Perspectives on diacylglycerol-induced improvement of insulin sensitivity in type 2 diabetes
Daoming Li, Yang Zhu, Yonghua Wang*, Qiong Zou, Jinzhu Duan,Dongxiao Sun-Watrhous, Baoguo Sun*
a Beijing Advanced Innovation Center for Food Nutrition and Human Health, Beijing Technology and Business University, Beijing 100048, China
b School of Food and Biological Engineering, Shaanxi University of Science and Technology, Xi’an 710021, China
c School of Food Science and Engineering, Guangdong Research Center of Lipid Science and Applied Engineering Technology,South China University of Technology, Guangzhou 510640, China
d Bioprocess Engineering, Wageningen University and Research, 6700 AA Wageningen, Netherlands
e Guangzhou Yong-h Special Nutrition Technology Co. Ltd, Guangzhou 510000, China
f Guangzhou Institute of Pediatrics, Guangzhou Women and Children's Medical Center, Guangzhou 510623, China
Keywords:
Diacylglycerols
Functional oil
Insulin sensitivity
Lipidomics
A B S T R A C T
Diacylglycerol (DAG)-based edible oils have attracted increasing research interest owing to their healthpromoting properties. Recent animal and human studies showed that an increased 1,2-DAG content in the liver and skeletal muscle may cause insulin resistance. However, earlier studies using animal models or humans reported that dietary DAGs with a 1,2-DAGs to 1,3-DAGs ratio of approximately 3:7 could improve insulin sensitivity in type 2 diabetic patients. This conflict raises the question of whether there is a link between the ingested DAGs and endogenous DAGs during their metabolism. To make a contribution to this field, this review provides an overview of the metabolic pathways of ingested DAGs and biological roles of DAGs (ingested and endogenous) in the change of insulin sensitivity. Accordingly, strategies for further investigations on the metabolism of DAGs are proposed.
1. Introduction
Diabetes, obesity and associated complications have become observably global metabolic diseases. Diabetes and obesity are closely linked. Obesity, which affects over one-third of the global population, is a major contributor to type 2 diabetes (T2D) [1,2].Long-term excessive caloric intake along with a sedentary lifestyle(insufficient physical activity) causes obesity [3]. Therefore, promoting a healthy diet and lifestyle is an effective approach to reduce the risk of developing obesity and diabetes. Functional oils with health-promoting components such asω-3 polyunsaturated fatty acids (FAs), mediumchain triacylglycerols (TAGs), and diacylglycerols (DAGs) can be part of a healthy diet. DAGs are present in almost all the vegetable oils. Commercially produced DAGs have gained popularity as cooking oils among the consumers who are motivated by the preliminarily demonstrated benefits of DAGs (such as their ability to suppress the accumulation of body fat) [4].
Further to the reports on suppressing the accumulation of body fat,a number of studies also indicated other health benefits of DAGs such as the reduction of postprandial serum TAG levels [5-7], suppression of postprandial hypertriglyceridemia along with hypertension [8],inhibition of the postprandial increase in very-low-density lipoproteins and insulin [9-11], and increase of insulin sensitivity [12]. Among these functions, the roles of DAGs in increasing insulin sensitivity have gained particular attention in recent years. Interestingly, dietary DAGs, but not TAGs, were found to reduce the concentration of insulin in the serum of both healthy and unhealthy mice or humans [11-14].Li et al. [15]showed that ingestion of DAGs (1,2-DAGs and 1,3-DAGs at a ratio of approximately 3:7) increased the insulin sensitivity in patients with T2D. Shulman’s group [16-18]reported that an increased DAG content in the liver cells could decrease insulin sensitivity in both animals and humans and the decrease of insulin sensitivity was found in close association with the occurrence of T2D.The different influences of the ingested DAGs and endogenous DAGs on insulin sensitivity in T2D have stimulated researchers to revisit the metabolic fates of the ingested DAGs and endogenous DAGs as well as their effects on the regulation and coordination of insulin signaling.To support such a research interest, this review was performed to examine the metabolic pathways of dietary DAGs, as well as the effects of the ingested DAGs and endogenous DAGs on insulin sensitivity. Strategies for future research to investigate the metabolism of DAGs and their effects on insulin sensitivity are also proposed.
