Explosion temperature mapping of emulsion explosives containing TiH2 powders with the two-color pyrometer technique
Yu-Le Yao ,Yang-Fan Cheng ,* ,Qi-Wei Zhang ,Yu Xia ,Fang-Fang Hu ,Quan Wang ,c ,Yuan Chen
a State Key Laboratory of Mining Response and Disaster Prevention and Control in Deep Coal Mines,Anhui University of Science and Technology,Huainan,232001,PR China
b School of Chemical Engineering,Anhui University of Science and Technology,Huainan,232001,PR China
c Anhui Engineering Laboratory of Explosive Materials and Technology,Anhui University of Science and Technology,Huainan,232001,PR China
d Quality Department,The 38th Research Institute of China Electronics Technology Group Corporation,Hefei,230088,PR China
Keywords:Two-color pyrometer Emulsion explosive Explosion temperature field Explosion heat
ABSTRACT In the study,the two-color pyrometer technique was used to measure the transient temperature field of emulsion explosives with different contents of TiH2 powders.The experimental results showed that the introduction of TiH2 powders could significantly increase the explosion temperature and fireball duration of emulsion explosive.When emulsion explosives were ignited,the average explosion temperature of pure emulsion explosive continuously decreased while emulsion explosives added with TiH2 powders increased at first and then decreased.When the content of TiH2 powders was 6 mass%,the explosion average temperature reached its maximum value of 3095 K,increasing by 43.7%as compared with that of pure emulsion explosive.In addition,the results of air blast experiment and explosion heat test showed that the variation trends of shock wave parameters,explosion heat and theoretical explosion temperature of emulsion explosives with different contents of TiH2 powders were basically consistent with that of explosion temperature measured by the two-color pyrometer technique.In conclusion,the two-color pyrometer technique would be conducive to the formula design of emulsion explosive by understanding the explosion temperature characteristics.
1.Introduction
Emulsion explosive has become one of the most widely used industrial explosives in the world for its advantages in water resistance,safety and environmental protection[1-5].As a kind of water-containing explosive,the addition of water could improve the safety of emulsion explosive but reduce its power,which seriously restricts its application and development.In order to improve the explosion power of emulsion explosives,metal powders,military explosives and hydrogen containing materials were usually added into explosives to improve the detonation performance[6-10].Previous studies on detonation performance of high-energy emulsion explosives mainly focused on power,detonation velocity and shock wave pressure [11-13] but seldom involved explosion temperature.However,as an important index to evaluate the performance of explosives,temperature could reflect the thermal damage property and energy release law of explosives [14].The explosion process of explosives was transient and accompanied with ultra-high temperature and pressure,and it was difficult to accurately measure the explosion temperature by sensors.Studies related to explosion temperature were mainly on basis of theoretical calculation[15].However,explosion temperature calculated by theoretical derivation was the estimated average temperature of whole explosion process,which could not reflect the space-time distribution of explosion temperature.In addition,the theoretical calculation of explosion temperature was mainly based on empirical formula,which may have a big deviation from the actual explosion temperature.Therefore,the theoretical calculation results of explosion temperature were inaccurate and could not be conducive to the in-depth study of explosive properties and detonation theory.
In order to obtain the accurate value of explosion temperature,researchers had been actively exploring the field of explosion temperature measurement.Frost et al.[16]used thermocouples to measure the fireball temperature of metallized explosives,and found that the temperatures of gases and combustion particles in the explosion fireball were up to 1800 K and 2700 K in the presence of aluminum particles,respectively.Lebel et al.[17] developed an optical fiber probe to measure the explosion fireball temperature of Detasheet-C explosives.However,when measuring the explosion temperature by the contact method,the thermocouples and optical fiber probes should be sticked to the measured objects,which would be destroyed easily after explosion.Furthermore,the measurement accuracy of the contact method was poor and its temperature response speed could not meet the requirement of the explosion transient process.Compared with the contact method of temperature measurement,non-contact method could detect the surface temperature of object with a wide temperature measurement range and quick response speed,which was more suitable for the explosion temperature measurement.Lewis et al.[18] used atomic spectroscopy to measure the explosion fireball temperature of RDX containing barium nitrate,and the time-averaged emission spectra indicated that the apparent temperature of explosion fireball was 3000 K,which was consistent with the theoretical prediction.Aduev et al.[19]established a thermal model of explosion luminescence of PETN containing iron nanoparticles by the spectroscopy thermometry method.Olokun et al.[20] studied the temperature distribution of polymer-coated HMX particles under impact load by a thermal imaging camera,and found that the temperature characteristics would be significantly affected by the particle size.Wang et al.[21]proposed a compensation method to adjust the explosion temperature of HMX measured by an infrared thermal imager,and found that the corrected temperature was close to its detonation temperature.However,due to the low accuracy of current experimental facilities and incomplete collection of spectroscopy radiation intensity,it would be difficult to measure the explosion temperature accurately using the spectroscopy method.In addition,the temperature measurement accuracy of the infrared thermometry method was greatly affected by the object emissivity and environmental radiation.In recent years,a novel temperature measuring technique on basis of colorimetric thermometry was developed.Goroshin et al.[22] experimentally measured the temperature history of explosives using a three-color optical pyrometer assisted by a qualitative spectroscopy.Densmore et al.[23,24] recorded the explosion flame images of TNT using a high speed camera and obtained the explosion temperature distribution by the two-color pyrometer technique.Chang et al.[25]compared the flame structure of dust explosion of micro-nano scale aluminum powders through a series of dust explosion experiments,and studied the flame temperature structures by the two-color pyrometer technique.Compared with the methods of spectroscopy thermometry and the infrared thermometry,the colorimetric thermometry method based on the black body radiation theory has the advantages of quick response speed,high measurement accuracy and strong anti-interference ability.Therefore,colorimetric thermometry method would have a broad application prospect in the field of explosion transient temperature measurement.
