Fabrication and characterization of mussel-inspired layer-by-layer assembled CL-20-based energetic films via micro-jet printing
Chun-yn Li ,Sheng Kong ,Dong-jie Lio ,Chong-wei An ,b,* ,Bo-yun Ye ,b ,Jing-yu Wng ,b,*
a School of Environment and Safety Engineering,North University of China,Taiyuan,030051,Shanxi,China
b Shanxi Engineering Technology Research Center for Ultrafine Powder,North University of China,Taiyuan,030051,Shanxi,China
c Taiyuan Institute of Technology,Taiyuan,030008,Shanxi,China
Keywords:CL-20-Based energetic film Micro-jet printing Polydopamine Self-assembly
ABSTRACT Three-dimensional (3D) micro-jet printing is a droplet deposition technique based on liquid-phase materials.To improve the deposition density and performance of energetic films with micro/nanoscale on an energetic chip,polydopamine (PDA) was utilized as a linker bridge to induce the in-situ self-assembly of CL-20-based energetic film via 3D micro-jet printing.The self-assembly was extensively characterized by confocal laser scanning microscopy (CLSM),SEM,power-XRD,XPS,and DSC.The performance of the self-assembled film was verified by the mechanical properties and detonation properties,and a possible self-assembly mechanism in the layer-by-layer micro-jet printing process was proposed.The results indicated PDA-induced self-assembly enhanced the physical entanglement between the binders and energetic crystal,reduced the porosity from 15.87% to 11.28%,and improved the elastic modulus and the detonation performance of the CL-20-based energetic film.This work proposes a novel and promising energetic film design and fabrication strategy to enhance the interaction between the energetic composite layers in the micro-jet printing process.
1.Introduction
Microelectromechanical systems (MEMS) pyrotechnics,including micro-pyrotechnic devices and micro-pyrotechnic trains,require small size,special-shaped structure,and compatibility with MEMS technology [1-3].Thus,energetic films,as part of micropyrotechnic trains and micro-energetic chips,need to achieve high energy output with a limited volume.For 2,4,6,8,10,12-hexanitro-2,4,6,8,10,12-hexaazaisowurtzitane (CL-20) crystals,as the primary material of the MEMS detonation agent,the safe fabrication and high detonation of energetic films are key problems to be solved.However,the fabrication of high-performance energetic films is still a core challenge in current research [4-6].Fortunately,additive manufacturing technology has proven to be an efficient method to realize the patterned design and micromanufacturing of energetic materials.Several methods have been developed to fabricate energetic films,such as direct printing,inkjet printing,and fused deposition modeling (FDM) [7-9].Compared with other 3D printing methods,micro-jet printing,which utilizes a novel bottom-up approach,has been employed to fabricate microscale patterns with high deposition accuracy[10,11].Drop-on-demand(DOD)inkjet printing technology can control the drop volume within 10 pL by controlling pressure pulses,enabling functional materials with micro/nano-level resolution [12].3D micro-jet printing is a micro-additive manufacturing method based on liquid-phase materials.Functional materials such as metal nanoparticle conductive materials [13],high molecular polymers [14],and medicinal materials [15] are deposited in liquid form in small sizes through piezoelectric or thermal control.The deposited droplets are cured by in-situ thermal curing or ultraviolet (UV)curing to fabricate complex devices layer by layer[16].Research on the 3D micro-jet printing of energetic materials has received extensive interest because of the rapid and precise fabrication of micro-sized parts to achieve a seamless combination of microelectronics and energetic materials [17-19].
3D micro-jet printing includes the formation and deposition of droplets.The formation of sediment includes surface wetting,diffusion,solvent evaporation,and entanglements between binders and crystals[20].However,energetic films fabricated by 3D microjet printing generally exhibit morphological nonuniformities due to diffusion,crystallization behavior,and weak physical entanglement during the deposition process,which is detrimental to the deposited density and performance of the energetic films.Evaporation rate,droplet volume,and droplet coalescence are used to determine the morphology of the printed energetic crystals [21].Essentially,the physical properties of ink droplets and the molecular structure of the solute are key variables that determine the uniform deposition and interlayer bonding of the composites.Several approaches improving the deposition quality have been proposed,such as single and dual solvent configurations [22],blending low molar mass organic nanoparticles with high molar mass polymers [23],and auxiliaries such as surfactants and functional additives [24,25].Among them,the addition of functional additives to improve the intermolecular binding force was found to be an effective way to enhance the adhesion between the energetic crystals and the polymer matrix in polymer-bonded explosive energetics.In recent years,polydopamine (PDA),a biomimetic material inspired by mussels,has been widely used in composite materials.PDA is a universal adhesive,adhering to various substrates during in-situ polymerization,and is an excellent interfacial modifier for the construction of nanocomposites [26,27].Recently,PDA has been applied to energetic materials,including aluminum,cyclo-tetra-methylene-tetra-nitramine (HMX),2,4,6-trinitrobenzene (TATB),and 2,6-diamino-3,5-dinitropyrazine-1-oxide (LLM-105) [28-30].Through its adhesive properties,reducibility,high thermal stability,and high carbon production,PDA is able to encapsulate these materials between layers or between energetic materials and binders.
CL-20,as one of the most energetic materials,combines the materials such as binders and aluminum to form different energetic composites,which are widely used in propellants,pyrotechnics,and booster agents for MEMSs [31,32].CL-20-based energetic film was reported to modify the internal structures of the system and improve the interlayer and intralayer bonding quality and the deposited density.The physical properties,deposition behavior,and binding mechanism of the molecule in the energetic films have been tuned to explore ink formulation that affects printing fluency and packing density[33,34].Herein,the CL-20-based energetic ink,combined with a composite binder,such as 3,3-bis(azidomethyl)oxetane-tetrahydrofuran (BAMO-THF) copolyether [35],or nitrocellulose (NC),was modified by adjusting the ratio of PDA to explore the interfacial reinforcement mechanism.The micromorphology of the CL-20-based energetic film fabricated by 3D microjet printing using optical microscopy(OM),laser scanning confocal microscopy (LSCM),and scanning electron microscopy (SEM) was observed to analyze the effects of PDA-induced self-assembly.The structure of self-assembled energetic films was verified by X-ray diffraction (XRD),X-ray photoelectron spectroscopy (XPS),and differential scanning calorimetry (DSC).Nanoindentation technology was conducted to test the mechanical properties of the energetic films.Finally,a miniature detonation sequence with a small size was prepared by 3D micro-jet printing using CL-20-based inks to verify the effect of molecular self-assembly on detonation performance.This is the first report on the application of PDA in improving interfacial interaction to achieve the powerful accumulation effect of energetic films with 3D micro-jet printing.The present work will potentially provide a novel approach for the high-quality printing of energetic composites.