2. The association of an increased intracellular DAG content in skeletal muscle or the liver with insulin resistance
It remains challenging to understand the molecular mechanisms of insulin resistance (how to trigger, enhance or inhibit), even though a number of hypotheses have been proposed, for example, inflammation has been linked to the alterations in adipocytokines and DAG-mediated insulin resistance [19-22]. In 2010, Erion and Shulman [23]proposed that an increased intracellular DAG content in skeletal muscle or the liver might lead to the reduction of insulin sensitivity through protein kinase Cθ(PKCθ) or PKCε-mediated phosphorylation of insulin receptor substrates. An animal study compared the 1,2-DAG content in livers from the genetically obese Zucker rats and lean Zucker rats, and results showed that the livers from the genetically obese Zucker rats contained approximately 1.8-fold higher 1,2-DAG content than the lean control rats [24]. Another report studied the hepatic insulin resistance in mice with overexpression of acylCoA:DAG acyltransferase 2, results demonstrated that the insulin sensitivity was significantly reduced along with an almost 12-fold increase in hepatic DAG content [25]. A subsequent study on mice with hepatocyte-specific SREBP-1c overexpression revealed that the increased C18:1-DAG content in the liver caused a decrease of insulin sensitivity [26]. Lyu et al. [27]demonstrated that plasma membrane-bound 1,2-DAGs caused hepatic insulin resistance in rats.The associations among DAG increase and insulin resistance were not only found in rats but also in humans. Kumashiro et al. [28]conducted a study using flash-frozen liver biopsies obtained from 37 obese,nondiabetic individuals, and found that the total hepatic DAG content showed a stronger correlation with HOMA-IR, compared with other variables such as body mass index, ceramide content, c-Jun N-terminal kinase phosphorylation, long-chain fatty acyl CoA content, multiple markers of ER stress, and multiple plasma inflammatory cytokine concentrations. It has demonstrated that only the 1,2-DAG isoform had the ability to activate PKCs, whereas 1,3 and 2,3 could not [29,30].The activation of PKCθor PKCεinduced by1,2-DAGs in skeletal muscle or liver cells of humans or mice was found in close association with the pathogenesis of insulin resistance [17,18,27,31,32].
In addition to DAG configuration, the localization of DAGs also affects its ability to activate PKCs [18,33,34]. Eichmann et al. [35]proposed a “3-pool model” of intracellular DAG compartmentation:Three distinct intracellular DAG pools may exist in the endoplasmic reticulum/Golgi network (“Pool I”), the lipid droplet (“Pool II”),and the plasma membrane (“Pool III”). Among them, Golgi DAGs may regulate novel PKC (nPKC) isoforms trafficking to the plasma membrane and drive DAG/nPKC-mediated insulin resistance [36].Lipolysis-derived DAGs represent “Pool II” (which locates in cytoplasmic lipid droplets). Moreover, 1,2-DAGs located on plasma membrane were found to be involved in DAG signaling events,executed by conventional PKC isoforms [35].
It is worth noting that besides DAG configuration and localization, the FA composition of DAGs also affects its ability to activate PKCs [33,37-43]. Montell et al. [37]demonstrated that with the increase of available saturated FAs, saturated FAs may accumulate as DAGs (whilst unsaturated FAs tend to be converted to TAGs), and such accumulation of DAGs is closely associated with the induction of insulin resistance in human muscle cells.Bergman et al. [33]showed the differences in DAG-associated insulin sensitivity among individuals with different health status and diet style. The total DAG concentration in the muscle was found higher in the obesity ((13.3 ± 1.0) pmol/μg protein) and T2D((15.2 ± 1.0) pmol/μg protein) groups, compared with the athlete group((10.0 ± 0.78) pmol/μg protein,P= 0.002), with far more membrane DAG species (C18:0/C20:4, di-C16:0and di-C18:0) found in the T2D group [33].Szendroedi et al. [38]performed lipid infusion studies and found that DAG species containing C16:0, C18:0, C18:1, C18:2or C20:4exhibited the closest relationship with PKCθactivation and insulin resistance in obese and T2D individuals. Lundsgaard et al. [39]reported that an unsaturated FA diet could increase 1,3-DAGs and intramyocellular TAGs in human muscle, compared with a carbohydrate diet. Nowotny et al. [40]showed that the content of membrane di-C18:2DAG doubled after an intravenous fat injection (infusion of Intralipids?, Fresenius Kabi GmbH, Bad Homburg, Germany), and seemed to correlate with PKCθactivation after oral consumption of fat (soybean oil) in insulinsensitive volunteers. Franko et al. [41]observed that I148M PNPLA3 carriers with a fatty liver had unaltered hepatic DAG (FA, C18:1)species and insulin sensitivity, whilst the subjects carrying wild-type PNPLA3 and having a fatty liver tended to have an elevated hepatic DAG (FA, C18:1) content and disrupted insulin signaling in the liver.More recently, Perreault et al. [42]reported a positive relationship between PKCεactivation and sarcolemma 1,2-DAG (C16:0–C18:2)in human skeletal muscle. Tonks et al. [43]found that di-C18:2was higher in obese insulin-resistant individuals. In conclusion, according to the above studies, although we found that many species of DAGs have the ability to modulate insulin resistance, there is no consensus on those that specifically alter insulin resistance.