In the study,the explosion temperature structures of emulsion explosives with different contents of TiHpowders were studied by the two-color pyrometer technique.Furthermore,the explosion shock wave parameters,explosion heat and theoretical explosion temperature were also discussed to verify the reliability of the twocolor pyrometer technique.
2.Experimental materials and methods
2.1.Experimental materials
Glass microspheres (GMs) with the bulk density of 0.25 g/cm,were purchased from the Minnesota Mining and Manufacturing Company of USA;Titanium hydride (TiH) with the hydrogen storage capacity of ca.3.85 mass%,was purchased from Thermo Fisher Scientific Company of USA;Tungsten halogen lamp was purchased from the Royal Philips Company of Netherlands;Ceramic crucibles with the diameters and height of 25 and 42 mm,respectively,were purchased from the Xi'an Modern Chemistry Research Institute of China;Emulsion matrix with the density of 1.31 g/cm,was produced by the Huainan Shun Tai Chemical Co.,Ltd.of China.The main components of emulsion matrix are listed in Table 1.
The particle size distribution and microstructure of GMs and TiHpowders were observed by a laser particle size analyzer and a scanning electron microscopy,respectively,as shown in Fig.1.GMs had smooth surfaces and a wide particle size distribution changed from 11.5 to 158.5 μm,and its average particle size was 55.6 μm(see Fig.1(a)).TiHparticles had rough surfaces with sharp edges and corners,and its particle size distribution was varied from 0.2 to 34.7 μm with the average particle size of 7.6 μm,(see Fig.1(b)).
2.2.Experimental facilities
Laser particle size analyzer (Mastersizer 2000,Malvern,UK),Scanning electron microscope (VEGA3,TESCAN,Czech Republic),High speed camera(Memrecam HX-3,NAC,Japan),High-resolution oscilloscope (LeCroy HDO4034,Teledyne LeCroy,USA),Constant current source (PCB,USA),PCB sensor (137B24B,PCB,USA).
3.Detonation parameter experiments
3.1.Calibration of two-color pyrometer system
The two-color pyrometer system was calibrated by a tungsten halogen lamp before measuring the explosion temperature distribution of emulsion explosives,and the calibration method had been referred in the previous studies [25,26].The steps of temperature calibration were as follows: firstly,the variation trend of correction coefficient with temperature was acquired through the calibration experiment of tungsten halogen lamp.Secondly,fitting the variation trend of temperature values with curve-fitting method (as shown in Fig.2),and then the mathematical relationship between the correction coefficient and temperature was obtained.
3.2.Air blast experiment
After the temperature calibration experiment,the explosion processes of emulsion explosives with different contents of TiHpowders were observed by a high-speed camera,and corresponding shock wave parameters were recorded by PCB pressure sensors in the air blast experiments,as shown in Fig.3.When the content of GMs in the emulsion explosive was 4 mass%,its explosion power would reach the maximum value [27].Therefore,the content ofGMs in each emulsion explosive sample was determined as 4 mass%,and the formulas of emulsion explosive samples were listed in Table 2.The mass of each emulsion explosive sample was 20 g,and they were wrapped by a polyethylene plastic film to form a spherical explosive charge.The center of the spherical explosive charge was 0.5 m above the ground and 0.7 m away from the pressure sensor,meanwhile,a high speed camera and a protective device were placed 20 m far from the spherical explosive charge.In the experiment,the explosive charge was initiated by a detonator,which was fixed in the center of the spherical explosive charge.Each emulsion explosive sample was tested over three times,and the effective data were averaged.The explosion process was recorded by a high speed camera with the frame rate and exposure time of 173,000 fps and 3.7 μs,respectively.The air blast experimental data was firstly collected by a PCB sensor (137B24B,PCB,USA),and recorded by HDO403A digital storage oscilloscope(LeCroy HDO4034,Teledyne LeCroy,USA) through a constant current source.
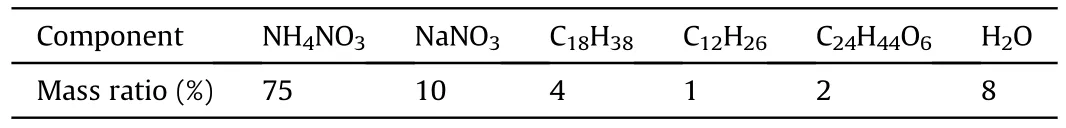
Table 1 Composition of emulsion matrix.