2.Experimental
2.1.Materials
ε-CL-20(mean particle size:30 μm)was provided by the China North Industries Group Corporation.BAMO-THF copolymer(BAMO/THF =5:5,hydroxyl value: 0.36 mmol/g,weight-average molecular weight: 5500 g/mol) was obtained from 806 Research Institute.NC with 11.9-12.4%N was produced by Sichuan Northern Nitrocellulose Co.Ltd.,China.Ethyl acetate (AR) was purchased from the Tianjin Fuchen Chemical Reagent Factory.Dopamine(DA)and trimethylaminopropane (tris) was purchased from Saran Chemical Technologies Co.,LTD.
2.2.Preparation of PDA
PDA was prepared via in situ polymerization,as shown in Fig.1(a).First,tris buffer solution (pH: 8.5) was prepared using hydrochloric acid,and then DA was dissolved in tris buffer solution to prepare a 2 mg/mL DA solution.Finally,PDA was obtained from DA self-aggregates using a magnetic stirrer(500 rpm,25C,24 h).PDA nanoparticles obtained by centrifugation and freeze-drying were stored in desiccators [28].
2.3.Preparation of printable energetic ink
All-liquid energetic ink was adopted.Ethyl acetate has a low boiling point,and its solubility with CL-20 can reach 45% [36].NC and BAMO-THF were selected as energetic binders and plasticizers,respectively,because of their thermal gelation characteristics,high adhesion,and high-energy properties.To satisfy both the viscosity and printing accuracy requirements,the physical properties of the adhesive inks were explored.The rigid fiber polymer(NC)and the flexible energetic polymer (BAMO-THF) were dissolved in ethyl acetate at five different NC/BAMO-THF ratios(0:10,3:7,5:5,7:3,and 10:0;the total polymer is fixed as 2.5 wt.%),and the binder inks were denoted as NP,NP,NP,NP,and NP,respectively.The hybrid mixture was found to be very stable with no evidence of separation.PDA is slightly soluble in ethyl acetate;thus,PDA with a mass fraction in the range of 0-0.5% was used to explore the stability of the ink.The basic CL-20-based energetic ink formulation was prepared by dissolving 22.5 wt.% CL-20 in ethyl acetate with the following binders: 0.75 wt.% NC and 1.75 wt.% BAMO-THF.Ultrasonic mixing was performed for 30 min in a sealed container,which was denoted as ink-1.The modified energetic inks were prepared by dissolving 0.1 wt.%,0.3 wt.%,and 0.5 wt.%of PDA in ink-1 and mixing for 30 min in a sealed container.Finally,after standing for 1 h,a filtration sieve with an aperture of 0.02 mm was used to remove impurities and to confirm that PDA was well-dispersed in the energetic ink.The completed mixtures were denoted as ink-2,ink-3,and ink-4,respectively(Fig.1(b)).
2.4.Fabrication of CL-20-based energetic film

Fig.1.Schematic diagram of 3D micro-jet printing molding process with energetic ink.
A 3D micro-jet printing device composed of a piezoelectric print-head (Nordson EFD,Westlake,Ohio;nozzle diameter:100 μm) and a three-axis positioning stage (Xiamen Twinwin Automation Technology Co.Ltd)was used to print the energetic ink.Fig.1(c) schematically illustrated the deposition process of energetic ink.The energetic ink was transferred to a 10 mL syringe,and the energetic film was printed at the horizontal position designated by the computer-controlled three-axis positioning stage.The printing speed and substrate temperature were set at 40 mm/s and 55C,respectively[34].Waveform parameters were adjusted to the optimal printing process by observing the deposited droplets on the glass substrate(spattered droplets or single droplets) using an optical microscope.The four inks can form stable droplets under a voltage amplitude of 120 V,a pulse width of 0.3 μs,and pulse displacement of 40%.Based on the optimized parameters for the droplets,a straight line was obtained at a frequency of 200 Hz.The complex pattern and 200 layers of 30 × 5 mmCL-20-based energetic film were obtained by 3D micro-jet printing and in-situ thermal evaporation on a glass substrate,as shown in Fig.1(d),and then dried in an oven at 55C for 24 h.The energetic films printed with ink-1,ink-2,ink-3,and ink-4 were marked as CPN,CPN-0.1%,CPN-0.3%,and CPN-0.5%,respectively.
2.5.Characterization and testing
The MCR302 rheometer (Anton Paar,Germany) was used to characterize the viscosity of the ink at a shear rate of 40 sat 25C,and the average viscosity was obtained through the instantaneous viscosity within 120 s.The surface tension was measured by a QBZY-3 automatic surface/interface tensiometer (Fangrui,China)using the platinum plate method,and the ink density was measured with an electronic densitometer (MZ-220SD).The average values of the surface tension and density were obtained by repeating the test in triplicate.The deposited surface morphology of the single-layer energetic film was observed by LSCM(Carl Zeiss LSM900),and the morphology of the multilayer energetic film was characterized by SEM (Czech TESCAN).The packing density and porosity of the printed energetic film were tested using an electronic densitometer(MZ-220SD).The packing density was tested in triplicate,and the average was taken.The crystalline properties of energetic films were characterized by XRD(DX-2700 X-ray,CHINA)using 40 kV and 30 mA Cu-Kα radiation.The films were characterized in the 2θ range from 5to 50with a step scan of 0.02.XPS(Thermo,ESCALAB 250Xi,USA) was used to precisely investigate the composition of CL-20-based energetic film.Mono Al-Kα(=1486.6 eV)ray was used,and operated at 12.5 kV and 16 mA,with the CAE scan mode.The full spectrum of passing energy was 50 eV and the step length is 0.05 eV.The pass energy and step length of the narrow spectrum test is 20,0.05 eV,respectively.All samples were fitted using Avantage XPS software with background lines added,and the fitting peak width of each peak was kept consistent.Thermal analyses were performed on a DSC (Setaram,DSC 131,France) at heating rates of 5,10,15 K/min,and 20 K/min from 25 to 350C under an Natmosphere with its flow of 30 mL/min.The low-load in-situ nanomechanical testing system (US,Hysitron) was adopted for mechanical performance testing.A wedge-shaped indenter was selected,and five test points were randomly selected on the prepared films.The maximum loading of the material was set as 300 mN to determine the elastic modulus and hardness of the energetic composites during the loading process [37].A microchannel detonation experiment was designed to test the detonation performances of the CL-20-based energetic film,including their critical size and velocity of detonation.The microchannel design is shown in Fig.2,and the width of the microchannel was set to 1 mm.The calculation method for the critical size and velocity of detonation can be found in the literature[38].
3.Results and discussions
3.1.Ink formulations and jetting performance
Proper jetting is related to different ink properties,namely viscosity,density,and surface tension.Among these properties,surface tension and viscosity are the most crucial [39].The Reynolds and Weber numbers define the behavior of the ink for droplet formation in 3D micro-jet printing.
