Intrathecal liproxstatin-1 delivery inhibits ferroptosis and attenuates mechanical and thermal hypersensitivities in rats with complete Freund’s adjuvant-induced inflammatory pain
Yi-Fan Deng, Ping Xiang, Jing-Yi Du, Jian-Fen Liang, Xiang Li,
Abstract Previous studies have confirmed the relationship between iron-dependent ferroptosis and a peripheral nerve injury-induced neuropathic pain model. However, the role of ferroptosis in inflammatory pain remains inconclusive. Therefore, we aimed to explore whether ferroptosis in the spinal cord and dorsal root ganglion contributes to complete Freund’s adjuvant (CFA)-induced painful behaviors in rats. Our results revealed that various biochemical and morphological changes were associated with ferroptosis in the spinal cord and dorsal root ganglion tissues of CFA rats. These changes included iron overload, enhanced lipid peroxidation, disorders of anti-acyl-coenzyme A synthetase long-chain family member 4 and glutathione peroxidase 4 levels, and abnormal morphological changes in mitochondria. Intrathecal treatment of liproxstatin-1 (a ferroptosis inhibitor) reversed these ferroptosis-related changes and alleviated mechanical and thermal hypersensitivities in CFA rats. Our study demonstrated the occurrence of ferroptosis in the spinal cord and dorsal root ganglion tissues in a rodent model of inflammatory pain and indicated that intrathecal administration of ferroptosis inhibitors, such as liproxstatin-1, is a potential therapeutic strategy for treating inflammatory pain.
Key Words: cell death; complete Freund’s adjuvant; dorsal root ganglion; ferroptosis; inflammatory pain; intrathecal delivery liproxstatin-1; spinal cord
Introduction 456 Methods 457 Results 458 Discussion 459
Graphical Abstract
Anti-ferroptosis effects of liproxstatin-1 on CFA-induced inflammatory pain pathology

Introduction
Inflammatory pain is a significant form of chronic pain that has become a major health problem and is primarily caused by trauma, arthritis, or pathogen infection, which can lead to allodynia, hyperalgesia, or even cognitive impairment (Ho et al., 2019). Tissue damage or inflammation may induce the overproduction of multiple pro-inflammatory compounds and dysregulation of pain-related genes, which may act as triggers for the emergence and maintenance of painful behaviors (Fattori et al., 2019). Currently, typical pharmacotherapy for inflammatory pain, such as opioids and cyclooxygenase inhibitors, is limited by partial and side effects (Volkow et al., 2018), and new drugs for inflammatory pain remain unavailable because of the obscure pathogenesis of inflammatory pain.
A cell-death disorder of the nervous system has been implicated in the occurrence of inflammatory pain. For example, peripheral complete Freund’s adjuvant (CFA) injection has the potential to promote neuronal apoptosis in the lumbar spinal cord and is a development that is thought to contribute to the imbalance of dorsal horn neuron excitability and eventually result in the enhancement of nociceptive signals and hyperalgesia (Liu et al., 2021). Moreover, the activation of spinal microglia following subcutaneous intraplantar CFA administration may be responsible for increased apoptosis by affecting the function of the phosphatase and tensin homologue deleted on chromosome 10/phosphatidylinositol-3-kinase/protein kinase B (PTEN/PI3K/Akt)-pathway function (Baniasadi et al., 2020). However, understanding the pathogenesis of inflammatory pain is complicated by the diverse forms of cell death, and the principal cell-death pathway that contributes to CFA-induced hyperalgesia remains unclear.
Ferroptosis is considered a unique oxidative and iron-driven class of programmed cell death because it does not commonly exhibit morphological changes in the nucleus or an enhancement of caspase-3 activity and is largely uninhibited by caspase suppressors; these features distinguish ferroptosis from previously proposed types of cell death (e.g., necrosis, apoptosis, and autophagy) (Dixon et al., 2012; Zhang et al., 2021a). Numerous studies have highlighted the potentially important roles of this iron-dependent mode of cell death in the occurrence and development of nervous system diseases (Tan et al., 2021; Yao et al., 2021). Previous preclinical investigations have reported that peripheral-nerve injury leads to iron overload (Xu et al., 2019), reactive-oxygen-species (ROS) accumulation (Valek et al., 2017), and mitochondrial dysfunction (Bae et al., 2018) in the spinal cord, which are considered crucial factors in ferroptosis induction. Moreover, a distinct set of ferroptosis-related genes, which include glutathione peroxidase 4 (GPX4), and anti-acyl-CoA synthetase long-chain family member 4 (ACSL4), has been found to be dysregulated in a neuropathic pain model (Guo et al., 2021; Wang et al., 2021). Using a high-throughput sequencing method, a recent study revealed the potential participation of a group of microRNAs and their target genes in the ferroptosis process (Jia et al., 2021). In addition, intraperitoneal injection of specific ferroptosis inhibitors (e.g., ferrostatin-1 and liproxstatin-1) was shown to suppress ferroptosis and alleviate pain-like behaviors in a chronic sciatic-nerve-constriction pain model (Guo et al., 2021; Wang et al., 2021). Taken together, the above evidence suggests that neuropathic pain is attributed to ferroptosis-like neurocyte death.
The overproduction of ROS, subsequent oxidative stress damage, and lipid peroxidation have been observed previously in a CFA-induced inflammatorypain model with hyperalgesia pathology (Singh and Vinayak, 2015). In addition, a recent review has reported that excess iron generates a considerable amount of damaging ROS via Fenton or Haber Weiss reactions, which may be involved in the progression of osteoarthritis (Sun et al., 2021). However, whether oxidative damage and iron accumulation lead to ferroptosis has not been demonstrated in inflammatory hyperalgesia. Therefore, we explored whether spinal cord and dorsal root ganglion (DRG) ferroptosis plays a causal role in inflammatory pain induced by CFA administration.
