LC-MS/MS method for the quantitation of serum tocilizumab in rheumatoid arthritis patients using rapid tryptic digestion without IgG purification
Tkshi Mohizuki ,Kito Shibt ,b,Tkfumi Nito ,b,*,Kumiko Shimoym ,Noriyoshi Ogw ,Msto Mekw ,Junihi Kwkmi
a Department of Hospital Pharmacy,Hamamatsu University School of Medicine,Hamamatsu,431-3192,Japan
b Department of Pharmacy,Shinshu University Hospital,Matsumoto,Nagano,390-8621,Japan
c Third Division,Department of Internal Medicine,Hamamatsu University School of Medicine,Hamamatsu,431-3192,Japan
d Department of Laboratory Medicine,Hamamatsu University School of Medicine,Hamamatsu,431-3192,Japan
Keywords:Tocilizumab LC-MS/MS Immobilized trypsin IgG purification Proteomics Rheumatoid arthritis
ABSTRACT The quantitation of serum tocilizumab using liquid chromatography tandem-mass spectrometry(LC-MS/MS)method has not been widely applied in clinical settings because of its time-consuming and costly sample pretreatments.The present study aimed to develop a validated LC-MS/MS method for detecting serum tocilizumab by utilizing immobilized trypsin without an immunoglobulin G purification step and evaluate its applicability in the treatment of rheumatoid arthritis(RA)patients administered intravenously or subcutaneously with tocilizumab.The tocilizumab-derived signature peptide was deciphered using a nano-LC system coupled to a hybrid quadrupole-orbitrap mass spectrometer.The serum tocilizumab was rapidly digested by immobilized trypsin for 30 min.The chromatographic peak of the signature peptide and that of the internal standard were separated from the serum digests for a total run time of 15 min.The calibration curve of serum tocilizumab concentration was linear with a range of 2-200μg/mL.The intra-and inter-day accuracy and relative standard deviation(RSD)were 90.7%-109.4%and<10%,respectively.The serum tocilizumab concentrations in the RA patients receiving intravenous
1.Introduction
Tocilizumab,a humanized immunoglobulin G(IgG)1κsubclass monoclonal antibody(mAb),suppresses systemic inflammation by inhibiting the binding of interleukin-6(IL-6)to its receptors[1,2].It is generally used to treat moderate to severe symptoms of remission-induced rheumatoid arthritis(RA)[3].The serum tocilizumab concentration has been associated with several prognostic factors of RA[4,5].The therapeutic drug monitoring(TDM)of serum tocilizumab is potentially helpful in remission in RA patients.However,a few analytical quantitation methods have been utilized in clinical settings for detecting serum tocilizumab.
Liquid chromatography tandem-mass spectrometry(LC-MS/MS)method has been developed to quantify therapeutic mAbs in human serum[6-9].However,this LC-MS/MS method for detecting serum therapeutic mAb requires complicated sample pretreatments,including complete tryptic digestion and IgG purification.The digestion process using conventional solubilized trypsin commonly takes 10-14 h[6,7].The concentration of solubilized trypsin increases with human serum and is limited to a certain level to avoid autolysis[10,11].In addition,the abundant peptides generated by the complete digestion cause ionization suppression,which interferes with the mass spectrometric analysis.Immobilized trypsin densely bound to a monolithic silica surface provides rapid centrifugal digestion[11,12].The IgG purification using protein A or G improves the sensitivity of the LC-MS/MS analysis by reducing ionization suppression[7,9,13,14].However,this costly purification causes quantitative variations in the serum levels of therapeutic mAbs.The time-consuming and costly processes are the major barriers to utilizing the existing LC-MS/MS methods in clinical settings.Therefore,an LC-MS/MS method for detecting the serum tocilizumab using rapid digestion without IgG purification needs to be developed.
Tocilizumab can be administered via different routes,intravenous or subcutaneous injection,depending on patient compliance.However,the tocilizumab dose and its administration interval are different for intravenous and subcutaneous injections[15].The administration route influences the entry of tocilizumab into the systemic circulation and its pharmacokinetic elimination.Serum tocilizumab concentrations show a significant variation in the RA patients receiving subcutaneous injections compared to intravenous injections[5,16].An LC-MS/MS method with a wide dynamic range is helpful for TDM of serum tocilizumab in RA patients whose administration route has been changed and who experience secondary failure to the treatment.
Some studies have reported an LC-MS/MS method that measured serum tocilizumab concentrations following intravenous and subcutaneous injections in clinical settings.The objective of this study was to validate an LC-MS/MS method for quantitating serum tocilizumab using rapid tryptic digestion without IgG purification.The LC-MS/MS method developed here was used to treat RA patients receiving intravenous or subcutaneous tocilizumab.