Reynolds number:
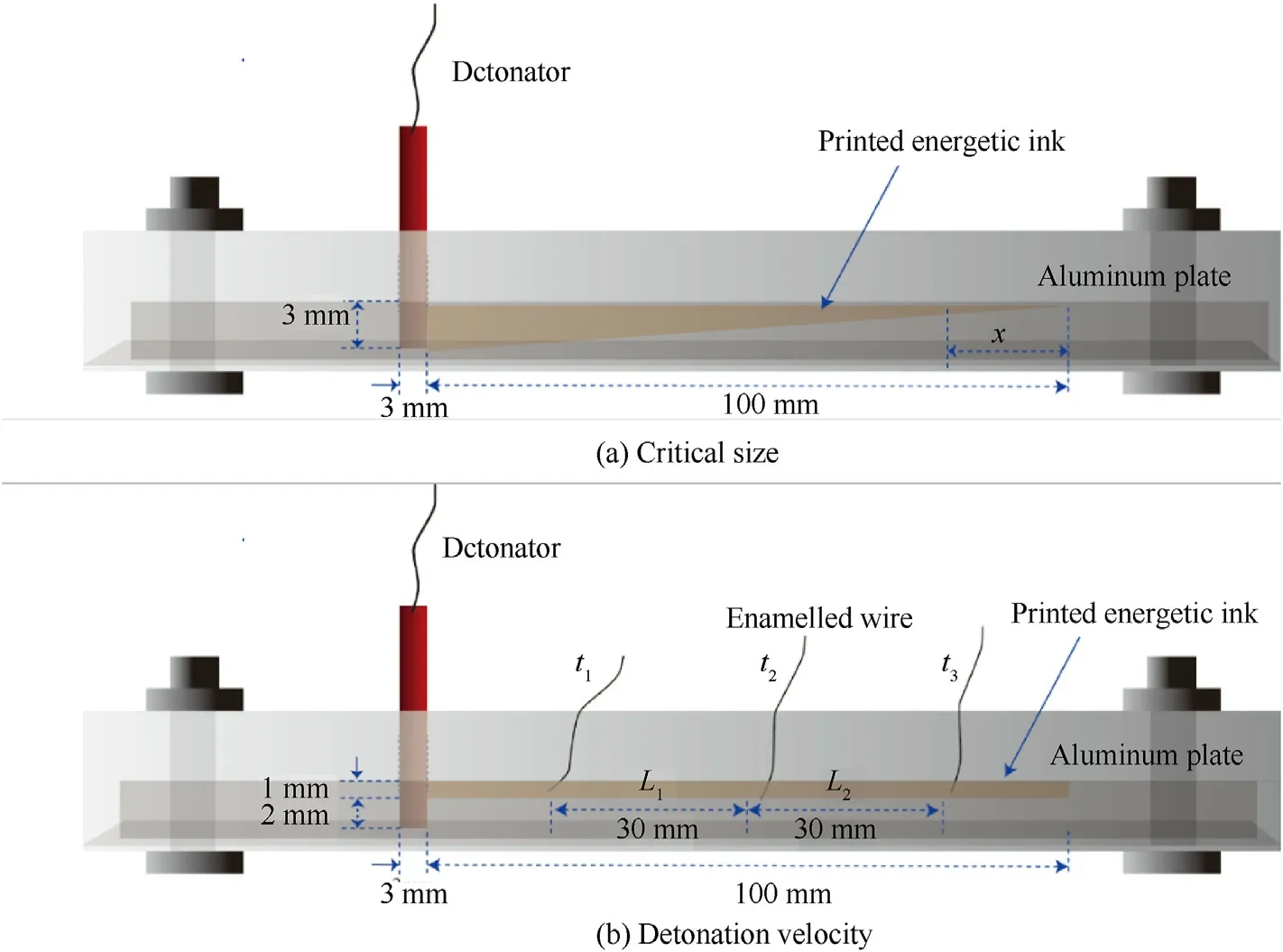
Fig.2.The detonation performance testing devices of the CL-20-based energetic film: (a) Critical size;(b) Detonation velocity.

Weber number:

Z value:

where ρ,υ,η,γ,andrepresent the density,velocity,viscosity,surface tension of ink,and nozzle diameter.,the number of Ohnesorge,is a non-dimensional number related to the viscous force,representing the surface tension and inertial force.Thevalue is the reciprocal of Ohnesorge,and the printable ink is characterized by a specific range of thevalue:1 <<10[40].The physical properties and calculation results of the binder and the energetic inks with different PDA content are listed in Table 1.
According to thevalue in Table 1,the printable range of the binder ink was between NPand NP.Combined with a previous work [33],the formulation was fixed to 3 wt.% NC and 7 wt.%BAMO-THF.Table 1 indicates that the viscosity of four energetic inks was~7 mPa·s,and the surface tension was~25 mN/m.PDA was shown to be have little effect on the physical properties because of the low concentration of PDA in the ink,and four inkswith≈7 could form stable droplets.Fig.3 shows the printed droplets of the four inks after drying at 55C;each had spread to an equilibrium diameter of~210 μm,which was consistent with the contact angle and was independent of the PDA content [41].As shown in Fig.3(a),the crystals tended to move outward and accumulated near a contact line to form a coffee-ring effect,which was induced by radial outward flow caused by the non-uniform evaporation rate in the radial direction.However,0.1 wt.% PDA reduced the coffee-ring effect and had no significant effect(Fig.3(b)).The deposition of the crystals was more uniform when 0.3 wt.% PDA was incorporated into the energetic ink,as shown in Fig.3(c).Fig.3(a1)-(d1) depicted the relatively uniform lines obtained with different inks,and the cross-sectional profile of the 3D image was quantitatively analyzed to obtain the coating depth of the crystals.As shown in Fig.3(a2)-(d2),the thickness was~8 μm and the width was~260 μm.The 0.5 wt% PDA droplets yielded a much more homogeneous pattern (Fig.3(d2)) than the 0.3 wt.%PDA droplets(Fig.3(c2)).The intensity of the PDA droplet gradually increased toward the droplet center,which may be caused by the strong Marangoni flow carrying most of the crystals toward the center.PDA particles exhibited strong radial inward and weaker outward movement in the droplets (Fig.3(b)-(d)) [42,43],indicating that the nano PDA particles promoted the uniform deposition and nucleation of energetic crystals in microdroplets.However,as shown in Fig.3(d1),the line of ink-4 showed a sawtooth shape,indicating an unstable state.According to the stability of ink drop formation and the quality of the printing line,the PDA content was determined to be 0.3 wt.%.
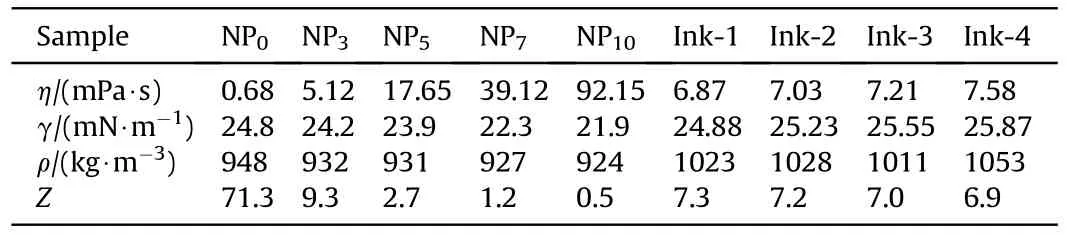
Table 1 Physical properties for CL-20-based energetic inks (20 °C).