Methods
Experimental animals
Responses to CFA administration between male and female rodents do not differ significantly (Bravo-Hernandez et al., 2016); thus, a total of 65 healthy male adult Sprague-Dawley (SD) rats (specific-pathogen-free [SPF] grade) weighing 180–220 g were recruited from SiPeiFu (Beijing) Biotechnology Co., Ltd (Production license number: SCXK [Jing] 2019-0010, Beijing, China) without any pretreatments. Rats were raised in an SPF environment at the Laboratory Animal Center of South China Agricultural University and housed in individual cages in the same room under standard and suitable conditions (12-hour light/dark cycle; 22 ± 2°C; 40–60% relative humidity) with freely available food (pellet diet) and water. All animal experimental procedures were approved by the Animal Care Committee of the South China Agricultural University (No. 2021d036) on May 15, 2021, and conducted in strict accordance with international laws and National Institutes of Health policies, including the Guide for the Care and Use of Laboratory Animals (eighth edition, 2011). This study is reported in accordance with the Animal Research: Reporting ofIn Vivo
Experiments 2.0 guidelines (Percie du Sert et al., 2020).Intrathecal catheterization
Intrathecal catheter implantation was performed as previously reported (Storkson et al., 1996). Under sufficient anesthetization, maintained with isoflurane (induction concentration: 3–4%, maintenance concentration: 1.5–2.5%; RWD Life Science Co, Shenzhen, Guangdong, China), a sterile polyethylene-10 catheter (0.28 mm inner diameter × 0.61 mm outer diameter) was inserted into the subarachnoid space of the rat through the L4–L5 intervertebral foramen. All incisions and saturation steps were carefully performed by an experienced researcher, and a heating pad was prepared in advance to minimize post-operative pain in the experimental rats. To minimize interference in DRG-tissue collection, we implanted the intrathecal catheter contralateral to the CFA injection side. According to a previously proposed method (Yang et al., 2021), the proper position of intrathecal catheterization was characterized by transient hind-limb paralysis induced by intrathecal lidocaine (2%, 20 μL) delivery following recovery from anesthesia. Before pain model construction, rodents spent 3 days in recovery from intrathecal catheterization surgery (Figure 1A
).Chronic inflammatory pain model construction and intrathecal drug
delivery
All experimental murine were randomly allocated to the following five groups using a random number table: (a) control (Con,n
= 15), (b) CFA with liproxstatin-1 (CFA-Lip,n
= 15), (c) CFA with vehicle (CFA-Veh,n
= 15), (d) CFA with no drug administration (CFA,n
= 15), and (e) CFA with minocycline (CFAMino,n
= 5). According to previous studies (Abbadie et al., 1996; Huang et al., 2019), we established an inflammatory pain model via subcutaneous intraplantar administration of 100 μL CFA (F5881, MilliporeSigma, Burlington, MA, USA) into the left hind paw. The control rats received an equivalent injection of saline (0.9%, 100 μL) in the comparable area of the left hind paw. Liproxstatin-1 is the firstin vivo
efficacious ferroptosis inhibitor and has been used extensively in preclinical research (Tang et al., 2019; Zheng et al., 2020). Liproxstatin-1 has been shown to suppress ferroptosis by subverting the lipidperoxidation process, and its anti-ferroptosis effect is considered superior to that of other lipid-autoxidation inhibitors (e.g., ferrostatin-1) (Zilka et al., 2017). Because we previously demonstrated the potential role of lipid peroxidation in spinal ferroptosis under chronic pain conditions (Guo et al., 2021), we selected this compound as the ferroptosis inhibitor in this study. For 3 consecutive days after CFA injection, 30 μL liproxstatin-1 (10 μg/μL, abs810754, Absin Bioscience Inc., Shanghai, China) or the same amount of vehicle (dimethyl sulfoxide, 10 μg/μL) was injected into the CFA-Lip or CFAVeh rats via the intrathecal route. The CFA and Con groups did not receive any drug (Figure 1A
). The above volume of liproxstatin-1 was selected because we observed in our pilot study that a higher volume of 40 μL liproxstatin-1 caused temporary lower limb paralysis (recovery occurred gradually within 3 days of the injection) in two of six rats, whereas there were no significant improvements in mechanical and thermal hypersensitivities when a dose of 20 μL was used (Additional Tables 1
and2
). Furthermore, we compared the anti-hyperalgesic effect of liproxstatin-1 with that of minocycline (positivecontrol pharmacotherapy), the latter of which has been shown to exert a promising therapeutic effect in a CFA-induced inflammatory-pain rodent model (Zhou et al., 2015; Li et al., 2022). According to previously reported methods (Zhou et al., 2015; Li et al., 2022), minocycline hydrochloride (100 μg; M9511, MilliporeSigma) was dissolved in 30 μL of 0.9% saline and administered daily for 7 successive days via the intrathecal route to the CFAMino group.Behavioral tests
To estimate painful behavior in rats, we performed pain-related behavioral tests based on tactile stimulus methods. Baseline values were measured 3 days before CFA administration. On the first, third, fifth, and seventh days after CFA injection, an independent investigator, who had no knowledge of the group allocation, measured the mechanical and thermal sensitivities of all five rats in the CFA-Mino group and five random rats in each of the remaining four groups before intrathecal drug administration (Figure 1A
). To allow acclimation to the testing environment and apparatus, each animal was separately placed into the testing boxes at least 30 minutes before the tests. In each rat, thermal sensitivity measurements were obtained 30 minutes after obtaining the mechanical sensitivity measurements.As described earlier (Guo et al., 2021), mechanical sensitivity was assessed by perpendicularly pushing multiple calibrated von Frey fibers (North Coast Medical, Morgan Hill, CA, USA) against the mid-plantar surface of the hind paw to increase stiffness (1.0, 1.4, 2.0, 4.0, 6.0, 8.0, 10.0, 15.0, and 26.0 g), and a positive sign was represented by a paw withdrawal in response to the tactile stimuli a minimum of three out of five testing performances. The lowest bending force that generated a positive response was considered the paw mechanical withdrawal threshold (PMWT).
Thermal sensitivity was quantified using radiant heat equipment (UGO BASILE Plantar Test Apparatus, Varese, Italy), which was placed under the rat’s hind paw. Based on our previous study (Guo et al., 2021), the maximum heating time was set to 25 seconds because a longer cutoff latency increases the risk of burns on the rat’s hind paw tissue. The thermal hyperalgesia test was repeated a minimum of five times at 10-minute intervals, and the paw thermal withdrawal latency (PTWL) was calculated as the mean latency of the withdrawal response, which was characterized by a lift or lick of the hind paw, across five tests.
Iron content and lipid peroxidation assays
On the seventh day after CFA or saline administration, the ipsilateral (CFA injection side) spinal cord enlargement and DRG tissues at L4–L6 were collected from three random rats per group. In line with previous studies (Ozen et al., 2011; Guo et al., 2021), iron content was measured using flame atomic absorption spectrophotometry, expressed as micrograms per gram sample, and estimated by contrasting with the absorbance to a series of standard concentrations of ferrous sulfate (FeSO4). Commercial assay kits were used to estimate the levels of malondialdehyde (MDA, Jiancheng Biology, Nanjing, Jiangsu, China, A003-1), superoxide dismutase (SOD, Jiancheng Biology, A001-1), and glutathione peroxidase (GSH-Px, Jiancheng Biology, A005-1) in line with the manufacturer’s handbook. ROS generation was determined using 2,7-dichlorodihydrofluorescein diacetate (Thermo Fisher Scientific, Waltham, MA, USA) in accordance with a previous publication (Chung et al., 2015).