Many factors could result in the increase of DAGs in liver and skeletal muscle. More than 20 years ago, Shmueli et al. [44]proposed that DAG level could be raised in different ways such asde-novosynthesis from phosphatidic acid, phosphatidylcholine hydrolysis, glycosyl-phosphatidylinositol hydrolysis, and hydrolysis of phosphatidylinositol 4,5-biphosphate (PIP2). Most recently,multiple pathological causes are believed to account for the high net intracellular DAG content in the liver and skeletal muscle, including over nutrition, defects in adipocyte metabolism (including lipid storage and lipolysis), defects in mitochondrial FA oxidation and gene variation in apolipoprotein C3 [23].
Based on above, we summarized the 1,2-DAG-induced PKCεactivation-mediated hepatic insulin resistance in Fig. 1. The increased 1,2-DAG content in liver cells stimulates the translocation of PKCεto the plasma membrane, causing the activation of PKCε. The activated PKCεcan phosphorylate Thr1160of the insulin receptor and impair the activity of insulin receptor tyrosine kinase. As a result,the insulin receptor substrates cannot be phosphorylated [17,18],phosphoinositide-3-kinase cannot be activated, and downstream insulin signaling is blocked. The molecular mechanisms behind the 1,2-DAG-mediated PKCθ-activation induced insulin resistance in skeletal muscle may resemble those of PKCε-induced insulin resistance in the liver [31]. Most recently, Jayasinghe et al. [45]integrated recent observational and mechanistic studies with the focus on the roles of DAGs in skeletal muscle’s insulin resistance, and concluded that whether DAGs play a causal role in skeletal muscle’s insulin resistance remains inconclusive.
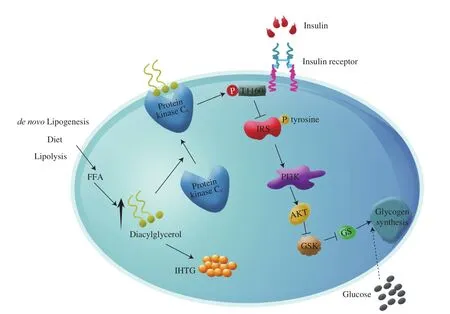
Fig. 1 Hepatic DAG-induced insulin resistance in liver cells. The increased DAG content in liver cells promotes the translocation of protein kinase Cε to the plasma membrane. The translocated protein kinase Cε can then phosphorylate Thr1160 of the insulin receptor and impair the activity of insulin receptor tyrosine kinase. As a result, the insulin receptor substrates cannot be phosphorylated, all the downstream hepatocellular insulin signaling processes are suppressed, and the glucose in serum cannot be metabolized causing hyperglycemia. FFA, free fatty acids; IRS, insulin receptor substrates; PI3K, phosphoinositide-3-kinase; AKT,protein kinase B; GSK, glycogen synthesis kinase; GS, glycogen synthase; IHTG, intrahepatic triglyceride.