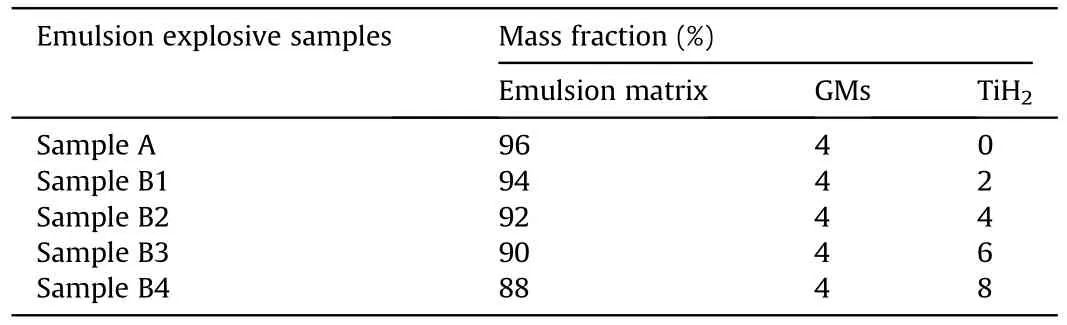
Table 2 Composition of emulsion explosive samples.
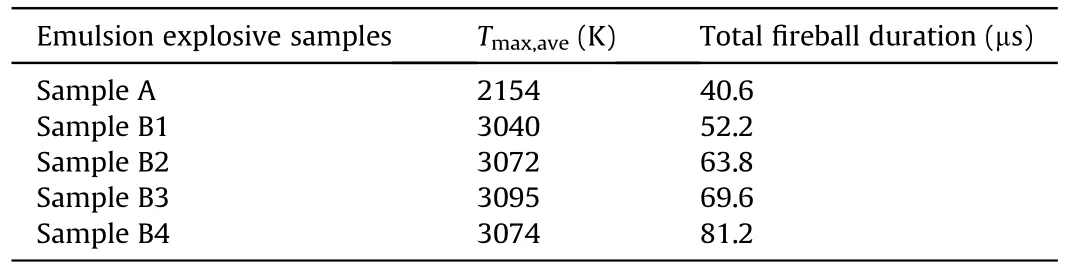
Table 3 Explosion temperature parameters of emulsion explosive samples.
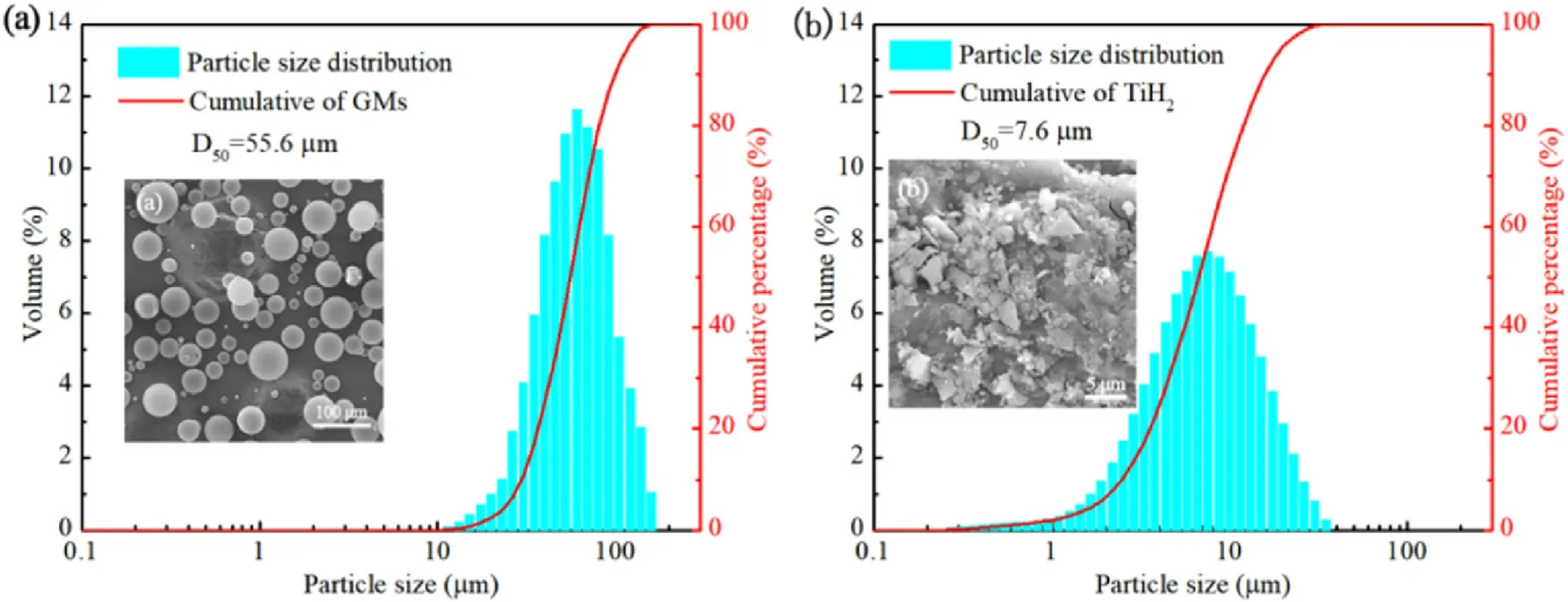
Fig.1.Particle size distributions of (a) GMs and (b) TiH2 powders.