Western blot analysis
The ipsilateral (CFA injection side) spinal cord enlargement and DRG tissues at L4–L6 of three random rats per group were collected 1 week after CFA or saline administration and homogenized in a lysis buffer that consisted of RIPA (radio immunoprecipitation assay) and protease inhibitor phenylmethanesulfonylfluoride (50:1, Beyotime Institute of Biotechnology, Shanghai, China). The western blotting analysis protocols were conducted in line with those reported previously (Cheng et al., 2019). Briefly, the total protein was extracted from the collected spinal cord and DRG tissues using RIPA lysis buffer (Solarbio, Beijing, China), and the protein concentration was evaluated using a BCA (bicinchoninic acid) protein assay kit (Bio-Rad, Hercules, CA, USA). Equal amounts of protein were separated in a 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel (Solarbio) and transferred to a nitrocellulose membrane after electrophoresis (Merck Millipore, Carrigtwohill, Co. Cork, Ireland). The membrane was blocked for 1 hour with 5% skim milk (Biofroxx, Einhausen, Germany) in TBST (tris buffered saline with tween 20) at room temperature and subsequently incubated with a primary antibody overnight at 4°C. The following primary antibodies were applied: rabbit polyclonal antibody to ACSL4 (1:1000 for the spinal cord and DRG; ABclonal, Wuhan, Hubei, China, Cat# A6826; RRID: AB_2767400), rabbit monoclonal antibody to GPX4 (1:1000 for the spinal cord and DRG; Abcam, Cambridge, UK, Cat# ab125066; RRID: AB_10973901), and rabbit monoclonal antibody to beta-actin (β-actin) (1:100,000 for the spinal cord and DRG; ABclonal, Cat# AC026; RRID: AB_2768234). Images were acquired using a Tanon 5500 chemiluminescent imaging system (Tanon, Shanghai, China), and the intensities of the protein bands were quantitatively determined by densitometry using ImagePro Plus (version 6.0, National Institutes of Health, Bethesda, MD, USA) and normalized according to the β-actin band intensities.
Immunofluorescence
The immunofluorescence analysis protocols were conducted in line with those of our previous study (Guo et al., 2021). After transcardial perfusion with 0.1 M phosphate-buffered saline (PBS), followed by 4% paraformaldehyde, the ipsilateral (CFA injection side) L4–L6 spinal cord enlargement and DRG tissues of three random rats per group were collected and sectioned into 10-μmthick specimens on a freezing microtome (MICROM, Walldorf, Germany, HM550VP). To determine the specific cellular distribution of the ferroptosis marker ACSL4 in the spinal cord and DRG tissues, we performed double staining using a rabbit polyclonal antibody to ACSL4 (1:200 for the spinal cord and 1:500 for the DRG, ABclonal, Cat# A6826, RRID: AB_2767400) and one of the following cell markers: neuronal nucleus (NeuN) as a specific neuronal marker (mouse monoclonal antibody, 1:200 for the spinal cord and 1:500 for the DRG, Abcam, Cat# ab104224, RRID: AB_10711040), glial fibrillary acidic protein (GFAP) as a hallmark of astrocyte or satellite glial cell (mouse monoclonal antibody, 1:200 for the spinal cord and 1:500 for the DRG, Abcam, Cat# ab10062, RRID: AB_296804), ionized calcium-binding adapter molecule 1 (Iba-1) as a hallmark of microglia (mouse monoclonal antibody, 1:200 for the spinal cord, Abcam, Cat# ab15690, RRID: AB_2224403), 2′3′-cyclic nucleotide 3′-phosphodiesterase (CNPase) as a hallmark of mature oligodendrocytes (mouse monoclonal antibody, 1:100 for the spinal cord, Abcam, Cat# ab6319, RRID: AB_2082593), and S100 calcium binding protein B (S100β) as a hallmark of Schwann cells (mouse monoclonal antibody, 1:200 for the DRG, Immunoway Biotechnology, Plano, TX, USA, Cat# YM0572) at 4°C overnight in a humid box. After three 5-minute washes with PBS, the slices were incubated with the following secondary antibodies: Alexa 594-labeled goat anti-mouse immunoglobulin G (IgG, 1:500, Abcam, Cat# ab150120, RRID: AB_2631447) or Alexa 488-labeled goat anti-rabbit IgG (1:500, Abcam, Cat# ab150081, RRID: AB_2734747) for 1 hour at room temperature. Sections were observed under a Thermo Fisher EVOS FL Auto laser confocal scanning microscope (Thermo Fisher Scientific Inc.). Five fields per rat were randomly selected for performing quantitative analysis using ImageJ (version 1.8.0, National Institutes of Health, Bethesda, MD, USA). As previously described (Batista et al., 2019; Zhang et al., 2022), the average fluorescence intensity of each pixel was normalized to the background intensity of the same image, and the ratio of overlapped pixels (ACSL4/cell marker) from the total cell marker+ pixels was used to measure the degree of colocalization in the spinal cord and DRG tissues.
Transmission electron microscope
On the seventh day following CFA/saline administration, three random rats per group were sacrificed and perfused with a mixed agent (2% paraformaldehyde and 2% glutaraldehyde in 0.1 M sodium cacodylate). Sequentially, the ipsilateral (CFA injection side) spinal cord enlargement and DRG tissues at L4–L6 were collected and post-fixed in 2% osmium tetroxide with 1.6% potassium ferrocyanide in 0.1 M sodium cacodylate. This was followed by sample cutting and dehydration with a graded ethanol (50%, 70%, and 90%) and acetone (100%) series. After embedding in Eponate 812 medium (90529–77–4, Structure Probe, Inc., West Chester, PA, USA), the sections were transferred to copper mesh grids and stained with 2% uranyl acetate and lead citrate. The ultrastructure of mitochondria was visualized in different types of spinal cord (neurons, astrocytes, microglia, and oligodendrocytes) and DRG-tissue (neurons, satellite glial cells, and Schwann cells) cells using transmission electron microscopy (Hitachi, Ltd., HT-7700, Hitachi, Tokyo, Japan). The ratio of mitochondria with aberrant architectural changes (e.g., rupture of cristae and membranes) to total mitochondria was calculated and averaged across five random fields per rat.
