Biomimetic chitosan scaffolds with long-term controlled release of nerve growth factor repairs 20-mm-long sciatic nerve defects in rats
Fa-Dong Liu, Hong-Mei Duan, Fei Hao, Wen Zhao, Yu-Dan Gao, Peng Hao, ,Zhao-Yang Yang, , Xiao-Guang Li, ,
Abstract Although autogenous nerve transplantation is the gold standard for treating peripheral nerve defects of considerable length, it still has some shortcomings, such as insufficient donors and secondary injury. Composite chitosan scaffolds loaded with controlled release of nerve growth factor can promote neuronal survival and axonal regeneration after short-segment sciatic nerve defects. However, the effects on extended nerve defects remain poorly understood. In this study, we used chitosan scaffolds loaded with nerve growth factor for 8 weeks to repair long-segment (20 mm) sciatic nerve defects in adult rats. The results showed that treatment markedly promoted the recovery of motor and sensory functions. The regenerated sciatic nerve not only reconnected with neurons but neural circuits with the central nervous system were also reconstructed. In addition, the regenerated sciatic nerve reconnected the motor endplate with the target muscle. Therefore, this novel biomimetic scaffold can promote the regeneration of extended sciatic nerve defects and reconstruct functional circuits. This provides a promising method for the clinical treatment of extended peripheral nerve injury. This study was approved by the Animal Ethics Committee of Capital Medical University, China (approval No. AEEI-2017-033) on March 21, 2017.
Key Words: axon; chitosan; functional recovery; myelin sheath; nerve growth factor; peripheral nerve injury; pseudorabies virus;regeneration; scaffold; sciatic nerve
Introduction
Although peripheral nerve injury remains prevalent after accidents, fortunately, short-distance peripheral nerve injuries can now be treated by surgery or tissue engineering scaffolds (Das and Srivastava, 2019; Wang et al., 2021; Zhang et al., 2021). However, for long (> 30 mm in humans, or> 10 mm in rats) peripheral nerve injury (Marquardt and Sakiyama-Elbert, 2013), direct surgical sutures are impossible because peripheral nerve elasticity is limited. At the same time, conventional scaffolds do not provide the necessary microenvironment for lengthy peripheral nerve regeneration.Thus, effectively bridging these kinds of peripheral nerve defects is difficult. At present, the gold standard for clinical treatment of extended peripheral nerve injury is still the autologous nerve graft (ANG) (Κornfeld et al., 2021). However,this procedure brings new trauma to the body region from which the graft is taken (Gu et al., 2011). In addition, the shapes of peripheral nerves in different parts of the body are very different, making sources limited (Rao et al., 2020).
Numerous studies have confirmed that composite scaffolds loaded with nerve growth factor (NGF) can promote the survival of neurons and accelerate axonal regeneration after sciatic nerve injury (Wang et al., 2012; Fu et al., 2013; Li et al., 2018; Mohamadi et al., 2018; Xia and Lv, 2018; Lackington et al., 2019). In rats, a chitosan-based nerve conduit loaded with NGF was capable of repairing 10-mm sciatic nerves and encouraging functional recovery that compared favorably with that of the autologous nerve grafts (Wang et al., 2012).However, the effectiveness of using chitosan scaffolds loaded with NGF for repairing longer distance (> 10 mm) nerve injury remains an open question. The objective of the present study was to determine whether a modified NGF-chitosan scaffold can repair 20-mm sciatic nerve gaps in adult rats.
Considering that hard chitosan scaffolds might cause compression and damage to surrounding tissue (Cao et al.,2018; Liu et al., 2021), we designed the elastic properties of natural sciatic nerves into an NGF-chitosan scaffold. Mosttime point-controlled release of NGF in previous studies had been less than 40 days (Kemp et al., 2011; Xu et al., 2011;Mohamadi et al., 2018), which might only be appropriate for short-distance (< 10 mm) peripheral nerve injury in rats. We recently showed that a stable regenerative microenvironment constructed with a biomimetic chitosan scaffold and long-term release of NGF (over a period of 8 weeks) would effectively promote the repair of 20-mm peripheral nerve injury. After 12 weeks, evaluation revealed that the sciatic nerve had successfully regenerated and that behavior had improved.In addition, functional reconstruction of neural circuits between regenerated peripheral nerves and the central nervous system were reported to facilitate the recovery of fine motion function and sensitive sensory function (Navarro et al., 2007). We studied the reconstruction of synaptic connections between regenerated sciatic nerves and the central nervous system using the cross-synaptic pseudorabies virus (PRV) tracing technique (Jia et al., 2019). We expected that the biomimetic NGF-chitosan scaffold (BCS) with controlreleased NGF would effectively promote nerve regeneration and functional recovery, so as to achieve the goal of replacing autogenous nerve transplantation and bring new hope for the repair of clinical nerve injuries.
Materials and Methods
Preparation of the BCS
In an improved method (Yang et al., 2015), under sterile conditions, a 2% (w/v) poly-N-acetyl glucosamine derived solution from 85% (w/v) deamidized chitosan (Sigma-Aldrich,St. Louis, MO, USA) in 100 mL of water containing 2% (w/v)acetic acid was plasticized by treatment with 1 g of lithium chloride. A 304 stainless steel mold (Newinvent, Wuxi, China)with a diameter of 1.8 mm and length of 20 cm was hung into the solution. The mold was rotated clockwise for five turns within 5 seconds, then the mold was removed vertically and placed diagonally into a 500-mL beaker (Shuniu, Chengdu,China). The beaker with the mold was placed in a 45°C oven and dried for 4–5 hours. This step was repeated nine times.NaOH (2 g) was then dissolved in 98 mL of pure water for preparing a 2% (w/v) NaOH solution, and the dried mold was soaked in a measuring cylinder containing the 2% (w/v)NaOH solution for 5–10 minutes. Deionized water was used to repeatedly clean the soaked NaOH mold, the outer chitosan tube was carefully removed from the mold, and the empty chitosan tube was cut to be 20 mm in length. The tube was soaked in 75% alcohol for 1 hour to dehydrate, and further soaked in anhydrous ethanol for 1 hour to fully dehydrate.Under aseptic conditions, 10 mg chitosan particles (Sigma-Aldrich) were immersed in 10 mL deionized water at pH 7.2,centrifuged for 6 hours, and the supernatant was discarded.The expanded chitosan particles were frozen at –20°C for 24 hours and then frozen at 4°C for 10 hours. One-hundred nanograms NGF (Sigma-Aldrich) was then mixed with the above-mentioned chitosan particles in a 4°C solution. The dried chitosan particles loaded with NGF were added to a type-I collagen solution, vacuumed, and freeze-dried. The diameter of the chitosan particles was about 300 μm. Before surgery, a 10-mg chitosan carrier loaded with NGF (100 ng)was evenly injected into the inner wall of the chitosan scaffold and stored at 4°C for use.