3.2.Density and morphology analysis
A 3D micro-jet printing device was used to deposit ink-1 and ink-3 to obtain energetic films,and the theoretical density (TMD),calculated using EXPLO5,was 1.941 g/cm.The packing density of ink-1 and ink-3 were tested to 1.63 g/cmand 1.722 g/cm,respectively.The porosity of CPN film reached 15.87%and may have been caused by solvent evaporation during ink deposition.Furthermore,weak intermolecular forces would create numerous pores between the crystals and polymer molecules,forming lowdensity composites.The porosity with ink-3 decreased to 11.28%in as the packing density increased,indicating that PDA acted as an intermediate molecular self-assembled filler and increased intermolecular interactions [44].
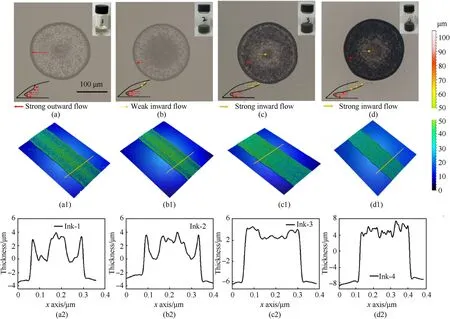
Fig.3.Optical microscope images of single droplets,3D images of uniform lines,and corresponding cross-sectional profiles obtained from energetic inks with different PDA contents using LSCM under the same conditions: (a)-(a2) Ink-1;(b)-(b2) Ink-2;(c)-(c2) Ink-3;(d)-(d2) Ink-4.
Fig.4 shows the surface and cross-sectional microstructure of CPN and CPNP-0.3%energetic films.As shown in Fig.4(a),a uniform and smooth energetic film surface was observed.Fig.4(a1) shows micro/nano-scale particles and also the specific pore structure on the surface of the CPNP film.The recrystallized ε-CL-20(Fig.4(a2))is a kind of polygon-shaped crystal with a particle size varying in the range of 0.6-1 μm.The different evaporation rate of the solvent at different positions during the ink drop deposition process led to a difference in the growth rate of the crystal nucleus [45],which is consistent with the uneven deposition of a single droplet shown in Fig.3.Moreover,the weak binding between the polymer binders and explosive crystals increased the voids in the specimens.Based on the dual network binder,the addition of PDA significantly improved the crosslinking density of the dual crosslinked polymer network (Fig.4(b1)).The recrystallized CL-20 (Fig.4(b2)) in the CPNP film is a flake-like crystal with a particle size of~200 nm.Compared with the CPNP film,the particles of the modified crystals on the CPNP film had significantly reduced in size,and their morphology and porosity had changed noticeably.Fig.4(b1)shows that the particle size distributions in the entire film surfaces were more uniform.The surface was almost free of defects,indicating a limited crystal nucleation rate and reduced micro-defects and pores generated by the energetic crystals [26,31].
The cross-sectional morphology of the CPN film is shown in Fig.4(c1),(c2),and (c3).The CPN film presented irregular shapes and large interlayer holes(Fig.4(c2)) with a single-layer thickness of~10 μm.It should be noted that under the same 3D micro-jet printing conditions,the uneven deposition between layers indicated weak physical entanglement and weak intermolecular bonding between molecules.Fig.4(d1) and (d2) show the SEM cross-sectional images of the CPNP-0.3%,from which the CPN layer structure was formed by bonding.The layer thickness was~8 μm,and there were no obvious pores or fault structures between layers.This indicated that CL-20 had achieved molecular assembly under the PDA interface material,forming more hydrogen bonds with PDA and binders [46].Compared with the differences noted in Fig.4(c2) and (d2),the surface and cross-section of the CPNP-0.3%film showed smaller crystal particle sizes.The layered structure was more compact and uniform,which can be attributed to the energetic ink modified by PDA.The presence of PDA improved interfacial interaction between the layers and increased physical entanglement and hydrogen bond self-assembly between the BAMO-THF molecular chain,the rigid NC molecular skeleton,and CL-20.
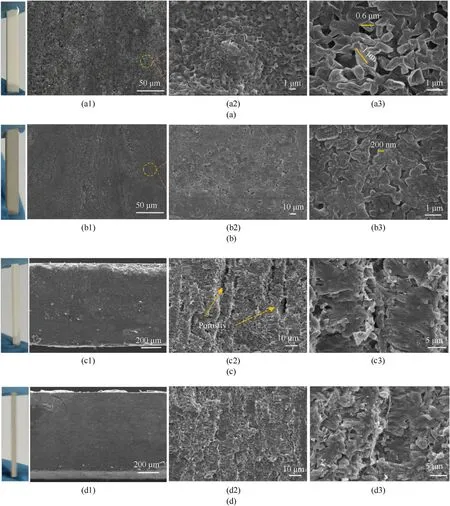
Fig.4.SEM images of the surface and cross-section of CL-20-based energetic film: (a) and (b): CPN film;(c) and (d): CPNP-0.3% film.
3.3.Analysis of crystal structures
XRD measurement was employed to investigate the phase transformation of CL-20 in the 3D micro-jet printing process.Fig.5 depicted the crystalline phase analysis of the raw CL-20 and two CL-20-based energetic film.Fig.5(a)showed the XRD spectra of raw CL-20,which was the same as the standard ε-CL-20 pattern (PDF Card 00-050-2045)[47],such as 12.7,13.9,and 30.4.As shown in Fig.5(b)and(c),the CPNP film and CPNP-0.3%film was analyzed to observe the change in the crystal phase.The characteristic peaks of the two films,such as 13.7,24.2,and 28.35,corresponding to the(111),(212),and(132)crystal planes,belonging to β phase(PDF Card 00-052-2432) [45],which revealed that the 3D micro-jet printing process brought about phase transition.PDA-induced self-assembly has no effect on the transformation of crystal form.Ethyl acetate with a large dipole moment and high solubility was used in ink.The formed solution with a large dipole moment had a greater induction effect on CL-20,resulting in the conversion to the β phase.When droplets were deposited on a heated substrate(50C),ethyl acetate was found to be a good solvent for CL-20,as the high evaporation rate was more conducive to the formation of a metastable β-CL-20 crystal phase.Meanwhile,the distance between the 3D micro-nozzle and the substrate was between 1 mm and 2 mm,which was not conducive to the transformation from the metastable β phase to the stable ε phase in time and space.
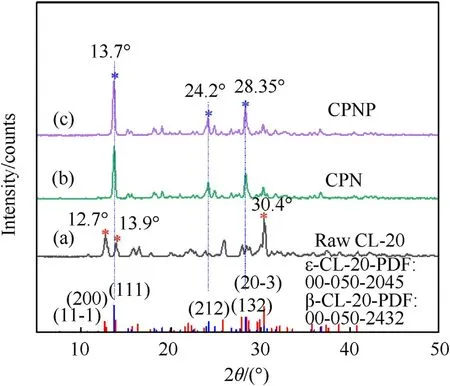
Fig.5.X-ray powder diffraction spectra of:(a)Raw CL-20;(b)CPN film;(c)CPNP-0.3%film.