2.Materials and methods
2.1.Signature peptide determination
Tocilizumab(Actemra;Chugai Pharmaceutical Co.,Ltd.,Tokyo,Japan)was digested using immobilized trypsin(MonoSpin Trypsin;GL Science,Tokyo,Japan)as reported previously[17].The tryptic digests containing tocilizumab-derived peptides were separated and measured using a nano-LC system coupled to a hybrid quadrupole-orbitrap mass spectrometer(Q Exactive,Thermo Fisher Scientific Inc.,Waltham,MA,USA).The signature peptide candidates were probed using the Proteome Discoverer Software Version 2.1(Thermo Fisher Scientific Inc.)with FASTA files(the International ImMunoGeneTics Information System?,IMGT/3Dstructure-DB,and IMGT/2Dstructure-DB Version 4.12.2[18])including the amino acid sequence of tocilizumab.The identified candidate peptides had an actualm/zvalue close to the theoreticalm/zpredicted by the FASTA file.The signature peptide was selected according to the below-mentioned criteria.First,the candidate peptide sequences should contain the complementarity determining region(CDR)of tocilizumab.Second,the peak displaying the candidate peptide can be isolated from the contaminant peaks of serum tryptic cleavage products.Third,no uncleaved residues of lysine or arginine should remain in the candidate peptide sequences.Last,there are no carbamidomethylated or oxidized residues in the candidate peptide sequences.
2.2.Sample preparation for LC-MS/MS quantitation
2.2.1.Preparation procedure
The 10μL of serum and 50μL of(1μg/mL)internal standard(IS)solution were mixed with 440μL of 50 mM ammonium bicarbonate(pH 8.1;Fujifilm Wako Pure Chemical Industries,Osaka,Japan).A 50μL of 10 mg/mL RapiGest SF surfactant(Waters,Milford,MA,USA)was added to the diluted serum,followed by incubation at 80°C for 10 min.The denatured serum was mixed with 25μL of 500 mM dithiothreitol(Thermo Fisher Scientific Inc.)and incubated at 60°C for 60 min.Alkylation was achieved by adding 50μL of 500 mM iodoacetamide(Fujifilm Wako Pure Chemical Industries)and storing it at room temperature under shaded conditions for 30 min.The mixture was loaded onto an immobilized trypsin column,passed through the column three times,and centrifuged at 100gat 37°C for 10 min.The trypsin column was cleaned-up with 300μL of 50 mM ammonium bicarbonate.In the digested products,the residual RapiGest SF surfactant was precipitated by adding 25μL of 2 M hydrochloric acid(pH 2.2;Nacalai Tesque,Kyoto,Japan),followed by centrifugation at 15,000gat 37°C for 30 min.The supernatant was desalinated using a solid-phase extraction column(Oasis HLB,3 cm3,60 mg of sorbent,30-μm particle size;Waters).The tryptic digests binding to the cartridge sorbent were eluted by 1 mL of 70% acetonitrile comprising 0.5% acetic acid(Fujifilm Wako Pure Chemical Industries),and subsequently,the elute was desiccated by evaporation at room temperature.The dried product was dissolved in 80μL of 14%acetonitrile comprising 0.1%acetic acid.After centrifugation at 10,000gat 4°C for 30 min,the supernatant was filtered,and 10μL of the solution was introduced into the high performance liquid chromatography(HPLC)system.
2.2.2.Optimization of sample preparation
The initial dilution ratio of the serum and the number of centrifugal digestions were examined.The initial dilution ratio of the serum was determined by comparing the peak areas of the signature peptides in three-,five-,six-,seven-,and eight-fold dilutions.The peak areas of the signature peptides were compared among one,two,three,and four centrifugal digestions.The digestion efficiency was evaluated as the ratio of the peak areas of signature peptides derived from tocilizumab digestion to those of the signature peptides added into the serum digests.
2.3.Characterization of trypsin digestion
2.3.1.Digestions using solubilized trypsin and immobilized trypsin
The tocilizumab products digested with immobilized trypsin were compared with the tocilizumab products digested with solubilized trypsin(Thermo Fisher Scientific Inc.).A 200μg of reduced-alkylated tocilizumab solution was added into 40μL of 100μg/mL solubilized trypsin,followed by incubation at 37°C for 12-14 h.The tryptic digestion was incubated with 25μL of 2 M hydrochloric acid.As for the immobilized trypsin,200μg of the reduced-alkylated tocilizumab was passed through the column once or three times.The digested products were introduced into the HPLC system coupled to a photodiode array(PDA)detector.