Mechanical characteristics of the BCS and sciatic nerve
The stress-strain curves for the complete NGF-chitosan scaffold (n= 3) and sciatic nerve (n= 3) were measured in rats with a mechanical tensile tester (Instron5565, INSTRON,Boston, MA, USA). Three specific-pathogen-free (SPF) adult female Wistar rats, weighing 230–250 g, 10-week-old, were provided by the Charles River Laboratories (Wilmington,MA, USA). Rats were anaesthetized with an intraperitoneal injection of 3% sodium pentobarbital solution (30 mg/kg body weight; Sigma-Aldrich). The sciatic nerve was exposed and removed. The NGF-chitosan scaffold and sciatic nerve were cut into 25-mm samples to be tested. The sample was placed into the measuring clamp, the tension was slowly loaded at a rate of 1 mm/min. We paid attention to any deformation of the sample in the process of stretching, and stopped testing when the sample fractured. For the nanoindentation test(UMT-2, Bruker, Billerica, MA, USA), a total of 3 NGF-chitosan scaffolds were measured, and five sites for detecting nanoindentation were randomly selected from each scaffold for testing.
Kinetics of NGF release
According to the instructions of the NGF-enzyme linked immunosorbent assay (X-Y BIOTECHNOLOGY, Shanghai, China),a 96-well plate was set up with blank wells and sample wells to be tested, and 100 μL phosphate buffer saline solution was added to each well. Then, a 1-mg chitosan carrier (10 ng NGF)was added to the phosphate buffer saline solution one well at a time. The NGF-enzyme linked immunosorbent assay was used to determine the kinetics of NGF release at 1, 3, 6, 12 hours, 1, 3 days, and 1–10 weeks. Absorbance was read at 450 nm using an enzyme linked immunosorbent assay plate reader(Model 680, Bio-Rad, Hercules, CA, USA).
Animal and surgical procedures
The study was by the ethical standards of the Animal Ethics Committee of Capital Medical University (approval No. AEEI-2017-033) on March 21, 2017. All experiments were designed and reported according to the Animal Research: Reporting ofIn VivoExperiments (ARRIVE) guidelines. One-hundred and three SPF adult female Wistar rats, weighing 230–250 g,10-week-old, were provided by the Charles River Laboratories(Wilmington, MA, USA). Rats were randomly divided into five groups: sham control (SHAM), lesion (LC), empty tube (ET),ANG, and BCS groups.
Animals were anesthetized with an intraperitoneal injection of 3% sodium pentobarbital solution (30 mg/kg body weight).The sciatic nerve was exposed by making a skin incision and splitting the underlying muscles in the left lateral thigh.In the BCS group (n= 27), after excising 20 mm of the left sciatic nerve, the BCS was implanted into the 20-mm gap and stitched to the ends of the remaining sciatic nerve with single 10/0 stitches (Ethilon?, Ethicon Inc., New Brunswick, NH, USA).For rats in the ET group (n= 17), an ET was implanted into the 20-mm gap. For rats in the ANG group (n= 21), a 20-mm-long segment of the left sciatic nerve was excised and not turned over, then immediately re-implanted with 10/0 stitches. For rats in the LC group (n= 17), 20 mm of the left sciatic nerve was excised, and rats received no treatment. For the SHAM group (n= 21), the left sciatic nerve was exposed but not injured in any way. Muscles and skin were closed in layers with 6/0 stitches. The skin of the operated area was disinfected with iodine. Rats were then intraperitoneally injected with 0.5 mL penicillin (1.0 × 104U; North China Pharmaceutical Co., Shijiazhuang, China) to prevent infection after surgery.The experimental rats were kept on a 12-hour light/dark cycle in a room at 24–26°C and a relative humidity of 35–45%.After surgery, all animals were housed and fed routinely, and monitored for changes in their general conditions.
Morphological evaluation of the regenerative sciatic nerve
To observe the number of regenerated nerves in the NGFchitosan scaffolds at acute time points, rats in the BCS group were sacrificed by intraperitoneal overdose of pentobarbital sodium solution (60 mg/kg body weight) at 7 and 10 days post-injury (n= 3 each day). The middle portion of the regenerated nerve tissue was cut into transverse sections on a cryostat microtome (CM1520, Leica, Frankfurt,Germany). Sections (four slices/rat) were immunostained with neurofilament-200 (NF200) antibody (chicken, 1:2000,Cat# AB5539, RRID: AB_11212161, Sigma-Aldrich) overnight at 4°C. The sections were then incubated in the secondary antibody (Alexa Fluor 488-conjugated goat anti-chicken IgY;1:200, Cat# A11039, RRID: AB_2534096, Thermo Fisher) for 6 hours at room temperature. The sections were then observed with a confocal microscope (TCS SP8, Leica). All axons visible in the cross sectioned (600 μm × 600 μm) confocal image were counted using Image-Pro Plus 6.0 software (Media Cybernetics, Rockville, MD, USA).
Twelve weeks after surgery, eight rats from each group were sacrificed by intraperitoneal overdose of pentobarbital sodium solution (60 mg/kg body weight). The injured region were then observed. Then, the sciatic nerves in the center of the injury area (3 mm) were cut into transverse sections (n= 4) for immunofluorescence staining. The sections were washed three times (3 minutes each time) with 0.01 M phosphate buffer saline. The sections (four slices/rat) were incubated in the blocking solution (ZSGB-BIO, Beijing, China) for 1 hour at room temperature, followed by incubation with NF200 antibody(chicken, 1:2000, Cat# AB5539, RRID: AB_11212161, Sigma-Aldrich) and S100 antibody (rabbit, 1:100, Cat# ab52642,RRID: AB_882426, Abcam, Cambridge, UK) overnight at 4°C.The sections were then incubated in secondary antibodies for 6 hours at room temperature. The secondary antibodies were Alexa Fluor 594-conjugated goat anti-chicken IgY (1:200, Cat#A32759, RRID: AB_2762829, Thermo Fisher) and Alexa Fluor 488-conjugated goat anti-rabbit IgG (1:200, Cat# A32731,RRID: AB_2633280, Thermo Fisher). 4′,6-Diamidino-2-phenylindole (1:1000, Sigma) was used to stain the nuclei. The sections were observed with a confocal microscope (TCS SP8,Leica). A field of view was randomly selected for each section to observe axon morphology. The number of axons visible in the whole cross section (1.2 mm × 1.2 mm) were counted using Image-Pro Plus 6.0 software. The fiber count generated by the software has been manually verified.
In addition, the sciatic nerves from the SHAM, ANG, and BCS groups were cut into longitudinal sections (n= 4). As mentioned above, sections were then stained with NF200 antibody and a secondary antibody. The sections were photographed under appropriate microscope settings (BX-61,Olympus, Tokyo, Japan).