Statistical analysis
Sample size was not predetermined using any statistical methods; however, our sample sizes were similar to those reported in a previous publication (Guo et al., 2021). We performed statistical comparisons using SPSS 20.0 statistical software (IBM Corp., Armonk, NY, USA). All values are expressed as means ± standard deviations (SDs). For the comparisons of PMWT and PTWL values, we used a two-way repeated-measures analysis of variance (ANOVA) followed by Bonferronipost hoc
tests. A one-way analysis of variance followed by Bonferronipost hoc
tests was used to compare other data among groups. In all cases, statistical significance was set atP
< 0.05.Results
Intrathecal liproxstatin-1 administration reduces CFA-induced painful behaviors
Baseline PMWT and PTWL values recorded before CFA/saline injection into the hind paw did not differ significantly among the groups of rats receiving different treatments (PMWT:P
= 0.320, PTWL:P
= 0.685). Compared with saline treatment, a single CFA injection induced hypersensitive behaviors in response to mechanical and thermal stimuli in rats during the entire experimental period (P
< 0.001 after CFA/saline injection;Figure 1B
andC
), which indicated that the chronic inflammatory-pain model was successfully constructed. After intrathecal administration of the potent ferroptosis inhibitor liproxstatin-1 for 3 consecutive days following CFA injection, the PMWT and PTWL of CFA-treated rats were dramatically elevated in comparison with those of the CFA-Veh rats (PMWT:P
< 0.01 on the third and fifth days andP
< 0.001 on the seventh day after CFA injection; PTWL:P
< 0.01 on the third and fifth days andP
< 0.001 on the seventh day after CFA injection;Figure 1B
andC
). Moreover, there were no significant differences in PMWT or PTWL between the liproxstatin-1- and minocycline-treated groups during the study period (Figure 1B
andC
).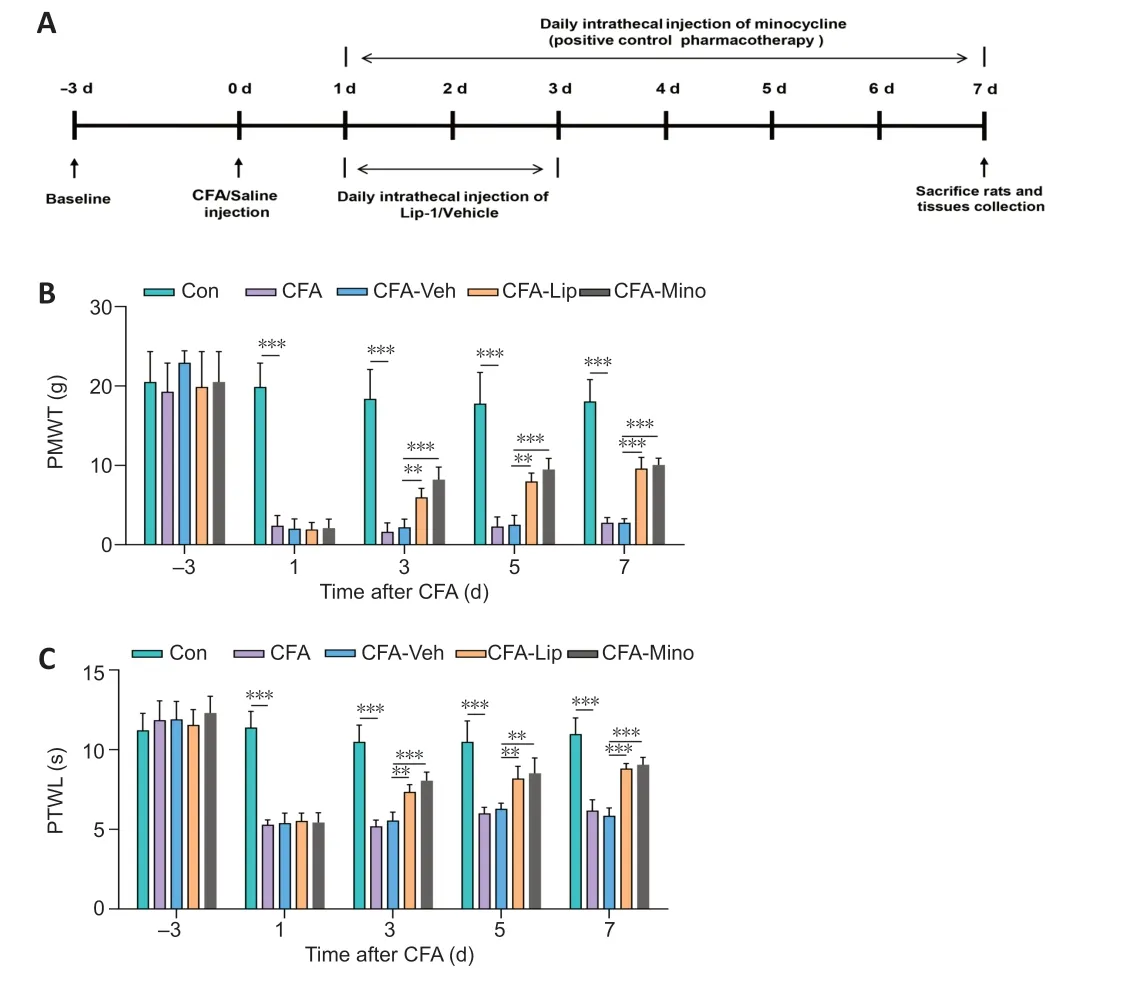
Figure 1|Attenuation of mechanical and thermal hypersensitivities following intrathecal liproxstatin-1 administration.(A) A diagram showing the experimental timeline. Baseline thresholds were obtained 3 days before complete Freund’s adjuvant (CFA) injection. On day 0, rats received 100 μL of CFA or saline as vehicle control to establish the inflammatory pain model. (B) PMWT and (C) PTWL values were reduced following CFA treatment, and intrathecal liproxstatin-1 or minocycline administration attenuated these mechanical and thermal hypersensitivities in CFA-treated rats. Data in bar charts are expressed as the mean ± SD and represent the results of three independent experiments in five rats per group. Data were analyzed using two-way repeated-measures analysis of variance followed by Bonferroni post hoc tests. **P < 0.01, ***P < 0.001. Lip: Liproxstatin-1; Mino: minocycline; PMWT: paw mechanical withdrawal threshold; PTWL: paw thermal withdrawal latency; Veh: vehicle
Liproxstatin-1 reduces levels of iron content and lipid peroxidation hallmarks in the spinal cord and DRG of rats treated with CFA
Iron overload and stimulation of lipid peroxisome proliferation are considered key regulators of ferroptosis (Guo et al., 2021). Thus, we subsequently determined the levels of iron content and several core factors of redox homeostasis in the spinal cord and DRG tissues. CFA treatment via the hind paw significantly enhanced iron levels in the rat spinal cord (P
< 0.001;Figure 2A
) and DRG tissues (P
< 0.001;Figure 2B
) compared with those in the Con group. Iron overload was significantly alleviated in the spinal cord (P
< 0.001;Figure 2A
) and DRG tissues (P
= 0.047;Figure 2B
) in CFA rats after intrathecal administration of ferroptosis inhibitor liproxstatin-1.In addition to iron accumulation, injection of CFA induced lipid peroxidation, as reflected by significant rises in ROS fold changes (spinal cord:P