2.3.2.Comparison of tryptic digestions
The tocilizumab-digested products were separated using a reversed-phase HPLC.The HPLC system with an SPD-M30A PDA detector(UFLCXR;Shimadzu Corporation,Kyoto,Japan)was provided with the LabSolutions software,version 5.73(Shimadzu Corporation).An Aeris WIDEPORE XB-C18analytical column(150 mm×2.1 mm i.d.,3.6μm;Phenomenex Inc.,Torrance,CA,USA)was used for the separation,and the column was maintained at 85°C.The gradient elution program was combined with the following mobile phase:A solution,0.3% trifluoroacetic acid in water;B solution,0.3% trifluoroacetic acid in isopropanol(Fujifilm Wako Pure Chemical Industries).The ratio of B solution to the mobile phase was increased from 5%to 35%linearly for 0-60 min.The isocratic mobile phase comprised 5% B solution from 60.1 to 70 min.The flow rate was 0.5 mL/min for 70 min.The absorption wavelength of the PDA detector was configured at 210 nm.The injection volume of the sample solution was 10μL.The peak profiles derived from tocilizumab digests using immobilized trypsin were compared with that from the tocilizumab digests using solubilized trypsin.
2.4.LC-MS/MS assay
2.4.1.Chromatographic conditions of LC-MS/MS
The HPLC system(Nexera X2;Shimadzu Corporation)was controlled using the LabSolutions software,version 5.91.An Aeris Peptide analytical C18column(150 mm×3.0 mm i.d.,2.6μm;Phenomenex Inc.)with a SecurityGuard Ultra cartridge(3.0 mm i.d.;Phenomenex Inc.)was used for the separation.The column was heated up to 85°C.The stepwise elution program was combined with the following mobile phase:A solution,0.1%acetic acid in the water,and B solution,0.1% acetic acid in acetonitrile.The mobile phase consisted of 14% B solution for the first 8 min,followed by 70%B solution from 8.1 to 11.0 min,and 14%B solution from 11.1 to 15.0 min.The flow rate was 0.3 mL/min for the first 8 min,and from 12.6 to 15.0 min,and 0.6 mL/min from 8.1 to 12.5 min.The valve position was switched to the flow path connected to the MS/MS system from 6.0 to 8.0 min.The outside of the injection needle in the HPLC system was rinsed with 30% methanol(Fujifilm Wako Pure Chemical Industries)in water.
2.4.2.Mass spectrometric conditions of LC-MS/MS
The signature peptide consisting of LLIYYTSR,and its stable isotope-labeled peptide[R(13C615N4)],the IS,were synthesized by the Scrum Inc.(Tokyo,Japan).In a triple quadrupole tandem mass spectrometer(LCMS8050;Shimadzu Corporation)coupled to an electrospray probe,the column effluent was detected using positive ion multiple reaction monitoring modes.The mass spectrometer was managed by the LabSolutions software,version 5.91.The ion transitions were monitored using a dwell time of 100 ms for each compound:LLIYYTSR as a signature peptide,m/z514.95/689.45(+);LLIYYTSR[R(13C615N4)]as IS,m/z519.90/699.30(+).The temperature inside the interface was set at 350°C through a turbo ion spray.A positive voltage of 4000 V was applied to the interface.The collision gas pressure was configured at 270 kPa.The flow rates for drying,nebulizer,and heating gas were set at 10,3,and 10 L/min,respectively.Collision energies for the signature peptide and IS were-19 and 20 V,respectively.
2.5.Method validation
The present method was validated according to the U.S.Food and Drug Administration(FDA)guidelines[19].Selectivity was tested by comparing the peaks of the signature peptides with those of the blank serum digests derived from six healthy subjects and six RA patients.The calibration plots were plotted using the peak area ratios of the signature peptides to the IS at serum tocilizumab concentrations of 2,5,10,20,50,100,150,and 200μg/mL.The lower limit of quantification(LLOQ)was defined as the lowest concentration at which the relative standard deviation(RSD)did not exceed 20%.The quality control(QC)samples were prepared at 4(low-quality control(LQC)),40(medium quality control(MQC)),and 160(high-quality control(HQC))μg/mL.The FDA guidelines defined the concentrations of three QCs.The intraand inter-day accuracy and RSD values were calculated for three QCs in the human serum.Accuracy was defined as the percentage deviation of the calculated concentration from the nominal concentration.