Morphological evaluation of the myelin sheath
Twelve weeks after surgery, four rats from each group were sacrificed by intraperitoneal overdose of pentobarbital sodium solution (60 mg/kg body weight). The sciatic nerves in the center of the injury area (3 mm) were cut into semithin sections. The sections were incubated with 1% toluidine blue dye (Solarbio, Beijing, China) for 10 minutes at room temperature, and observed under a microscope (BX-51,Olympus). The remaining nerve specimens were cut into ultrathin sections with a thickness of 60 nm and stained with lead citrate and uranyl acetate. Stained sections were observed using a transmission electron microscope (HT7700, Hitachi,Tokyo, Japan).
PRV retrograde tracing
Twelve weeks after surgery, PRV retrograde tracing was performed. The titer of PRV-enhanced green fluorescent protein (BrainVTA (Wuhan) Co., Ltd., Wuhan, China) was 2.0× 109plaque forming units/mL. Eight rats in each group were anesthetized by intraperitoneal injection of 3% pentobarbital sodium (30 μg/g body weight). Then, 2 μL PRV was injected into the sciatic nerve, 5 mm away from the injured area. All injections were made with a 36 GA injection needle (World Precision Instruments, Sarasota, FL, USA) at a rate of 1 μL/min.The needle was held in place for an additional 30 seconds following the injection. Five days after PRV injection, the rats were over anesthetized (pentobarbital sodium solution,60 mg/kg body weight) to death and perfused through the heart. The spinal cord, dorsal root ganglion (DRG), and brain were sectioned on a cryostat microtome (DMR, Leica). The DRG sections were incubated with calcitonin gene-related peptide (CGRP) antibody (goat, 1:400, Cat# ab36001, RRID:AB_725807, Abcam) at 4°C for 24 hours. The secondary antibody was Alexa Fluor 594-conjugated donkey anti-goat IgG (1:200, Cat# A32758, RRID: AB_2762828, Thermo Fisher).4′,6-Diamidino-2-phenylindole was used to stain the nuclei.The sections (five slices/rat) were observed under a confocal microscope. The number of neurons at several locations was analyzed using Image-Pro Plus 6.0 software. PRV-positive motor neurons located in the left anterior horn of the spinal cord were counted. Neurons (CGRP/PRV double positive cells)in the entire DRG section (1.2 mm × 1.2 mm) were counted.PRV-positive neurons in the reticular nucleus, vestibular nucleus, and M1 region of motor cortex were counted separately in the sections.
Behavioral tests
For assessing nerve function, we used the sciatic nervefunction index (SFI). Five rats were selected from each group for testing at 2, 4, 6, 8, 10, and 12 weeks after surgery.Cardboard was used to make a 60 cm × 10 cm × 10 cm carton that was open at both ends. For each test, white paper was cut to the same length and width as the carton and laid at the bottom. After dipping the left hindfeet in red ink and the right hindfeet in blue ink, rats in each group were placed in the carton. Rats were placed at one end of the carton and then walked to the other end, leaving clear footprints on the white paper. The SFI was calculated using the following formula(Bain et al., 1989): SFI = –38.3 (EPL – NPL) / NPL + 109.5 (ETS –NTS) / NTS + 13.3 (EIT – NIT) / NIT – 8.8. The PL (print length)is the distance from the heel to the third toe, the TS (toe spread) is the distance from the first to the fifth toe, and the intermediary IT (toe spread) is the distance from the second to the fourth toe. EPL, ETS and EIT represent the records of the injured foot, while NPL, NTS and NIT represent the records of the non-operative foot. A value of negative 100 indicates total impairment.For sensory recovery in rats, an Austerlitz pin (Entomoravia,Austerlitz, Czech) was used to evaluate high threshold mechanical sensitivity responsiveness of the Wistar rats, as has been previously described (Sakuma et al., 2016). After sciatic nerve injury, we gently pricked the surface of the affected hindfoot with the pin, without penetrating the skin or moving the claw. The outermost part of the plantar of the hind claw (the sensory area for the sciatic nerve) was divided into 5 areas. The needle test was performed from the outermost toe to the heel. When the animal significantly moved its claws, the response was considered positive. A value of 1 was assigned for the area, and then the next area was tested. If all pinpricks got a positive response, the total score was 5. After the surgery, five rats in each group received the pinprick assay each week.
Analysis of muscle atrophy
Twelve weeks after surgery, four rats from each group were sacrificed to determine the muscle wet-weight ratio between the injured and uninjured side. Both anterior tibial muscles were harvested and immediately weighed with an electronic balance (MP200-1, Mettler-Toledo, Zurich, Switzerland). The left tibialis anterior muscle was sectioned and stained with traditional hematoxylin-eosin (HE). In brief, the sections were put into hematoxylin dye (ZSGB-BIO) and incubated at room temperature for 10 minutes. The sections were washed with double distilled water to remove the dye and then placed in running tap water for 10 minutes. The sections were then placed in eosin solution (ZSGB-BIO) at room temperature for 30 seconds, dehydrated with gradient alcohol, made transparent with xylene, and sealed with neutral gum. The sections were photographed using a light microscope.
Analysis of motor endplate
Twelve weeks after surgery, the mid-belly of the tibialis anterior muscle (n= 5) was sliced longitudinally with a cryostat microtome. All sections were divided into 2 groups:group 1 for acetylcholinesterase (AChE) staining, and group 2 for immunohistochemical staining. Group 1 sections (five slices/rat) were incubated in AChE buffer (Baso, Zhuhai,China) for 2 hours. The sections were then dehydrated with gradient alcohol, made transparent with xylene, and sealed with neutral gum. The sections were then photographed using a light microscope. The integrated optical density (IOD)of the AChE in the image was calculated. Group 2 sections(five slices/rat) were stained with fluorescent Synapse1 (SYN)antibody (rabbit, 1:200, Cat# 2312S, RRID: AB_2200102,Millipore) and α-Bungarotoxin (BTX) (bungarus multicinctus,1:500, Cat# B13423, RRID: AB_2891137, Molecular Probes).Immunofluorescence staining following the same procedure as the AChE staining. Images were captured using a confocal microscope. The mean area of the motor endplate and the IOD of AChE were measured using Image-Pro Plus 6.0 software.
Statistical analysis
All data are expressed as the mean ± standard deviation (SD)and were statistically analyzed using SPSS 16.0 software (SPSS,Inc., Chicago, IL, USA). Significance was determined by oneway analysis of variance followed by Fisher’s least significant difference test. The Student’st-test was used to compare the two-sample means. Values were considered significantly different atP< 0.05.
Results
Characterization of the NGF-chitosan scaffold
The total length of the NGF-chitosan scaffold used for transplantation was 20 mm. The inner diameter of the NGFchitosan scaffold was 1.8 mm, the outer diameter was 3.0 mm, and the thickness of the tube wall was 0.6 mm (Figure1A).