3.4.Elemental analysis
The surface element analysis of three samples was measured by XPS to further detect the PDA-induced self-assembly of CPNP film.Fig.6(a) depicts the full spectra of the chemical state of CPN film and the CPNP film after 200 layers of printing.The raw ε-CL-20 was used as a comparison and surface element analysis was performed.Comparing the CPNP-0.3%film with the CPN film and the raw ε-CL-20,the intensity of C1s increased,while the intensity of N1s and O1s decreased,illustrating the presence of PDA on the CPNP film[27].Representative high-resolution spectra of the C1s,N1s,and O1s regions of the specimens were presented in Fig.6(b),(c),and(d).As shown in Fig.6(b),the peaks of the C1s region in raw ε-CL-20 were fitted to two peaks:C-N(288.7 eV)and C-C/C-H(284.8 eV).The peaks of N1s region in raw -CL-20 appeared at 406.8 eV and 401.2 eV,which were attributed to the-NOand N-N,respectively,in the ammonium nitrate functional group.The peak at 533.8 eV in the O1s spectrum of raw ε-CL-20 was assigned to-NO,which was consistent with the literature [32].Compared with characteristic peaks of raw ε-CL-20,new bands were observed in the C1s,N1s,and O1s spectra of CPN film and CPNP film.For the C1s spectrum,the new peak assigned to the C-O component appeared at 286 eV,corresponding to the ether bonds of BAMO-THF and NC.After PDA modification,CPNP film (0.486) exhibited a lower N/C ratio than CPN film (0.597),which was the values observed for the CPN film and PDA [28,32].The C-O peak intensity of CPNP film was higher than that of CPN film owing to the presence of C-OH.For the N1s spectrum,the N=N+=N-peak belonging to BAMO-THF appeared at 404.2 eV in the N1s peaks,demonstrating successful permeation between explosive particles.The peak at 401 eV was assigned to O-NO,which corresponded to the nitrate ester group of NC.The emerged peak at 400.3 eV from N1s spectrum was attributed to-NHof PDA.The O1s spectrum of the CPN film presented three characteristic peaks at 533.8,532.8,and 532 eV,respectively,which were consistent with the O element in the -NO,C-OH,and C-O bond,respectively [27].After the addition of PDA,the peak intensity of C-OH in CPNP film was attributed to PDA,which proved the successful self-assembly of CL-20 and PDA.
3.5.Thermal stability properties
To characterize the compatibility and thermal stability of the CPNP-0.3%film,the DSC curves in Fig.7 and the kinetic parameter in Table 3 demonstrated the thermal decomposition performance of the CL-20-based energetic film under four heating rates(5,10,15,and 20 K/min).As shown in Fig.7(a) and b,the decomposition behavior of raw ε-CL-20 included an endothermic process of crystalline transition and the strongly exothermic decomposition process.The first peaks appeared at 160-175C,which was related to the ε-γ phase transition temperature of raw CL-20 [47],and the strong exothermic peak at 245-270C was caused by thermal decomposition of the carbon skeleton.For BAMO-THF/NC composite binder,two distinct exothermic peaks were observed in Fig.7(c).The