Traditional Chinese medicine as monotherapy or combined with oseltamivir in the treatment of H1N1 influenza: a systematic review and meta-analysis
Chun-Ping Gu,Dong-Wei Ye,Xin Mao,Shu-Wen Liu*
1 Clinical Pharmacy Center,Nanfang Hospital,Southern Medical University,Guangzhou 510515,China;
2 Guangdong Provincial Key Laboratory of New Drug Screening,School of Pharmaceutical Sciences,Southern Medical University,Guangzhou 510515,China.
Abstract Objective This study aimed to assess the efficacy and safety of traditional Chinese medicine,alone or in combination with oseltamivir,in patients with H1N1 Influenza.Methods In the present study,we searched the Cochrane Central Register of Controlled Trials,PUBMED,EMBASE,Chinese Biomedical Literature Database,China Science and Technology Journal Database,China National Knowledge Infrastructure Database,and WanFang Data for studies published in or before February 8,2022.Data were extracted and checked by two investigators.Review Manager 5.4 and STATA statistical software 16.0 were used for the data analysis.Results We identified 22 individual studies reporting data from 2292 individuals with H1N1 influenza.Compared with oseltamivir,the fever clearance duration[MD=-3.99,95% CI (-6.89,-1.09)] and sore throat relief time [MD=-5.39,95% CI (-10.19,-0.59)] in the intervention group of traditional Chinese medicine monotherapy or combined with oseltamivir were shorter.Maxingshigan was the primary component of Lianhuaqingwen.The subgroup analyses indicated that Maxingshigan shortened fever clearance time [MD=-3.23,95% CI (-5.60,-0.85)],and also had certain advantages in relieving sore throat [RR=-4.55,95% CI (-10.04,0.95)].However, as for the effective rate,fever duration,cough disappearance time,hospital length of stay,clinical symptoms time as well as viral shedding duration,there were no significant differences between the two groups. Besides, no serious adverse effects were reported in the included studies.Conclusion Although we couldn’t get a definitive conclusion due to the small sample sizes and high risk of bias in the included studies,most traditional Chinese medicine showed similar effects to oseltamivir in treating H1N1 influenza.Some were showed to have a statistically significant shorter time of fever clearance and sore throat remission when they were used alone or in combination with oseltamivir and were well-tolerated treatment,such as Maxingshigan.
Keywords: traditional Chinese medicine;maxingshigan;oseltamivir;H1N1 influenza;meta-analysis
Background
Influenza is an acute respiratory illness,with significant morbidity and mortality [1].An estimated 5%-10% of adults and 20%-30% of children worldwide get influenza every year,causing annually about 290,000-650,000 deaths before the severe acute respiratory syndromecoronavirus 2 (SARS-CoV-2) epidemic [2].The influenza syndrome is typically of sudden onset and is characterized by fever,cough and sore throat [3].Children and adolescents with certain underlying medical conditions have a high risk of complications from influenza [4-7].For the 2020-2021 influenza season,it will be particularly important to understand the effect of SARS-CoV-2 and influenza virus cocirculation on the epidemiology and morbidity of influenza [8-10].Influenza viruses are RNA viruses,including influenza A,B and C.Currently,influenza A/H1N1,A/H3N2,and influenza type B viruses are circulating in humans.Previous study reported that H1N1 virus can cause severe illness [11].
Influenza vaccination is an important intervention to protect vulnerable populations and reduce the burden of respiratory illnesses.Oral oseltamivir remains the antiviral drug of choice for the management of illness caused by influenza virus infections [9].Oseltamivir is available in capsule and oral suspension formulations.It should be taken within 48 hours after the onset of symptoms.However,the Influenza Resistance Information Study found an association between prolonged use of oseltamivir due to persistent viral shedding and subsequent development of oseltamivir resistance [12,13].In adverse event data collected systematically in prospective trials,vomiting was the only adverse effect reported more often with oseltamivir.In addition,clinicians are also concerned about oseltamivir-attributable neuropsychiatric adverse effects [14,15].
In China,traditional Chinese medicine (TCM) has been used against influenza for thousands of years.Furthermore,TCM has widespread use outside of China by both emigrants and an increasing number of Western patients.When the H1N1 was reported in Mexico and the United States in April 2009,the Chinese government released three editions of a document entitled “Recommended Schemes for Pandemic Influenza A Diagnoses and Treatments” [16].Except for oseltamivir,TCM prescriptions were recommended for treating H1N1 infection in the third edition [16].According to the TCM theory,influenza is differentiated into two types: Windcold Syndrome and Wind-heat Syndrome [17].Lianhuaqingwen (LH) has been widely used in treating influenza and shown a broad spectrum of effects on a series of influenza viruses,including H1N1 virus [18].LH comes from two prescriptions,Maxingshigan and Yinqiaosan,and the main effective components have been identified in recent years [19].Maxingshigan decoction is composed ofEphedraceae Herba,Armeniaca vulgarisLam,Gypsum FibrosumandGlycyrrhiza glabraL.It has multiple functions of antiviral,anti-inflammatory,immune regulation as well as fever reduction [20].