After our modification, the BCS has similar physical properties to the rat sciatic nerve (Figure 1B). The maximum stress withstood by the BCS was 2.656 ± 0.214 MPa and the maximum stress withstood by sciatic nerve was 2.094 ±0.185 MPa (P< 0.05,n= 3). The elastic modulus was 9.692± 0.122 MPa for the BCS and 9.644 ± 0.228 MPa in normal sciatic nerves. In addition, breaking-point elongation was 32.1± 4.2% for the BCS and 31.8 ± 6.5% for the sciatic nerves.There was no difference in elastic modulus (P= 0.689,n=3) and breaking elongation (P= 0.946,n= 3) between the two groups. This biomimetic elastic design might avoid any abnormal sensation caused by the scaffold around the sciatic nerve. Nanoindentation testing the BCS (Figure 1C), showed that the hardness at maximum load was 0.123 ± 0.044 GPa,the modulus at maximum load was 1.068 ± 0.164 GPa, and the displacement at maximum load was 4244.5 ± 511 nm. This indicated that the micromechanical properties of chitosan scaffolds also could deform quite a bit.
NGF was released at a rate of 15.7 ± 2.08% within the first 1 hour (Figure 1D). At 12 hours, total NGF release reached 34.0± 5.57%. This facilitated rapid deployment of large amounts of NGF in the microenvironment to promote nerve regeneration.At the following time point, NGF release began to slow, and by the 8thweek, the total sustained release rate of NGF had reached 96 ± 2.65%. No release of NGF was detected at weeks 9 or 10. This indicates that the chitosan carrier could release NGF stably for 8 weeks, showing its potential to provide longterm nutritional support for repair of lengthy peripheral nerve injury.
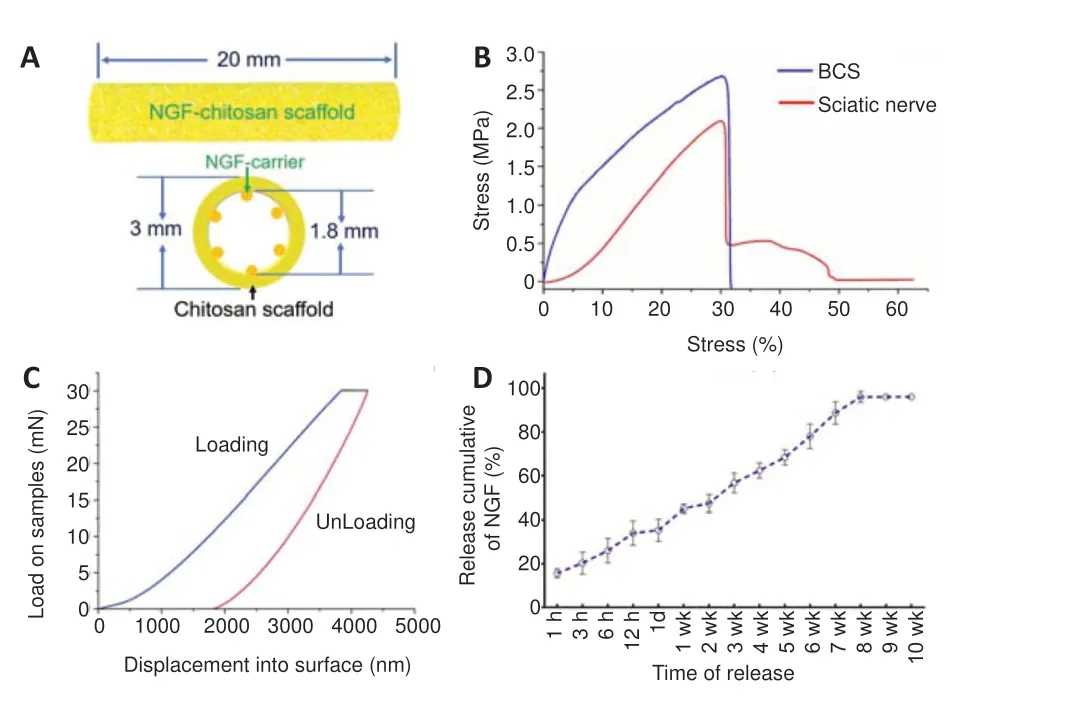
Figure 1|Characterization of the BCS.
General observation of the regenerated sciatic nerve
Twelve weeks after surgery, the injured areas were observed in each group (Figure 2AandB). Macroscopic signs of neuroma formation, seroma, and inflammation were absent. In the SHAM group, the intact sciatic nerve was observed, while no significant sciatic nerve regeneration was observed in the LC group. No significant neural tissue regeneration was observed in the chitosan tube of the ET group. The nerve defect was successfully bridged by autologous nerve transplantation in the ANG group. The regenerated nerve tissue in the BCS group was able to bridge the 20-mm gap and showed normal peripheral nerve features. Thus, both BCS and ANG can be used to repair a 20-mm sciatic nerve defect in rats, but an empty tube alone failed to bridge the nerve defect (Figure2C).
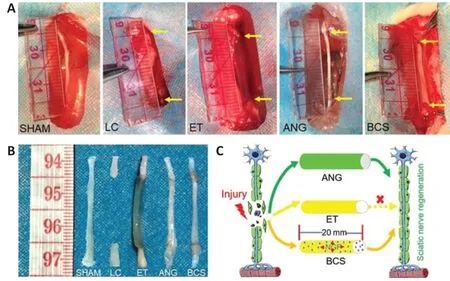
Figure 2|General observation of the regenerated sciatic nerve 12 weeks after surgery.
BCS facilitates sciatic nerve regeneration
To observe the regenerated sciatic nerve in the BCS (Figure3A), axons were labeled with NF200 antibody at 7 or 10 days after operation. Numerous regenerated axons were thus visible in the transverse sections of regenerated tissue(Figure 3B). Statistical analysis showed that the number of regenerated nerve fibers increased significantly between 7 and 10 days after operation in the BCS group (7 days: 1256.7 ±54.0; 10 days: 723.3 ± 43.0;P< 0.001,n= 3;Figure 3C).
Twelve weeks after surgery, longitudinal section analysis showed that the regenerated nerves successfully bridged the 20-mm injured area. In the ANG and BCS groups,NF200+regenerated nerve fibers were observed in the distal stump of the injured area (Additional Figure 1). Numerous axons and myelin sheaths were observed in the transverse sections (Figure 3D), and the microscopic structure of the myelin sheath around the axons could be observed in high magnification images (Figure 3E). In the BCS group, the chitosan carrier had been completely degraded and a large number of NF200 positive axons were found in the chitosan scaffold (Additional Figure 2). There was no significant difference in the number of regenerated axons between the ANG and BCS groups (ANG: 8605.3 ± 340.1; BCSS: 8546.5 ±383.7;P= 0.859,n= 4;Figure 3F), but both were less than the total number of axons in the SHAM group (11729.5 ± 665.9,P< 0.001). In addition, observation of the myelin sheath via toluidine blue staining and transmission electron microscopy showed a large amount of regenerated myelin in the ANG and BCS groups (Additional Figure 3).
