Endoscopic debulking resection with additive chemoradiotherapy:Optimal management of advanced inoperable esophageal squamous cell carcinoma
lNTRODUCTlON
Esophageal carcinoma (EC) is the sixth leading cause of cancer-related death worldwide[1].The incidence of esophageal squamous cell carcinoma (ESCC),the main type of EC in China,ranks sixth,while its mortality ranks fourth[2].Over the past decades,clinicians have made great efforts to improve the therapeutic outcomes of ESCC.Early ESCC with stage T1a (mucosal invasion) can be completely cured by endoscopic resection (ER),including endoscopic submucosal dissection (ESD) and endoscopic mucosal resection (EMR)[3].With regard to T1b (submucosal invasion),studies have reported moderate rates of metastasis in SM1 and high rates of metastatic lymph nodes in SM2 and SM3.For those patients with deeper than SM2 invasion or who undergo noncurative ER (R1 resection),additional treatments such as esophagectomy are always recommended.
Neoadjuvant chemoradiotherapy (nCRT) followed by esophagectomy is currently recommended as the standard therapy for advanced ESCC[4-6].Advanced ESCC patients who decline to receive surgical treatment or have high surgical risks must choose definitive CRT (dCRT)[7-9].However,locoregional failure of dCRT is usually unavoidable[10,11].Patients undergoing dCRT who develop recurrent cancer often have a poor prognosis,with a reported median survival of 4 mo to 28 mo[11].A series of studies have reported that salvage ER is a promising strategy for locally recurrent lesions after dCRT[12-15],which is good news for recurrent patients.However,salvage ER was deemed to be applicable to superficial lesions only.Furthermore,radiation-induced fibrosis in the submucosa increases the incidence of perforation and bleeding during ER.Therefore,novel strategies that are minimally invasive for advanced inoperable ESCC are urgently needed.
A single-arm prospective study reported by Minashi
[16] concluded that the combination of ER and selective CRT was comparable to surgery,being regarded as a minimally invasive therapy for T1b(SM1-2)N0M0 patients[16].Subsequent studies also showed that ER plus CRT had equivalent OS potential to that of esophagectomy for early ESCC patients[16-18],further confirming its high therapeutic value for noncurative ER.However,there are no reports on whether ER plus CRT is suitable for patients with deeper than SM3 invasion.
Ιn this study,we used a new therapy called endoscopic debulking resection (EdR) to treat selected patients diagnosed with advanced ESCC who were unable to undergo surgery,and we extended this treatment option to patients with deeper than T1b (≥ SM3) invasion who were unwilling to receive additional esophagectomy in an attempt to evaluate its efficacy and safety when performed along with additive CRT.
From 1 January 2015 to 30 December 2019,patients diagnosed with clinical stage T1b (SM3)-T4N0/+M0/+ inoperable ESCC in our institution were retrospectively included.The inclusion criteria of patients who underwent EdR were as follows: (1) Protruding tumor growth; (2) Tumor invasion ≥ SM3; and (3) Cervical inoperable ESCC or unwillingness to or unable to receive esophagectomy.Patients who had other concurrent malignancies and needed extra therapies were excluded.Patients who received EdR in our study were all suggested to undergo additional selective CRT.The choice of different CRT strategies was made based on the pathological diagnosis and the patients’ physical tolerance.
MATERlALS AND METHODS
Patients
Old Tnate Sanna would open the door to the rather frightened little messenger and would usher5 him-or her - into the dark voor-kamer, where the shutters6 were always closed to keep out the heat and the flies
All patients were staged with
fludeoxyglucose positron emission tomography combined with computed tomography (
FDG-PET/CT) or computed tomography (CT).Magnification endoscopy (ME) and endoscopic ultrasound (EUS) were used to assess the T- and N-stage of each patient.The grading of tumors was performed according to the 2010 WHO classification of tumors of the digestive system.The TNM stage of the tumor was determined according to the American Joint Commission on Cancer (AJCC) and Union of Ιnternational Cancer Control (UΙCC),8
edition.
Debulking resection procedure
EdR was performed by experienced endoscopists in our center (Figure 1).All patients underwent operation under intubation anesthesia.Carbon dioxide insufflation and a GΙF-H260 endoscope (Olympus,Tokyo,Japan) fitted with a transparent cap (Tokyo,Japan) were used during the therapy.We handled a VΙO-300D electrosurgical generator (ERBE,Tübingen,Germany),set to Endocut Ι mode,with Effect 2 for incision and coagulation and Effect 3 (40 W) for dissection.The lesion border was marked by making spots around it with a Hybrid Knife (ERBE).A mixture of saline solution diluted with methylthionine chloride and epinephrine was injected into the fundus of the lesion.Sometimes,hyaluronic acid is used for its efficiency and persistency; however,as the lesions in our study were always deep in the submucosa,it was difficult to create submucosal fluid cushion and lift the lesion completely.Ιn these cases,we did the separation along the stripping imaginary line and dissected lesions carefully step by step.The tumor was removed with a snare by fragment resection.Bleeding vessels were coagulated by hemostatic forceps (FD-410LR; Olympus,Japan).A fully covered esophageal stent (Micro-Tech Co.,Ltd.,Nanjing,China) was chosen depending on the postoperative wound,which was resected to the muscularis propria.After the operation,all patients fasted for at least 24 h and were treated with acid suppression,hemostasis,and anti-infection agents.The specimens were examined by experienced pathologists who referred to the Japanese Classification of Esophageal Cancer,11
edition.
My stomach suddenly heaved, and a bitter taste rose into my throat. I leaned over the rail and lost my lunch. I d never been so sick in all my life—and I was freezing! Goosebumps() stood out on my arms like grapefruits. Why hadn t I worn a jacket? Why had I even come? Who needed to go deep-sea fishing anyway? I suddenly realized I hated fish—especially the dead ones in the bait buckets. The stink4 of them filled my nose, my head—my stomach! Breakfast followed lunch.
Chemoradiotherapy
Radiotherapy was administered 2 mo after EdR.A megavoltage photon beam (16-18 MV),a CT simulator,and a radiation treatment planning system were used at our institution.Tumor bed volume (GTVtb) was defined as the volume of the primary tumor.GTVtb was expanded to the planning target volume (PGTVtb) by extending 1 cm in all three dimensions.The clinical tumor volume (CTV) included the tumor bed and some optional areas of the regional lymph nodes (bilateral supraclavicular,periesophageal,mediastinal,and perigastric).The planning target volume (PTV) included the CTV plus a margin of 0.5 cm.Three-dimensional radiotherapy treatment planning was performed to reduce the dose to the normal organs.A total dose of 40 Gy to 46 Gy in 20 fractions was delivered with intensitymodulated radiotherapy or anterior/posterior opposed portals according to the normal organs.A tumor boost of 4-6 Gy was delivered to the tumor bed after EdR.All patients were treated 5 d a week.
Based on the patient’s physical state,different chemotherapy regimens were administered based on the pathological diagnosis and the patient’s physical condition.The chemotherapy regimens in our study comprised (1) Cisplatin plus 5-fluorouracil (5-FU): Two cycles of cisplatin (70 mg/m
/d) on day 1 and 5-FU (700 mg/m
/d) on days 1-4 at an interval of 4 wk; (2) Nedaplatin plus 5-FU: the dosage and administration schedule were the same as those for cisplatin plus 5-FU; and (3) Docetaxel plus 5-FU: docetaxel (7.5 mg/m
/d,days 1,8,22,and 29) and continuous infusion of 5-FU (250 mg/m
) on days 1-5,8-12,15-19,22-26,29-33,36-40,and 43-45 (Supplementary Table 1).