< 0.001;
Figure 2C
; DRG:P
< 0.001;
Figure 2D
) and MDA concentrations (spinal cord:P
< 0.001;Figure 2E
; DRG:P
< 0.001;
Figure 2F
) and decreases in SOD (spinal cord:P
= 0.002,Figure 2G
; DRG:P
< 0.001;Figure 2H
) and GSH-Px levels (spinal cord:P
< 0.001;
Figure 2I
; DRG:P
= 0.004;Figure 2J
) in both the spinal cord and DRG tissues. Intrathecal delivery of liproxstatin-1 to CFA rats significantly lowered the rise in ROS (spinal cord:P
< 0.001;
Figure 2C
; DRG:P
= 0.001;Figure 2D
) and MDA levels (spinal cord:P
= 0.020;Figure 2E
; DRG:P
= 0.019;Figure 2F
) but activated the decrease in SOD (spinal cord:P
= 0.001;Figure 2G
; DRG:P
= 0.008;Figure 2H
) and GSH-Px levels (spinal cord:P
= 0.012;Figure 2I
; DRG:P
= 0.042,Figure 2J
) in the spinal cord and DRG tissues.Liproxstatin-1 treatment diminishes ACSL4 protein levels and increases
GPX4 protein levels
Various proteins, such as ACSL4 and GPX4, are considered crucial ferroptosisregulating factors. Therefore, we focused on the levels of ACSL4 and GPX4 in the spinal cord and DRG tissues. CFA treatment significantly upregulated ACSL4 levels and downregulated GPX4 levels in the spinal cord (ACSL4:P
< 0.001; GPX4:P
= 0.026;Figure 3A–C
) and DRG tissues (ACSL4:P
= 0.001; GPX4:P
= 0.002,Figure 3D–F
). Intrathecal liproxstatin-1 delivery restored ACSL4 and GPX4 expression disorders secondary to CFA injection in both the spinal cord (ACSL4:P
= 0.003; GPX4:
P
= 0.002;Figure 3A–C
) and DRG tissues (ACSL4:P
= 0.002; GPX4:P
= 0.019;Figure 3D–F
).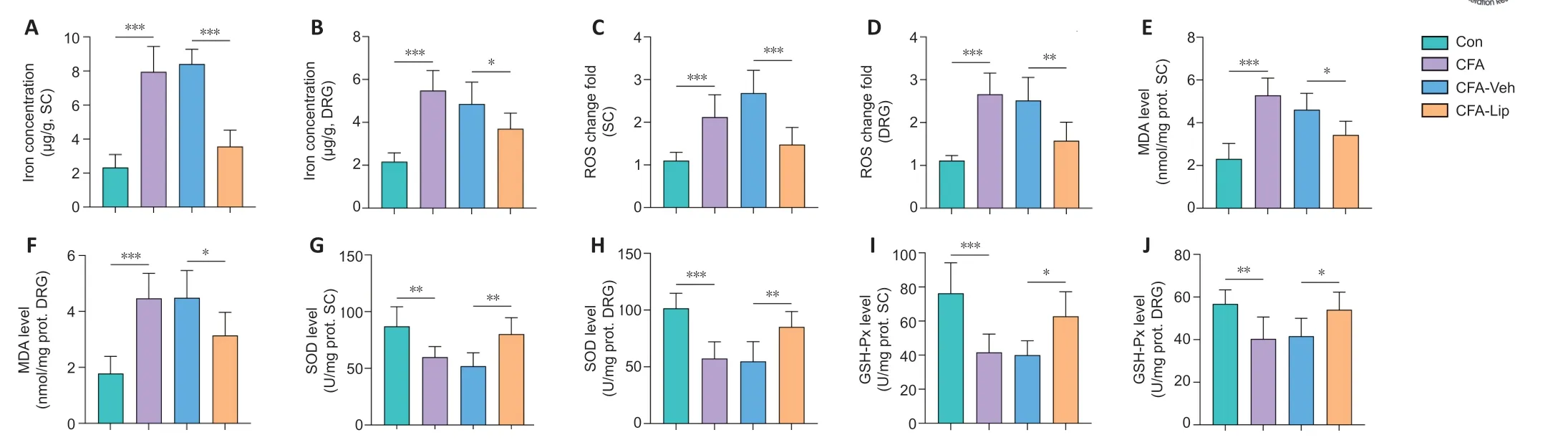
Figure 2|Restoration of CFA-induced iron overload and lipid peroxidation, which contributed to pain-like behaviors following intrathecal liproxstatin-1 administration.Levels of iron content and lipid peroxidation hallmarks in the spinal cord (SC) and DRG tissues. Intrathecal liproxstatin-1 administration decreased iron, reactive oxygen species (ROS), and malondialdehyde (MDA) levels and increased superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) levels in the spinal cord and DRG of CFA rats. Data are expressed as the mean ± SD and represent the results of three independent experiments in three rats per group. Data were analyzed using one-way analysis of variance followed by Bonferroni post hoc tests. *P < 0.05, **P < 0.01, ***P < 0.001. CFA: Complete Freund’s adjuvant; DRG: dorsal root ganglion; GSH-Px: glutathione peroxidase; Lip: liproxstatin-1; MDA: malondialdehyde; Mino: minocycline; prot: protein; ROS: reactive-oxygen-species; SOD: superoxide dismutase; Veh: vehicle.
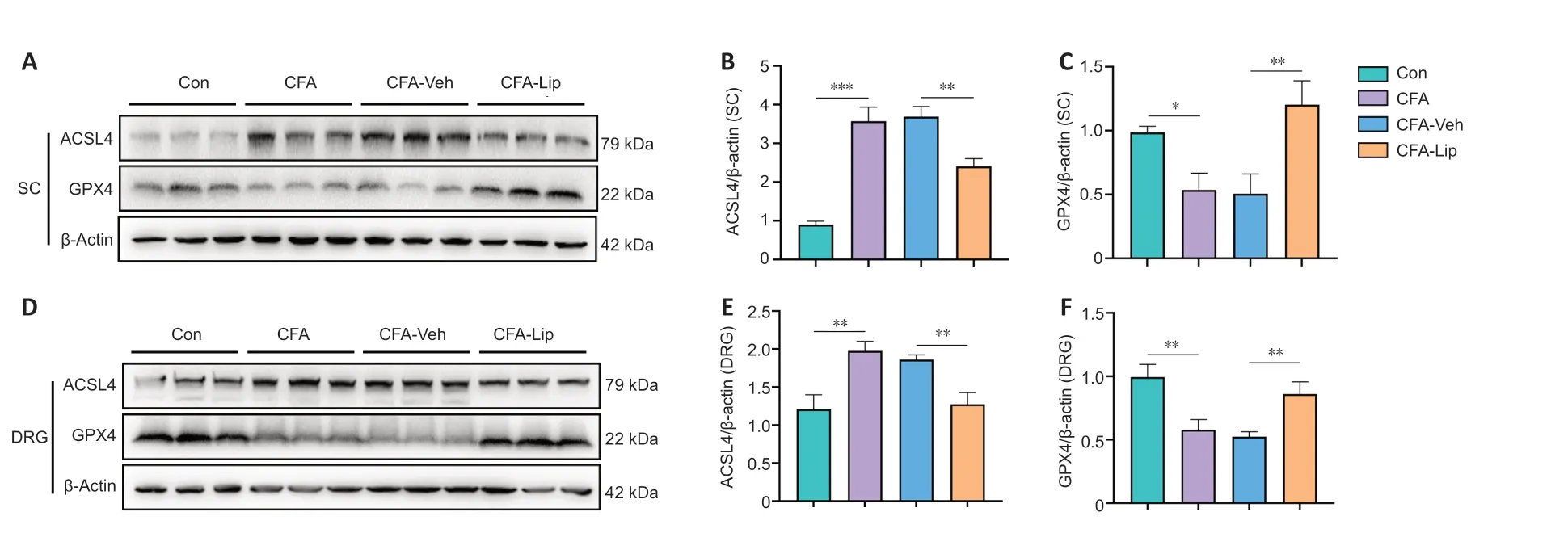
Figure 3|Anti-acyl-CoA synthetase long-chain family member 4 (ACSL4) decreases and glutathione peroxidase 4 (GPX4) increases in CFA rats following intrathecal liproxstatin-1 administration(A–C) The representative bands and quantitative evaluations of ACSL4 and GPX4 protein levels demonstrated that liproxstatin-1 restored the abnormal levels of ACSL4 and GPX4 in the spinal cord (SC) of CFA rats. (D–F) The representative bands and quantitative evaluations of ACSL4 and GPX4 protein levels demonstrated that liproxstatin-1 restored the abnormal levels of ACSL4 and GPX4 in the dorsal root ganglion tissue of CFA rats. Data in bar charts are expressed as the mean ± SD and represent results of three independent experiments in three rats per group. Data were analyzed using one-way analysis of variance followed by Bonferroni post hoc tests. *P < 0.05, **P < 0.01, ***P < 0.001. ACSL4: Anti-acyl-coenzyme A synthetase long-chain family member 4; CFA: complete Freund’s adjuvant; GPX4: glutathione peroxidase 4; Lip: liproxstatin-1; Mino: minocycline; Veh: vehicle.