Matrix factor and recovery rate were examined using the synthesized signature peptide prepared at concentrations equivalent to three QCs.The matrix factor was obtained by comparing peak areas of the signature peptide after spiking blank serum from six healthy subjects with those after spiking distilled water.The recovery rate was evaluated by comparing the peak areas of the signature peptide with and without passing through the solidphase extraction cartridge in the presence of human serum digests.The stability of tocilizumab in human serum was assessed for LQC and HQC at room temperature and 4°C for 24 h,at-80°C for 1 month,and after three freeze-thaw cycles.The stability of the signature peptide in the mobile phase was investigated after storage in an autoinjector system at 4°C for 24 h.The stability values were expressed as the accuracy calculated from the calibration curve generated by freshly prepared samples.The carry-over effect was evaluated by comparing the peak areas of the signature peptide in blank serum digests just after the measurement of 200μg/mL tocilizumab-spiked serum digests with those in serum digests of 2μg/mL tocilizumab.
2.6.Ethics and clinical application
The present study received an institutional review board approval by the Ethics Committee of Hamamatsu University School of Medicine(Authorization code:19-130).This study comprised 22 Japanese patients treated with tocilizumab(Actemra)for RA at the Hamamatsu University Hospital(Hamamatsu,Japan).Tocilizumab was administered by intravenous injection at 8 mg/kg every 4-5 weeks or subcutaneous injection at 162 mg/body every two weeks.Blood specimens were collected just before dosing after at least six intravenous injections or were collected within one week before dosing after at least twelve subcutaneous injections.The serum specimens were stored at-80°C until analysis.
2.7.Comparison of LC-MS/MS and enzyme-linked immunosorbent assay(ELISA)methods
The serum tocilizumab concentrations in the RA patients treated with intravenous or subcutaneous injection were quantified by LCMS/MS and ELISA methods(ImmunoGuide,TANI Medikal Ltd.,Ankara,Türkiye).The Pearson correlation coefficient was used to investigate the association of the LC-MS/MS method with the ELISA method.The Bland-Altman plots were obtained to assess the statistical bias between the LC-MS/MS and ELISA methods[20].The IBM SPSS 23(IBM Japan Ltd.,Tokyo,Japan)was utilized for all statistical analyses.AP<0.05 was considered statistically significant.
3.Results
3.1.Signature peptide determination
A hybrid quadrupole-Orbitrap mass spectrometer detected 37 candidate peptides derived from tocilizumab.The variable regionderived candidates consisted of twelve peptides(Table 1).Five candidate peptides(TL 46-53,TL 25-42,TL 25-45,TH 83-98,and TH 103-123)possessed the CDR of tocilizumab.The study excluded two peptides(TL 25-42 and TL 25-45)containing missing cleavage regions.Two peptides(TH 83-98 and TH 103-123)having chemically modified residues were unsuitable for the signature peptide.The TL 46-53 peptide(LLIYYTSR)was determined as a signature peptide for the quantitation of serum tocilizumab.

Table 1 Characteristics of candidate peptides derived from variable regions of tocilizumab identified by hybrid quadrupole-Orbitrap mass spectrometry.
3.2.Optimization of sample preparations
The peak area ratios of the signature peptides in three-,six-,seven-,and eight-fold diluted serum compared to five-fold diluted one were 14.4%,77.7%,83.6%,and 79.7%,respectively.The peak areas of the signature peptides with one,two,and four centrifugal digestions compared to three declined to 47.9%,70.4%,and 89.6%,respectively.The rate of digestion efficiency in three centrifugal digestions was 50.1%(n=3;RSD:5.2%).
3.3.Comparison of digests between solubilized trypsin and immobilized trypsin
Intact tocilizumab and its signature peptides were eluted at 45.7 and 6.2 min,respectively(Fig.S1A).The peak of the intact tocilizumab was not detected from its digests using solubilized and immobilized trypsin.The number of peaks at 30.0-35.0 min in tocilizumab digests with immobilized trypsin was more significant than that of the peaks obtained using solubilized trypsin.The peak profiles of tocilizumab digests at 0-25.0 min with immobilized trypsin were similar to those with solubilized trypsin(Figs.S1B-D).With respect to the number of centrifugal digestions,the intensity of the peaks at 0-15.0 min for three digestions was higher than that for one digestion(Figs.S1C and D).
3.4.Chromatographic separation and selectivity
The signature peptides and the IS chromatograms in the human serum provided by the LC-MS/MS are shown in Fig.1.The peaks derived from the signature peptide and IS were isolated from the serum digests.The signature peptide's and IS's retention time was 7.6 min,respectively.No peaks interfering with the signature peptide or IS were observed from the serum digests of six healthy subjects and six RA patients(not treated with tocilizumab)(data not shown).