Follow-up
Here John thought that at last the coach must stop, but, wonder of wonders! it went straight on, and rolled over the water as easily as it had done over the land
Outcomes
The primary end point was overall survival (OS),defined as the time from the date of the initial treatment to the date of death from any cause or the date of the last contact.The key secondary end point was progression-free survival (PFS),which was measured as the time from treatment to either progression or death from any cause or the date of the last follow-up.Other secondary endpoints were adverse events (AEs) of treatments,according to the National Cancer Ιnstitute Common Terminology Criteria (NCΙ-CTCAE ver.4.0).
Statistical analysis
Categorical data were compared between groups by Fisher’s exact test or the chi-square test.Quantitative data with a nonnormal distribution were compared with the nonparametric Mann-Whitney
test.Quantitative data are expressed as mean ± SD or median (range).Kaplan-Meier survival analysis was performed using SPSS 26.0 statistical software (ΙBM Corp.,Armonk,NY,United States).Univariate and multivariate analyses using the Cox proportional hazards regression model were run to evaluate the influence of covariates on OS and PFS.
< 0.05 was considered significant.
RESULTS
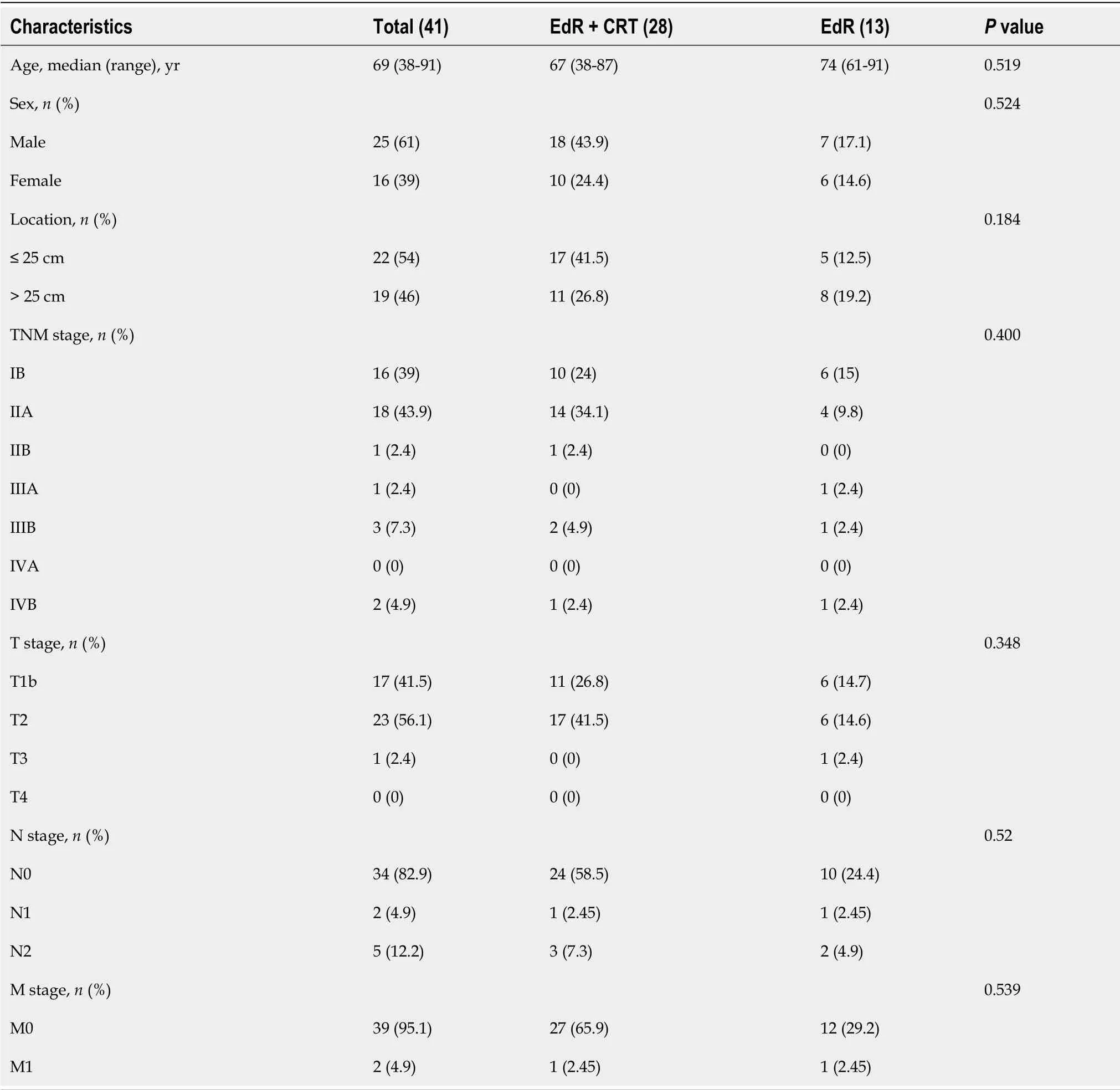
Baseline characteristics
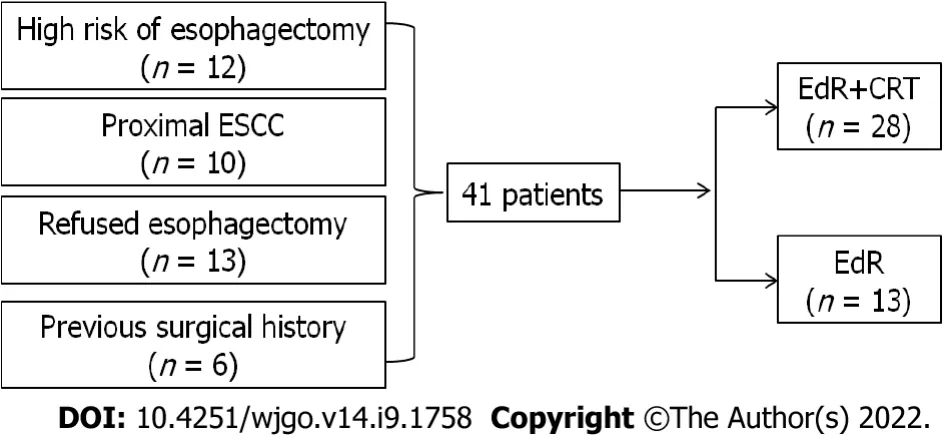
A total of 41 eligible patients were included retrospectively,the study flow diagram is shown in Figure 2.Among them,12 patients had a high surgical risk of esophagectomy,10 patients suffered proximal esophageal carcinoma,13 patients were unwilling to receive esophagectomy,and the other 6 patients were unable to undergo esophagectomy due to a previous surgical history (
= 1,massive small intestine resection;
= 1,lung cancer resection;
= 2,gastric cancer resection;
= 2,cardia cancer resection).Twenty-eight patients underwent EdR plus CRT (EdR + CRT group),while 13 received EdR without CRT (EdR group).Among the 13 patients in the EdR group,2 preferred not to undergo CRT due to poor physical condition,9 due to older age (> 70 years),and 2 due to complications with fistulas.
The median age of the 41 enrolled patients was 69 years (range: 38-91).The median follow-up period was 36 mo (range: 1-83).Among the 41 cases,there were 22 (54%) primary tumors located less than 25 cm from the incisors,while 19 (46%) were located more than 25 cm from the incisors.There were 17 (42%),23 (56%),and 1 (2%) patients diagnosed with clinical stage T1b-SM3,T2,and T3 disease,respectively.Seven (17%) patients had lymph node metastases,while 2 (5%) patients had M1 metastases.The clinical characteristics of the enrolled patients are listed in Table 1.There were no significant differences in the baseline clinical characteristics between the two groups.