temperature ranged of the first and second exothermic stages are 199-218C and 251-274C,which were consistent with the exothermic peaks of NC and BAMO-THF,respectively [35].After being physically cross-linked with CL-20 by 3D micro-jet printing,the decomposition peak temperature of NC was delayed by about 20C.Meanwhile,the transformation of the crystal phase increased the decomposition temperature of CL-20 by 5C.The phase transition temperature of CPN film and CPNP-0.3% film disappeared,as shown in Fig.7(d) and (e).A possible reason was the transition of the ε-β phase during recrystallization [45].The decomposition temperature range of CPNP-0.3% film was 242-262C.Compared with CPN film,the change of decomposition temperature was less than 1C,indicating that PDA had good compatibility with CL-20.Besides,Fig.7(e)showed that the two exothermic peaks tended to be parallel,which may be due to the interfacial interaction that promoted PDA to transfer hot to CL-20 more effectively under the heating process.These results were attributable to the interfacial transfer provided by the PDA for the heating process.Therefore,the thermal stability of the composites also can be remarkably improved by thermal decomposition kinetic parameters.
Based on the measurement results,the kinetic parameters of thermal decomposition reactions,which are listed in Table 2,were calculated using Kissinger,Ozawa,and Starink method [48].
Kissinger:
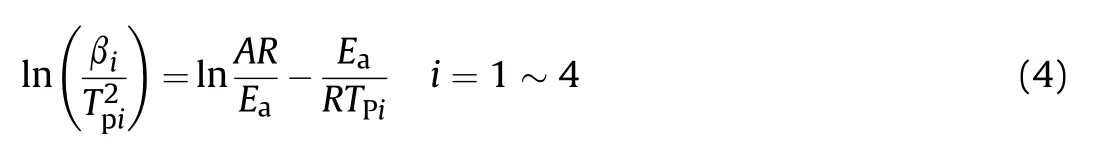
Flann-Wall-Ozawa:
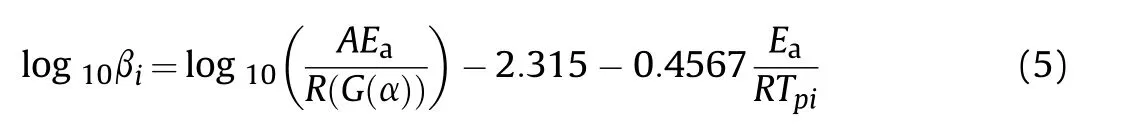
Starink:

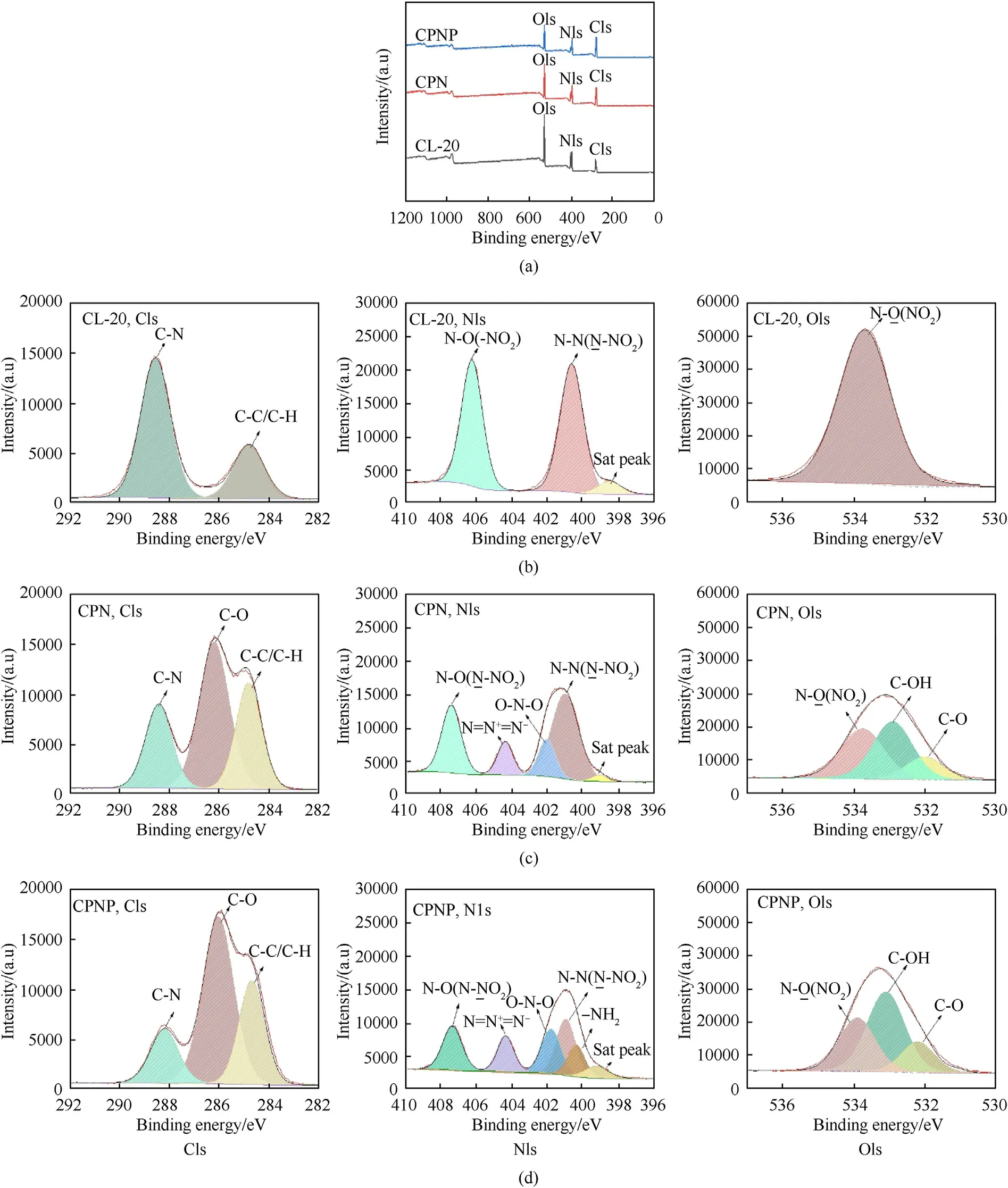
Fig.6.XPS spectra of film coatings: (a) Survey of elements;(b) C1s,N1s,and O1s spectrum of raw CL-20;(c) CPN film;(d) CPNP-0.3% film.
where βis the heating rate,K/min;is the initial peak temperature,K;is the molar gas constant,8.314 J/(mol·K).is the activation energy,andis the pre-exponential factor.The Kissinger method(Eq.(4))[47]is used to perform linear regression fitting of ln (β/) to 1/;the results are shown in Fig.7(e) and (f).is a goodness-of-fit measure.
Table 2 depicted that the activation energy of the CPNP film was reduced by 9.23 kJ/mol compared with the raw CL-20.The possible reason is that the crystal grain size in the CPNP film decreases,which leads to an increase in specific surface area and reactivity.Meanwhile,CL-20 crystals mainly exist in the metastable β phase,which reduces the thermal stability.The activation energy of CPNP film is higher than that of CPN film(3.55 kJ/mol).After adding PDA,the intermolecular force increased and the intensity of the decomposition peak decreased.The intermolecular force reduced the internal porosity,intermolecular surface energy,and atomic vibration,resulting in weakening of the heat transfer capacity of the sample and improving the stability of the material.
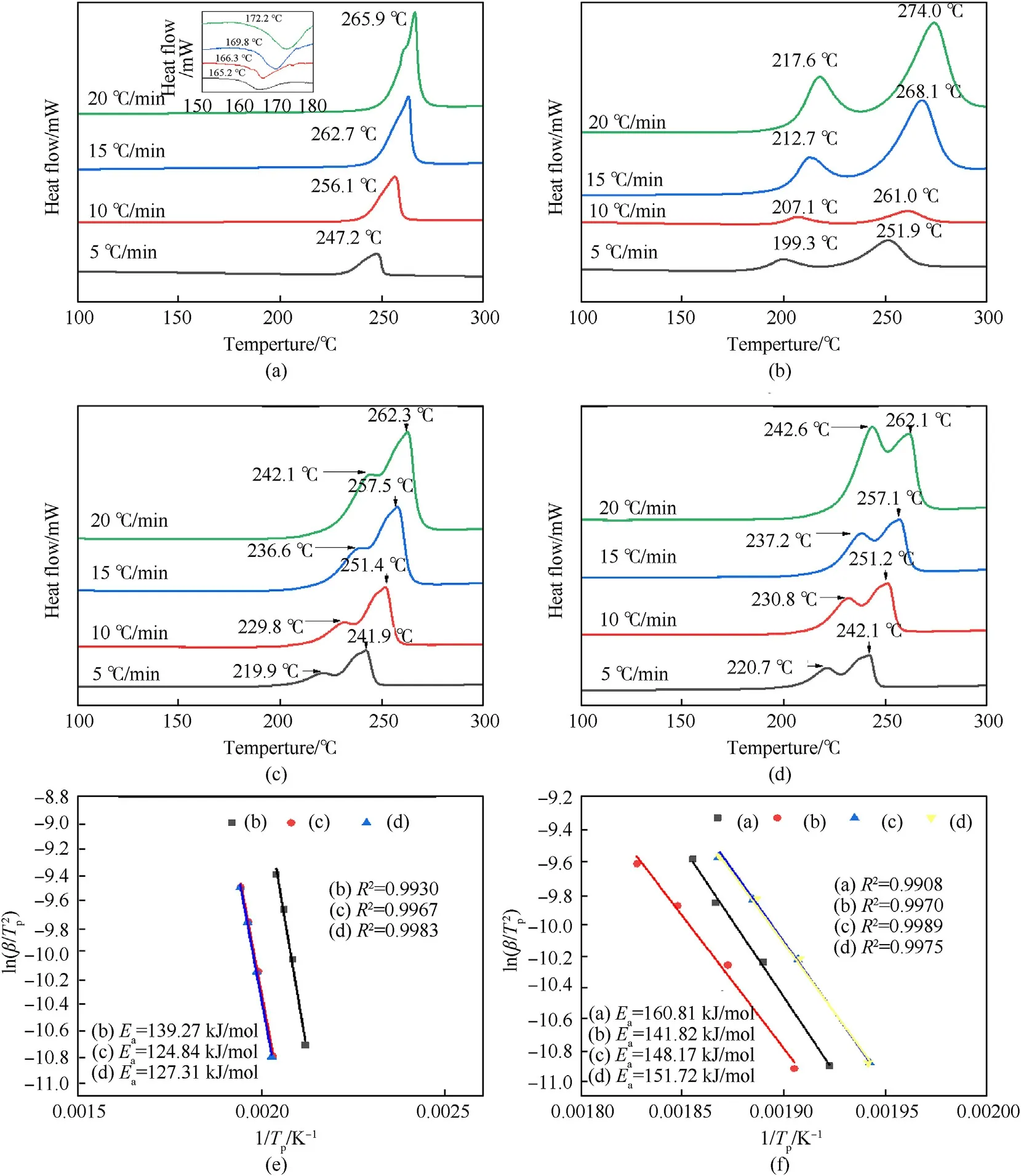
Fig.7.DSC curves of samples at different heating rates: (a) Raw CL-20;(b) BAMO-THF/NC;(c) CPN;(d) CPNP;(e) and (f) Kinetic studies on the thermal decomposition of four materials by Kissinger method.