Currently,there are only three meta-analyses discussing applications and efficacy and safety of TCM in treating H1N1 influenza [21-23].One of the meta-analyses aimed to evaluate the efficacy and safety of TCM in the treatment of H1N1 infection [21].The another one reviewed the results of studies on the efficacy of LH capsule compared with oseltamivir in treating H1N1 influenza [22].The third one was a systematic review on potential benefits and harms of Chinese herbal medicines for the treatment of Type A H1N1 influenza [23].However,there have been no comprehensive meta-analyses of TCM or Maxingshigan compared with oseltamivir for the treatment of H1N1 influenza.Thus,the objective of this review and meta-analysis was to evaluate and update the efficacy and safety of TCM with or without oseltamivir,in alleviating symptoms caused by H1N1 influenza.
Methods
Protocol registration
This systematic review protocol has been registered in the PROSPERO network (No.CRD42021253876).Although this was a review of published literature,ethics committee approval was also obtained from the Nanfang hospital’s ethics committee (No.NFEC-2021-123).
We searched the following databases from the date of inception until February 8,2022: Cochrane Central Register of Controlled Trials,PUBMED,EMBASE,Chinese Biomedical Literature Database,China Science and Technology Journal Database,China National Knowledge Infrastructure Database,and WanFang Data.We used the following search strategy: ‘H1N1 Influenza’AND ‘traditional Chinese medicine’ AND ‘Oseltamivir’NOT (animals[mh] NOT humans[mh]) AND ‘randomized controlled trial’.We did not use any publication or language restrictions.
Participants
This study included patients who had been clearly diagnosed with H1N1 influenza.There were no strict restrictions on gender and severity of the disease.
Intervention
The experiment group uses TCM alone or combined with oseltamivir.TCM includes Lianhuaqingwen capsules or granules,Maxingshigan decoction,modified Maxingshigan decoction,Maxingshigan-Yingqiaosan decoction,and so on.The control group can be treated with oseltamivir.There are no obvious restrictions on the dosage of therapeutic drugs and specific intervention routes.
Primary outcomes
The primary outcome measures (efficacy) were the length of time from the start of medication to resolution of influenza symptoms (fever,sore throat,cough),effective rate,clinical symptoms time,and viral shedding duration.
Secondary outcomes
The secondary outcome measures were the side effects and the adverse reactions,such as nausea,vomiting,diarrhea,rash,discontinued treatment and developed pneumonia.
Studies selection
The titles,abstracts and keywords of every record retrieved were scanned,and the full articles were located for further assessment when the information given suggested that the study: (1) included patients with uncomplicated H1N1 influenza;(2) compared Chinese medicinal herbs with placebo or other active drugs;(3) assessed one or more relevant clinical outcome measure.Review articles,case studies,basic research,short reports,or comments were excluded in this study.
Data collection and analysis
Two authors (Gu CP and Mao X) independently extracted,checked and made a review table of the articles.The extracted data comprised the (1) authors,(2) year of publication,(3) details of control interventions,(4)outcomes.For binary outcomes,we extracted the number of events and total number in each group.For continuous outcomes,we extracted the mean,standard deviation and sample size from each group.
The reporting quality of each trial was assessed by Review Manager 5.4,based largely on the quality criteria specified by Cochrane Handbook for Systematic Reviews of Interventions [24].The authors resolved any differences of opinion by discussion.We performed a meta-analysis when data for a given outcome were available in more than two of the included studies.The unit of analysis was the individual participants.The present meta-analysis was performed using STATA statistical software 16.0.
The heterogeneity was examined through visual inspections of forest plots,a standard χ2test,and the I2statistic.To combine studies,we used the fixed-effects model when the studies in the subgroup were sufficiently similar (P> 0.10,I2statistic<50%).Heterogeneity was to be tested for using theI2statistic with significance being set atP<0.10.When heterogeneity was suggested,a random-effects model was used to analyze whether heterogeneity may have led to the different effects among studies.The risk ratio (RR) and its 95% confidence interval(CI) are used as the index of discontinuous data,and the weighted men difference (WMD) and its 95% CI are used as the index of continuous data.