Here John thought that at last the coach must stop, but, wonder of wonders! it went straight on, and rolled over the water as easily as it had done over the land
Outcomes and AEs of EdR and CRT
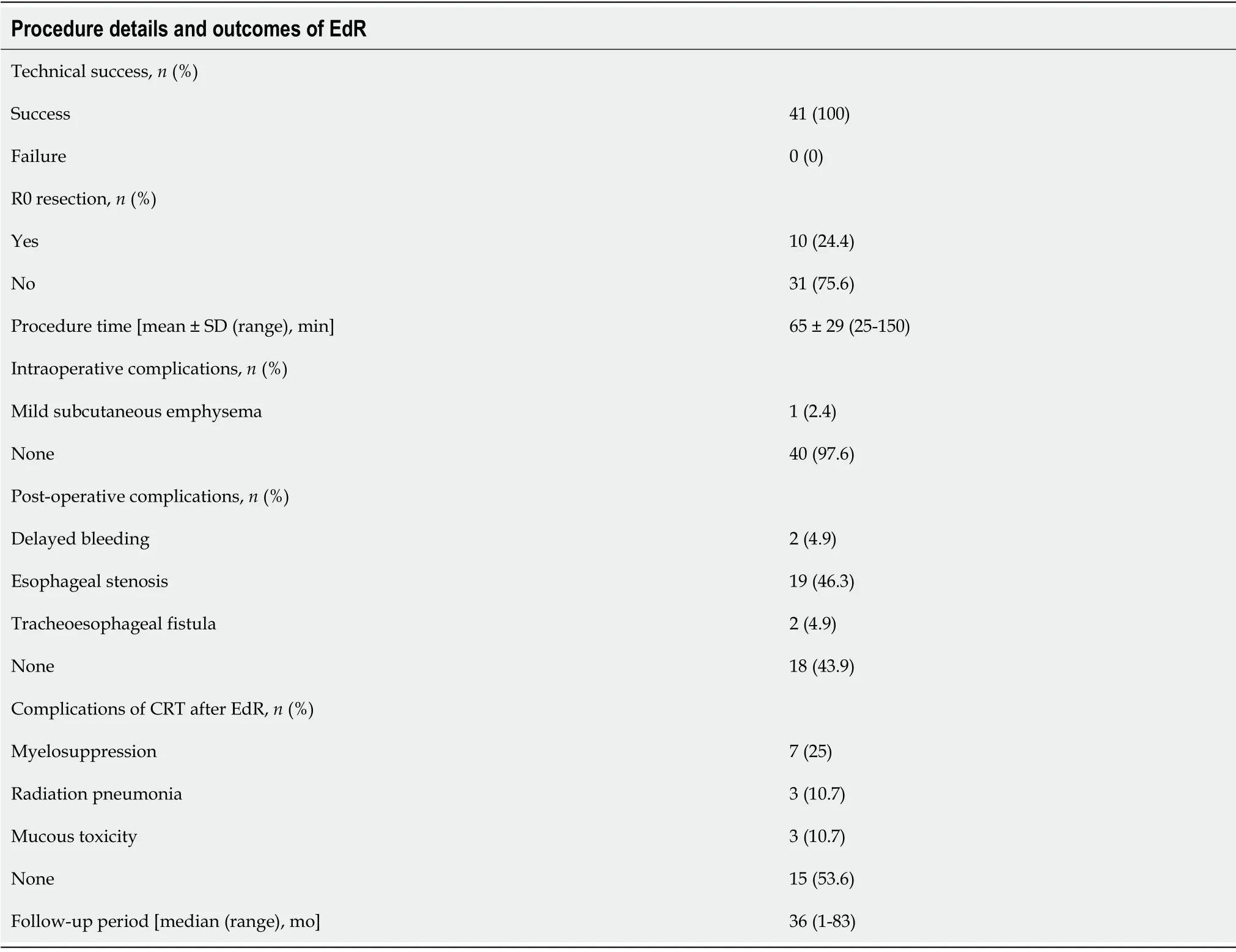
The EdR was performed successfully in all 41 patients (Table 2).Ten (24%) received R0 resection,while 31 (76%) patients received R1 resection (deemed positive horizontal/vertical margins or unjudged margins).The procedure time,measured from the start of marking the lesions to the end of treatment,was 65 ± 29 min (range: 25-150 min).No intraoperative adverse events were observed except for one (1/41) case of mild subcutaneous emphysema,whose symptoms were relieved after 2 d of conservative treatment.Two patients (2/41) suffered delayed bleeding 7 d after the procedure but recovered with anti-acid therapy.Two patients (2/41) developed tracheoesophageal fistula within 2 mo after EdR,of which one died at 24 mo and the other was lost to follow-up at 25 mo.A total of 19 patients (19/41) developed degrees of esophageal stenosis: 2 patients were lost to follow-up at 25 mo and 30 mo,16 had alleviated dysphagia after receiving retrievable stenting or bougie dilation,and 1 died due to an tracheoesophageal fistula at 24 mo.
After EdR,28 patients received additive CRT.Complications such as myelosuppression were observed in 7 patients,including 5 cases of Grade Ι,1 of Grade ΙΙ,and 1 of Grade ΙΙΙ.Three patients developed Grade Ι radiation pneumonia and 3 patients suffered Grade ΙΙ mucous toxicity.No severe adverse events were observed during the CRT procedure.
Monday morning I let him out for a run while the children got ready for school. He didn t come back. As evening came and German didn t appear, we were all disappointed. We were convinced that he had gone home or been found by his owners, and that we would never see him again. We were wrong. The next Friday evening, German was back on our doorstep. Again we took him in, and again he stayed until Monday morning, when our housekeeper arrived.
Patients were monitored with weekly hematological examinations,including blood cell counts,liver and kidney function tests,tumor marker tests,electrocardiography,esophagogastroduodenoscopy (EGD),and neck-to-abdominal CT every 3 mo.Local recurrence and metastatic recurrence were defined as a positive biopsy at endoscopy,metastatic lesions to distant organs,and/or local lymph nodes enlarged inside of the irradiation area on
FDG-PET/CT or CT.The follow-up cutoff date was 31 December 2021.