3.5.Analytical performance
3.5.1.Calibration curve and sensitivity
The calibration curve of the serum tocilizumab concentration was linear within a range of 2-200μg/mL.The present study's correlation coefficient with the weighting factor 1/xwas 0.999.The LLOQ for the serum tocilizumab was 2μg/mL.The accuracy and RSD ranges of LLOQ with five replicates in three runs were 89.6%-100.5% and 8.5%-18.0%,respectively.
3.5.2.Assay accuracy and RSD
Table 2 shows the intra-and inter-day accuracy and RSD values of the serum tocilizumab using three QCs(n=5).The intra-and inter-accuracy ranges were 90.7%-109.4% and 97.5%-101.3%,respectively.The intra-and inter-RSD ranges were 2.3%-8.5% and 2.9%-6.1%,respectively.
3.5.3.Matrix effect and solid-phase extraction recovery
The matrix factor and solid-phase extraction recovery rate were calculated for three QCs in six healthy subjects.The matrix factors of the signature peptide with LQC,MQC,and HQC were 50.4%(RSD:7.3%),51.8%(2.7%),and 50.8%(4.5%),respectively.The IS-corrected matrix factors with LQC,MQC,and HQC were 99.6%(RSD:3.7%),97.4%(2.0%),and 101.0%(2.4%),respectively.The solid-phase extraction recovery rates of signature peptide with LQC,MQC,and HQC were 96.5%(RSD:4.5%),93.8%(1.6%),and 94.5%(3.2%),respectively.
3.5.4.Stability and carry-over effect
Table 3 shows the stability of tocilizumab in human serum.Storage at room temperature and 4°C for 24 h did not affect the stability of tocilizumab.There was no difference in the stabilities between one,two,and three freeze-thaw cycles.Tocilizumab in the human serum was stable at-80°C for one month.The peak area ratio of the signature peptide to IS did not change after storage in an autoinjection system at 4°C for 24 h(n=3;percentage of initial value:98.8%(LQC)and 100.7%(HQC)).No peaks derived from the signature peptide were detected in the blank serum digests just after measuring 200μg/mL of tocilizumab-spiked serum digests(Fig.S2).There was no carry-over effect causing contamination.

Table 2 Intra-and inter-day accuracy and imprecision of tocilizumab in human serum.
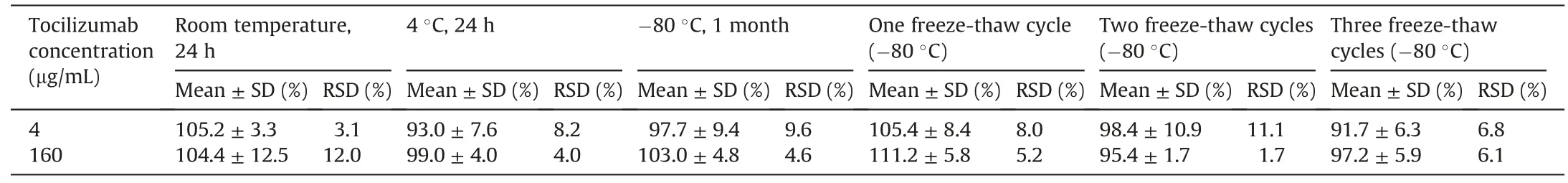
Table 3 Stability of tocilizumab in human serum under different conditions(n=3).
3.6.Quantitation of serum tocilizumab in RA patients
Intravenous and subcutaneous tocilizumab was administered in eight and fourteen RA patients.The range of serum tocilizumab in the patients receiving intravenous and subcutaneous injections was 5.8-28.9(mean value±SD,15.9±8.5)and 2.4-63.5(25.6±19.6)μg/mL,respectively(Fig.2).The serum tocilizumab concentrations in all the patients were measured using the calibration curve.
3.7.Comparison of LC-MS/MS and ELISA methods
The association of serum tocilizumab concentration between the LC-MS/MS and ELISA methods was evaluated using twenty-two specimens of the RA patients.The serum tocilizumab concentrations measured by the LC-MS/MS method had a positive correlation with that by the ELISA method(y=0.55x-0.33,r=0.82,P<0.01)(Fig.3).The Bland-Altman plots indicated that the mean bias between the LC-MS/MS and ELISA methods was 69.8%.The 95%confidence interval of the mean bias ranged from 47.9% to 91.7%,and a statistically systematic error was observed between the LCMS/MS and ELISA methods.