Results
Literature search results
In this updated review,290 studies were found in the 7 electronic databases.A total of 238 articles were initially included after duplicate publications were excluded,and 169 records were removed because they were (1) review articles,(2) case studies,(3) basic research,(4) short reports,(5) comments.We further excluded 36 literatures due to the following reasons: (1) oseltamivir was not taken as the control group or TCM monotherapy or combined with oseltamivir was not taken as the treatment group (n=6);(2) the clinical trials with small sample size (n=30).Ultimately,22 eligible studies were included in this review(Figure 1).
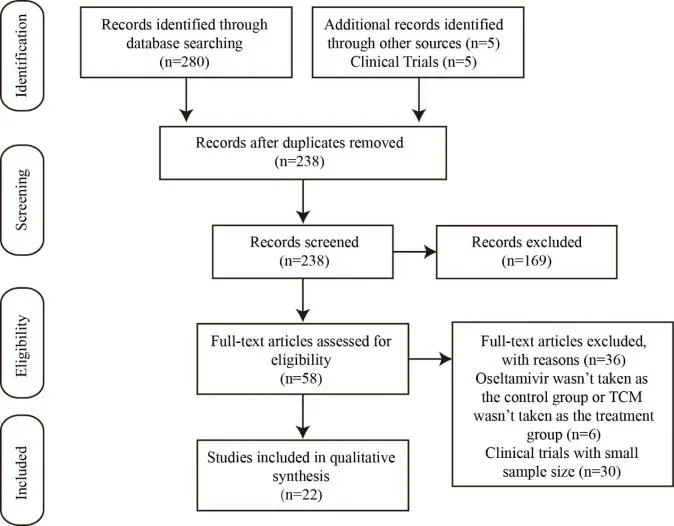
Figure 1.The specific process of literature screening.A total of 290 articles were retrieved,and 22 studies were selected.
Basic characteristics of the included literature
A total of 2292 participants were included in these 22 trials[25-46],with numbers of patients in each trial varying from 33 to 307.All trials enrolled patients with H1N1 influenza alone.Details of the included studies are shown in Table 1.
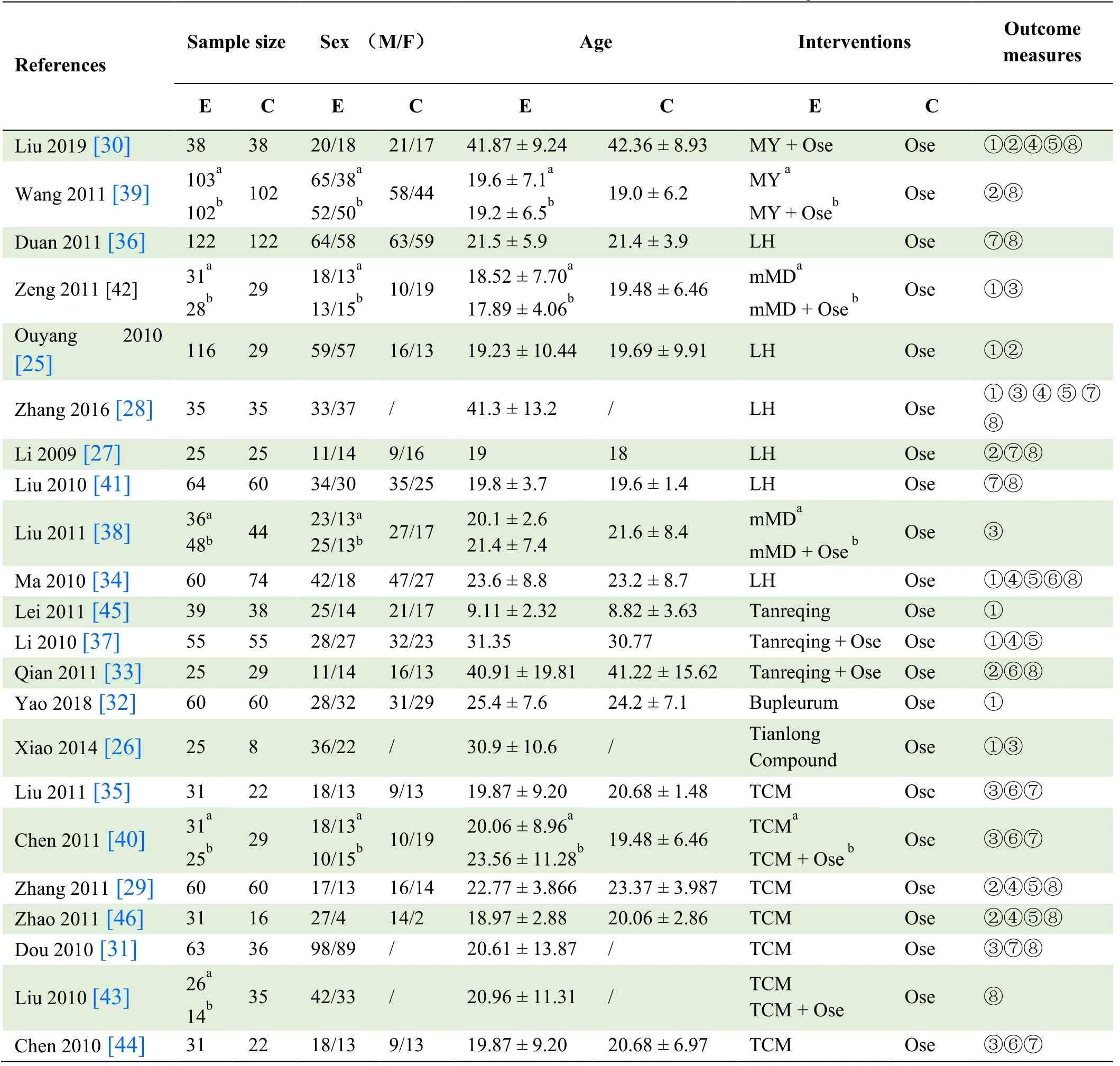
