health[1]. Italy has the highest prevalence of HCV-positive patients in Europe, as well as the highest rate of complication-related deaths such as cirrhosis and hepatocellular carcinoma[2]. These conditions are associated with significant costs, particularly in terms of therapies, hospital admissions, hepatic transplantations, and associated extrahepatic manifestations and complications[3-6].
Direct-Acting Antivirals (DAAs) have demonstrated a high response rate as an anti-HCV treatment, resulting in effective curative therapy in 95%-96% of cases, and is an affordable and cost-effective treatment[7].
The World Health Organization (WHO) has set ambitious targets for the global elimination of HCV, including an 80% reduction in new chronic infections, a 90% reduction in the incidence of new infections, and an 80% increase in treatment by 2030[8].
People who inject drugs (PWIDs) currently bear the heaviest burden of HCV infection in Italy, with a prevalence of around 60%, increased morbidity and mortality, and limited access to care[9]. Microelimination in this subgroup of HCV patients is becoming a public healthcare priority in order to reduce circulation[10]. According to WHO data, PWIDs account for 23% of new infections, but this population is considered “hard to reach” for several reasons. Indeed, access to care is extremely limited for these citizens due to social stigma, multifactorial frailty (i.e.,low compliance, alcoholic abuse, co-infection with HBV and HIV, homelessness, lack of a caregiver, and psychological and psychiatric issues), and poor use of standard medical channels[11,12].
Direct-Acting Antivirals are proven to be safe and effective in individuals with active substance abuse and those receiving opioid substitution therapy[13-15]. The SIMPLIFY study found that 92.5% of PWIDs with ongoing drug abuse achieved an overall sustained virologic response (SVR) following treatment with sofosbuvir and velpatasvir[16].
In Italy, approximately 50% of the population receiving assistance for substance use disorder is positive for HCV infection. These services in Italy could be considered a “hot spot” for HCV screening and treatment because they avoid the typical health care pattern, create a dedicated link to a care strategy for patient retention, curing the condition, and improving adherence to therapy and follow-up[11,17].
This approach would allow marginalized patients to undergo treatment in a standard care setting, accompanied throughout the whole process, thereby reducing loss to follow-up and low adherence. Since PWIDs are one of the mainreservoirsof HCV infection, eradicating the virus in this “key subgroup” is crucial for halting its spread, improving public health, and reducing costs for the National Health System[18].
This study aims to prospectively describe the experience of our HCV-dedicated multidisciplinary program at the SSUD of Trento (Northeast Italy), which focuses on providing anti-HCV therapy in infected PWIDs with an active or past history of substance abuse.
MATERlALS AND METHODS
Study design and endpoints
This study, which had a prospective observational design and retrospective data analysis, included patients who met the following criteria: PWIDs attending our SSUD in Trento, having positive HCVantibodies and HCV-RiboNucleic Acid (RNA) > 15 UI/mL, and were current or previous drug users.
The exclusion criteria were: age < 18 years old and the absence of informed consent.
The primary endpoint was the efficacy of our dedicated program in terms of therapy completion and PWIDs’ adherence to post-treatment controls. Secondary endpoints included HCV eradication, the rate of HCV reinfection after treatment, and DAA-related toxicity.
In compliance with local legislation, the study protocol was approved by the local ethical committee (N. A785), and patients provided their informed consent for data acquisition.
The study is reported in accordance with the STROBE guidelines for observational studies and follows the criteria of the Declaration of Helsinki.
SSUD activities
In October 2020, a dedicated team was set up at the SSUD in Trento to provide opioid agonist therapy and DAA therapy to HCV RNA-positive patients with an active or past history of substance abuse.
The team consists of a hepatologist, a facility physician, and four dedicated nurses. The synergistic collaboration between the different specialists resulted in the development of a health education program, which included counseling on how to avoid reinfection.
Subjects are closely guided and monitored throughout treatment and follow-up. While some patients can take the treatment on their own and only attend the SSUD once a week, the vast majority receive daily treatment directly at the SSUD or a therapeutic rehabilitation facility. The team plans and organizes all the blood tests and visits, while also monitoring and enforcing adherence.
Opioid use disorder was diagnosed following the Diagnostic and Statistical Manual of Mental Disorders 5, and its severity was assessed before and after eradication therapy. Patients were classified as: (1) “Abstinent”: The absence of other drugs other than opioid agonist maintenance therapy or occasional consumption; (2) “User”: constant use without severe impairment of health and quality of life; and (3) “Abuser”: constant consumption severely compromising health and quality of life and unable to maintain a normal social and work routine.