Table 2 Thermal decomposition kinetic parameters.
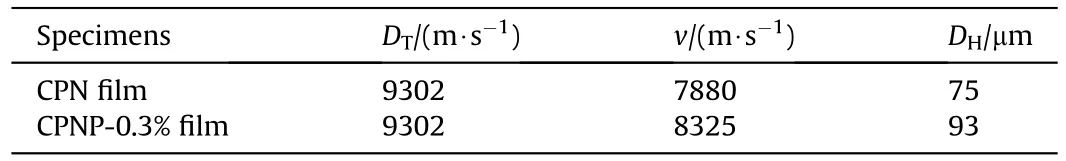
Table 3 Detonation properties of the CL-20-based energetic film.
3.6.Mechanical properties

Fig.8.Mechanical properties of CPN film and CPNP-0.3% film: (a) Load-displacement curves;(b) Modulus and hardness values.
Mechanical properties of energetic films are important parameters,which are mainly controlled by their microstructural characteristics such as porosity,pore structure,and crystal bonding[37].Nanoindentation technology for mechanical testing range in the micro/nanometer scale,including elastic modulus and hardness,was used to investigate the mechanical PDA reinforcement mechanism.The proper dispersion and strong interfacial interaction of PDA in the CPN hybrid structure were expected to greatly improve the mechanical properties of the composite.The non-deformability of films,which was universally measured by loading-unloading tests,was used to demonstrate the resistance to the loss of deformation energy.The curves of CPN film and CPNP-0.3% film was obtained by measuring the load acting on the films and the depth of the film surface,from which the elastic modulus of energetic films and hardness as the mean contact pressure in nanoindentation can be calculated,as shown in Fig.8(a) and (b).
As shown in Fig.8(a),the CPN film exhibited significant mechanical hysteresis from the loading-unloading curves,originating from the occurrence of the internal fracture [37].Compared with CPN,the indentation depth on the surface of CPNP-0.3% film decreased from 9 μm to 7 μm under the load condition,indicating that the interaction force between molecules was enhanced,and the internal friction between molecules or crystal grains was sufficiently reduced.Fig.8(b) depicted the elastic modulus and hardness at the maximum load,and the elastic modulus and hardness of CPN film were about 6.2 GPa and 0.164 GPa,respectively.The elastic modulus of CPNP-0.3% was ≈7.8 GPa,and the average hardness was ≈0.252 GPa,exhibiting an increase of 25.8%,and 53.7%,respectively,indicate that the resistance of an object to elastic deformation,plastic deformation,or failure was enhanced.The construction of PDA-induced self-assembly microstructure can yield higher strength than CPN based on sample physical entanglement.The smooth and compact microstructure was perfectly eliminating the weakness of interfacial bonding.The interfacial reinforcement effect by interactional bonding between layers was considered as the enhanced mechanism of mechanical properties.
3.7.Detonation performance
To evaluate the packing density under the small size structure,a microchannel detonation experiment was designed to test the detonation performance of the CPN film and the CPNP-0.3% film.The critical size and the detonation velocity are the important parameters for the detonation performance of explosives.The critical size was tested by depositing the energetic ink in the wedgeshaped groove using 3D micro-jet printing technology for describing the dynamic behavior of explosive detonation propagation [38,47].The corresponding critical size of explosive detonation was shown in Fig.9.Similarly,the detonation velocity of the two films was tested,as shown in Fig.10.The calculated theory of detonation velocity(),actual detonation velocity(),and critical size () of detonation were listed in Table 3.
As shown in Table 3,the energetic films fabricated by 3D microjet printing spread stably in a microgroove with a size of 100×1×(0-3)mmand exhibited excellent microscale explosion transferability.The detonation wave of the CPN film,detonated by the detonator,propagated rapidly along the wedge-shaped groove to 97.5 mm.The critical size of the detonation was determined to be 75 μm.There was a significant increase (from 75 to 93 μm) in the critical size of the explosive detonation with the addition of PDA,indicating the decreased porosity between the layers that eroded the formation of hot spots,which resulted in a slower volumetric combustion rate,a relatively wide chemical reaction zone,and increased lateral compression on the surrounding aluminum substrate,subsequently causing a large energy loss,which results in increased critical detonation size,which is consistent with the packing density.
Detonation velocity is a key indicator for testing the energy performance of energetic films.The detonation velocity of the CPNP-0.3%film after stable detonation is 8325 m/s,which is 89.5%of the theoretical detonation velocity.Compared with the CPN film,the detonation velocity has increased by 445 m/s,which is consistent with the density.It has been confirmed that PDAinduced self-assembly increases the packing density in the trench.
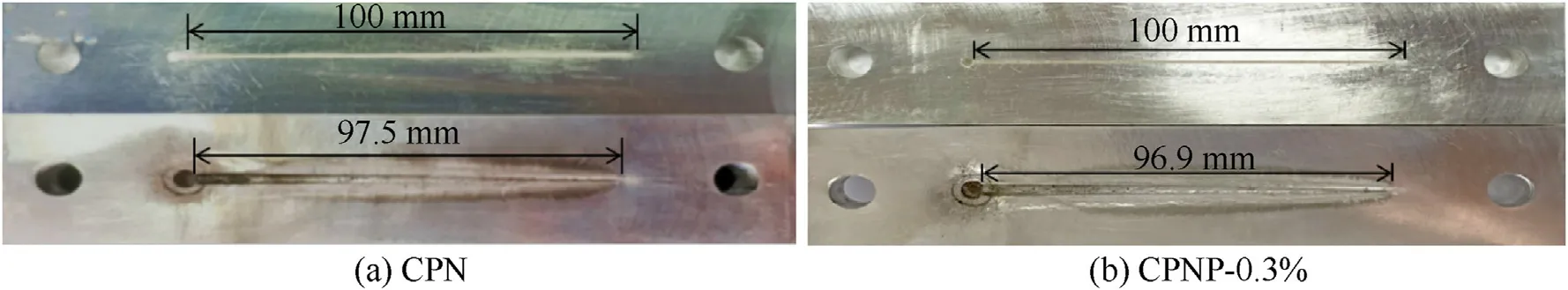
Fig.9.Photographs obtained from the detonation critical size test of energetic films: (a) CPN;(b) CPNP-0.3%.