In summary, there are still some unclear points about 1,2-DAG-induced insulin resistance in human skeletal muscle or liver cells.For example, although it has demonstrated that only the 1,2-DAGs in specific subcellular localizations in skeletal muscle or liver cells could activate PKCθor Cε, there is no consensus on the role of DAGs with different localizations in insulin resistance. Additionally, there is no consensus on how the FA composition of 1,2-DAGs in skeletal muscle or liver cells affects the insulin resistance. Further studies are still need to clarify the unclear points.
3. Dietary DAG-induced insulin sensitivity increasing in patients with T2D
TAGs in the liver mainly come from 3 sources: 1) Approximately 60% of the TAGs in the liver originate from FAs that are produced through lipolysis of peripheral adipose tissue; 2) 15% –20% of the TAGs in the liver are from dietary fats; 3) 5% –23% of the TAGs in the liver are fromde novolipogenesis [46].
As shown in Fig. 2A, an important physiological function of the insulin in white adipose tissues (WATs) is to suppress lipolysis [36,47]. In the patients with T2D, adipose insulin resistance promotes adipose lipolysis, and subsequent macrophage infiltration into WATs releases cytokines that favor lipolysis. For instance,TNF-α can lower perilipin expression, and presumably enhance lipolysis [48,49]. Accordingly, excessive adipose lipolysis can lead to an increased release of FAs that are delivered to the liver at an accelerating rate [50]. Consequently, the esterification of FAs to DAGs and TAGs likely increases. Furthermore, an increased adipose lipolysis was also found to stimulate hepatic gluconeogenesis, which would promote hepatic insulin resistance [50]. Hepatic insulin signaling-dependent hepaticde novolipogenesis can be reduced as a result of impaired hepatic insulin signaling in T2D patients [34].Dietary lipids rich in TAGs are contributors to the accumulation of liver TAGs in the hepatocytes of T2D patients. Notably, the TAG and DAG contents in the liver cells can be significantly increased in the insulin resistance state [18,51], and the secretion of remnant-like particle cholesterol (RLP-C) can also be increased greatly in these patients [10]. However, as shown in Fig. 2B, when the dietary lipids rich in TAGs are substituted by DAGs in T2D patients, insulin sensitivity may increase. The mechanisms behind the effect of DAG intake on the change of insulin sensitivity in T2D patients remain unclear. That is, what would happen in humans may or may not resemble those occurring in rats. In rats, there might be fewer chylomicrons formed and the formed chylomicrons could be removed at a relatively fast rate [52,53], thereby causing fewer FAs or residual particles to enter the liver (which may reduce the formation of lipid intermediates such as DAGs and subsequently increase insulin sensitivity) [54]. Perry et al. [16]reported that a very low-calorie diet could reduce hepatic DAG content and PKCεtranslocation, thereby improving hepatic insulin sensitivity. Since DAGs behave like low-calorie fat [55], dietary DAGs may be able to improve insulin sensitivity in the liver through reducing the local formation of DAGs.Table 1 summarizes the recent studies examining the effects of dietary DAGs on diabetic animals and human subjects.
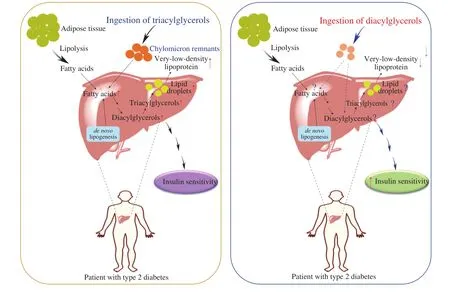
Fig. 2 Dietary DAGs increase insulin sensitivity in patients with T2D. (a) Liver lipids in type 2 diabetic patients come mainly from 1) lipolysis of adipose tissue(which is greatly enhanced in T2D patients, compared with healthy people); 2) de novo lipogenesis of two-carbon precursors (which is reduced owing to the defective hepatic insulin signaling); 3) dietary sources. (b) Substitution of normal dietary fat rich in TAGs with fat rich in DAGs can increase the insulin sensitivity of patients with T2D, although the mechanisms remain unclear.

Table 1Animal and human studies on how dietary DAGs improve insulin sensitivity.