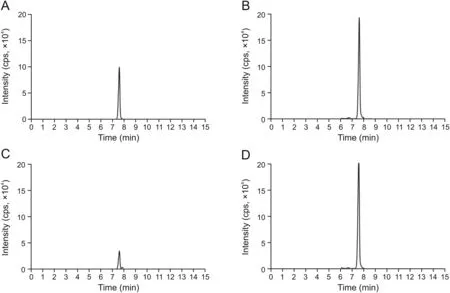
Fig.1.Selective reaction monitoring chromatograms of the signature peptide derived from tocilizumab.Drug-free human serum with 160μg/mL tocilizumab:(A)signature peptide and(B)1μg/mL internal standard(IS).Serum of rheumatoid arthritis patients treated subcutaneously with tocilizumab:(C)signature peptide and(D)1μg/mL IS.The IS concentration is comparable to the signature peptide concentration derived from approximately 360μg/mL of tocilizumab.
4.Discussion
A rapid and robust measurement method for detecting serum tocilizumab is essential for maintaining remission in RA patients in clinical settings.In the present study,immobilized trypsin was substituted for solubilized trypsin,and the centrifugal digestion was completed within 30 min.The serum tocilizumab concentration calibration curve had a dynamic range of 2-200μg/mL.The sensitivity and reproducibility of this method were sufficient to measure the trough concentration of serum tocilizumab.To the best of our knowledge,this is the first study that has led to the development of a rapid LC-MS/MS method for quantitating the serum tocilizumab with immobilized trypsin and without IgG purification in RA patients receiving intravenous or subcutaneous tocilizumab.
The signature peptide for quantitating the serum tocilizumab was determined using a nano-LC system coupled to a hybrid quadrupole-orbitrap mass spectrometer.Five candidate peptides had CDR,and four out of five possessed analytical characteristics unsuitable for the signature peptide.The peptides containing missed cleavage regions might not have amino acid sequences specific to tryptic digestion.Chemical modifications during sample pretreatment changed the molecular weight of peptides.The occurrence of missed cleavage and chemical modifications caused quantitative variations in the serum levels of therapeutic mAb.Incompletely digested peptides with internal lysine or arginine and structurally altered peptides with carbamidomethylated cysteine or oxidized methionine were excluded from the study.The LLIYYTSR was the signature peptide without any missed cleavage or chemically modified residues.
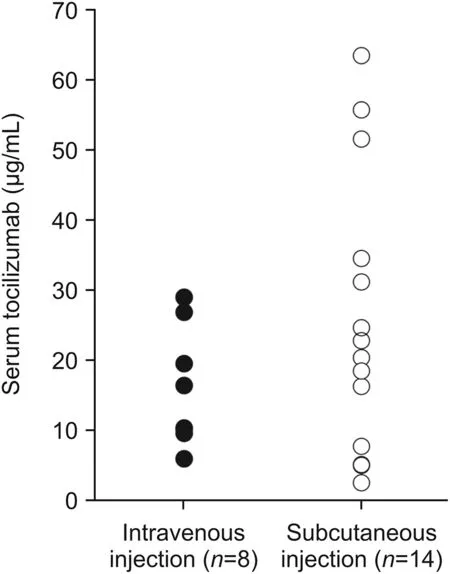
Fig.2.Serum tocilizumab concentrations in twenty-two rheumatoid arthritis patients.The patients were administered intravenously with tocilizumab at 8 mg/kg every 4-5 weeks(closed circle)or subcutaneously with tocilizumab at 162 mg/body every two weeks(open circle).

Fig.3.The relationship between the serum tocilizumab concentrations quantitated by the liquid chromatography tandem-mass spectrometry(LC-MS/MS)and enzyme-linked immunosorbent assay(ELISA)methods in twenty-two rheumatoid arthritis patients.(A)Scatter plots of the serum tocilizumab concentrations quantitated by the LC-MS/MS and ELISA methods.(B)Bland-Altman plots of the serum tocilizumab concentrations quantitated by the LC-MS/MS and ELISA methods.There were eight rheumatoid arthritis patients treated with intravenous tocilizumab(closed circles),and fourteen were treated with subcutaneous tocilizumab(open circles).The solid,dotted,and heavy dotted lines in the Bland-Altman plots are mean bias,zero,and 95% confidence intervals,respectively.