Table 1.Basic characteristics of included trials and subjects
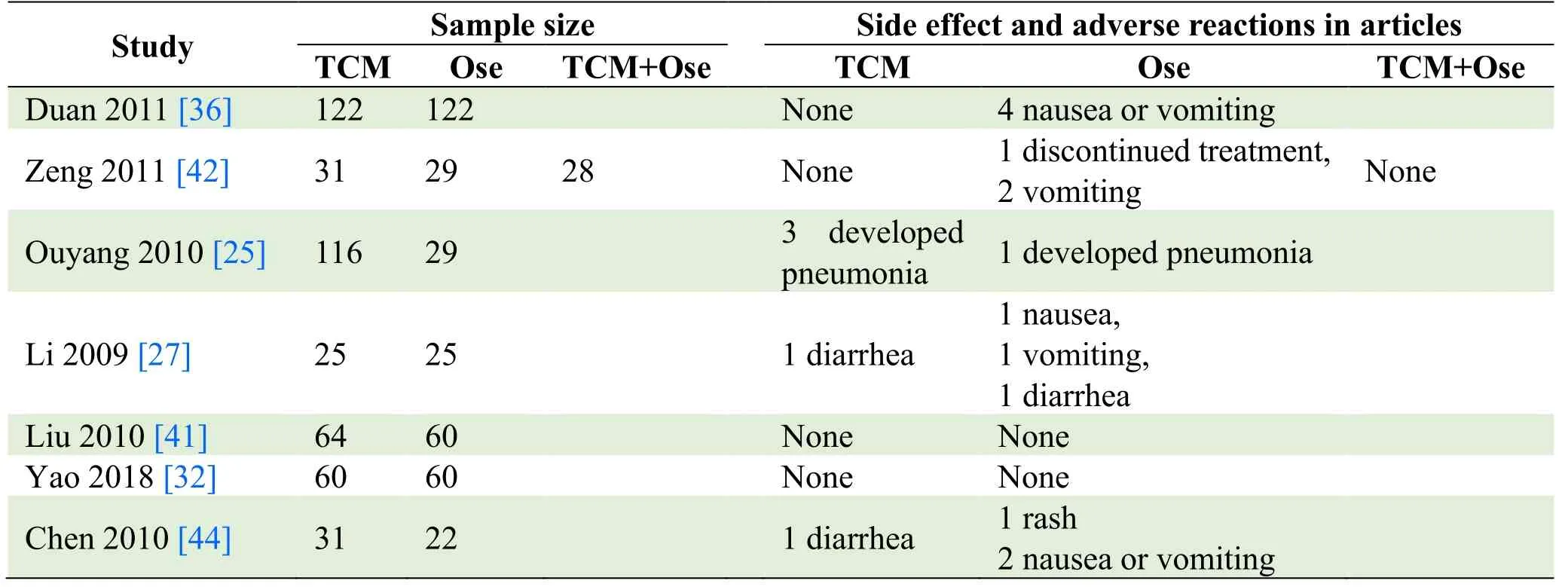
Table 2.Side effects and adverse reactions
Risk of bias in included studies
The quality of included studies was evaluated by Cochrane risk of bias tool.The results of the quality assessment for each of the included studies are presented in Figure 2 and 3.We deemed 2 studies to be at low risk of bias [36,39].We revealed that 6 trials described the adequate random sequence generation [35,36,39,40,42,44],but only 1 trial provided the methods used for allocation concealment[39].Twelve trials reported adequate blinding of participants and personnel and blinding of outcome assessment [26,29,31,32,35,40,42-44,46].Most studies dealt with incomplete outcome data adequately.However,we judged one trial to be at high risk of bias due to participant Withdrawal [42].Most trials did not publish a protocol paper and thus were considered to be at unclear risk of bias for selective reporting,with the exception of two studies [36,39].We rated one study to be at low risk of bias regarding other biases [39].Overall,the quality of the included RCTs was low.