Cellular localization of ACSL4 in the spinal dorsal horn and DR
GUsing the double-immunofluorescence staining method, we investigated the ACSL4 protein distribution in defined cellular compartments in the rat spinal cord and DRG tissues. After CFA injection, ACSL4 colocalized primarily with the microglial marker Iba-1 (P
= 0.008), the astrocyte marker GFAP (P
< 0.001), and oligodendrocyte marker CNPase (
P
< 0.001) in the spinal tissues (
Figure 4
). In addition, CFA administration enhanced the expression of ACSL4 in the satellite glial cells labeled by GFAP (P
= 0.002) and Schwann cells labeled by S100β (P
< 0.001) in DRG tissues (Figure 5
). These results suggested that the injection of CFA into the hind paw predominantly causes the widespread presence of ferroptosis in glial and Schwann cells in the rat spinal cord and DRG tissues. Notably, intrathecal liproxstatin-1 delivery led to a significant reduction in ACSL4 immunoreactivity in astrocytes (P
= 0.001), microglia (P
= 0.002), and oligodendrocytes (
P
= 0.013) in the spinal cord (Figure 4
), as well as satellite glial (P
= 0.018) and Schwann cells (P
= 0.001) in the DRG (Figure 5
).Liproxstatin-1 suppresses CFA-induced abnormal morphological changes in the mitochondria in the spinal cord and DRG
One of the classical pathological changes during ferroptosis is the abnormal morphological characteristics of the mitochondria (Dixon et al., 2012). In response to CFA injection, the mitochondria showed unusual morphological features, such as cristae rupture and greater membrane density in the spinal cord (Figure 6
) and DRG tissues (Figure 7
). Interestingly, although representative ferroptosis-like changes in the mitochondria were observed in the spinal and DRG neurons, quantitative analysis revealed higher ratios of mitochondria that represented morphological disorders in response to CFA administration in the non-neural cells of the spinal cord (P
< 0.001 for astrocytes, microglia, and oligodendrocytes;Figure 6
) and DRG (P
< 0.001 for satellite glial and Schwann cells;Figure 7
). Moreover, intrathecal injection of liproxstatin-1 into CFA rats not only reversed the abnormal mitochondrial changes but also resulted in a significant reduction in the abnormal mitochondria ratio in the non-neural spinal cord (P
< 0.001 for astrocytes,
P
= 0.003 for microglia, andP
< 0.001 for oligodendrocytes;Figure 6
) and DRG cells (P
< 0.001 for satellite cells andP
= 0.023 for Schwann cells;
Figure 7
).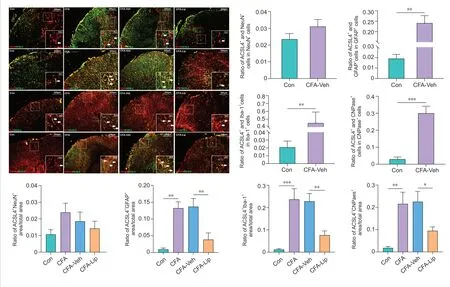
Figure 4|Distribution of anti-acylcoenzyme A synthetase long-chain family member 4 (ACSL4) in distinct cell types in the spinal cord.Representative double-immunofluorescence staining images show the distribution of ACSL4 in the spinal cord tissue. The white arrows indicate spinal cells stained with distinct cell markers. Scale bar: 200 μm (50 μm in the magnified boxes). Quantification analyses of cells colocalized by ACSL4 revealed that CFA injection into the hind paw caused widespread ferroptosis in spinal astrocytes, microglia, and oligodendrocytes. Intrathecal treatment of liproxstatin-1 dramatically decreased the ratio of ACSL4 immunoreactivity area to astrocyte/microglia/oligodendrocyte marker immunoreactivity area in spinal cord. Data in bar charts are expressed as the mean ± SD and represent results of five sections per rat with three rats per group. Data were analyzed using one-way analysis of variance, followed by Bonferroni post hoc tests. *P < 0.05, **P < 0.01, ***P < 0.001. ACSL4: Anti-acyl-coenzyme A synthetase long-chain family member 4; CFA: complete Freund’s adjuvant; Lip: liproxstatin-1; Mino: minocycline; Veh: vehicle.
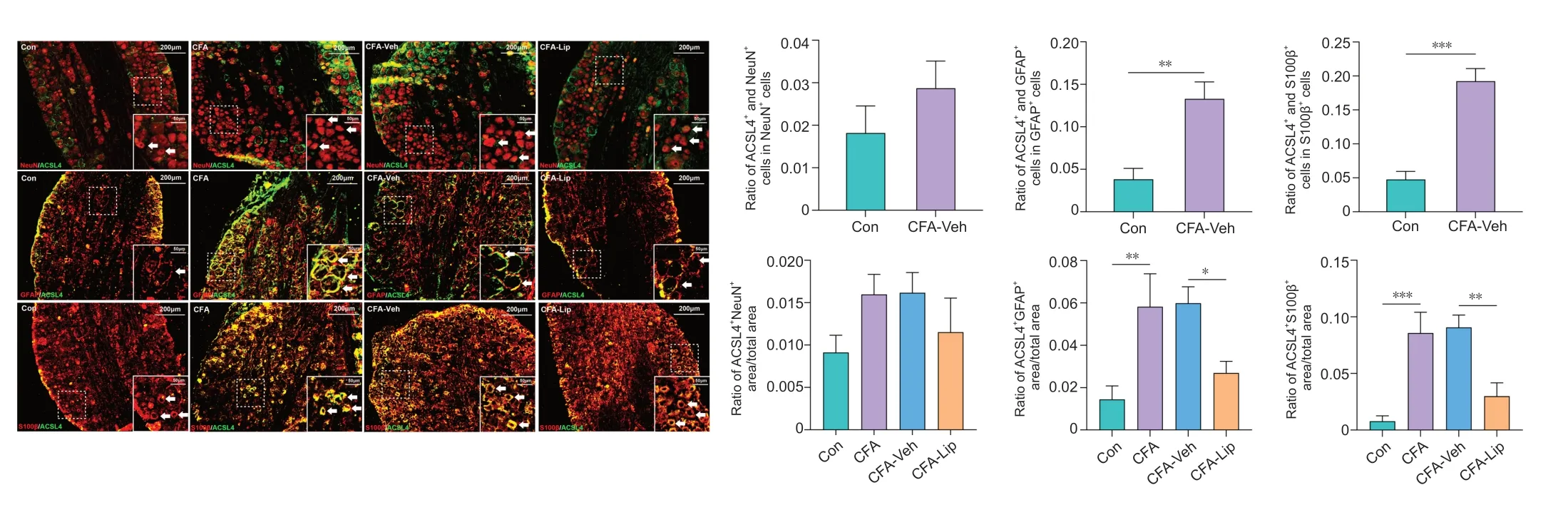
Figure 5|Distribution of anti-acyl-coenzyme A synthetase long-chain family member 4 (ACSL4) in distinct cell types in the dorsal root ganglion (DRG) cells.Representative double-immunofluorescence staining images show the distribution of the ACSL4 protein in the DRG tissues. The white arrows indicate DRG cells stained with distinct cell markers. Scale bar: 200 μm (50 μm in the magnified boxes). Quantification analyses of cells colocalized by ACSL4 revealed that CFA injection into the hind paw caused widespread ferroptosis in the satellite glial and Schwann cells of the rat DRG. Intrathecal treatment of liproxstatin-1 dramatically decreased the ratio of ACSL4 immunoreactivity to satellite glial cell/Schwann cell marker immunoreactivity area in DRG. Data in bar charts are expressed as the mean ± SD and represent results from five sections per rat with three rats per group. Data were analyzed using one-way analysis of variance followed by Bonferroni post hoc tests. *P < 0.05, **P < 0.01, ***P < 0.001. ACSL4: Anti-acyl-coenzyme A synthetase long-chain family member 4; CFA: complete Freund’s adjuvant; DRG: dorsal root ganglion; Lip: liproxstatin-1; Mino: minocycline; Veh: vehicle.