Fig.2.Calibration experiment with a tungsten halogen lamp: (a) Schematic diagram of experimental facilities and (b) Temperature-R/G ratio fitting curve.
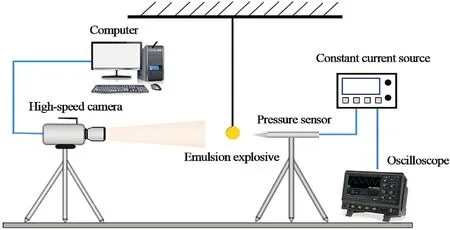
Fig.3.Schemic diagram of measuring system of air blast experiment.
3.3.Explosion heat test
In order to verify the reliability of the two-color pyrometer technique,the explosion heats of emulsion explosive samples were experimentally studied by explosion heat testing system,as shown in Fig.4.The steps of explosion heat test were as follows:Firstly,the emulsion explosive samples with the mass of 20 g and diameter of 8 mm were ignited in explosion heat testing system under the pressure of -0.094 MPa.Secondly,the distilled water with the volume of 16.5 L was as temperature measuring medium,and obtained the constant volume explosion heat by the heat capacity and temperature rising value of explosion heat testing system.Each sample was tested three times,and its measurement deviation was controlled within 3%.In the experiment,the temperature of outer bucket was adjusted by temperature controller to make that of inner bucket stable,that is,the temperature change of inner bucket within 15 min was not over 0.003C.The temperature of outer bucket was subsequently raised up to a stable value by temperature controller,that is,the temperature of outer bucket fluctuated no more than 0.02C within 15 min.
4.Results and discussion
4.1.Effects of TiH2 powders content on explosion temperature field

Fig.4.Schemic diagram of heat-insulating type explosion heat testing system.
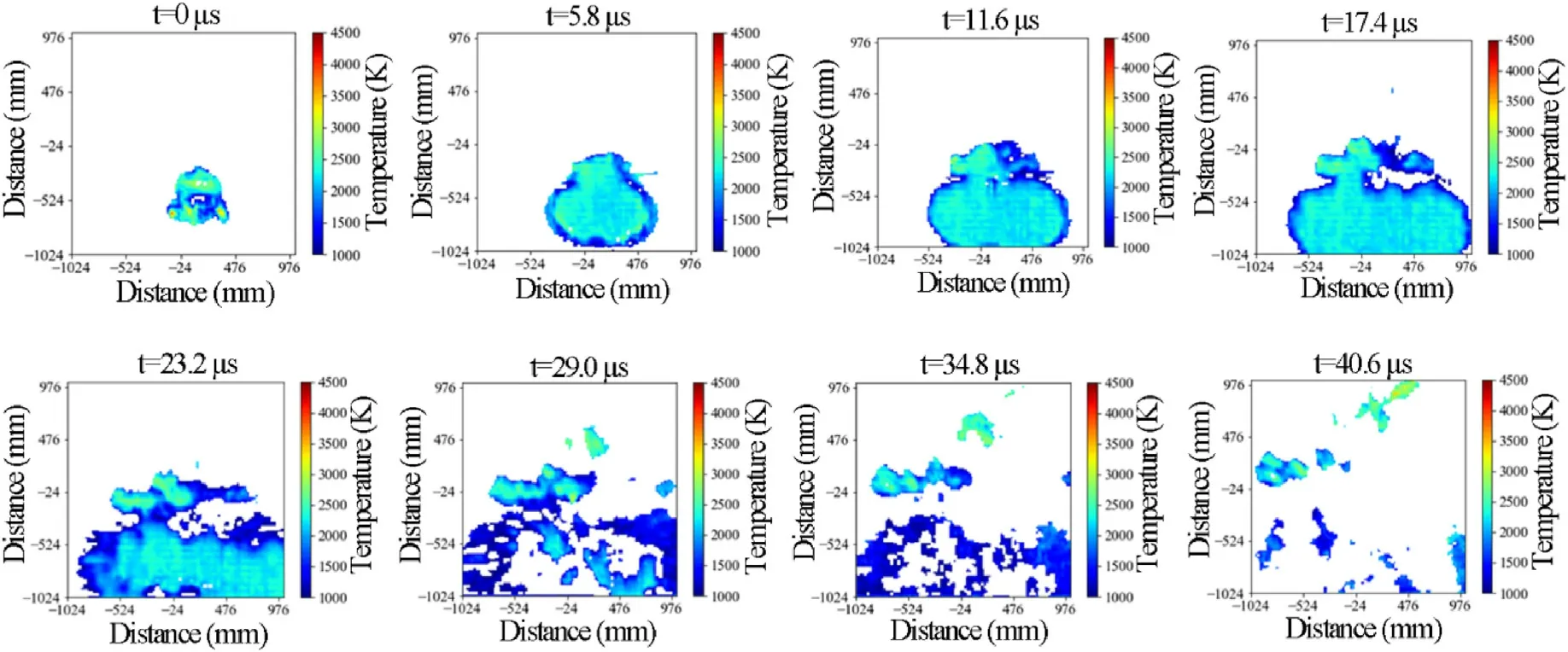
Fig.5.Explosion temperature distribution development of emulsion explosive without TiH2 powders.