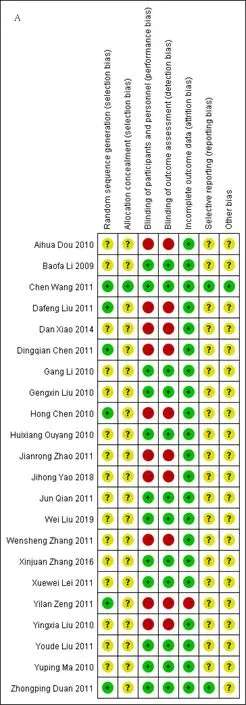
Figure 2.Risk of bias summary.
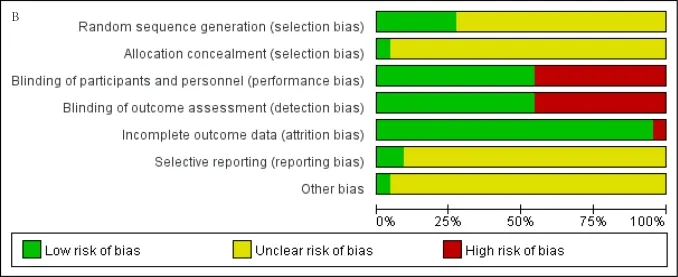
Figure 3.Risk of bias graph.
Meta-analysis
Trials showing statistical significances between the interventionandcomparison.(1) Time to defervescence,Eight studies compared the time to defervescence [27,29,30,33,34,36,39,41],including 1049 patients.There was heterogeneity among the studies by comparing the defervescence time (P=0.00<0.05,I2=99.73%) with the random-effects model used.The time to defervescence was significantly shorter in patients administered TCM plus oseltamivir or those administered TCM alone [MD=-3.99,95% CI (-6.89,-1.09),P=0.00<0.05],as shown in Figure 4A.Results of subgroup analyses of the TCM components for the fever clearance time were shown in Figure 4B.When the TCM included Maxingshigan,it can shorten the defervescence time compared with oseltamivir [MD=-3.23,95% CI (-5.60,-0.85),P=0.00<0.05].The heterogeneity was significant(P=0.00<0.05,I2=99.42%),and we chose a randomeffects model for this outcome (Figure 4B).More importantly,patients received Maxingshigan plus oseltamivir tended to have shorter defervescence duration[MD=-5.76,95% CI (-8.30,-3.22),P=0.19].(2) Time to sore throat relief,Nine studies demonstrated the time to sore throat relief from 866 individuals [27-30,34,36,37,41,46],and found a significantly shorter time to sore throat relief [MD=-5.39,95% CI (-10.19,-0.59),P=0.00<0.05] in TCM plus oseltamivir or TCM monotherapy(Figure 5A).There was heterogeneity among the studies(P=0.00<0.05,I2=99.78%),and the random effect model was used.
In the Maxingshigan subgroup,the time to sore throat relief tended to be shorter in patients treated with Maxingshigan plus oseltamivir or Maxingshigan alone.[RR=-4.55,95% CI (-10.04,0.95),P=0.00<0.05],as shown in Figure 5B.
Trials showing no statistically significant differences between the intervention and comparison.Eleven studies [25,26,28-30,32,34,37,40,42,45] demonstrated the effective rate between patients received TCM alone or in combination with oseltamivir and those received oseltamivir alone.The outcome indicated the difference wasn’t statistically significant [RR=1.02,95% CI (0.93,1.13),P=1.00] (Figure 6A).The heterogeneity wasn’t significant (P=1.00,I2=0.00%),and we chose a fixedeffects model.Besides,as for the fever duration [26,28,35,37,38,42] [MD=0.02,95% CI (-2.03,2.08),P=0.00<0.05],cough disappearance time [27-30,34,36,37,41,46] [MD=-2.42,95% CI (-5.26,0.42),P=0.00<0.05],hospital length of stay [29,33-36,39,40,42,44,46] [MD=-0.01,95% CI (-0.08,0.06),P=0.52],clinical symptoms time [28,31,35,39,40,42,44,45] [MD=-0.13,95% CI (-1.66,1.40),P=0.00<0.05] and viral shedding duration [27-31,33,34,36,41,42,46] [MD=-0.28,95% CI (-0.75,0.20),P=0.00<0.05],there were no significant differences between patients treated with TCM alone or TCM plus oseltamivir and those received oseltamivir monotherapy,as shown in Figure S1A-S5A.