More research is needed to examine how dietary DAGs improve insulin sensitivity, given the facts that FAs can compete with glucose for substrate oxidation at the mitochondrial level, and the change of insulin sensitivity is likely fat depot-specific. Recent studies reported that the composition and localization (subcellular location)of DAGs, along with certain lipid species located in cell organelles,could influence insulin sensitivity via different mechanisms [56].This means that not only the accumulation of lipids like DAGs and sphingolipids can mediate and promote insulin resistance via altering cellular signaling specific to their location, but also other cellular and subcellular activities can affect insulin sensitivity.Nuclear DAGs may act as nuclear lipid ligands to promote PPARα activation. Specific species of mitochondrial/ER DAGs may be positively related to insulin sensitivity and promote mitochondrial fission and mitochondrial dynamics [14]. While 1,3- and 2,3-DAGs unlikely affect insulin sensitivity in humans, the accumulation of sarcolemmal 1,2-DAGs was found in close association with PKCεactivation (although other PKC isoforms might also be activated in a transitory manner) [38,42]. Moreover, the roles of the β cells and the change of their state (from a glucose-responsive differentiated state to a proliferative state) deserve attention, especially the two receptors of the β cell, insulin receptors A and B (which can exert different biological functions). It was recently found that insulin-producing cells may be insulin resistant and insulin sensitive at the same time.With the aid of the “switch” PI3K-C2α, insulin may still activate a signaling pathway for β cell proliferation, despite that the insulin receptor B is insulin insensitive for another signaling pathway [57]. It is therefore of high interest to examine in the future the participation of DAGs in such a signaling pathway switch event. Furthermore,insulin sensitivity is well known for its partial dependence on insulin-mediated nitric oxide release. Accordingly, future work ?should also be directed towards the understanding of which type of DAGs can increase nitric oxide bioavailability and consequently reduce insulin resistance.
While Zheng et al. [12]showed that dietary DAGs could improve insulin sensitivity in T2D patients with body weights in the normal range (BMI < 25), some other studies reported that dietary DAGs could also improve insulin sensitivity in overweight (BMI < 25) patients with T2D [9,58]. Taken together, it remains debatable whether DAGs are capable of improving the conditions of diabetic patients. Xu et al. [59]conducted a meta-analysis of randomized controlled trials to assess the association between DAG intake and the concentrations of fasting serum glucose and insulin. The obtained findings suggested that DAGs might be beneficial in preventing atherosclerosis and related cardiovascular diseases, and the consumption of DAG-rich oil by humans might improve their impaired glucose tolerance and insulin resistance.
The beneficial effects of dietary DAGs on insulin sensitivity are attributed to their unique metabolic pathway(s). Fig. 3 shows the metabolic pathways of DAGs and TAGs. Dietary DAGs can lead to a significantly lower TAG concentration in the intestinal epithelial cells, compared with dietary TAGs. In the case of dietary DAGs,TAGs could be synthesized via the glycerol-3-phosphate pathway(which is significantly less efficient than the 2-monoacylglycerol pathway) [52]. The re-synthesized TAGs in the intestinal epithelial cells are subsequently combined with apoproteins to form chylomicrons before being transferred to the serum via lymphatic transport. In rats, the transport of chylomicrons could be significantly delayed and reduced after DAG ingestion as compared to TAG ingestion [60]. The chylomicrons formed after DAG ingestion in male C57BL/6J mice were easier metabolized in the serum, compared with the chylomicrons formed after TAG ingestion. This phenomenon was likely due to the more efficientin vitroLPL-mediated lipolysis and apolipoprotein E-mediated hepatic endocytosis for the DAG-derived chylomicrons [53]. Consequently, relatively less chylomicron remnants were retained in the serum after DAG ingestion as compared to TAG ingestion.
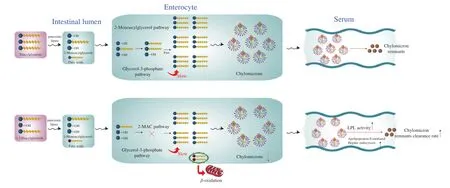
Fig. 3 Metabolic pathways of TAGs and DAGs in the digestive tract and serum. Dietary TAGs are digested to 2-monoacylglycerols (2-MAGs) and FAs in the intestinal lumen, and both are then absorbed by enterocytes and used for the re-synthesis of TAGs through the 2-MAG pathway (main) and the glycerol-3-phosphate pathway (secondary). In comparison, dietary DAGs are digested into 1-MAGs and FAs, and the generated 1-MAGs are poor substrates for the re-synthesis of TAGs through the 2-MAG pathway in enterocytes. Thus, the glycerol-3-phosphate pathway for the re-synthesis of TAGs is enhanced in this case.As a result, fewer TAGs are produced and less chylomicrons are formed, eventually causing fewer chylomicron remnants in the serum.