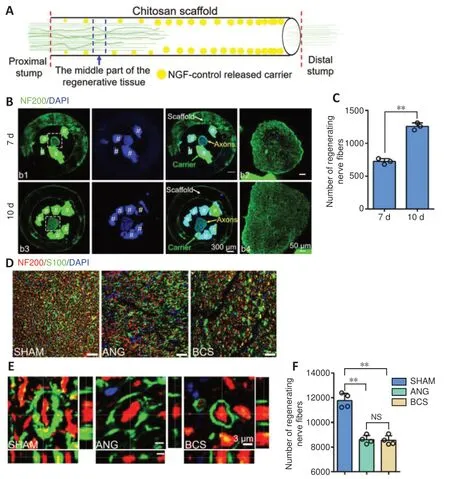
Figure 3|Sciatic nerve was successfully regenerated after BCS implant.
BCS promotes functional circuit reconstruction
PRV-labeled motoneurons were observed in the L4 spinal cord of the SHAM group, ANG group, and BCS group (Figure 4A).We counted the number of PRV-labeled motor neurons, and found no significant difference between the ANG and BCS groups (ANG: 7.3 ± 0.9; BCS: 7.1 ± 1.1;P= 0.776,n= 8,Figure4B). However, the number of PRV-labeled motoneurons was significantly lower in the ANG and BCS groups than in the SHAM group (SHAM: 11.1 ± 1.0,P< 0.001). The sciatic nerve is known to contain many sensory fibers that are directly connected to cell bodies located in the DRG (Dayawansa et al.,2016). We found that many DRG neurons were labeled in the SHAM, ANG and BCS groups (Figure 4C). The number of PRVlabeled DRG neurons was significantly lower in the BCS group than in the SHAM group (SHAM: 106.1 ± 7.8; BCS: 63.6 ± 8.4;P< 0.000,n= 8,Figure 4D). There was no significant difference between the BCS and ANG groups (BCS: 63.6 ± 8.4; ANG: 64.6 ±10.2;P= 0.793,n= 8,Figure 4D). On this basis, we performed immunofluorescence staining for CGRP positive cells in the DRG. CGRP positive cells are mainly responsible for conducting nociceptive and temperature sensations (McCoy et al., 2013).We found many CGRP positive cells labeled in the DRG (Figure 4CandE). Many DRG cells were co-labeled with both CGRP and PRV in the ANG and BCS groups (Figure 4CandF).
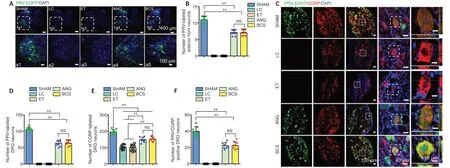
Figure 4|PRV-labeled motor and sensory neurons 12 weeks after surgery in the rats with sciatic nerve injury repaired with BCS.
It is worth investigating whether the regenerated sciatic nerve established functional connections with the brain. Brain sections from the SHAM, ANG, and BCS groups showed that PRV-labeled neurons were mainly located in the reticular nucleus (Figure 5A), vestibular nucleus (Figure 5B), and cerebral cortex (Figure 5C). In addition, because of the transsynaptic ability of PRV and the complex connections between brain regions, PRV labeled neurons were also observed in the medial preoptic area, lateral hypothalamus,periaqueductal gray, red nucleus, and locus coeruleus (Figure5DandE). However, no PRV labeled neurons were observed in the LC or ET groups. There was no statistically significant difference in the number of PRV-labeled neurons in the reticular nucleus (BCS: 354.6 ± 29.5; ANG: 347.8 ± 35.9;P=0.779,n= 4), vestibular nucleus (BCS: 67.8 ± 2.2; ANG: 65.5± 3.4;P= 0.494,n= 4), and M1 region of motor cortex (BCS:167.8 ± 18.9; ANG: 172.3 ± 24.0;P= 0.744,n= 4) between the BCS and ANG groups (Figure 5F–H). The number of PRVlabeled neurons in all three nuclei was lower in the ANG and BCS groups than in the SHAM group (allPs< 0.01).
BCS enhances sensory and motor functional recovery
The left and right footprints of rats in each group were collected after surgery (Figure 6AandB). The SFI in the SHAM group remained close to –8.8 for the 12 weeks after surgery(Figure 6C), indicating that the motor function in this group was unaffected. From 1 to 12 weeks after operation, the SFI in the LC and ET groups remained around –100. In the ANG group, the SFI began to increase the 4thweek after surgery,and continued to increase gradually through the 12thweek.In the BCS group, the SFI began to increase the 6thweek after surgery, and continued to increase gradually through the 12thweek. Twelve weeks after surgery, the SFI did not differ between the ANG and BCS groups (ANG: –52.96 ± 6.29; BCS:–53.34 ± 5.02;P= 0.901,n= 5,Figure 6C). This suggests that with the passage of time, motor function in the BCS group gradually recovered to the same levels as are achieved with an autogenous nerve graft.
We used a pinprick assay to evaluate the recovery of sensory function in the plantar skin of each group (Figure 6D). The five response areas (from A to E) in the SHAM group responded quickly and forcefully to the pinprick, resulting in a pinprick score of 5 (Sakuma et al., 2016). In the LC and ET groups, the pinprick score was 0 throughout the experimental period.At the 4thweek after surgery, one of the five reaction areas of the injured foot in the ANG group began to respond to the pinprick. Over weeks 4 to 12, the number of areas that responded gradually increased. Results were similar in the BCS group, with initial responses in one area beginning at the 5thweek after surgery. At the 12thweek, all designated areas of the injured foot in the BCS group responded to the pinprick.
BCS relieves muscle atrophy
Twelve weeks after surgery, we removed the left and righttibialis anterior muscles from rats in each group (Figure 7A).Atrophy of the muscle on the injured side was severe in the LC and ET groups. Tibialis anterior atrophy also appeared in rats from the ANG and BCS groups, but was less than in the groups that were not treated (Figure 7C). The muscle wet weight ratio for the BCS group was significantly worse than that of the SHAM group (BCS: 0.684 ± 0.036; SHAM: 1.005± 0.049;P< 0.01,n= 4,Figure 7C), but better than those of the LC (0.292 ± 0.025,P< 0.01) and ET groups (0.285 ± 0.061,P< 0.01). There was no significant difference in muscle wet weight ratio between the ANG and BCS groups (BCS: 0.684± 0.036; ANG: 0.708 ± 0.068;P= 0.497,n= 4,Figure 7C).Next, HE staining was performed on the transverse section of the left tibialis anterior muscle to observe changes in muscle fiber diameter (Figure 7B). The diameters of muscle fibers in the SHAM, LC, and ET groups were 84.0 ± 8.98, 11.0 ± 2.58 and 12.0 ± 3.65 μm, respectively. There was no significant difference between the ANG and BCS groups (ANG: 63.5 ± 7.72μm; BCS: 61.0 ± 7.87 μm;P= 0.604,n= 4,Figure 7D). The muscle fiber diameter in both groups was significantly larger than what was observed in the LC or ET groups (P< 0.01), but significantly smaller than that of the SHAM group (P< 0.01).These results indicate that wet muscle weight is maintained,and muscle fiber diameter is larger in the ANG and BCS groups than in untreated rats, thus reducing muscular atrophy.