In the experiments,the explosion processes of emulsion explosives were observed by a high speed camera,and the related explosion temperature distributions were calculated using a python code on basis of two-color pyrometer technique.In order to study the effect of TiHpowders on the explosion temperature field of emulsion explosives,the temperature distributions of pure emulsion explosive(Sample A)and emulsion explosive with 4 mass% TiHpowders (Sample B2) were compared,as shown in Figs.5 and 6.In addition,to express the changes of explosion temperature field more clearly,the moment that first recorded the explosion image was determined as=0 μs.Since the frame rate of high speed camera was set as 173,000 fps,the time interval between two adjacent explosion images was 5.8 μs.Fig.5 indicates that after the pure emulsion explosive (Sample A) being ignited,the volume of explosion fireball continuously increased whereas its temperature gradually decreased from=0 to=17.4 μs.The explosion fireball began to break and gradually extinguished and its temperature continuously decreased between=17.4 and 40.6 μs.Therefore,the maximum average temperature of explosion fireball was determined as 2154 K at=0 μs.The reasons for this phenomenon could be concluded as follows:Firstly,explosives would work and release energy by compressing the surrounding air in the explosion process.Secondly,after explosive being ignited,it would release energy in the form of sound,light,heat and vibration et al.Thirdly,the explosion products were driven by the shock waves to diffuse around and release heat through rapid heat exchange with the external environment.Due to the above three reasons,the explosion energy of explosives continuously decreased during the explosion process,resulting a continuous temperature reduction of explosion fireball during the whole explosion process.

Fig.7.Average explosion temperature curves of emulsion explosives with different content of TiH2 powders.
Fig.6 demonstrates that after the emulsion explosive with 4 mass% TiHpowders (Sample B2) being ignited,the volume and temperature of explosion fireball gradually increased from=5.8 to=23.2 μs.When the time was between=23.2 and 34.8 μs,the explosion fireball gradually broke up while its temperature continuously increased.In addition,the maximum average temperature of explosion fireball was reached at=34.8 μs,then the explosion fireball gradually extinguished and its temperature continuously decreased.Compared with the pure emulsion explosive,the explosion fireball temperature of emulsion explosive with 4 mass% TiHpowders increased at first and then decreased,and the maximum average temperature and duration of fireball were increased by 42.7% and 57.1%,respectively.The reasons for this phenomenon could be summarized as follows: after the emulsion explosive with 4 mass% TiHpowders being ignited,part of TiHpowders participated in the detonation reaction of emulsion explosive as the high energy fuels,which increased the explosion output energy.Then the rest of TiHparticles and the explosion products turbulently