The present method does not include a step for IgG fraction extraction prior to tryptic digestion.The IgG purification using protein A or G having high affinity for the Fc domain of an antibody is commonly utilized to reduce the ionization suppression[7,9,13,14,17].However,this complicated procedure potentially leads to artificial variation in the serum levels of the therapeutic mAb[21].The complexity and variability in IgG purification are barriers to utilizing the LC-MS/MS method for quantitating the serum therapeutic mAb.In our evaluation,the moderate matrix effect of the signature peptide was fully corrected by the IS.The solid-phase extraction reduced the non-targeted tryptic digests introduced into the MS/MS system.The present method without IgG purification provided the LLOQ level required to measure the serum trough concentration of tocilizumab in the RA patients.
The initial dilution ratio of the serum and the number of centrifugal digestions were investigated to maximize the peak area of the signature peptide.Serum with high viscosity was diluted to promote the passage through the immobilized trypsin columns.The limited amount of serum and the number of centrifugal digestions were minimized to suppress the generation of abundant non-targeted peptides.The maximum peak area of the signature peptide and its reproducibility were obtained by a five-fold dilution of the serum and three centrifugal digestions.
Immobilized trypsin was used for the rapid tryptic digestion of the serum tocilizumab.A centrifugal column with monolithic silica densely binds to trypsin and can minimize autolysis and accelerate digestion[10,11].Lysine or arginine residues buried inside the protein are difficult to cleave by the immobilized trypsin,resulting in a decrease in serum digests.In the chromatograms with a PDA detector,the peak profiles of low molecular weight peptides derived from tocilizumab with immobilized trypsin were similar to the peak profiles obtained with solubilized trypsin.In contrast,the number of peaks derived from high molecular weight peptides with the immobilized trypsin was more significant than the peak profiles obtained with solubilized trypsin.The single centrifugal digestion using the immobilized trypsin was insufficient to generate signature peptides for serum tocilizumab quantitation.The optimal digestion condition in the present method was three centrifugal digestions using immobilized trypsin.
Chromatographic conditions were optimized to detect the signature peptide specifically and shorten the run time.A core-shell technology column was used to obtain high-resolution separation of the signature peptide[22].The column was heated at 85°C for fast and sensitive separation by reducing the mobile phase viscosity and facilitating the diffusion of the peptide.A stepwise elution program using a mobile phase containing 70% acetonitrile was adopted for cleaning up the analytical column with residual protein digests,allowing for continuous quantitation of serum tocilizumab.The peaks matching the signature peptide and IS were not detected in the serum digests of six healthy subjects and six RA patients.
The dynamic range of the calibration curve was set at 2-200μg/mL to monitor the serum tocilizumab in the RA patients receiving intravenous or subcutaneous injections.The accurate and precise quantitation of serum tocilizumab met the international bioanalytical method validation guidelines set.The MQC is slightly lower than 30% of the maximum concentration of the standard curve.The analytical reproducibility must be confirmed by assessing three QCs and the calibration points when this method is applied to clinical settings.The stability of tocilizumab and its signature peptides were maintained during sample pretreatments on the bench top in the LC-MS/MS method.The temperature for long-term storage of serum specimens was-80°C.Up to three freeze-thaw cycles of serum specimens were allowed.The carryover effect was avoided by using a standard rinsing program for an injection needle of the HPLC system.
The present method was applied to the RA patients administered with an intravenous or subcutaneous tocilizumab.This study collected the blood specimens corresponding to the trough or nontrough concentrations of serum tocilizumab.The degree of serum tocilizumab variation in the patients receiving subcutaneous injections was greater than for intravenous injections.This study observed a higher concentration of serum tocilizumab than that of a phase III study in the RA patients receiving intravenous(12.4±7.9μg/mL)and subcutaneous injections(10.6±7.8μg/mL)[23].The serum tocilizumab concentrations measured in the present study were within the calibration curve.This LC-MS/MS method applies to clinical settings independent of the administration route and blood sampling points.
The serum tocilizumab concentrations determined using the LCMS/MS method correlated with the serum concentrations obtained using the ELISA method.In contrast,the LC-MS/MS method showed statistically higher serum tocilizumab concentrations than the ELISA method.The present ELISA method quantitated serum tocilizumab not bound to the IL-6 receptors.An LC-MS/MS method based on the determination of signature peptide measured unbound and bound forms of tocilizumab[24].The difference in quantitative results between the LC-MS/MS and ELISA methods was potentially influenced by the target analytes and their detection processes.The serum tocilizumab concentrations quantitated by these methods need to be interpreted carefully.