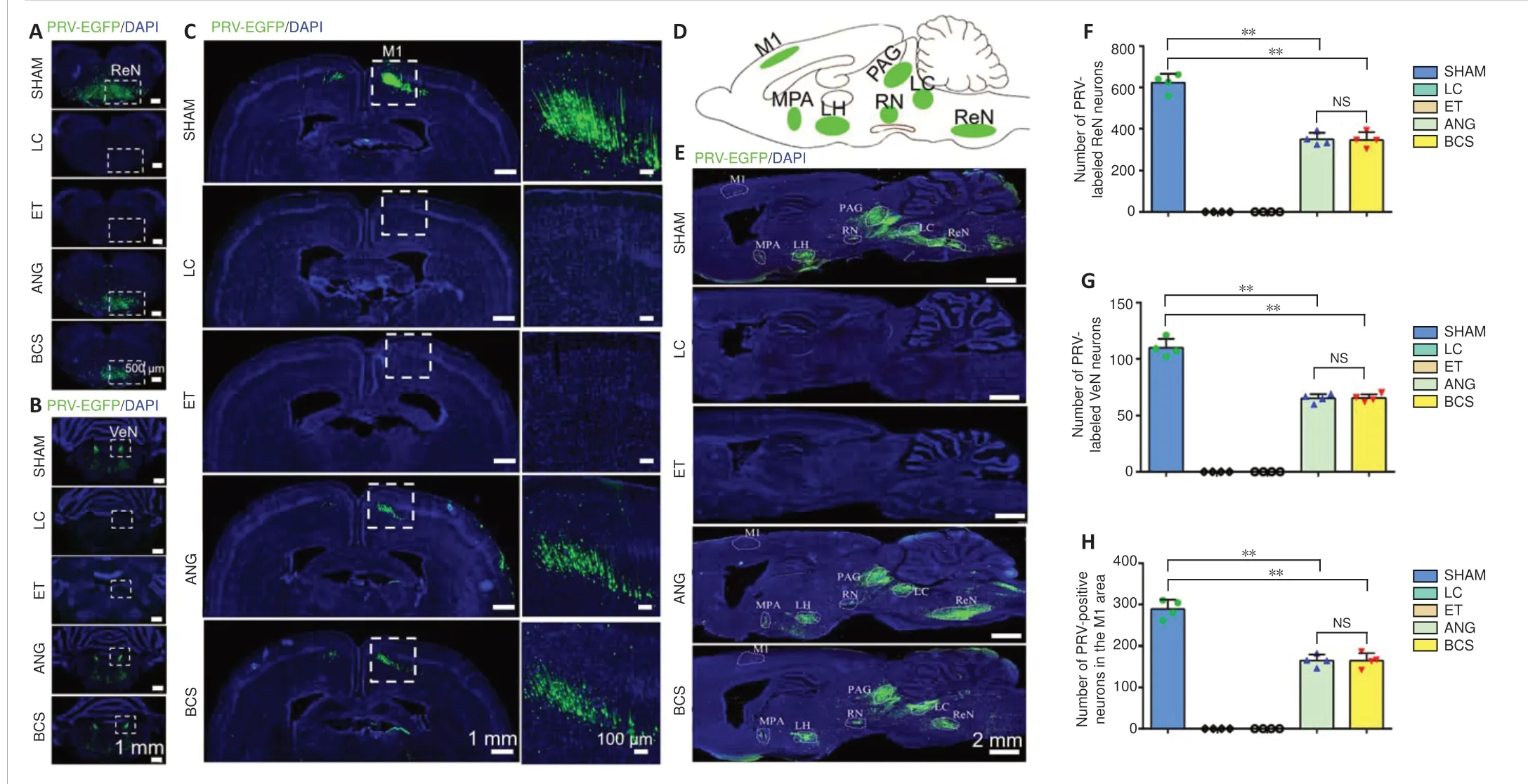
Figure 5| PRV-labeled neurons in the brains of rats with sciatic nerve injury repaired via BCS.
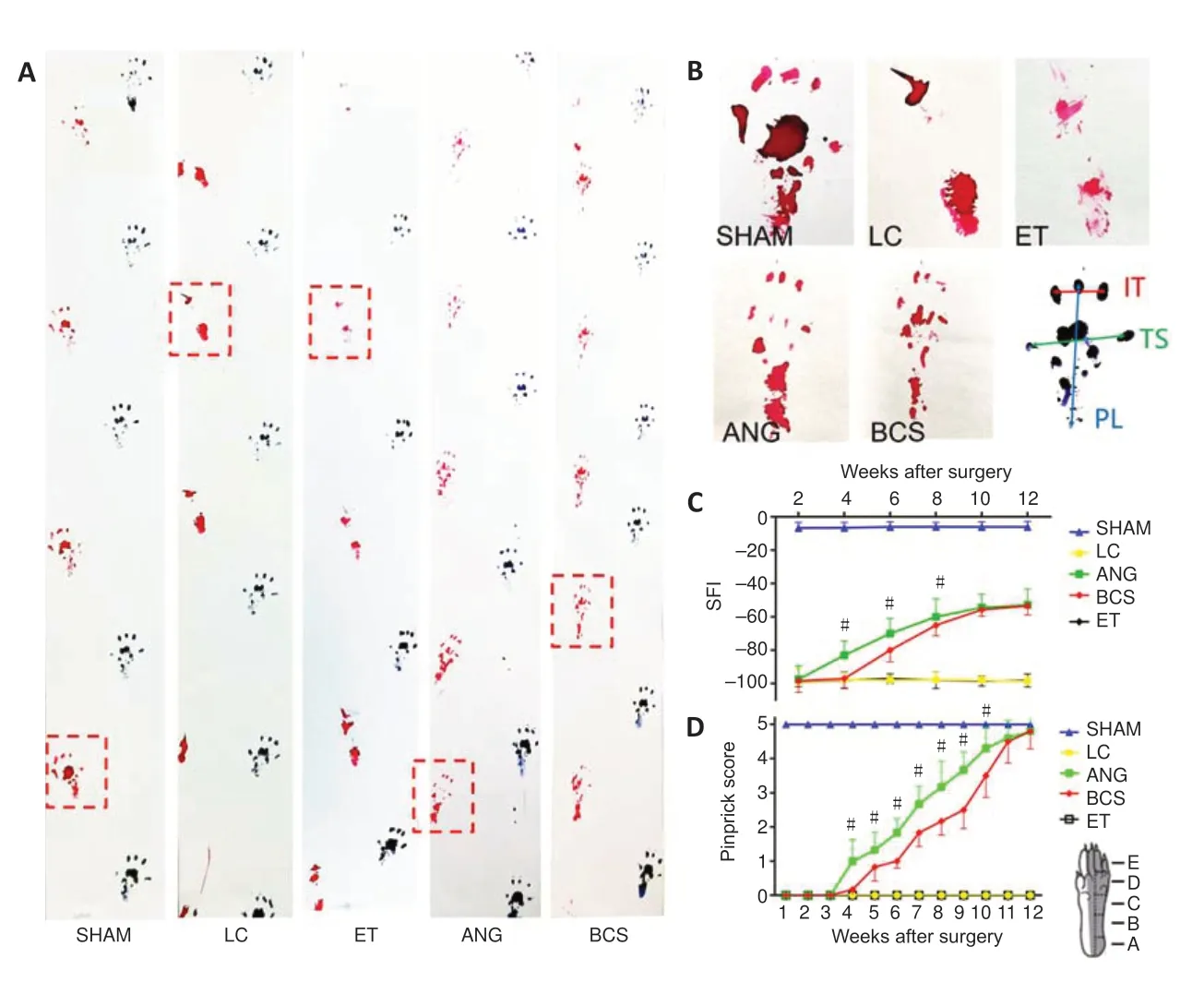
Figure 6|Behavioral motor and sensory functional recovery after sciatic nerve injury with BCS repair.