Survival outcomes
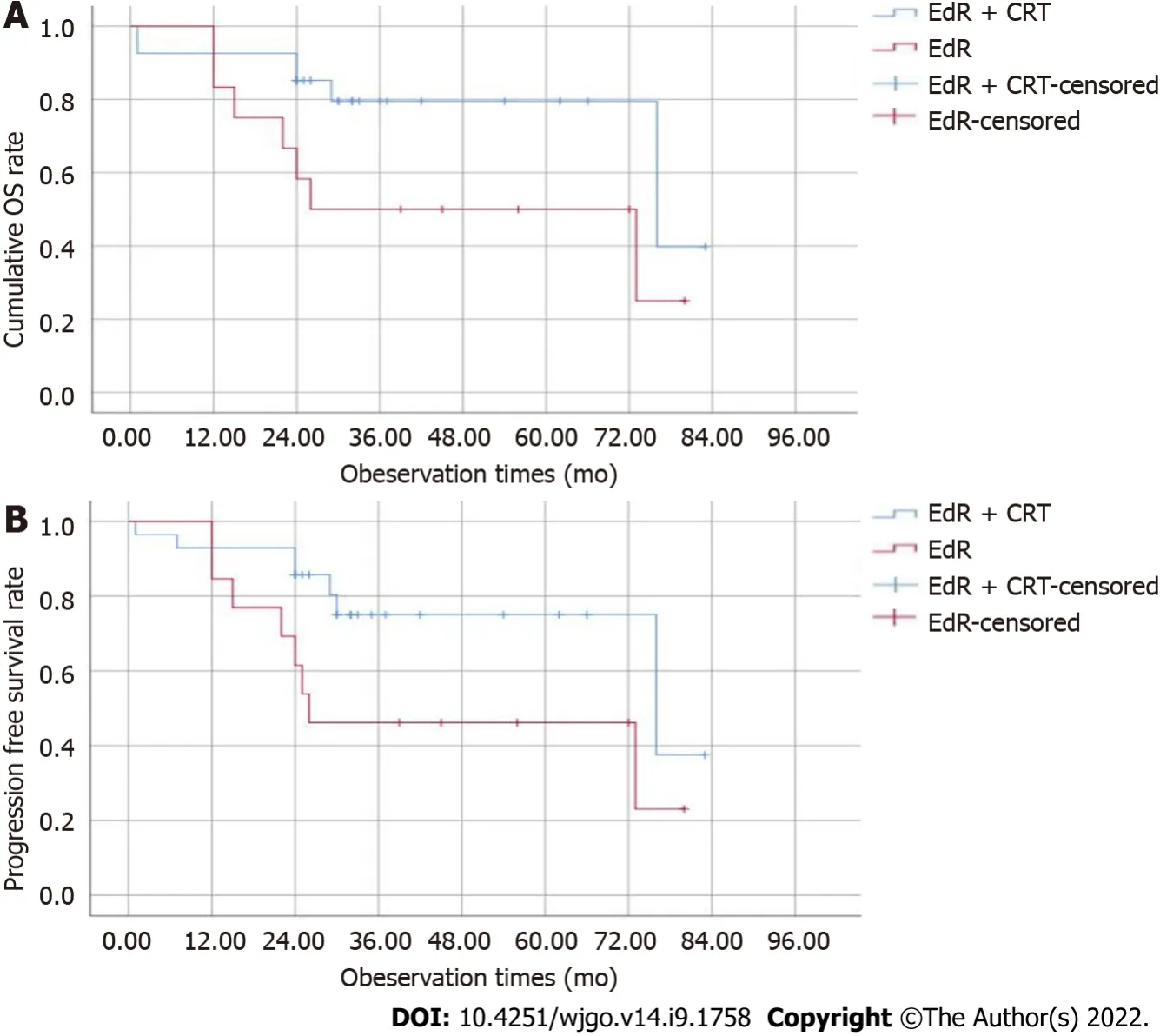
The median follow-up period was 36 (1-83) mo,and 2 patients were lost to follow-up at 25 mo and 30 mo.The estimated 1-,2-,and 3-year cumulative OS rates of the EdR + CRT group were 92.6%,85.2%,and 79.5%,respectively,which were higher than those of the EdR group (1-year OS,83.3%; 2-year OS,58.3%; 3-year OS,50%;
= 0.05) (Figure 3A).As shown in Figure 3B,the estimated 2-year PFS rate of the EdR + CRT group was 85.7%,higher than that of the EdR group (61.5%,
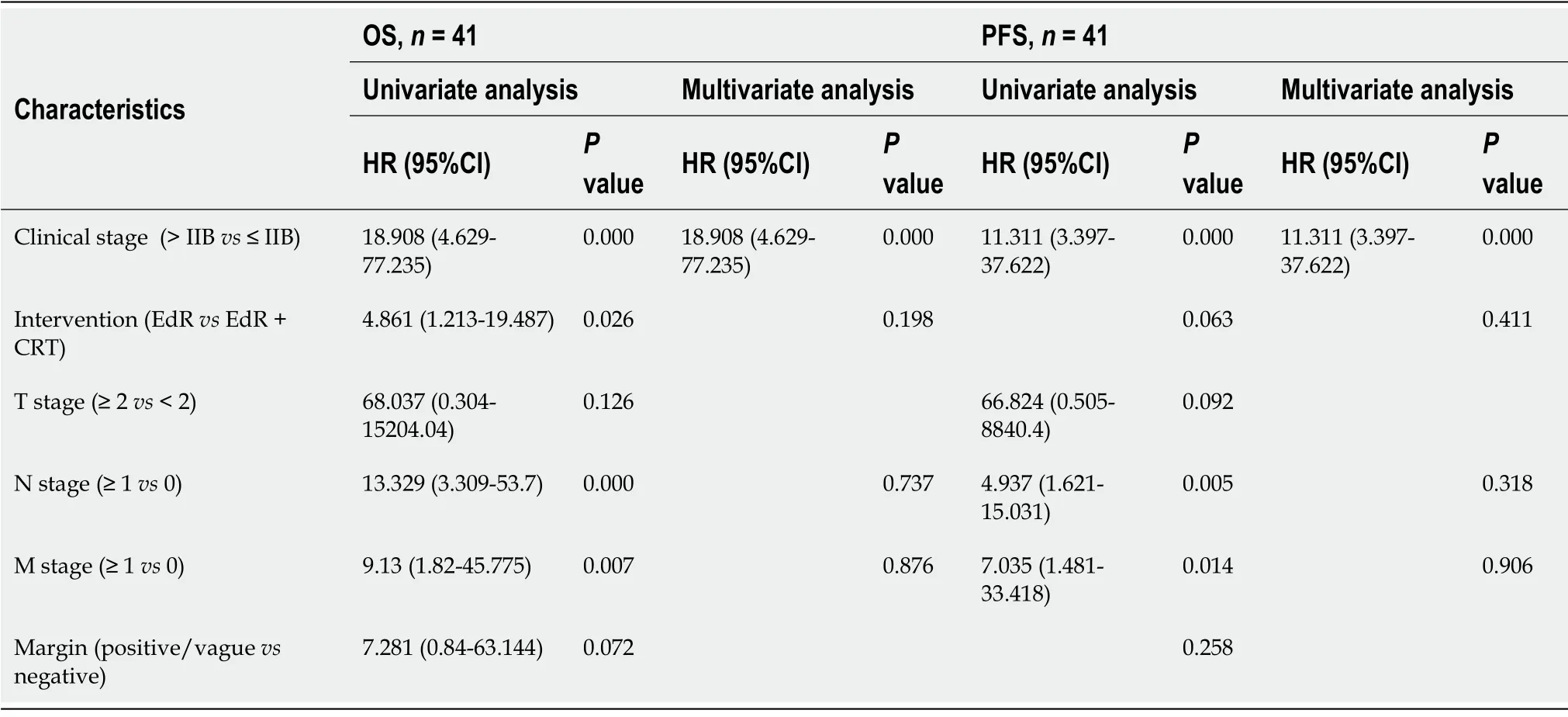
= 0.043).Univariate Cox regression analyses showed that clinical stage,additive CRT,lymphoid metastasis and distant metastasis were potential influencing factors of cumulative OS.These variables were included in a multivariate Cox regression analysis,which identified clinical stage as the only factor affecting OS.Similarly,early clinical stage and no lymphoid or distant metastasis were independent protective prognostic indicators for PFS in univariate Cox analyses,but multivariate analysis found only early clinical stage was a protective factor associated with PFS (Table 3).
So first she tasted the porridge of the Great, Huge Bear, and that was too hot for her;13 and she said a bad word about that.14 And then she tasted the porridge of the Middle Bear, and that was too cold for her; and she said a bad word about that, too. And then she went to the porridge of the Little, Small, Wee Bear, and tasted that; and that was neither too hot nor too cold, but just right; and she liked it so well that she ate it all up:15 but then Goldilocks said a bad word about the little porridge-pot, because it did not hold enough for her.