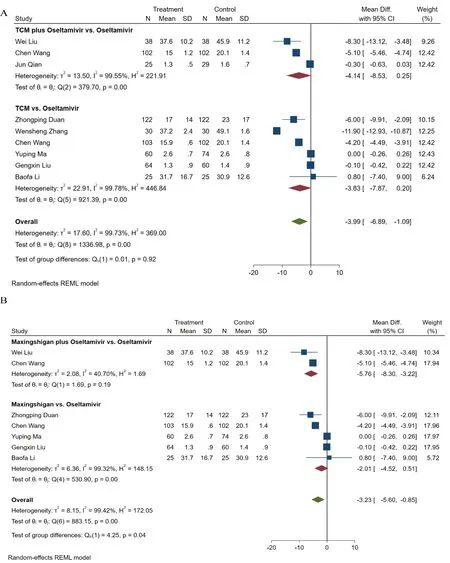
Figure 4.Meta-analysis of time to defervescence.(A) TCM alone or combined with oseltamivir versus oseltamivir,(B)Maxingshigan alone or combined with oseltamivir versus oseltamivir.
As a subgroup analysis,we divided the studies according to the type of the TCM (Maxingshigan and Tanreqing).This subgroup analysis did not significantly alter the combined results.Meta-analyses revealed that Maxingshigan [RR=1.02,95% CI (0.88,1.18),P=0.97](Figure 6B) and Tanreqing [RR=1.03,95% CI (0.83,1.29),P=0.77] (Figure 6C) did not display any differences in the effective rate.In addition,the fever duration [MD=0.59,95% CI (-1.57,2.76),P=0.00<0.05],cough disappearance time [MD=-3.20,95% CI (-7.58,1.19),P=0.00<0.05],hospital length of stay [MD=-0.02,95%CI (-0.09,0.05),P=0.88],clinical symptoms time [MD=1.26,95% CI (-0.64,3.15),P=0.00<0.05] as well as viral shedding duration [MD=-0.18,95% CI (-0.57,0.20),P=0.01] weren’t significantly different between the two groups (Figure S1B-S5B).
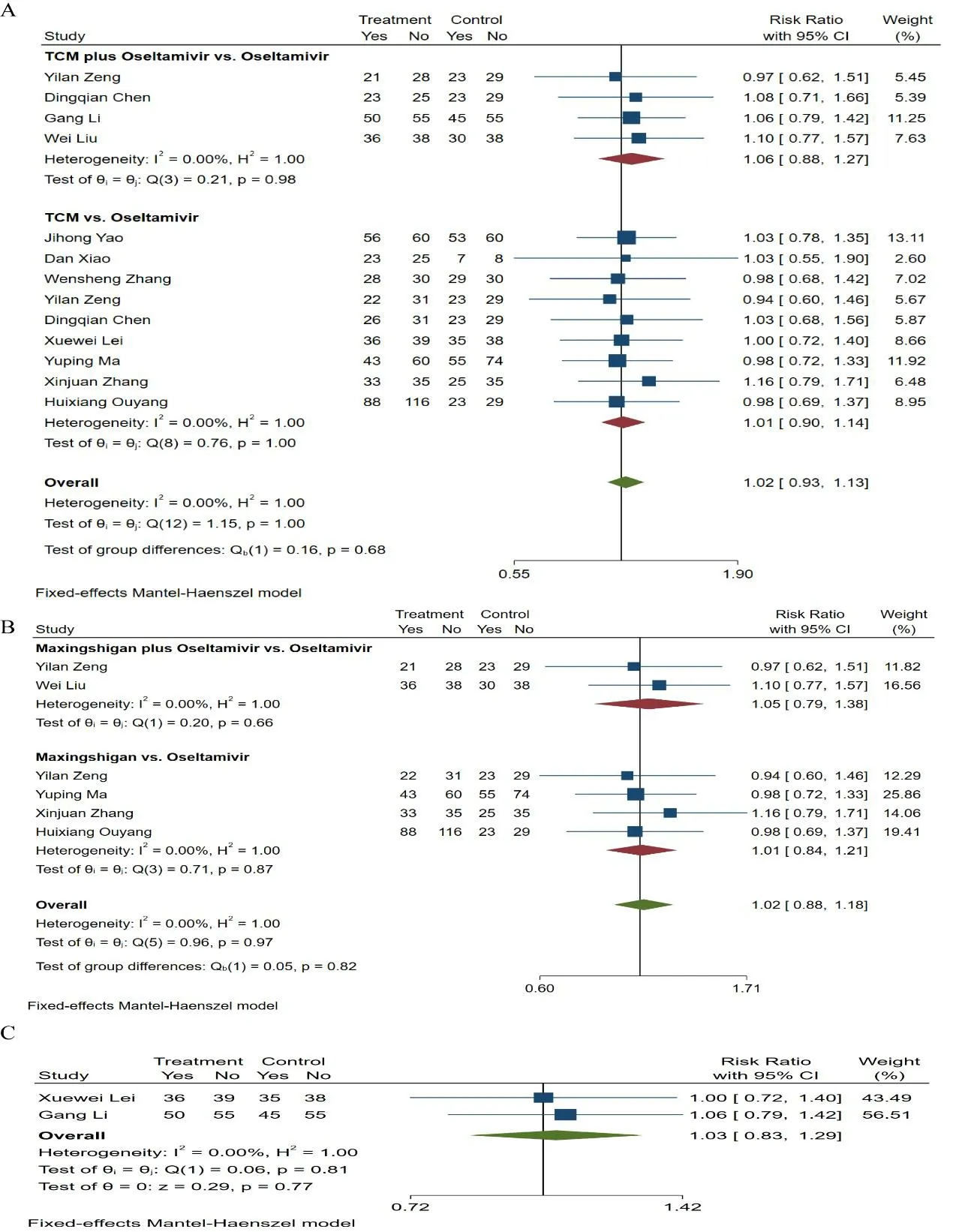
Figure 6.Meta-analysis of effective rate.(A) TCM alone or combined with oseltamivir versus oseltamivir,(B)Maxingshigan alone or combined with oseltamivir versus oseltamivir,(C) Tanreqing alone or combined with oseltamivir versus oseltamivir.
Safety of the interventions
In this review,7 trials reported adverse reactions [25,27,32,36,41,42,44].All reactions were mild,and no severe adverse effects were identified.Among them,2 studies identified no adverse effect in both TCM and oseltamivir groups [32,41],and oseltamivir was discontinued in 1 study [42].Adverse effects in the rest 4 trials included gastrointestinal reactions (diarrhea,nausea,vomiting),pneumonia,and rash [25,27,36,44].None of the studies reported mortality,as shown in Table 2.
Discussion
Although we could not reach a reliable conclusion because the included studies had a high risk of bias,this metaanalysis suggested that TCM may shorten the duration of fever clearance and sore throat relief when used alone or in combination with oseltamivir and that TCM may be a well-tolerated approach for alleviating H1N1 influenza symptoms.In the subgroup analysis,the resolution of fever and sore throat was faster in patients received Maxingshigan monotherapy or combined with oseltamivir.Nonetheless,the differences in the effective rate,fever duration,cough disappearance time,hospital length of stay,clinical symptoms time and viral shedding duration weren’t statistically significant.Furthermore,it should be noted that the quality of evidence for this conclusion was low.In the future,more well-designed trials will be required to evaluate the efficacy and safety of TCM for H1N1 influenza.