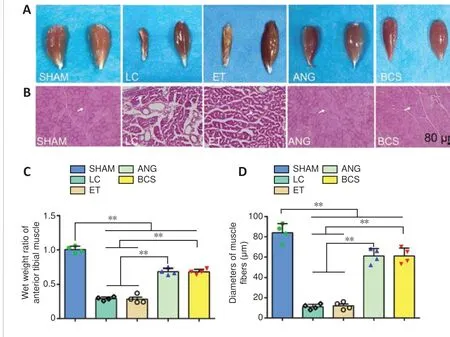
Figure 7|Atrophy of the anterior tibia muscle 12 weeks after the sciatic nerve injury and BCS repair.
BCS increases reinnervation of the motor endplate
Twelve weeks after surgery, stained AChE was brown,smooth edged, and had a clear structure in the SHAM group(Figure 8A). In the LC and ET groups, stained AChE was only observed occasionally. In the ANG and BCS groups, stained AChE was numerous, indicating the recovery of AChE activity. There was no significant difference in the IOD of AChE staining between the two groups (ANG: 848.6 ± 79.1;BCS: 829.2 ± 93.7;P= 0.745,n= 5,Figure 8B), but it was less for both than for the SHAM group (1457.8 ± 166.7,P<0.01).
To detect the remodeling of motor endplates in the rats,sections of the left tibialis anterior muscle were stained with SYN and BTX and analyzed for immunofluorescence. In the SHAM group, clear motor endplate structures were observed,including SYN+presynaptic membranes and BTX+postsynaptic membranes (Figure 8C). Similarly, numerous marked motor endplates were observed in the ANG and BCS groups.In contrast, the structures were rarely observed in the presynaptic and post-synaptic membranes of the LC and ET groups, indicating a severe loss of motor function. There was no significant difference in the reconstructed motor endplate areas between the ANG and BCS groups (ANG: 2018.0 ±347.2 μm2; BCS: 1812.2 ± 148.6 μm2;P= 0.128,n= 5,Figure8D), but both areas were smaller than what was seen in the SHAM group (2679.6 ± 258.8 μm2,P< 0.01). This suggests that regenerated nerves and muscles can help rebuild the motor endplate, thus restoring the contractive function of muscles.
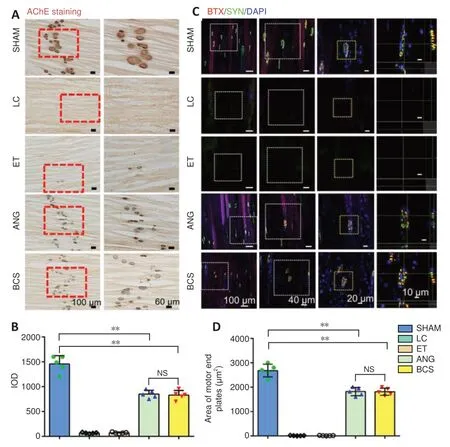
Figure 8|Reconstruction of motor end plates 12 weeks after sciatic nerve injury and BCS repair.
Discussion
Repair of extended peripheral nerve injury remains a great clinical challenge. Current approaches for treatment are promising, but remain insufficient compared with the functional recovery that can be achieved by gold standard autologous nerve transplantation. Although previous work has demonstrated the effectiveness of chitosan scaffolds in improving the quality of nerve regeneration and reducing muscle atrophy in rats (Brown et al., 2015), the studies were either examining short injuries or the degree of peripheral nerve repair was unsatisfactory. The new chitosan scaffold should be able to support axonal growth and nerve regeneration over an extended period of time and over long distances, with the goal of replacing autologous nerve transplantation (Meena et al., 2021). Because the empty nerve conduit lacks the growth factors to induce axons to extend to their targets, the regenerated nerve cannot cross large gaps in injured nerves (Yannas et al., 2007). NGF can promote regenerated axons to grow across the damaged area and finally extend their growth cones into the target organ to restore motor and sensory function (Makwana and Raivich, 2005). Both chitosan scaffolds and NGF have been studied in a number of experiments, and it is predictable that combining both leads in greater safety inin vivoapplication,avoiding ethical disputes and unnecessary immune reactions.However, the activity of NGF decreases rapidlyin vivoand cannot support axonal regeneration for extended periods of time (Fang et al., 2020). To solve the above problems,our group developed a new type of controlled release biomaterial, which can slowly release NGF in chitosan scaffolds and provide continuous nutritional support for axonal regeneration. The controlled release technology was based on a controlled release system for neurotrophic factors that was developed in previous studies (Hao et al., 2017; Rao et al., 2018; Oudega et al., 2019).In vivo, lysozyme hydrolyzes chitosan to oligosaccharides containing N-acetyl-glucosamine and glucosamine, thus achieving the degradation of chitosan(Pangburn et al., 1982). For the NGF-chitosan scaffold, NGF was able to be released in the chitosan scaffolds for 8 weeks,and the chitosan carrier then began to naturally degrade. The complete degradation of the chitosan scaffold took at least two years in rats.
