Injectable self-healing nanocellulose hydrogels crosslinked by aluminum: Cellulose nanocrystals vs.cellulose nanofibrils
Zhongxin Lin,Renliang Huang,Jiangjiexing Wu,*,Anastasia Penkova,Wei Qi,Zhimin He,Rongxin Su,,*
1 State Key Laboratory of Chemical Engineering,Tianjin Key Laboratory of Membrane Science and Desalination Technology,School of Chemical Engineering and Technology,Tianjin University,Tianjin 300072,China
2 Zhejiang Institute of Tianjin University,Ningbo 315201,China
3 School of Marine Science and Technology,Tianjin University,Tianjin 300072,China
4 St.Petersburg State University,7/9 Universitetskaya nab.,199034 Saint Petersburg,Russia
Keywords:Cellulose nanocrystals Cellulose nanofibrils Surface heterogeneities Gels Fabrication Mechanical properties
ABSTRACT With excellent biocompatibility and unique physiochemical properties,nanocelluloses including cellulose nanocrystals(CNCs)and cellulose nanofibrils(CNFs)are promising candidates for preparing biomedical hydrogels.CNCs and CNFs are different in morphology and surface charges.Herein,CNCs and two CNFs(CNFs-C,Carboxylated CNFs;CNFs-P,Phosphorylated CNFs)were synthesized and applied to fabricate hydrogels through metal crosslinking.Aluminum crosslinking was found to be the best choice for enhancing the strength.This study systematically compared the morphologies,storage modulus,loss factor,continuous shear ramp,self-healing,swelling, in vitro degradation and injectable properties of the fabricated hydrogels.Further,a radar chart is summarized as guidelines to direct the rational selection to meet the specific requirements of further biomedical applications.At the same nanocellulose concentration and after Al3+ crosslinking,CNCs hydrogels had strong water holding capacity twice as much as that of CNFs hydrogels.While CNFs hydrogels showed higher hardness and stronger resistance to degradation than that of CNCs.These results provide detailed insights into nanocellulose hydrogels,making it possible to use these guidelines to select hydrogels for desired performance.
1.Introduction
With three-dimensional hydrophilic networks,hydrogels are promising candidates in fields such as drug delivery [1],sorbents[2],sensors[3],and lubrication[4],due to their excellent mechanical stability,controllable morphology,biodegradability,and high biocompatibility.However,the high moisture content in hydrogels makes them mechanically fragile and brittle.Combining with other mechanically reinforced nanofilms or composites to improve the mechanical strength is a feasible strategy[5-7].As the main component of lignocellulose biomass and the most abundant natural polymer on earth,cellulose is rapidly gaining popularity among industrial and academic researchers [8,9].The global annual production around 75-100 billion tons made it a promising alternative to petroleum-based materials [10].In particular,the excellent intrinsic physical and chemical properties of cellulose,such as high tensile strength and elastic modulus (130-150 GPa),high specific surface area (hundreds of m2·g-1),low density (1.6 g·cm-3),and biodegradability [11],have endowed nanocellulose hydrogels more unique properties,consequently leading to wide applications,such as flame retardant material[12],antibacterial materials[13],efficient adsorption[14],nano generator[15],super capacitor[16],sustainable energy devices [17],microwave absorption and shielding [18].
Despite the significant progress that have been achieved for nanocellulose hydrogels,most of them were hybrid hydrogels with nanocellulose as a filler to improve the mechanical property[19,20].Only a few studies with hydrogels solely prepared from nanocellulose have been reported [21].Until now,there is no detailed comparison of hydrogels made by different nanocelluloses,which makes the choice of a suitable nanocellulose to prepare hydrogels with desired properties difficult.It should be noted that different synthesized methods would give a totally different size,morphology,and surface groups of nanocellulose.Normally,according to the size and morphology,cellulose nanomaterials are mainly divided into cellulose nanocrystals(CNCs)and cellulose nanofibrils(CNFs)[22,23].And the sulfate half ester surface groups could be introduced into the CNCs by sulfuric acid hydrolysis,which made the surface of CNCs with negative charge [24].During the preparation of CNFs,2,2,6,6-Tetramethylpiperidine 1-oxyl (TEMPO)-mediated oxidation could selectively oxidize the approachable C6 primary hydroxyl groups on CNFs and produced high-density carboxylates on the surface of CNFs [25].Similarly,the phosphorylation pretreatment of cellulose could modify the phosphate group on the surface of CNFs [26].However,a comparison of these nanocellulose with morphology heterogeneities and their effect on the hydrogels have not yet been accomplished.Adjusting the expansion and mechanical properties of a hydrogel to meet the needs of specific applications is critical to create high-performance and multifunction materials [27].Therefore,a compressive investigation on the effect of these nanocellulose on the hydrogels is highly desirable to achieve precise choice of suitable cellulose.