Antiviral therapy
Indications for DAA therapy followed the WHO criteria[19]. The HCV genotype was assessed before the start of treatment and the stage of liver disease was evaluated using transient elastography[20]. When this method was not feasible due to social and welfare-related reasons, liver stiffness was assessed using serum markers, such as the “Fibrosis 4 Score” (Fib-4), as recommended by the EASL Guidelines[20].
The treatment regimen was based on the standard 12-week oral schedule with Sofosbuvir/ Velpatasvir or an 8-week oral Glecaprevir/Pibrentasvir regimen[20].
The patients received clinical monitoring each month throughout the treatment. Treatment efficacy was evaluated by detection of negative HCV RNA at the end of treatment and after 12 wk (SVR12), when possible, or by “delayed SVR” (SVR evaluated at any time after 12 wk).
Statistical analysis
Prospectively collected variables were extracted from electronic information flows and paper-based patient records and entered into an anonymous database. They included demographic information, concomitant substance abuse, comorbidities, adherence to the therapy schedule and follow-up controls, HCV RNA levels at the start and end of therapy, SVR12 or delayed SVR, rate of HCV reinfection, treatment pause, and side effects.
Statistical analysis was performed using a dedicated software program (Medcalc 15.6.1, www.medcalc.be).
The distribution of continuous variables was reported as the median and range.
SVR rates were evaluated through intention-to-treat analysis (considering all missing data as failures).
RESULTS
Patient characteristics
A total of 42 patients met this study’s inclusion criteria. The final analysis included a total of forty patients, since two patients are still undergoing treatment. Patient characteristics are detailed in Table 1.
The majority of patients were male (77.5%). Elastography for the staging of liver disease could not be used in 5 patients, and the Fib-4 score was used instead for their evaluation. Four out of 35 patients (11.4%) were already suffering from liver cirrhosis at the start of treatment: three patients were diagnosed with compensated cirrhosis (Child-Pugh A) and one was described as decompensated (Child-Pugh B7).