Fig.10.Photographs obtained from the energetic film detonation velocity test of before and after detonation,respectively: (a) CPN;(b) CPNP-0.3%.
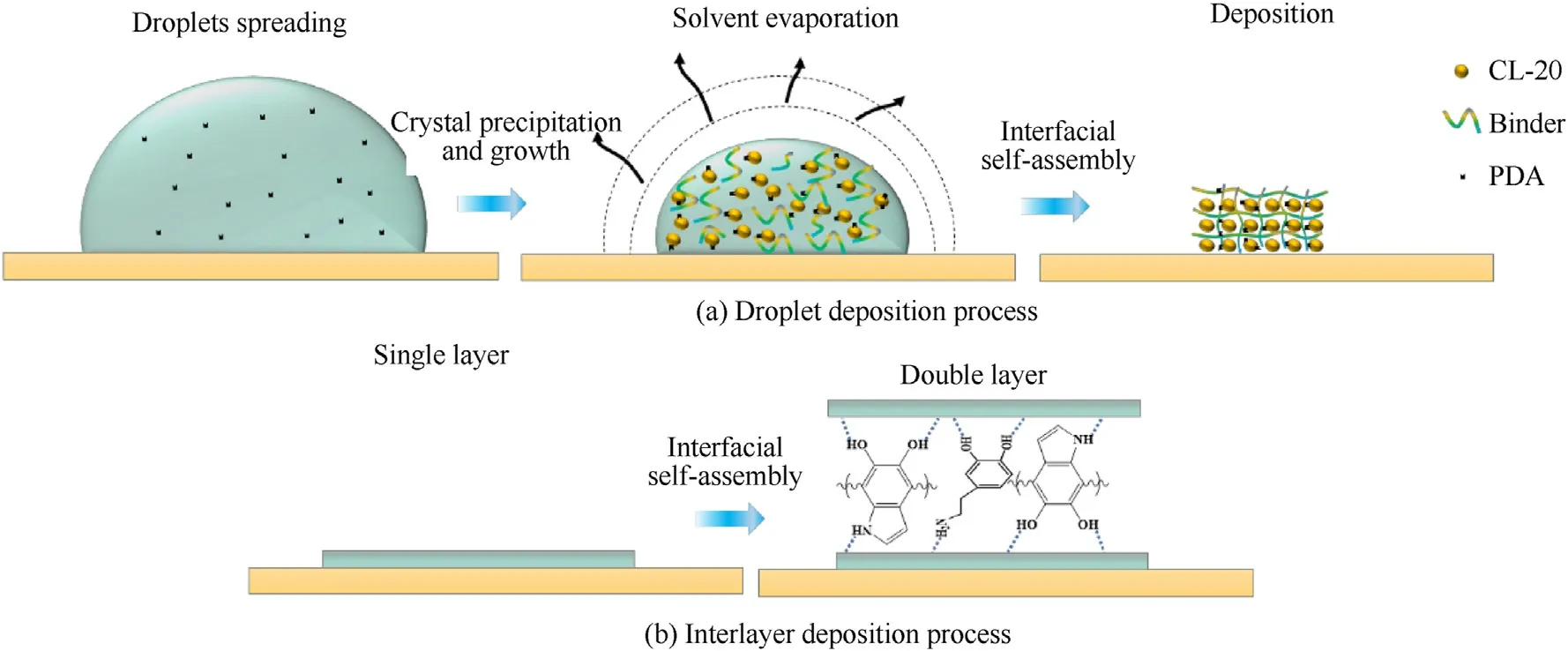
Fig.11.Schematic illustration of self-assembly: (a) Droplet deposition process;(b) Interlayer deposition process.
3.8.Proposed self-assembly mechanisms
For the CPN film,the volatilization of the solvent caused the ink droplet to become supersaturated and resulted in the primary nucleation and subsequent growth of the CL-20 crystals.Uneven droplet evaporation,uneven binder dispersion,and weak bonding force lead to disorderly growth of the CL-20 crystals,resulting in high interlayer porosity in the CPN film.Cyclic loading at the elastic zone causes high internal friction to occur between the internal microstructures.This results in significantly retarded elasticity,and fractures at the relatively weak interface of the film layer,leading to elastic failure [27].For the CPNP film,adding nano-PDA to the energetic ink causes it to approach the crystal surface during crystal nucleation and growth,as shown in Fig.11(e) and (f),resulting in the modification of the grain boundary energies and hydrogen bonding [49,50].These changes isolate the continuous growth of the crystal plane to a certain extent,inhibit the crystal nucleation rate,and form an orderly assembled microstructure,as shown in Fig.11(a).
For the multilayer films,PDA acts as an interfacial platform,constructing a bridge between the layers by forming hydrogen bonds between the molecules (Fig.11(b)),which results in a tight connection between layers.Under compression and cyclic loading,the compact microstructure completely dissipates the load energy[51].Thus,the PDA-induced self-assembly enhanced the intermolecular bonding between the layers in the layer-by-layer deposition of the 3D micro-jet,and effectively improved the mechanical strength of the interfaces.The self-assembly enhancement mechanism of the interfacial layer is an important factor for the mechanical enhancement of the multilayer printed composites [52].Therefore,PDA can act as an efficient linker bridge to enhance the interfacial interaction between the energetic film layers fabricated by 3D micro-jet printing,and achieve high detonation and mechanical strength.
4.Conclusion
In summary,PDA as a mussel-inspired adhesive has been demonstrated to efficiently improve the interface structure between energetic film layers.The CPN film used for micro-scale detonation in MEMSs fabricated by 3D micro-jet printing and PDA-induced self-assembly exhibited high detonation and high mechanical performance.The CPNP ink showed good printability and a high deposition resolution of 200 μm.With efficient selfassembly,the printed CPNP film has compact microstructure with a porosity of 11.28%.DSC and XPS data showed that PDA had good compatibility with the CL-20 crystals,resulting in uniform aggregation on the surface of the film.In conclusion,a strategy combining 3D micro-jet printing and PDA-induced self-assembly deposition to prepare micro-scale energetic film has been developed,which can provide a very promising means for the preparation of new micro-miniaturized devices.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
- Defence Technology的其它文章
- Establishment,simulation and verification of firepower safety control model
- Burning characteristics of high density foamed GAP/CL-20 propellants
- Cell-type continuous electromagnetic radiation system generating millimeter waves for active denial system applications
- Sandwich structure for enhancing the interface reaction of hexanitrohexaazaisowurtzitane and nanoporous carbon scaffolds film to improve the thermal decomposition performance
- Ablation characteristics of insulator under high-temperature gas dualpulse erosion
- Influence of shaped charge structure parameters on the formation of linear explosively formed projectiles