mixed with the atmospheric oxygen when diffusing with shock waves to the external environment,which caused a rapid combustion reaction and released a large number of combustion heats[28,29].Therefore,the explosion temperature of emulsion explosive with 4 mass% TiHpowders did not decrease but keep increasing in the initial stage.However,with the end of combustion reaction and the further diffusion of explosion products,the combustion particles and explosion products gradually cooled,which showed that the explosion fireball temperature began to drop.Therefore,when TiHpowders were introduced into emulsion explosives as high energy additives,the explosion fireball temperature and duration could be significantly improved.
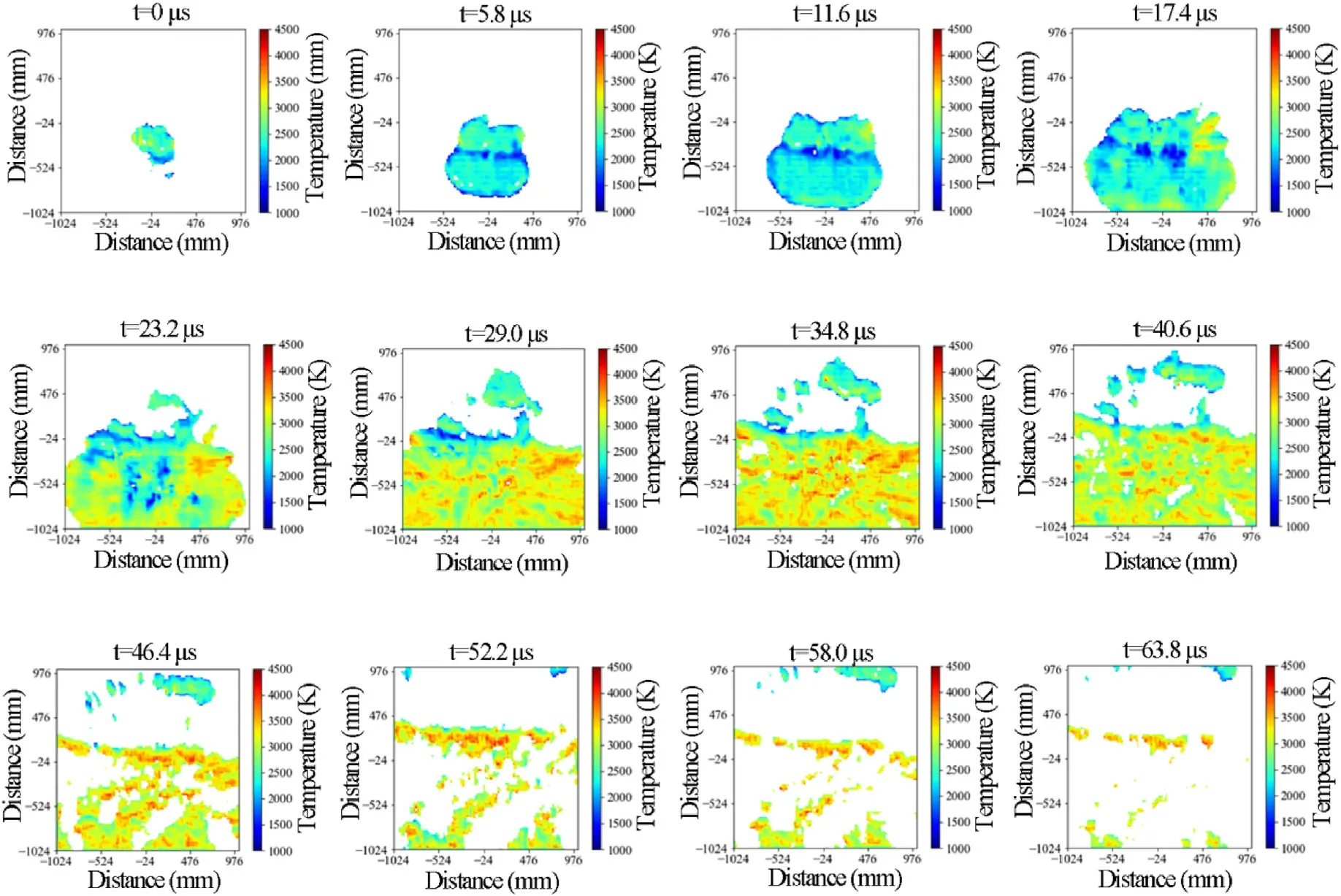
Fig.6.Explosion temperature distribution development of emulsion explosive with 4% TiH2 powders.
In order to obtain the emulsion explosive with optimum thermal damage effects,the explosion temperature variation of emulsion explosives with different contents of TiHpowders was studied.As Fig.7 shows,after the emulsion explosives being ignited,the average explosion temperature of pure emulsion explosive continuously decreased while that of other emulsion explosive samples increased at first and then decreased.In addition,the maximum average explosion temperature and fireball duration of Sample A,Sample B1,Sample B2,Sample B3 and Sample B4 were 2154,3040,3072,3095,3074 K and 40.6,52.2,63.8,69.6,81.2 μs,respectively,as shown in Table 3.When the contents of TiHpowders in emulsion explosives were 6 mass% (Sample B3) and 8 mass%(Sample B4),the average explosion temperature and fireball duration reached their maximum value of 3095 K and 81.2 μs,respectively.The reasons for this phenomenon could be concluded as follows: On the one hand,TiHpowders participated in the detonation reaction of emulsion explosives and increased the explosion temperature as hydrogen storage alloy powders.On the other hand,TiHpowders in the emulsion explosives could cause a violent post-combustion reaction,which delayed the temperature attenuation of explosion fireball.Therefore,when the amount of TiHpowders added in emulsion explosive was less,the explosion temperature gradually increased with the increase of TiHpowders.However,when the amount of TiHpowders was excessive,the oxygen balance of emulsion explosives was changed,resulting in the decrease of explosion temperature[28,29].
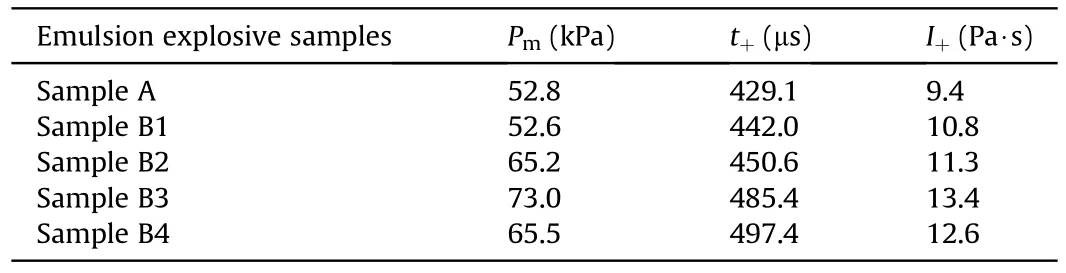
Table 4 Air blast parameters of emulsion explosive samples.
4.2.Effects of TiH2 powders content on shock wave parameters
In the study,the shock wave parameters of emulsion explosives with different contents of TiHpowders were studied by the air blast experiment.The waveforms collected by pressure sensors could not be used directly for there were plenty of interference factors in the air blast experiment,such as the system and environmental noises.The modified-Friedlander three-parameter fitting formula(Formula 1)was used to fit the collected waveforms to obtain more accurate data [30],as shown in Fig.8.