Although DAG ingestion may be able to increase the insulin sensitivity in T2D patients, there is little information available to demonstrate clearly how the liver lipids are affected by the chylomicron remnants formed after DAG ingestion. The aforementioned studies already reported that the increased DAG content in skeletal muscle or liver cells could lead to insulin resistance. These effects of ingested and endogenous DAGs on insulin sensitivity are still of concern and several questions can be raised, for example, whether the ingested DAGs would influence the insulin sensitive in T2D patients through mediating the DAG content in skeletal muscle or liver cells? Although DAG ingestion can reduce the secretion of very-low-density lipoprotein by the liver [11], to date, there is no direct evidence for the effect of dietary DAGs on intracellular DAG content. Also, no information is available on how DAG ingestion influences lipid metabolism in the liver or skeletal muscle. Thus, further studies are still needed to gain direct evidence and several approaches (as shown in Fig. 4) can be adoptedto achieve this goal. Among which, lipidomics can be used in combination with isotope tracer techniques [61-63]to gain insight into the metabolic fates of ingested DAGs (differing in FA chains, degree of saturation,and spatial configurations) in healthy people, and patients with T2D and/or obesity.
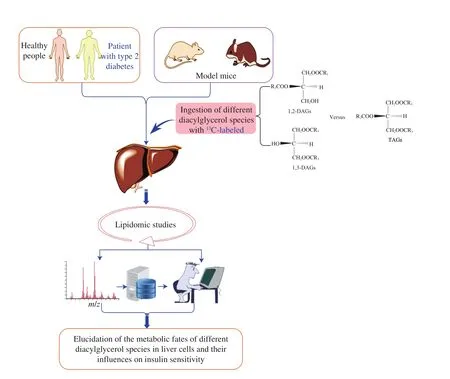
Fig. 4 Proposed research approaches for future studies on the metabolic pathways of different DAG species in the liver and their effects on insulin sensitivity:The combined use of an isotopic tracer technique (e.g. DAG species with different FA chains, degree of saturation, and spatial configurations are labeled with 13C)and lipidomics to track the changes in lipid profiles. Healthy people and patients with T2D, as well as corresponding model mice, are subjected to treatments with different dietary DAG products.
4. Conclusion
DAGs have attracted increasing interest owing to their preliminarily confirmed health benefits, as well as physicochemical properties similar to those of TAGs. Although the functional benefits of DAGs have been studied for more than 30 years, a number of critical aspects remain unclear. Further animal and clinical studies will be needed to answer several important and intriguing questions about the metabolism of dietary DAGs in the liver or skeletal muscle and the possible link to the occurrence of T2D. Eventually, these findings would guide the production of specially designed DAG products (including those used as functional oils) and their uses in a healthy diet for humans.
Funding sources
This work was supported by Chinese National Natural Foundation for Distinguished Young Scholars (31725022), Beijing Advanced Innovation Center for Food Nutrition and Human Health, Beijing Technology and Business University (BTBU)(20171049), and the Science & Technology Department of Shaanxi Province (2019JM-333).
Conflict of interest
All authors declare that they have no conflicts of interest.
- 食品科學與人類健康(英文)的其它文章
- Potential application of proteolysis targeting chimera (PROTAC) modification technology in natural products for their targeted protein degradation
- Effects of vegetarian diet-associated nutrients on gut microbiota and intestinal physiology
- Recent progress in preventive effect of collagen peptides on photoaging skin and action mechanism
- Effect of bacteriocin-producing Pediococcus acidilactici strains on the immune system and intestinal flora of normal mice
- A new Lactobacillus gasseri strain HMV18 inhibits the growth of pathogenic bacteria
- Formation of advanced glycation end products in raw and subsequently boiled broiler muscle: biological variation and effects of postmortem ageing and storage