In this study, the chitosan scaffold not only provided a traditional regeneration environment, but also improved the elasticity of the chitosan scaffold, which helped it avoid compressing and damaging the surrounding tissue. General observation and immunofluorescence staining of the regenerated nerve showed that the large gap (20 mm) in the BCS group was bridged successfully. In most studies of using scaffolds to repair peripheral nerve injury, the tracers that were used did not cross the synapse, such as fluorogold, Dil,cholera toxin B subunit, and hydrogen peroxidase (Han et al.,2015; Bozkurt et al., 2016; Zhang et al., 2020). In our study,we used a retrograde tracer which was helpful for clearly observing whether the regenerated nerve has successfully reconnected with the central nervous system. The PRV results showed that the regenerated sciatic nerves in the ANG and BCS groups reconnected with anterior horn motoneurons and sensory neurons in the DRG, and established formed synapses with neurons in the central nervous system. Xing et al. (2020)previously found that whole brain activity was significantly reduced in a rat model of sciatic nerve injury, and alterations in connectivity between resting-state networks verified the damage in motor-related functional neural circuits. In contrast, the regenerated sciatic nerves in the current study successfully established functional neural circuits similar to those of normal sciatic nerves. Combined with the results of the behavioral analysis, our results indicated that the function of the damaged nerves was partially restored in the ANG and BCS groups. We speculate that the decrease in brain activity observed in Xing et al. (2020) was mainly due to a decrease in the number of connections between the brain and neurons in the injured nerve. In that case, sensory and motor functions of the damaged nerve could not fully recover after peripheral nerve injury.
The pinprick assay showed that the rats receiving the BCS transplant had a response to mechanically induced nociceptive sensation 5 weeks after surgery. At this time, it can be speculated that the regenerated nerve has crossed the large gap in the injured area and re-connected with nociceptors in the skin. The SFI results showed that motor function in the BCS group began to recover by the 6th week.This suggests that the regenerated sciatic nerve crossed the injured area and re-innervated the muscles. It is worth noting that sensory function in rats recovered earlier than motor function, which is likely because the structure of sensory nerve endings is relatively simpler than that of motor endplates.Additionally, unlike motor fibers, nociceptor sensory fibers are not myelinated. It is possible that sensory and motor fibers regenerate at similar rates, but that subsequent myelination leads to the recovery-time differences that we observed. As a result, sensory functions can be recovered earlier and more easily, which is consistent with the results of Ma et al. (2011).
The motor endplate structure includes the presynaptic membrane, synaptic space, and postsynaptic membrane(Rudolf et al., 2019). Acetylcholine is released from the presynaptic membrane into the synaptic space and reaches the postsynaptic membrane to bind to its receptor, then it is quickly hydrolyzed by AChE to prevent continuous muscle excitation (Güller et al., 2020). If the motor endplate structure of the muscle is destroyed because of denervation and no release of acetylcholine, AChE will gradually be metabolized and disappear (Zhou et al., 2020). Therefore, we used AChE staining and immunofluorescence staining to assess the functional recovery of the motor endplate. What cannot be ignored is that animals that have physical impairments are likely to experience atrophy on the non-experimental side as well as the experimental side because of a reduced amount of overall movement. This can potentially bias the measurements and lead to a higher wet-weight ratio of muscles. The effect of unilateral sciatic nerve injury on contralateral muscle atrophy needs to be verified by further experiments.
Although the BCS transplant was able to repair extensive sciatic nerve injury in rats, the clinical application of the BCS needs to be verified in experiments using larger animals, such as sheep or rhesus monkeys. In addition, although a large number of studies have been conducted on the molecular mechanism through which NGF promotes nerve regeneration,this study focused only on the outcomes of animal experiments, but did not verify the molecular mechanisms involved in BCS-related recovery. To further improve the outcomes of chitosan scaffolds in repairing peripheral nerve injury, future BCS-based chitosan scaffolds can combine multiple neurotrophic factors.
In conclusion, chitosan scaffolds have been studied extensively in previous experiments. However, for treating peripheral nerve injury in rats, the previous chitosan scaffolds loaded with NGF either failed to reach the level of repair that can be achieved via ANG, or the length of the nerve damage was short (< 20 mm). At the same time, the duration of NGF release in chitosan scaffolds was relatively short (< 40 days), which may not be suitable for treating long (20 mm)sciatic nerve defect in rats. The current study was the first test using a chitosan scaffold containing control-released (8 weeks) NGF for repairing 20-mm gaps in the sciatic nerve of Wistar rats. The SFI and pinprick-assay score gradually improved after BCS transplant. By 12 weeks after injury,a large number of regenerated NF200+axons and S100+myelin sheaths were observed. The regenerated sciatic nerve successfully established a functional neural connection with the reticular nucleus, vestibular nucleus, red nucleus, and motor cortex. Muscle wet weight ratio was maintained,and muscular atrophy was improved. SYN+presynaptic membranes and BTX+postsynaptic membranes were observed in immunofluorescent stained sections, indicating the reconstruction of neuromuscular junctions between the regenerated nerve and muscle. Therefore, implanting a BCS led to a level of repair that was equivalent to what can be achieved with autologous nerve transplantation. Thus, BCS represents a potential new treatment option for patients with peripheral nerve injury, which has a prospect for broad clinical application.
Author contributions:Study conception? design and definition of intellectual content: FDL? PH? ZYY? XGL; literature search and experimental studies: FDL? HMD; data acquisition and analysis: FDL? HMD? FH? WZ?YDG; manuscript preparation? editing and review: FDL? PH? ZYY? XGL. All authors read and approved the final manuscript.
Conflicts of interest:The authors declare that they have no conflicts of interest.
Financial support:This study was supported by the National Natural Science Foundation of China? Nos. 31900749 (to PH)? 31730030 (to XGL)?81941011 (to XGL)? 31971279 (to ZYY)? 31771053 (to HMD); and the Natural Science Foundation of Beijing of China? No. 7214301 (to FH). The funding sources had no role in study conception and design? data analysis or interpretation? paper writing or deciding to submit this paper for publication.
Institutional review board statement:This study was approved by the Animal Ethics Committee of Capital Medical University (approval No.AEEI-2017-033) on March 21? 2017.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal? and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License? which allows others to remix?tweak? and build upon the work non-commercially? as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewers:Artur S.P. Varej?o? University of Trás-os-Montes e Alto Douro? Portugal; Zhengchao Guo? Universiteit Twente? Netherlands.
Additional files:
Additional Figure 1:The sciatic nerve was successfully regenerated at 12 weeks after sciatic nerve injury.
Additional Figure 2:Image of successful regeneration of injured sciatic nerve in the biomimetic nerve growth factor-chitosan scaffold (BCS).
Additional Figure 3:Morphological analysis of regenerated myelin sheaths at 12 weeks after sciatic nerve injury.
Additional file 1:Open peer review reports 1 and 2.
- 中國神經(jīng)再生研究(英文版)的其它文章
- Notice of Retraction
- The emerging world of subcellular biological medicine: extracellular vesicles as novel biomarkers, targets,and therapeutics
- Embolic stroke of undetermined source: identification of patient subgroups for oral anticoagulation treatment
- Activity-dependent remodeling of genome architecture in engram cells facilitates memory formation and recall
- Can lithium enhance the extent of axon regeneration and neurological recovery following peripheral nerve trauma?
- Antipsychotics preserve telomere length in peripheral blood mononuclear cells after acute oxidative stress injury