The included studies were generally of low quality for a variety of reasons.First,only two studies were characterized as high quality [36,39].The common weaknesses of the RCTs are “Blinding of participants and personnel” and “Uncertainty in blinding of outcome assessment”.Second,most of trials were reported as double-blinded,but the methods for blinding were poorly described.Indeed,it would be difficult to find appropriate placebos for TCM that had a similar color and taste [47].Third,the included studies had small sample sizes.Of the 22 included studies,2 trials had a sample size of fewer than 50 individuals [26,46].The imprecision due to the small number of events is a major limitation that hampers the generalizability of these results.
One known systematic review assessed the efficacy and safety of TCM for the treatment of H1N1 influenza [21].The results showed that the mean time to defervescence and viral shedding in the TCM treatment group was significantly shortened than noted in the control group,and no obvious adverse events were reported in the included studies.The other meta-analysis concluded that LH capsule was superior to oseltamivir in improving the symptoms of H1N1 influenza infection [22].As a TCM prescription,LH exerted broad-spectrum effects on a variety of influenza viruses,such as H1N1,and particularly regulated the immune response of virus infection [18].In summary,previous studies indicated that treatment of some Chinese herbs may have beneficial immunomodulatory effects for rapid recovery from H1N1 viral infections [48,49].Consistent with previous systematic reviews of TCM for H1N1 influenza [21-23],we found that the time to defevescence is lower in the TCM treatment.Previous study has consistently demonstrated that Lianhuaqingwen was superior to oseltamivir in improving the symptoms of influenza A virus infection[22].Nevertheless,this study differs from those three reviews [21-23] in two aspects.Firstly,we systematically compared the duration of sore throat relief and found that TCM treatment had significantly advantages in relieving sore throat.Secondly,we conducted subgroup analyses in Maxingshigan treatment that time to fever clearance and sore throat relief were shorter in patients treated Maxingshigan with or without oseltamivir.