Twenty-eight patients (70.0%) were treated with Sofosbuvir/ Velpatasvir, while 12 (30.0%) received Glecaprevir/Pibrentasvir. Table 2 reports treatment outcomes.
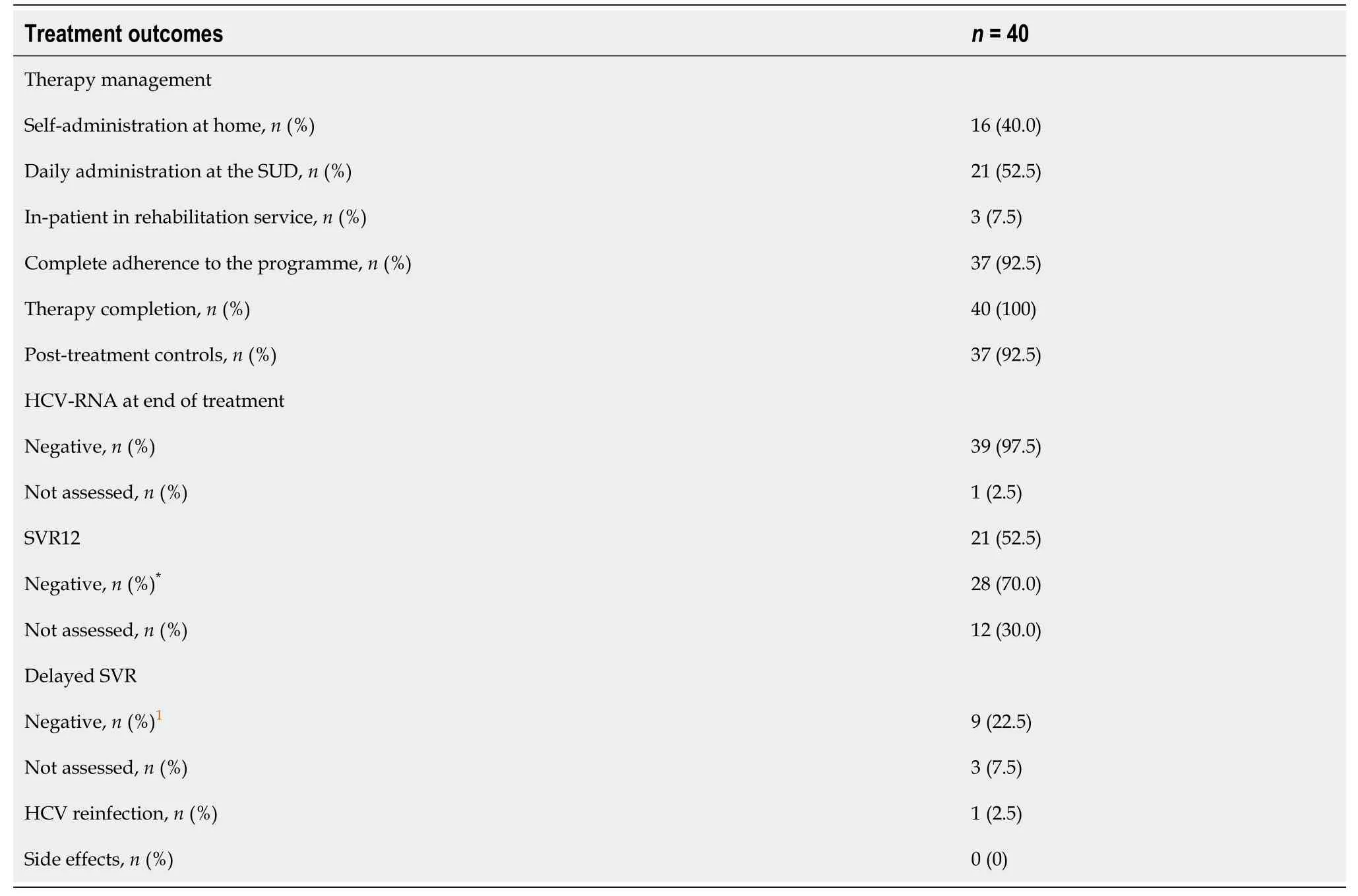
Table 2 Management and outcomes of anti-hepatitis C virus treatment
Recruited individuals had an active or past history of drug consumption and other psychoactive substances (i.e.,benzodiazepines).
Thirty-six of the 40 recruited patients were receiving opioid agonist maintenance therapy at study inclusion, whilst another 4 had just completed treatment.
A significant proportion of the study population (37.5%) had a history of alcohol consumption, whilst 42.5% received concomitant psychiatric treatment. Patients were generally prescribed benzodiazepines and neuroleptics, but six patients were prescribed selective serotonin reuptake inhibitors.
Treatment outcomes
Treatment outcomes are detailed in Table 2. The therapy cycle was completed by 40 patients (100%). All tested patients presented a negative viral load at the end of treatment and a sustained virologic response was observed in 92.5% (SVR12 + delayed SVR). Therapy was well-tolerated, except in two cases where the patients temporarily discontinued treatment and refused subsequent lab tests. Another patient elected to only be tested for HCV RNA at the end of treatment but refused all post-therapy controls. No significant drug interactions with commonly used psychiatric treatments or side effects were observed. One patient died of an overdose, another patient died of cirrhosis complications following HCV eradication and one reinfection was observed ten months after SVR12.
DlSCUSSlON
The present study demonstrates that a multidisciplinary, dedicated program to assist PWIDs during anti-HCV treatment can be a safe and effective method to improve their adherence to therapy and follow-up schedule. In detail, our service achieved 100% therapy completion, 92.5% adherence to posttreatment follow-up, and one loss at follow-up despite a negative HCV RNA 4 wk after treatment ended. Furthermore, 92.5% of cases responded to treatment with good tolerability of DAAs (100.0%). Therapy efficacy was also observed in people with concurrent drug and alcohol abuse, without any significant drug interactions with commonly used psychiatric treatments.