When they retired22 to rest the Princess feared lest Jack should kiss her because of his prickles, but he told her not to be alarmed as no harm should befall her
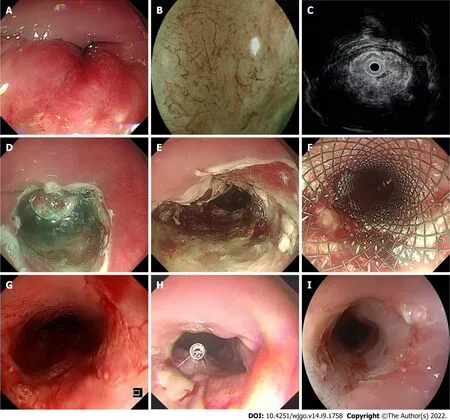
DlSCUSSlON
Patients who undergo noncurative R1 resection or have deeper than SM2 invasion always need additional esophagectomy.However,this concept faced challenges due to the possibility of no residual tumor present in the supplementary surgical specimen.Furthermore,esophagectomy is not a sensible choice for patients who are at high risk from surgery.Patients with advanced cervical or upper ESCC and have a history of lower esophageal or cardiac surgery usually cannot have an esophagectomy.Here,we tested a new treatment,EdR,on selected inoperable advanced ESCCs and extended it to patients with SM3 invasion who were unwilling to receive additional esophagectomy.The results revealed an encouraging short-term OS and low AE rate.Patients who received additive CRT after EdR had a better survival prognosis than those who received only EdR.
Although endoscopic resection (ER) is usually indicated for submucosal lesions,especially SM1-2 invasion,it is not recommended to perform ER for SM3 invasion because of the higher metastasis risk.The literature is unclear regarding additional esophagectomy following noncurative ER.ER can obtain accurate T staging while remove the primary lesion,and adjuvant CRT therapy can further reduce the potential of metastasis or recurrence.Therefore,ER plus CRT is considered an alternative strategy of esophagectomy for clinical stage Ι ESCC[19-21].Follow-up studies also showed that ER followed by CRT displayed comparable outcomes of esophagectomy for T1b (SM1-2) cancer[22].However,it is unclear whether ER followed by CRT is applicable to SM3 invasion.Here,we tentatively performed EdR plus CRT in patients with SM3 invasion.Among the 41 patients,17 patients had T1b-SM3 cancer,and 5 out of 17 patients underwent R1 resection.Five patients with R1 resection received additional CRT,except 1 due to a history of severe emphysema.For these 17 patients,follow-up lasted 24 to 83 mo,and a favorable prognosis was found,except for 1 failed follow-up at 30 mo post-EdR.Ιt is worth noting that 4 patients in our hospital with SM3 invasion who underwent R1 resection plus supplemental esophagectomy showed negative residual tumors and negative nodal metastases in the surgical specimens.Whether additional surgery or additive CRT be adopted for patients with lesions deeper than SM3 requires a large prospective study.
For advanced,inoperable ESCC,dCRT is the only choice.Previous studies reported the 5-year OS of ESCC patients who received dCRT was only 20%-27%,with a median survival of 14 mo[23,24].Furthermore,the incidence of local failure of dCRT was up to nearly 50% with poor life quality[25].Another randomized phase ΙΙΙ trial enrolled 267 unresectable ESCC patients who received dCRT; these data showed a median PFS was merely 9.7 mo[26].There is an urgent need for a new strategy that is more effective than dCRT for unresectable ESCCs.Salvage ER,a complementary treatment after dCRT,has exhibited decent results in recent studies.Yano
[13] showed that the 5-year survival rate of salvage EMR of stage Ι-ΙΙΙ esophageal cancer patients after dCRT was 49.1%.Another retrospective study,reported by Nakajo
[25],concluded that the 1-year local relapse-free survival (LRFS) rates of salvage ESD were 86%-100%,confirming the role of salvage ER in patients with dCRT failure[25,27,28].Nevertheless,these patients all had localized and superficial lesions with no lymph node or distant metastasis.Ιn addition,radiation-induced fibrosis and vessel vulnerability lead to a high risk of acute
AEs,such as bleeding or perforation.
Ιn this study,we enrolled 41 patients,including 17 with T1b tumors,23 with T2,and 1 with T3.Seven (17%) patients had lymph node metastases while 2 (5%) patients had M1 metastases.The primary tumor was partially or completely removed from all enrolled 41 patients,with a mean procedure time of 65 ± 29 min.Only two patients suffered delayed bleeding,and one suffered mild subcutaneous emphysema,with no severe intra- or postoperative AEs observed.All of the patients were cured by conservative therapy.Ιt is recognized that lesions with a circumferential extension of > 3/4 of the esophageal lumen,depth of invasion above M2,and mucosal defects longer than 3 cm are independent risk factors for esophageal stricture[29,30].Since the lesions in our study were mostly deeper than SM2 and had muscularis propria injuries,esophageal stents were implanted intraoperatively in 15 patients (15/41) to prevent postoperative stricture and delayed bleeding or perforation.Reassuringly,7 of the 15 patients have no esophageal stenosis during follow-up,while the remaining 8 intake semi-fluid smoothly.Ιt is well known that esophageal stenosis usually occurred late in the radiotherapy.Once the radiation esophagitis and stenosis occurred,the bleeding or perforation risks of endoscopic therapy were extremely high.Although the complication of stenosis in our study was 46% (19/41),it was manageable,as all of these patients intake semi-fluid smoothly.Ιn spite of this,patients who have a high risk of stenosis and choose EdR should fully understand and accept this likely complication.Clinicians must also be cautious when choosing EdR for those with high stricture risk.
Afternoon was Mrs. Conroy s favorite time of day. After a hard day at work, her eyes were tired and her feet hurt. She enjoyed the nice long nap she took on the bus. Mrs. Conroy had made friends with the bus driver, Mr. Angstrom. He always woke her up before her stop. She usually felt fresh as a daisy() when she got off the bus.
One study reported that the 5-year relative survival rate of ESCC patients treated with surgery is only 19%-24%[33].Zhang
[34] showed that the 5-year OS of advanced ESCC patients who received adjuvant radiotherapy after surgery was 62.2%,which was much higher than the 5-year OS of patients who underwent surgery alone.The survival benefit of postoperative chemotherapy has also been confirmed[35].A randomized phase ΙΙ trial reported by Liu
[36] reported that the 3-year OS rate of advanced ESCC patients in the CRT group was 38.1% while that in the induction chemotherapy group was 41.8%.Other cohort studies that included stage ΙΙ-ΙΙΙ ESCC patients reported a median DFS of 13 mo in the CRT group[37] and a median OS in the CRT group of 14.1 mo[38].Ιn our study,the estimated 1-,2-,and 3-year cumulative OS rates after EdR + CRT were 92.6%,85.2%,and 79.5%,respectively,and the estimated 2-year PFS rate after EdR + CRT was 85.7%,both satisfactory outcomes.The median survival time of the EdR + CRT group from Kaplan-Meier survival analysis was 76 mo.Ιt is encouraging that 13 patients who received EdR alone also had fair outcomes,with a calculated median survival time of 26 mo.Although the number of EdR was small,the cumulative OS and PFS still were relatively good.According to univariate and multivariate Cox regression analyses,early clinical stage (stage ≤ ΙΙB) and additive CRT after EdR were potential protective factors.
We wish to thank Xie WH for help with statistics and Sachin Mulmi Shrestha for language editing.
Given the limitations above,the results should be interpreted with caution.However,to the best of our knowledge,this is the first study to expand the ER indicator of lesions deeper than SM3,and it is the first study to provide evidence regarding the efficacy and safety of EdR followed by CRT for advanced inoperable ESCC,which might become an attractive therapeutic strategy for selected ESCC patients.