This study had several limitations.First,the method using immobilized trypsin without IgG purification exhibited low digestion efficiency for the serum tocilizumab.The partial digestion may cause variations in the measurement values and a decrease in the measurement sensitivity.This method showed a slight variation in the digestion efficiency and can be applied to RA patients with a low concentration of the serum tocilizumab.Second,this method did not evaluate RA patients with hyperproteinemia caused by the overproduction of immunoglobulins[25,26].The abundant proteins might have caused a decrease in the digestion efficiency of the serum tocilizumab and an increase in ionization suppression.The RA patients in this study had close to normal serum total protein levels(64-73 g/L).Analytical validation of this method in populations with abnormally high serum total protein levels is required in the future.Third,the applicability of this method was not confirmed in RA patients using tocilizumab at short administration intervals.Subcutaneous tocilizumab is administered weekly in the refractory RA for early remission.In this study,the serum tocilizumab in the patients receiving biweekly subcutaneous injections ranged from 2 to 64μg/mL.The present calibration range is wide enough to quantitate the serum tocilizumab given once a week.Fourth,the reproducibility of this method in patients with abnormal physical conditions and juvenile RA patients is yet to be identified.The median values of age,body weight,serum albumin,and globulin in the patients were 69(range 36-78)years,53(39-81)kg,43(36-48)g/L,and 26(22-35)g/L,respectively.All the patients continued to receive tocilizumab treatment for at least six months and were considered to maintain remission in RA patients.The age and physical conditions of the patients are typical in clinical settings and are unlikely to influence the accuracy of this method.In contrast,the ELISA method's associations of crossreactivity with age and physical conditions remain unclear.The difference in quantitative results between the LC-MS/MS and ELISA methods might change in the RA patients with abnormal physical conditions and juvenile RA patients.Fifth,the number of the enrolled patients receiving intravenous or subcutaneous tocilizumab injections was small.In the present study,all patients who provided informed consent under tocilizumab treatment for a certain period were enrolled without patient exclusion.Physicians chose the administration route of tocilizumab according to the patient's lifestyle.The age distribution in each group of the patients who received intravenous or subcutaneous tocilizumab injections was similar to that in the Japanese RA patients.There were no differences in age,body weight,or disease activity between the patients administered intravenously or subcutaneously.In the future,the validation of this method in the expanded cohort is necessary for its application in patients with a wide range of clinical characteristics.
5.Conclusions
This study developed a validated LC-MS/MS method for rapidly quantifying the serum tocilizumab with immobilized trypsin and without IgG purification.Based on the bioanalytical validation,this method allowed accurate and precise quantitation and had a sizable dynamic calibration range.Serum trough and non-trough tocilizumab exhibited a significant variation in the study.The present method can be applied to evaluate the pharmacokinetics of intravenous and subcutaneous tocilizumab in RA patients.This study provides a new and acceptable method for TDM of the serum tocilizumab in clinical settings.
CRediT author statement
Takashi Mochizuki:Conceptualization,Methodology,Validation,Formal analysis,Investigation,Data curation,Writing-Original draft preparation,Funding acquisition;Kaito Shibata:Conceptualization,Methodology,Validation,Investigation,Writing-Original draft preparation,Reviewing and Editing;Takafumi Naito:Conceptualization,Methodology,Validation,Writing-Original draft preparation,Reviewing and Editing,Project administration;Kumiko Shimoyama:Resources;Noriyoshi Ogawa:Resources;Masato Maekawa:Resources;Junichi Kawakami:Writing-Reviewing and Editing,Supervision.
Declaration of competing interest
The authors declare that there are no conflicts of interest.
Acknowledgments
This study was supported by the Japan Society for the Promotion of Science KAKENHI(Grant No.:JP19H00349).
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jpha.2022.08.003.
 Journal of Pharmaceutical Analysis2022年6期
Journal of Pharmaceutical Analysis2022年6期
- Journal of Pharmaceutical Analysis的其它文章
- Potential use of a dried saliva spot(DSS)in therapeutic drug monitoring and disease diagnosis
- Rabdosia serra alleviates dextran sulfate sodium salt-induced colitis in mice through anti-inflammation,regulating Th17/Treg balance,maintaining intestinal barrier integrity,and modulating gut microbiota
- Identification of potential anti-pneumonia pharmacologicalcomponents of Glycyrrhizae Radix et Rhizoma after the treatment with Gan An He Ji oral liquid
- Discovery of pulmonary fibrosis inhibitor targeting TGF-βRI in Polygonum cuspidatum by high resolution mass spectrometry with in silico strategy
- Development of a radiolabeled site-specific single-domain antibody positron emission tomography probe for monitoring PD-L1 expression in cancer
- A highly efficient protein corona-based proteomic analysis strategy for the discovery of pharmacodynamic biomarkers