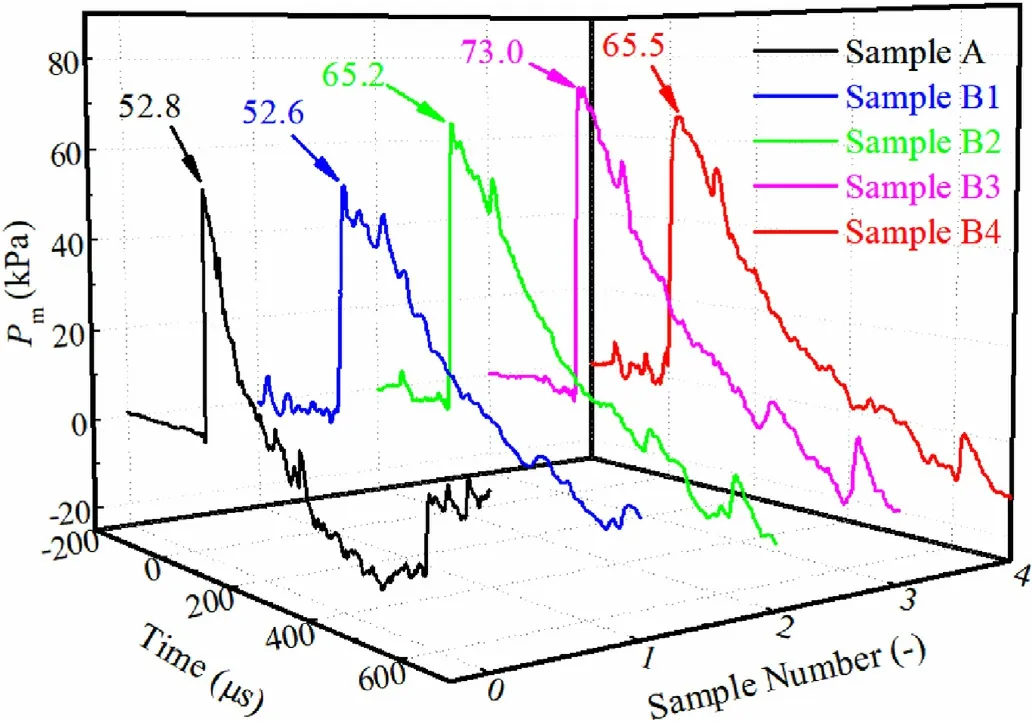
Fig.8.Pressure-time curves of emulsion explosives with different contents of TiH2 powders.

where,represents the pressure of shock wave,represents the peak pressure of shock wave,represents the time history,represents the positive pressure duration,α represents the wave coefficient of shock wave[31].
In addition,the pressure curves of shock waves were linearly fitted to obtain the positive pressure duration of emulsion explosives.The corresponding positive impulse was obtained by Formula 2 [32].
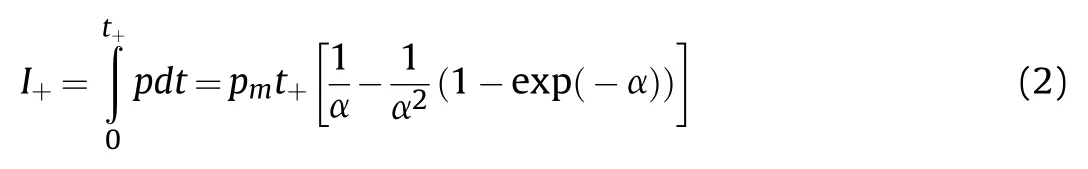
Fig.8 demonstrates that the peak pressure of shock wave increased at first and then decreased with the increase of TiHpowders.The reasons for this phenomenon could be concluded as follows: the mass of each emulsion explosive sample was fixed at 20 g in the experiment,so the quantity of emulsion matrix would decrease with the introduction of TiHpowders.When the content of TiHpowders was not too many,the promotion effect of TiHpowders on the shock wave pressure would exceed the weakening effect caused by the decreased emulsion matrix.As the content of TiHpowders kept rising,the explosion pressure would be weaken owing to the dominant effects of decreasing emulsion matrix and negative oxygen balance,in spite of the positive effect of TiHpowders[28,29].The pressure curves of shock waves after the noise reduction were calculated to acquire the corresponding positive pressure duration and impulse parameters,as listed in Table 4.Table 4 shows that the positive pressure duration of shock waves continuously increased with the increasing TiHpowders inemulsion explosives.That was because TiHpowders would release hydrogen gas in the detonation process,which could participate in the detonation reaction to increase the explosion power and delay the attenuation of shock waves.As the content of TiHpowders was 6 mass% in emulsion explosive,the peak pressure and positive impulse of shock wave reached their maximum value of 73.0 kPa and 13.4 Pa·s,that were,increasing by 38.3% and 42.6% when compared with the pure emulsion explosive,respectively.Therefore,the air blast experimental results showed that the pressure variation trend of shock waves were basically consistent with that of explosion temperature fields.