In comparison to previous studies, good adherence to treatment and the follow-up program was observed in our series, with no drop-outs due to toxicity[21-25]. In particular, Avramovicet al[24] reported a similar rate of virological response (92%) in PWIDs. This rate would have been even higher in our series (100.0%vs96% for Avramovicet al[24]) if a “per-protocol” analysis had been performed by excluding any missing data or drop-outs from the calculation. Avramovicet al[24] also reported a high rate of loss to follow-up (17%) and reinfection (3.5%) in PWIDs with ongoing drug use treated for HCV. Adherence was higher in our series, with only one case of reinfection involving a patient who became homeless and had a history of ongoing alcohol abuse and psychiatric comorbidities. Our encouraging results are a direct outcome of the dedicated work of the SSUD of Trento’s multidisciplinary team and its efforts to increase this fragile population’s adherence to the anti-HCV program through a more vigilant and attentive approach. Adherence was difficult to maintain during the follow-up period, owing to the patients’ general condition, poor access to the healthcare system, and a low peripheral venous heritage. However, our results demonstrate that a tailored treatment and follow-up plan, accompanied by close monitoring and constant contact to avoid alienation, can also be successful in treating HCV in PWIDs.
In the SIMPLIFY multicenter design study, Cunninghamet al[21] demonstrated high adherence to anti-HCV therapy in PWIDs, measured using an electronic blister pack. Furthermore, a correlation was observed between non-adherence and recent stimulant injecting before and during DAA therapy, but with no impact on response to therapy. Our study corroborates these findings, as loss of compliance or delayed SVR was frequently associated with deterioration in the psychological/psychiatric situation and wealth status (i.e.,homelessness, self-isolation, loss of job, and the absence of a caregiver). However, the two subjects who discontinued DAA therapy for an extended period due to a boost in drug abuse associated with a worsening of their psychiatric conditions may also achieve negative HCV RNA at the end of treatment.
Two subjects in our population died following SVR12, including one who began the treatment with decompensated cirrhosis (Child-Pugh B7). Our patients were predominantly young (median age 46.5 years old), male (in line with the literature[26]), and mostly without clinically significant fibrosis. Eradicating HCV in a population with a high prevalence of infection and no or early-stage liver disease has a significant impact on public health by interrupting the vicious cycle of viral spread, progression to cirrhosis, and its complications, with significant cost-effectiveness.
Despite the strength of a real-world setting and prospective design, this study has a major limitation. The findings are the result of intensive and time-consuming work, with the program having been applied to a small population on a local scale thus far. The question then becomes whether this type of model could be scaled up to a larger and more complex field while maintaining reasonable costs and demand for human resources. Based on the encouraging results achieved thus far, our next step will be to apply this multidisciplinary anti-HCV program to a larger PWID population and validate it in a larger-scale real-world setting.
CONCLUSlON
In conclusion, targeted anti-HCV programs involving vulnerable infected patients, such as PWIDs, can be effective at improving patient compliance and eradicating infection with good tolerability. However, a larger prospective study is required to definitively confirm the efficacy of this initiative.
ARTlCLE HlGHLlGHTS
Research background
Hepatitis C virus (HCV) infection has an incidence of around 1.1% worldwide, making it one of the main issues in the area of public health. Direct-Acting Antivirals (DAAs) have demonstrated a high response rate as an anti-HCV treatment, resulting in effective curative therapy in 95%-96% of cases, and is an affordable and cost-effective treatment. In Italy, approximately 50% of the population receiving assistance for substance use disorder (SSUDs) is positive for HCV infection. These services in Italy could be considered a “hot spot” for HCV screening and treatment because they avoid the typical health care pattern, create a dedicated link to a care strategy for patient retention, curing the condition, and improving adherence to therapy and follow-up.
Research motivation
To prospectively describe the experience of our HCV-dedicated multidisciplinary program at the SSUD of Trento (Northeast Italy), which focuses on providing anti-HCV therapy in infected "people who inject drugs" (PWIDs) with an active or past history of substance abuse.
Research objectives
To show the efficacy of our dedicated program in terms of therapy completion and PWIDs’ adherence to post-treatment controls. Secondary endpoints included HCV eradication, the rate of HCV reinfection after treatment, and DAA-related toxicity.
Research methods
This study included: PWIDs attending our SSUD in Trento, with HCV-antibodies and HCV-RiboNucleic Acid (RNA) > 15 UI/mL, with history of substance abuse. In October 2020, a dedicated team was set up at the SSUD in Trento to provide opioid agonist therapy and DAA therapy to HCV RNA-positive to these patients. The team provided health education program, including counseling, planning of blood tests and visits. Indications for DAA therapy followed the World Health Organization criteria. The HCV genotype was assessed before treatment start and the stage of liver disease by transient elastography or Fibrosis 4 Score”, as recommended by the EASL Guidelines. The treatment regimen was based on the standard 12 wk oral schedule with Sofosbuvir/Velpatasvir or an 8 wk oral Glecaprevir/Pibrentasvir regimen. Treatment efficacy was evaluated by negative HCV RNA at the end of treatment and after 12 wk (SVR12), or by “delayed SVR” (SVR evaluated at any time after 12 wk).