Patients with influenza typically experience most common symptoms including fever,chills,headache,myalgias,cough,nasal congestion,sore throat and fatigue.A recent observational study in Chinese patients showed that the most common symptoms were fever (in 67.4% of the patients),cough (69.5%),and sore throat [50].This meta-analysis revealed that TCM treatment could significantly shorten the time to defervescence and remarkably evaluate sore throat,compared with oseltamivir.These results suggested that TCM has potential effects for accelerated recovery resulting from infection.As one of the primary components of LH,Maxingshigan can improve clinical symptoms (fever,sore throat,headache,etc.) caused by influenza [51].Animal pharmacological experiments have shown that the thrombin and Toll-like receptor (TLR) signaling pathway were suggested to be essential pathways for Maxingshigan decoction mediated anti-inflammatory effects [52].Interestingly,this study suggested that Maxingshigan decoction,the core component of whole recipe may have a major contribution to the overall efficacy of Lianhuaqingwen to prevent the H1N1 influenza pandemic.However,there are many possible limitations in this study,such as the intervention choice.Except for Maxingshigan decoction or modified Maxingshigan decoction,there are many different TCM prescriptions in the treatment of H1N1 influenza.Due to the limited number of eligible clinical trials for meta-analyses,we did not include other TCM prescriptions for evaluating the TCM as monotherapy or combined with oseltamivir in the treatment of H1N1 influenza.
Also,our study found that there was no significant difference on hospital length of stay,which is an important quality metric for total influenza infection.Many researchers observed that oseltamivir was associated with shorter length of stay in patients admitted with influenza[53,54].Since effective compounds in TCM were found to have potential neuraminidase (NA) inhibitory activity,we assessed the impact of TCM therapy on length of stay in hospitalized patients with H1N1 influenza.Prolonged hospital length of stay is one of factors which predispose patients to severe respiratory tract infections.We found that patients treated with TCM monotherapy or combined with oseltamivir did not undergo a prolonged length of stay.
Conclusion
The current evidence is too weak to draw a conclusion which supports or rejects the use of any Chinese medicinal herbs for treating H1N1 influenza.The results of welldesigned RCTs with large sample sizes in the future may confirm or refute our conclusions.In general,most Chinese medical herbs or combined with oseltamivir in the included studies showed similar effects to oseltamivir in treating H1N1 influenza.Some were showed to have a statistically significant shorter time to fever clearance and remission the sore throat than oseltamivir,such as Maxingshigan.
Data availability or Code availability
Supplementary data are available online at TMR Modern Herbal Medicine.
Abbreviations
CBM,Chinese Biomedical Literature Database;CCRCT,Cochrane Central Register of Controlled Trials;CNKI,China National Knowledge Infrastructure Database;CI,confidence interval;CSTJ,China Science and Technology Journal Database;LH,Lianhuaqingwen;NA,neuraminidase;RANTES,regulated upon activation,normal T Cell expressed and presumably secreted;RCT,randomized controlled trial;RR,risk ratio;SARS-CoV-2,severe acute respiratory syndrome-coronavirus 2;TCM,traditional Chinese medicine;WMD,weighted mean difference.
Acknowledgments
This work was supported by the National Administration of Traditional Chinese Medicine Project (2019XZZXLG04) and Chinese Academy of Traditional Chinese Medicine Project (ZZ13-035-02) to S.L.
Author contributions
Gu CP contributed to the data extraction,statistical analysis and manuscript writing.Ye DW and Mao X evaluated the methodological quality of each trial.Liu SW contributed to the study design and manuscript writing.
Competing interests
The authors have declared that there is no conflict of interest.
 TMR Modern Herbal Medicine2022年3期
TMR Modern Herbal Medicine2022年3期
- TMR Modern Herbal Medicine的其它文章
- The genus Strobilanthes: phytochemistry and pharmacology
- Niaoduqing granules inhibits TGF-β1-induced epithelial-mesenchymal transition in human renal tubular epithelial HK-2 cells
- Standardization and Quality control parameters of Kayam Churna
- Effects of Euphorbiasteroid on gene expression in lung cancer cells based on gene chip
- A review of the ethnobotanical value,phytochemistry,and pharmacology of Physalis pubescens L.