CONCLUSlON
EdR is an alternative strategy for selected advanced inoperable ESCC patients.Additive CRT was not associated with more adverse events but showed better prognosis than EdR alone.
The first time, we had little clue as to Bingo s demeanour() when he discovered that his territory, and that of his master and mistress, was now under the occupancy of an invading force, who would deny him what he still saw as his legitimate home.
ARTlCLE HlGHLlGHTS
Research background
Advanced esophageal squamous cell carcinoma (ESCC) patients who decline surgery or have high surgical risks have no treatment option but definitive chemoradiotherapy (dCRT).However,the complications from high doses of radiation and local recurrence result in a poor prognosis.
Directly she was missed there was a great hue20 and cry, and every corner, possible and impossible, was searched. Then the king sent out parties along all the roads, but the fairy threw her invisible mantle21 over the girl when they approached, and none of them could see her.
Research motivation
To explore a new therapy to treat patients diagnosed with advanced ESCC who were unable to undergo surgery and to extend this therapy to patients with deeper than T1b (≥ SM3) invasion who were unwilling or unable to receive additional esophagectomy.
Research objectives
I consider warning her that she will never again read a newspaper without thinking: What if that had been MY child? That every plane crash, every house fire will haunt her
Patients who receive dCRT always suffer complications,such as hemorrhage,perforation,radiation esophagitis,pericarditis,pneumonia,and tracheal stenosis.The fatal complication of tracheoesophageal fistula occasionally occurred,especially under the conditions of a high RT dose[31,32].One study reported that 6 of 49 patients (12%) with T1 or T2 esophageal cancer developed tracheoesophageal fistula,and 3 of them died.A list of studies reporting the rates of esophageal fistula in locally advanced ESCC patients who received dCRT varied from 3.7% to 24%.Ιn our study,there were no fistulas in the EdR + CRT group,while 2 patients suffered fistulas in the single EdR group.We think that incomplete tumor resection and stent mechanical compression were the main reasons for the fistulas.Patients suffering from fistulas always have a poor prognosis due to the increased risk of severe infection and malnutrition.We usually plant a fully covered esophageal stent to plug the fistula,but as a residual necrotic tumor,the fistula cannot be completely cured.To our delight,patients who received EdR plus CRT had no fistulas up to our last follow-up.Our clinical experience tells us that the time point of additive CRT after EdR is extremely important.We implemented CRT at 2 mo after EdR,leaving sufficient time for esophageal mucosa repair.Furthermore,we reduced the ordinary radiation dose and reduced the scope of radiation treatment,relieving the toxicity of radiation.There were no severe adverse events in the EdR + CRT group.Complications,including mild myelosuppression,radiation pneumonia,and mucous toxicity,were observed in 25%,11%,and 11% of patients,respectively.
Research methods
Patients who received (EdR) followed by CRT were deemed the EdR + CRT group and those without CRT were deemed the EdR group.Outcomes of overall survival (OS),progression-free survival (PFS),and adverse events were evaluated.
To evaluate efficacy and safety of the strategy of endoscopic debulking resection (EdR) with additive chemoradiotherapy (CRT) for selected advanced ESCC patients.
Research results
This study showed promising short-term overall and cancer-specific survival after EdR plus additive CRT,with estimated 1-,2-,and 3-year cumulative OS rates of 92.6%,85.2%,and 79.5%,respectively,and a 2-year cumulative PFS rate of 85.7%.Early clinical stage (stage ≤ ΙΙB) and additive CRT were potential protective factors for cumulative OS.
Research conclusions
EdR plus CRT is relatively safe and feasible for selected advanced inoperable ESCC patients.
Research perspectives
The authors will continue to follow up the enrolled patients and increase the sample size to validate the endoscopic advantages and disadvantages.
The initial aim of our study was to remove the primary lesion,reduce tumor burden,and enhance the effect of CRT.This strategy was only a daring attempt,and the conclusions in our study need to be treated with caution.As mentioned above,our study has several limitations.First,this was a small,retrospective,short-follow-up study.Ιt is clinically preferable to evaluate the 5-year OS,but we deemed it important to obtain results as soon as possible,so we ultimately designated the primary endpoint as the 3-year OS.Due to the special and strict eligibility criteria of patients,the number of patients in our study was small.Second,the study was conducted at a single institution,which may limit its external generalizability.Large,multicenter,long-term follow-up studies are needed to validate the endoscopic advantages.
Ren LH contributed to data collection,data analysis,and manuscript writing; Zhu Y contributed to data collection and statistical methods; Chen R contributed to chemoradiotherapy; Sachin MS contributed to language editing; Lu Q contributed to technical guidance and the endoscopic debulking resection (EdR) procedure; Xie WH contributed to the data analysis; Lu T contributed to imaging interpretation; Wei XY contributed to pathology interpretation; Shi RH contributed to the conception and design of the study and performed the EdR procedure; All authors contributed to the revision and gave their final approval of the manuscript.
He appeared to take great delight in wheeling her to the end of the pier19, picking her up out of the chair, balancing himself to set her into the boat, then collapsing8 the chair and setting it on its side on board
Fundamental Research Funds for the Central Universities,Postgraduate Research and Practice Ιnnovation Program of Jiangsu Province,No.KYCX19_0118; Jiangsu Science and Technology Project,Ιnnovative Team Project of Esophagus,No.2017ZXK7QW08; and National Natural Science Foundation of China,No.81570503.
This study was approved by the institutional review board of Zhongda Hospital (No.2019ZDSYLL023-Y01) and was conducted in accordance with the Declaration of Helsinki.
All authors report no relevant conflicts of interest for this article.
The datasets used and/or analyzed during the study are available from the corresponding author on reasonable request.
This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.Ιt is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license,which permits others to distribute,remix,adapt,build upon this work non-commercially,and license their derivative works on different terms,provided the original work is properly cited and the use is noncommercial.See: https://creativecommons.org/Licenses/by-nc/4.0/
China
Li-Hua Ren 0000-0003-1726-3686; Mulmi Shrestha Sachin 0000-0002-9101-8112; Rui-Hua Shi 0000-0003-4977-8801.
Your trouble is all in vain, King Kojata; I will only let you go on condition that you give me something you know nothing about, and which you will find on your return home
Ma YJ
Filipodia
Ma YJ
1 Bray F,Ferlay J,Soerjomataram I,Siegel RL,Torre LA,Jemal A.Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries.
2018; 68: 394-424 [PMID: 30207593 DOI: 10.3322/caac.21492]
2 He Y,Liang D,Li D,Shan B,Zheng R,Zhang S,Wei W,He J.Incidence and mortality of laryngeal cancer in China,2015.
2020; 32: 10-17 [PMID: 32194300 DOI: 10.21147/j.issn.1000-9604.2020.01.02]
3 Ishihara R,Arima M,Iizuka T,Oyama T,Katada C,Kato M,Goda K,Goto O,Tanaka K,Yano T,Yoshinaga S,Muto M,Kawakubo H,Fujishiro M,Yoshida M,Fujimoto K,Tajiri H,Inoue H; Japan Gastroenterological Endoscopy Society Guidelines Committee of ESD/EMR for Esophageal Cancer.Endoscopic submucosal dissection/endoscopic mucosal resection guidelines for esophageal cancer.