Research results
Forty patients were included in the study, with active or past history of drug consumption and other psychoactive substances (i.e.,benzodiazepines), and 37.5% with history of alcohol consumption. Twenty-eight patients (70.0%) were treated with Sofosbuvir/Velpatasvir, 12 (30.0%) received Glecaprevir/Pibrentasvir. The therapy cycle was completed by 40 patients (100%). All tested patients presented a negative viral load at the end of treatment and a sustained virologic response was observed in 92.5% (SVR12 + delayed SVR). Therapy was well-tolerated, except in two cases where the patients temporarily discontinued treatment and refused subsequent lab tests. Another patient elected to only be tested for HCV RNA at the end of treatment but refused all post-therapy controls. No significant drug interactions with commonly used psychiatric treatments or side effects were observed. One patient died of an overdose and another of cirrhosis complications following HCV eradication. One reinfection was observed ten months after SVR12.
Research conclusions
In conclusion, targeted anti-HCV programs involving vulnerable infected patients, such as PWIDs, can be effective at improving patient compliance and eradicating infection with good tolerability. However, a larger prospective study is required to definitively confirm the efficacy of this initiative.
Research perspectives
Despite the strength of a real-world setting and prospective design, this study has a major limitation. The findings are the result of intensive and time-consuming work, with the program having been applied to a small population on a local scale thus far. The question then becomes whether this type of model could be scaled up to a larger and more complex field while maintaining reasonable costs and demand for human resources. Based on the encouraging results achieved thus far, our next step will be to apply this multidisciplinary anti-HCV program to a larger PWID population and validate it in a larger-scale real-world setting.
FOOTNOTES
Author contributions:Elettra E and Menotti E shared the first authorship; Pravadelli C, Branz G, and Merola E designed the research and served as guarantors; Menotti E, Seligmann S, and Branz G participated in the data acquisition; Merola E, Pravadelli C, and Pertile R participated in the data analysis; all the authors participated in the interpretation of the data; Merola E, Menotti E, Pravadelli C, Branz G, and Michielan A drafted the initial manuscript; all the authors participated in a critical review of the article’s important intellectual content and approved the final version.
lnstitutional review board statement:In accordance with local legislation, the study protocol was approved by the local ethical committee (N. A785), and informed consent was obtained from patients for data acquisition.
lnformed consent statement:All study participants, or their legal guardian, provided informed written consent prior to study enrollment.
Conflict-of-interest statement:All authors declare that they have no conflict of interest.
Data sharing statement:Participants gave informed consent for data sharing
STROBE statement:The authors have read the STROBE Statement-checklist of items, and the manuscript was prepared and revised according to the STROBE Statement-checklist of items.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:Italy
ORClD number:Elettra Merola 0000-0001-9553-7684; Andrea Michielan 0000-0003-1353-0935; Sonia Seligmann 0000-0002-5045-0013; Flora Agugiaro 0000-0003-4119-6014; Luisa Moser 0000-0003-4929-4030; Giovanni Vettori 0000-0002-6507-9317; Giovanni de Pretis 0000-0001-7636-0422; Cecilia Pravadelli 0000-0003-1392-466X.
S-Editor:Wang LL
L-Editor:A
P-Editor:Wang LL
 World Journal of Hepatology2022年7期
World Journal of Hepatology2022年7期
- World Journal of Hepatology的其它文章
- Retraction Note: Screening and identification of bioactive compounds from citrus against non-structural protein 3 protease of hepatitis C virus genotype 3a by fluorescence resonance energy transfer assay and mass spectrometry
- Challenge of managing hepatitis B virus and hepatitis C virus infections in resource-limited settings
- Gut microbiota contribution to hepatocellular carcinoma manifestation in non-alcoholic steatohepatitis
- “Starry liver” - Von Meyenburg complex clinical case presentation and differential diagnosis discussion: A case report
- Hepatitis B virus markers in hepatitis B surface antigen negative patients with pancreatic cancer: Two case reports
- Volumetric assessment of hepatic grafts using a light detection and ranging system for 3D scanning: Preliminary data