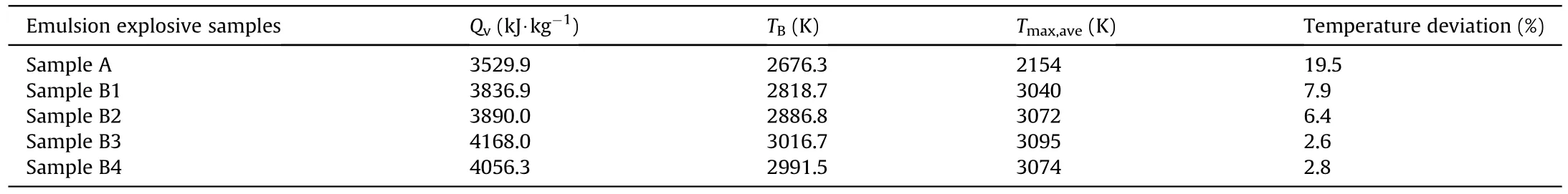
Table 5 Explosion heat results of emulsion explosive samples.
4.3.Effects of TiH2 powders content on explosion heat
In the study,the explosion heat of emulsion explosives with different contents of TiHpowders were studied by an explosion heat testing system.In addition,the theoretical explosion temperatures () of corresponding emulsion explosives were calculated by equation (3) [33] for comparing,as listed in Table 5.

where,represents the explosion temperature,K.represents the constant volume explosion heat,kJ·kg.A and B represent the algebraic sum of constant term and coefficient of first order term,respectively,in the function of heat capacity and temperature of explosion products.
Table 5 shows that the explosion heat of emulsion explosives enhanced at first and then decreased with the increasing content of TiHpowders.When the content of TiHpowders in emulsion explosive was 6 mass%,the explosion heat reached its maximum value of 4168 kJ·kg.That was because moderate content of TiHpowders would improve the post-combustion reaction to release more energy in the explosion process,and finally increased the explosion heat of emulsion explosives.However,when the content of TiHpowders was excessive,the explosion energy of emulsion explosives would be affected by the negative oxygen balance and the decreasing amount of emulsion matrix [28,29].
The theoretical explosion temperature of emulsion explosives was positively correlated with the explosion heat according to equation (3),so their variation trends were basically consistent.In order to verify the reliability of the two-color pyrometer technique,the theoretical and experimental values of explosion temperatures were compared.The law of experimental explosion temperature varied with the content of TiHpowders was in accordance with that of theoretical explosion temperature,as shown in Table 5,the temperature deviation between experimental measurement and theoretical calculation of emulsion explosive samples were less than 8%,except the pure emulsion explosive with a temperature deviation of 19.5%.Furthermore,previous studies showed that the explosion temperature of pure emulsion explosive was ca.2070 K[34],which was basically consistent with the results of colorimetric thermometry experiment.In addition,the composition of emulsion matrix in Ref.[34] was as follows: the oxidant was an aqueous solution of ammonium (AN) and sodium (SN) nitrates,94 mass%;the fuel was a mixture of industrial oil,paraffin,and emulsifying agent,6 mass%.It could be found that the contents of the oxidant and fuel between the emulsion matrix and Ref.[34] in the paper were almost consistent,which further verified the reliability and accuracy of the two-color pyrometer technique.
5.Conclusion
In the study,the transient temperature fields of emulsion explosives with different contents of TiHpowders were measured using the two-color pyrometer technique.The experimental results showed that the addition of TiHpowders in emulsion explosives could increase the explosion temperature and delay the temperature attenuation of the explosion fireball.When the content of TiHpowders in emulsion explosive changed from 0 to 8 mass%,the average explosion temperature increased at first and then decreased with the increasing TiHpowders.When the content of TiHpowders was 6 mass%,the average explosion temperature reached its maximum value of 3095 K,increasing by 43.7% as compared with that of the pure emulsion explosive.The air blast experimental results demonstrated that the peak pressure and positive impulse of shock waves increased at first and then decreased as the content of TiHpowders progressively increased,and reached their maximum value of 73.0 kPa and 13.4 Pa·s,that were,increasing by 38.3% and 42.6% as compared with pure emulsion explosive,respectively.In addition,the explosion heat testing results indicated that the variation trends of explosion heat and theoretical explosion temperature of emulsion explosives were basically consistent with that of explosion temperature in colorimetric thermometry experiments,which further verified the reliability of the two-color pyrometer technique.Therefore,the twocolor pyrometer technique could effectively measure the transient temperature field,which was helpful for studying the thermal damage effect and energy release law of emulsion explosives.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Many thanks to Prof.Ritsu Dobashi,Assoc.Prof.Toshio Mogi,and Dr.Po-Jul Chang of The University of Tokyo for their kindness help with the two-colour pyrometer technique.This work was supported by the National Natural Science Foundation of China(No.11972046),Outstanding Youth Project of Natural Science Foundation of Anhui Province (No.2108085Y02),Major Project of Anhui University Natural Science Foundation (No.KJ2020ZD30) and Anhui University of Science and Technology Postgraduate Innovation Fund (No.2020CX2066),and the authors would like to thank these foundations for their financial support.
- Defence Technology的其它文章
- Establishment,simulation and verification of firepower safety control model
- Burning characteristics of high density foamed GAP/CL-20 propellants
- Cell-type continuous electromagnetic radiation system generating millimeter waves for active denial system applications
- Sandwich structure for enhancing the interface reaction of hexanitrohexaazaisowurtzitane and nanoporous carbon scaffolds film to improve the thermal decomposition performance
- Ablation characteristics of insulator under high-temperature gas dualpulse erosion
- Influence of shaped charge structure parameters on the formation of linear explosively formed projectiles