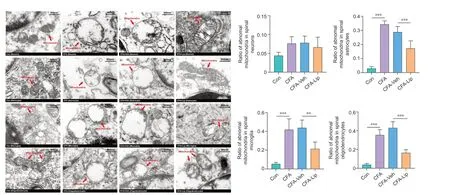
Figure 6|Liproxstatin-1-induced protection against mitochondrial morphological changes caused by CFA in the spinal cord.Representative electron micrographs of the mitochondrial ultrastructure (red arrows) in the spinal cord across four groups. Scale bar: 500 nm. Quantification analyses showed that intrathecal liproxstatin-1 administration to CFA rats significantly reduced the ratios of abnormal mitochondria to the total mitochondria in spinal astrocytes, microglia, and oligodendrocytes. Data in bar charts are expressed as the mean ± SD and represent results of five sections per rat with three rats per group. Data were analyzed using one-way analysis of variance followed by Bonferroni post hoc tests. **P < 0.01, ***P < 0.001. CFA: Complete Freund’s adjuvant; Lip: liproxstatin-1; Mino: minocycline; Veh: vehicle.

Figure 7|Liproxstatin-1-induced protection against mitochondrial morphological changes caused by CFA in dorsal root ganglion (DRG) cells.Representative electron micrographs of the mitochondrial ultrastructure (red arrows) in the DRG across four groups. Scale bar: 500 nm. Quantification analyses showed that intrathecal liproxstatin-1 administration to CFA rats significantly reduced the ratios of abnormal mitochondria to the total mitochondria in DRG satellite glial and Schwann cells. Data in bar charts are expressed as the mean ± SD and represent results of five sections per rat with three rats per group. Data were analyzed using one-way analysis of variance followed by Bonferroni post hoc tests. *P < 0.05, ***P < 0.001. CFA: Complete Freund’s adjuvant; DRG: dorsal root ganglion; Lip: liproxstatin-1; Mino: minocycline; Veh: vehicle.
Discussion
As a response to inflammatory factors, inflammatory pain is considered a global clinical problem that diminishes the quality of life of patients (Conaghan et al., 2019). Several studies have revealed that the inflammatory response and ferroptosis share several common features, such as iron accumulation and lipid peroxidation (Singh and Vinayak, 2015; Sun et al., 2021). However, the role of oxidative and iron-driven ferroptosis from an inflammatory pain perspective remains unclear. The current study is the first to identify ferroptosis-like cell death in the spinal cord and DRG in an inflammatorypain rodent model. Moreover, although previous studies have demonstrated the effects of liproxstatin-1 and ferrostatin-1 (delivered intraperitoneally at a dose of 10 mg/kg in a neuropathic-pain model) (Guo et al., 2021; Wang et al., 2021), whether the administration of ferroptosis inhibitors via the intrathecal route induces an effective analgesic effect against inflammatory pain remains inconclusive. In this study, we found that injection of liproxstatin-1 into the spinal intrathecal space at a relatively low dose (10 μg/μL and 30 μL) effectively hinders spinal ferroptosis and alleviates CFA-induced mechanical allodynia and thermal hyperalgesia. Compared with other drug administration routes, intrathecal drug delivery is particularly advantageous for precisely delivering drugs into the spinal intrathecal space, without the need to consider the permeability of the blood-brain barrier or toxicity to other organs (Cui et al., 2015; Hou et al., 2016). Thus, our results offer a reasonable method to clinically alleviate inflammatory pain. Remarkably, intrathecal injection of 40 μL liproxstatin-1 caused temporary lower limb paralysis in some rats, which suggested that the adverse effects of intrathecal liproxstatin-1 injection were dose-related. Therefore, additional preclinical investigations that clarify the prospects of intrathecal liproxstatin-1 administration for the treatment of inflammatory pain are warranted. It has been shown that DRG tissues transmit nociceptive stimuli to the spinal cord and brain in the form of electrical action potentials (Cui et al., 2021; Ma et al., 2021; Guo et al., 2022). Compelling evidence has indicated that the differential occurrence of multiple pain-related pathophysiological changes in the spinal cord and DRG acts as a trigger during the pathological process of nociceptive hypersensitivity of chronic inflammatory pain (Garcia et al., 2021; Li et al., 2021). For example, changes in programmed cell death, such as autophagy (Chen and Lu, 2017; Yin et al., 2018) and apoptosis (Kahya et al., 2017; Dai et al., 2020), are consistent across both spinal cord and DRG tissues under chronic pain conditions. Moreover, researchers have verified the mechanisms of spinal ferroptosis under neuropathic pain conditions (Guo et al., 2021; Wang et al., 2021); however, implications for ferroptosis in the DRG after painful injuries remain unclear. Our results suggest that ferroptosislike cell death is enhanced simultaneously in the DRG and spinal cord of CFA rats and is characterized by mitochondrial morphological abnormalities, overwhelming accumulation of iron and lipid peroxisome proliferation, and disorders of expression levels of several ferroptosis biomarkers (i.e., ACSL4 and GPX4). In addition, our data showed that intrathecal injections of liproxstatin-1 successfully alleviated CFA-induced pain-like behaviors, partly via inhibition of the ferroptosis process in the spinal cord and DRG. Taken together, we conclude that ferroptosis plays a crucial role in the CFA-induced sensitization of both DRG and spinal cord tissues.
Although the exact mechanism underlying ferroptosis remains elusive, iron overload has been identified as the most likely trigger of cell death (Dixon et al., 2012). Given its role as a predominant trace element, iron is essential for maintaining cell functions and mediates various important physiological processes, which include but are not limited to hemoglobin synthesis, oxygen transport, and adenosine triphosphate production (Koleini et al., 2021). Iron overload may generate excessive ROS production via Fenton or Haber Weiss reactions, which can induce oxidative stress injury to DNA and proteins and even ferroptosis-like cell death (Alves et al., 2021). Moreover, emerging evidence has established a link between iron homeostasis and the occurrence of inflammatory pain (Sun et al., 2021). For instance, the release of proinflammatory cytokine in the cavum articulare was reported to result from the deposit of hemosiderin, an intracellular iron complex that is closely associated with joint pain (Heiland et al., 2010; van Vulpen et al., 2015; Sun et al., 2021). Accordingly, the current study revealed significant iron accumulation in the spinal cord and DRG tissues in CFA rats that exhibited painful behaviors, which suggested that iron overload contributes to ferroptosis activation during CFAinduced inflammatory pain.