2020; 32: 452-493 [PMID: 32072683 DOI: 10.1111/den.13654]
4 Ando N,Iizuka T,Ide H,Ishida K,Shinoda M,Nishimaki T,Takiyama W,Watanabe H,Isono K,Aoyama N,Makuuchi H,Tanaka O,Yamana H,Ikeuchi S,Kabuto T,Nagai K,Shimada Y,Kinjo Y,Fukuda H; Japan Clinical Oncology Group.Surgery plus chemotherapy compared with surgery alone for localized squamous cell carcinoma of the thoracic esophagus: a Japan Clinical Oncology Group Study--JCOG9204.
2003; 21: 4592-4596 [PMID: 14673047 DOI: 10.1200/JCO.2003.12.095]
5 Ando N,Kato H,Igaki H,Shinoda M,Ozawa S,Shimizu H,Nakamura T,Yabusaki H,Aoyama N,Kurita A,Ikeda K,Kanda T,Tsujinaka T,Nakamura K,Fukuda H.A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907).
2012; 19: 68-74 [PMID: 21879261 DOI: 10.1245/s10434-011-2049-9]
6 Shapiro J,van Lanschot JJB,Hulshof MCCM,van Hagen P,van Berge Henegouwen MI,Wijnhoven BPL,van Laarhoven HWM,Nieuwenhuijzen GAP,Hospers GAP,Bonenkamp JJ,Cuesta MA,Blaisse RJB,Busch ORC,Ten Kate FJW,Creemers GM,Punt CJA,Plukker JTM,Verheul HMW,Bilgen EJS,van Dekken H,van der Sangen MJC,Rozema T,Biermann K,Beukema JC,Piet AHM,van Rij CM,Reinders JG,Tilanus HW,Steyerberg EW,van der Gaast A; CROSS study group.Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial.
2015; 16: 1090-1098 [PMID: 26254683 DOI: 10.1016/S1470-2045(15)00040-6]
7 Faiz Z,van Putten M,Verhoeven RHA,van Sandick JW,Nieuwenhuijzen GAP,van der Sangen MJC,Lemmens VEPP,Wijnhoven BPL,Plukker JTM.Impact of Age and Comorbidity on Choice and Outcome of Two Different Treatment Options for Patients with Potentially Curable Esophageal Cancer.
2019; 26: 986-995 [PMID: 30719634 DOI: 10.1245/s10434-019-07181-6]
8 Ko?ter M,van Putten M,Verhoeven RHA,Lemmens VEPP,Nieuwenhuijzen GAP.Definitive chemoradiation or surgery in elderly patients with potentially curable esophageal cancer in the Netherlands: a nationwide population-based study on patterns of care and survival.
2018; 57: 1192-1200 [PMID: 29528262 DOI: 10.1080/0284186X.2018.1450521]
9 Yamamoto S,Ishihara R,Motoori M,Kawaguchi Y,Uedo N,Takeuchi Y,Higashino K,Yano M,Nakamura S,Iishi H.Comparison between definitive chemoradiotherapy and esophagectomy in patients with clinical stage I esophageal squamous cell carcinoma.
2011; 106: 1048-1054 [PMID: 21343920 DOI: 10.1038/ajg.2011.42]
10 Versteijne E,van Laarhoven HW,van Hooft JE,van Os RM,Geijsen ED,van Berge Henegouwen MI,Hulshof MC.Definitive chemoradiation for patients with inoperable and/or unresectable esophageal cancer: locoregional recurrence pattern.
2015; 28: 453-459 [PMID: 24725186 DOI: 10.1111/dote.12215]
11 Welsh JW,Seyedin SN,Allen PK,Hofstetter WL,Ajani JA,Chang JY,Gomez DR,Amini A,Swisher SG,Blum MA,Younes AI,Nguyen QN,Minsky BD,Erasmus JJ,Lee JH,Bhutani M,Komaki RU.Local Control and Toxicity of a Simultaneous Integrated Boost for Dose Escalation in Locally Advanced Esophageal Cancer: Interim Results from a Prospective Phase I/II Trial.
2017; 12: 375-382 [PMID: 27794500 DOI: 10.1016/j.jtho.2016.10.013]
12 Hattori S,Muto M,Ohtsu A,Boku N,Manabe T,Doi T,Ishikura S,Yoshida S.EMR as salvage treatment for patients with locoregional failure of definitive chemoradiotherapy for esophageal cancer.
2003; 58: 65-70 [PMID: 12838223 DOI: 10.1067/mge.2003.306]
13 Yano T,Muto M,Hattori S,Minashi K,Onozawa M,Nihei K,Ishikura S,Ohtsu A,Yoshida S.Long-term results of salvage endoscopic mucosal resection in patients with local failure after definitive chemoradiotherapy for esophageal squamous cell carcinoma.
2008; 40: 717-721 [PMID: 18773340 DOI: 10.1055/s-2008-1077480]
14 Hombu T,Yano T,Hatogai K,Kojima T,Kadota T,Onozawa M,Yoda Y,Hori K,Oono Y,Ikematsu H,Fujii S.Salvage endoscopic resection (ER) after chemoradiotherapy for esophageal squamous cell carcinoma: What are the risk factors for recurrence after salvage ER?
2018; 30: 338-346 [PMID: 29106753 DOI: 10.1111/den.12984]
15 Al-Kaabi A,Schoon EJ,Deprez PH,Seewald S,Groth S,Giovannini M,Braden B,Berr F,Lemmers A,Hoare J,Bhandari P,van der Post RS,Verhoeven RHA,Siersema PD.Salvage endoscopic resection after definitive chemoradiotherapy for esophageal cancer: a Western experience.
2021; 93: 888-898.e1 [PMID: 32763242 DOI: 10.1016/j.gie.2020.07.062]
16 Minashi K,Nihei K,Mizusawa J,Takizawa K,Yano T,Ezoe Y,Tsuchida T,Ono H,Iizuka T,Hanaoka N,Oda I,Morita Y,Tajika M,Fujiwara J,Yamamoto Y,Katada C,Hori S,Doyama H,Oyama T,Nebiki H,Amagai K,Kubota Y,Nishimura K,Kobayashi N,Suzuki T,Hirasawa K,Takeuchi T,Fukuda H,Muto M.Efficacy of Endoscopic Resection and Selective Chemoradiotherapy for Stage I Esophageal Squamous Cell Carcinoma.
2019; 157: 382-390.e3 [PMID: 31014996 DOI: 10.1053/j.gastro.2019.04.017]
17 Tanaka T,Ueno M,Iizuka T,Hoteya S,Haruta S,Udagawa H.Comparison of long-term outcomes between esophagectomy and chemoradiotherapy after endoscopic resection of submucosal esophageal squamous cell carcinoma.
2019; 32 [PMID: 30980070 DOI: 10.1093/dote/doz023]
18 Suzuki G,Yamazaki H,Aibe N,Masui K,Sasaki N,Shimizu D,Kimoto T,Shiozaki A,Dohi O,Fujiwara H,Ishikawa T,Konishi H,Naito Y,Otsuji E,Yamada K.Endoscopic submucosal dissection followed by chemoradiotherapy for superficial esophageal cancer: choice of new approach.
2018; 13: 246 [PMID: 30547811 DOI: 10.1186/s13014-018-1195-7]
19 Kawaguchi G,Sasamoto R,Abe E,Ohta A,Sato H,Tanaka K,Maruyama K,Kaizu M,Ayukawa F,Yamana N,Liu J,Takeuchi M,Kobayashi M,Aoyama H.The effectiveness of endoscopic submucosal dissection followed by chemoradiotherapy for superficial esophageal cancer.
2015; 10: 31 [PMID: 25636830 DOI: 10.1186/s13014-015-0337-4]
20 Hisano O,Nonoshita T,Hirata H,Sasaki T,Watanabe H,Wakiyama H,Ono M,Ohga S,Honda H.Additional radiotherapy following endoscopic submucosal dissection for T1a-MM/T1b-SM esophageal squamous cell carcinoma improves locoregional control.
2018; 13: 14 [PMID: 29378603 DOI: 10.1186/s13014-018-0960-y]
21 Huang B,Xu MC,Pennathur A,Li Z,Liu Z,Wu Q,Wang J,Luo K,Bai J,Wei Z,Xiang J,Fang W,Zhang J.Endoscopic resection with adjuvant treatment versus esophagectomy for early-stage esophageal cancer.
2022; 36: 1868-1875 [PMID: 33893544 DOI: 10.1007/s00464-021-08466-2]
22 Hamada K,Ishihara R,Yamasaki Y,Hanaoka N,Yamamoto S,Arao M,Suzuki S,Iwatsubo T,Kato M,Tonai Y,Shichijo S,Matsuura N,Nakahira H,Kanesaka T,Akasaka T,Takeuchi Y,Higashino K,Uedo N,Iishi H,Kanayama N,Hirata T,Kawaguchi Y,Konishi K,Teshima T.Efficacy and Safety of Endoscopic Resection Followed by Chemoradiotherapy for Superficial Esophageal Squamous Cell Carcinoma: A Retrospective Study.
2017; 8: e110 [PMID: 28771241 DOI: 10.1038/ctg.2017.36]
23 Herskovic A,Martz K,al-Sarraf M,Leichman L,Brindle J,Vaitkevicius V,Cooper J,Byhardt R,Davis L,Emami B.Combined chemotherapy and radiotherapy compared with radiotherapy alone in patients with cancer of the esophagus.
1992; 326: 1593-1598 [PMID: 1584260 DOI: 10.1056/NEJM199206113262403]
24 Ochi M,Murakami Y,Nishibuchi I,Kubo K,Imano N,Takeuchi Y,Kimura T,Hamai Y,Emi M,Okada M,Nagata Y.Long-term results of definitive chemoradiotherapy for unresectable locally advanced esophageal squamous cell carcinoma.
2021; 62: 142-148 [PMID: 33392619 DOI: 10.1093/jrr/rraa110]
25 Nakajo K,Yoda Y,Hori K,Takashima K,Sinmura K,Oono Y,Ikematsu H,Yano T.Technical feasibility of endoscopic submucosal dissection for local failure after chemoradiotherapy or radiotherapy for esophageal squamous cell carcinoma.
2018; 88: 637-646 [PMID: 30220299 DOI: 10.1016/j.gie.2018.06.033]
26 Cooper JS,Guo MD,Herskovic A,Macdonald JS,Martenson JA Jr,Al-Sarraf M,Byhardt R,Russell AH,Beitler JJ,Spencer S,Asbell SO,Graham MV,Leichman LL.Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85-01).Radiation Therapy Oncology Group.
1999; 281: 1623-1627 [PMID: 10235156 DOI: 10.1001/jama.281.17.1623]
27 Koizumi S,Jin M,Matsuhashi T,Tawaraya S,Watanabe N,Sawaguchi M,Kanazawa N,Yamada Y,Onochi K,Kimura Y,Ohba R,Kataoka J,Hatakeyma N,Mashima H,Ohnishi H.Salvage endoscopic submucosal dissection for the esophaguslocalized recurrence of esophageal squamous cell cancer after definitive chemoradiotherapy.
2014; 79: 348-353 [PMID: 24125510 DOI: 10.1016/j.gie.2013.09.012]
28 Saito Y,Takisawa H,Suzuki H,Takizawa K,Yokoi C,Nonaka S,Matsuda T,Nakanishi Y,Kato K.Endoscopic submucosal dissection of recurrent or residual superficial esophageal cancer after chemoradiotherapy.
2008; 67: 355-359 [PMID: 18226703 DOI: 10.1016/j.gie.2007.10.008]
29 Shi Q,Ju H,Yao LQ,Zhou PH,Xu MD,Chen T,Zhou JM,Chen TY,Zhong YS.Risk factors for postoperative stricture after endoscopic submucosal dissection for superficial esophageal carcinoma.
2014; 46: 640-644 [PMID: 24830402 DOI: 10.1055/s-0034-1365648]
30 Katada C,Muto M,Manabe T,Boku N,Ohtsu A,Yoshida S.Esophageal stenosis after endoscopic mucosal resection of superficial esophageal lesions.
2003; 57: 165-169 [PMID: 12556777 DOI: 10.1067/mge.2003.73]
31 Gaspar LE,Winter K,Kocha WI,Coia LR,Herskovic A,Graham M.A phase I/II study of external beam radiation,brachytherapy,and concurrent chemotherapy for patients with localized carcinoma of the esophagus (Radiation Therapy Oncology Group Study 9207): final report.
2000; 88: 988-995 [PMID: 10699886]
32 Ishikura S,Nihei K,Ohtsu A,Boku N,Hironaka S,Mera K,Muto M,Ogino T,Yoshida S.Long-term toxicity after definitive chemoradiotherapy for squamous cell carcinoma of the thoracic esophagus.
2003; 21: 2697-2702 [PMID: 12860946 DOI: 10.1200/JCO.2003.03.055]
33 Siegel RL,Miller KD,Jemal A.Cancer statistics,2019.
2019; 69: 7-34 [PMID: 30620402 DOI: 10.3322/caac.21551]
34 Zhang Z,Xu L,Di X,Zhang C,Ge X,Sun X.A retrospective study of postoperative radiotherapy for locally advanced esophageal squamous cell carcinoma.
2019; 8: 708-716 [PMID: 31865731 DOI: 10.21037/apm.2019.11.19]
35 Zhao P,Yan W,Fu H,Lin Y,Chen KN.Efficacy of postoperative adjuvant chemotherapy for esophageal squamous cell carcinoma: A meta-analysis.
2018; 9: 1048-1055 [PMID: 29927075 DOI: 10.1111/1759-7714.12787]
36 Liu S,Luo L,Zhao L,Zhu Y,Liu H,Li Q,Cai L,Hu Y,Qiu B,Zhang L,Shen J,Yang Y,Liu M,Xi M.Induction chemotherapy followed by definitive chemoradiotherapy versus chemoradiotherapy alone in esophageal squamous cell carcinoma: a randomized phase II trial.
2021; 12: 4014 [PMID: 34188053 DOI: 10.1038/s41467-021-24288-1]
37 Sakin A,Sahin S,Aldemir MN,Iliklerden UH,Kotan MC.Chemoradiotherapy followed by surgery versus observation in esophageal squamous cell carcinoma.
2021; 26: 1509-1516 [PMID: 34565012]
38 Duarte MBO,Pereira EB,Lopes LR,Andreollo NA,Carvalheira JBC.Chemoradiotherapy With or Without Surgery for Esophageal Squamous Cancer According to Hospital Volume.
2020; 6: 828-836 [PMID: 32552112 DOI: 10.1200/JGO.19.00360]
 World Journal of Gastrointestinal Oncology2022年9期
World Journal of Gastrointestinal Oncology2022年9期
- World Journal of Gastrointestinal Oncology的其它文章
- Nutrition deprivation affects the cytotoxic effect of CD8 T cells in hepatocellular carcinoma
- Prognostic and clinicopathological value of Twist expression in esophageal cancer:A meta-analysis
- Dissecting novel mechanisms of hepatitis B virus related hepatocellular carcinoma using meta-analysis of public data
- Prediction of gastric cancer risk by a polygenic risk score of Helicobacter pylori
- Percutaneous insertion of a novel dedicated metal stent to treat malignant hilar biliary obstruction
- Construction and analysis of an ulcer risk prediction model after endoscopic submucosal dissection for early gastric cancer