Another contributory mechanism of ferroptosis is lipid peroxidation (Lee et al., 2021). At the molecular level, the contributions of GPX4 and ACSL4 to lipid peroxidation injury during ferroptosis have been demonstrated recently (Doll et al., 2017; Tang et al., 2019; Zhang et al., 2021b). When cells are treated with a ferroptosis inducer, the reduced expression of GPX4 and enhanced expression of ACSL4 that respond to an excessive iron level may suppress GSH activity and increase polyunsaturated fatty acyl phospholipids, phospholipids, and oxidized phosphatidylethanolamine levels, which would eventually elicit membrane lipid peroxidation and oxidative stress damage (Doll et al., 2017; Zhang et al., 2021b). Our current work showed that injection of CFA negatively regulates GPX4 level and positively regulates ACSL4 level in the spinal cord and DRG tissues. Consistent with a previous study (Singh and Vinayak, 2015), we also found disordered levels of several lipid peroxidation hallmarks, including the downregulated expression of MDA and GSH-Px levels and the upregulated expression of ROS and MDA levels. Administration of a ferroptosis inhibitor restored GPX4 and ACSL4 levels and alleviated the lipid peroxidation that resulted from CFA treatment. These findings indicated that ferroptosis caused by CFA is involved in GPX4 and ACSL4 dysfunctioninduced lipid peroxidation in the context of mechanical allodynia and thermal hyperalgesia.
Our previous study identified ferroptosis-like cell death throughout spinal neurons and glial cells in a neuropathic-pain rodent model (Guo et al., 2021). Interestingly, in the present study, we demonstrated that ferroptosisrelated ACSL4 dysregulation and mitochondrial changes were detected primarily in non-neural cells in the spinal cord and DRG under a CFA-induced inflammatory pain condition. Recently, the possible pathogenesis of nonneural ferroptosis in nervous system diseases has gained attention (Wang et al., 2021). For example, a previous study reported the proinflammatory effect of ferroptosis on glial cells: ferroptotic primary astrocytes in mice release excessive amounts of pro-inflammatory factors (e.g., interleukin (IL)-6 and IL-1β), and ferrostatin-1 treatment inhibits inflammatory-factor overproduction (Li et al., 2021). Moreover, inhibiting ferroptosis in Schwann cells via the over-expression of c-Jun may contribute to the recovery of peripheral-nerve function, which highlights the role that ferroptosis plays in nerve-function injury and repair (Gao et al. 2022). Taken together, these findings indicate that non-neural ferroptosis is closely associated with central nervous system inflammation and impairment of peripheral nerve function and that further studies to unmask the exact role of non-neural ferroptosis in CFA-induced inflammatory pain are warranted.
Diminished mitochondrial cristae and increased mitochondrial membrane density usually occur during ferroptosis; moreover, mitochondrial abnormalities have been detected in an inflammatory-pain rodent model (Guo et al., 2013). Our study identified significant disorders of mitochondrial morphological characteristics (e.g., collapsed cristae and disrupted mitochondrial membrane) throughout the non-neural cells in the spinal cord and DRG tissues of CFA-treated rats. Mitochondria have been recognized as a pivotal part of lipid and iron metabolism, and there is a growing interest in understanding the mechanism of mitochondria in ferroptotic cell death (Gan, 2021; Otasevic et al., 2021). Previous studies have identified two enzymes located in mitochondria, citrate synthase and acyl-coenzyme A (CoA) synthetase family member 2, as crucial mediators of erastin-induced ferroptosis owing to their ability to stimulate fatty acid activity and supply precursors during the lipid peroxidation process (Dixon et al., 2012). Moreover, accumulating evidence has suggested that several iron homeostasis-related factors in mitochondria, such as mitoferrin and voltagedependent anion channels, are functionally relevant to ferroptosis occurrence and that the suppression of two gene-encoding proteins may result in an intracellular accumulation of free iron and promote ferroptosis-like cell death (Shoshan-Barmatz et al., 2010; Otasevic et al., 2021). Our study showed that administration of the ferroptosis suppressor liproxstatin-1 restored the abnormal morphological changes in mitochondria induced by CFA, which suggests that the aberrant mitochondrial morphological characteristics in the spinal cord and DRG tissues are involved in ferroptosis in rats treated with CFA.
This study has several limitations. First, we exclusively focused on the effects of one ferroptosis inhibitor on an inflammatory-pain animal model, and further investigations of other ferroptosis inhibitors are necessary to confirm the role of ferroptosis in inflammatory pain. Second, this preliminary investigation did not completely determine the specific cellular mechanism underlying ferroptosis in the inflammatory-pain process and thus, further investigation is warranted.
Overall, based on an inflammatory-pain animal model with intrathecal CFA injection, our study provided evidence for the occurrence of ferroptosis in the rat spinal cord and DRG tissues, and clarified the effectiveness of intrathecal liproxstatin-1 administration for treating inflammatory pain-like behaviors. These findings highlight the possible link between non-neural ferroptosis and the occurrence of nociceptive responses caused by CFA injection, and demonstrate that this unique form of programmed cell death is a promising therapeutic target for inflammatory pain.
Acknowledgments:
The authors thank Dr. Cui-Cui Xiao from the Third Affiliated Hospital, Sun Yat-sen University for participating in the discussion of the manuscript, and Dr. Jia Chen from the Laboratory Animal Center of South China Agricultural University for assistance in the animal husbandary.
Author contributions:
Study design: XL; experiment implementation and data acquisition: YFD, JYD; data analysis: PX, JFL; manuscript draft: XL, YFD; manuscript review: XL. All the authors read and approved the final version of manuscript.
Conflicts of interest:
The authors declare no conflicts of interest.
Availability of data and materials:
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Open access statement:
This is an open access journal, and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Additional files:
Paw withdrawal threshold values (g) of rats that were administered different volumes of liproxstatin-1.
Paw thermal withdrawal latency (s) of rats that were administered different volumes of liproxstatin-1.
 中國(guó)神經(jīng)再生研究(英文版)2023年2期
中國(guó)神經(jīng)再生研究(英文版)2023年2期
- 中國(guó)神經(jīng)再生研究(英文版)的其它文章
- Nicotinic acetylcholine signaling is required for motor learning but not for rehabilitation from spinal cord injury
- Commentary on “PANoptosis-like cell death in ischemia/reperfusion injury of retinal neurons”
- Understanding the timing of brain injury in fetal growth restriction: lessons from a model of spontaneous growth restriction in piglets
- Kainate receptors in the CA2 region of the hippocampus
- Advances in quantitative analysis of astrocytes using machine learning
- MicroRNA-based targeting of the Rho/ROCK pathway in therapeutic strategies after spinal cord injury