In this work,three types of nanocellulose including cellulose nanocrystals (CNCs),carboxylated cellulose nanofibrils (CNFs-C),and phosphorylated cellulose nanofibrils hydrogels (CNFs-P) were chosen and used to prepare the injectable hydrogels through an aluminum crosslinking strategy(Fig.1).The properties of the three nanocellulose hydrogels,such as rheological properties,selfhealing,swelling andin vitrodegradation were investigated,compared,and summarized as a radar chart.
2.Experimental
2.1.Materials
Medical skimmed cotton(Grade A)was purchased from Jiangxi Huazhong Textile and Chemical Co.Ltd.and crushed.Concentrated sulfuric acid (98% (mass)) was purchased from Tianjin Jiangtian Chemical Technology Co.Ltd.and diluted to 62% (mass) before use.Softwood and cardboard were bought from Dalian Yangrun Trading Co.Ltd.2,2,6,6-tetramethylpyperidine-1-oxyl (TEMPO,98%)and other chemicals were purchased from Aladdin(Shanghai,China),and used as received without further treatment.
2.2.Preparation of CNCs and CNFs
CNCs were prepared through sulfuric acid hydrolysis [28].In a typical procedure,20 g crushed cotton was mixed with 200 ml of 62% (mass) sulfuric acid solution and magnetically stirred for 130 min at 50°C.Then the mixture was poured into 2000 ml of distilled water to stop the hydrolysis.After 12 h incubation,the precipitate was obtained and washed through centrifugation (10000 r·min-1).When the upper suspension after centrifugation became turbid,the suspension was collected and dialyzed in distilled water to achieve a neutral pH.
CNFs-C were synthesized according to a previous work [29].Briefly,0.25 g TEMPO and 2.5 g NaBr were dissolved in deionized water and the pH of the solution was adjusted to 10 with NaOH(8 mol·L-1).Then 20 g pulp was added to the solution and stirred for 1 h to impregnate the oxidation chemicals in the fiber structure through fiber swelling.The TEMPO-mediated oxidation was initiated by adding 8.8 mmol NaClO per gram of pulp at room temperature.The pH was maintained at 10 until no NaOH consumption was observed.The TEMPO-oxidized pulp was washed with deionized water by vacuum filtration.
CNFs-P were synthesized as follows [30].The phosphorylation process consisted of impregnation and curing.During impregnation,20 g softwood pulp was soaked in 70 ml of aqueous solution containing 8.5 g urea and 36.3 g NH4H2PO4.The fibers were then dried in an oven at 105 °C,followed by curing at 150 °C for 20 min.The phosphorylated pulp was then washed with deionized water to remove unreacted chemicals.The pulp content was controlled at 0.5% (mass) by dilution with deionized water,and the pH of the suspension was set to 9.5 by adding a dilute NaOH solution for complete dissociation of carboxylate and phosphate groups.
The TEMPO-oxidized pulp and phosphorylated pulp dispersions were mechanically disintegrated by a high-pressure homogenizer(AH-NANO,China)at 90-100 MPa for eight passes and ten passes,respectively.
The chemical structures of CNCs and CNFs were determined by Fourier transform infrared spectrometer (Nicolet 6700,USA).The FTIR spectrum were obtained in the spectral range of 650-4000 cm-1.The morphologies of CNCs and CNFs were scanned by atomic force microscope (Dimensionicon,Germany).The images were processed by NanoScope Analysis 1.9.The zeta potential of CNCs and CNFs suspensions were measured by Zetasizer (Nano ZS,UK).
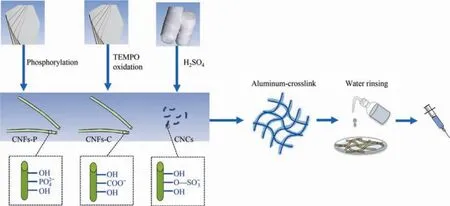
Fig.1.Illustration of the preparation of hydrogels with three different nanocelluloses (CNCs,CNFs-C,and CNFs-P).
2.3.Preparation of hydrogels
Hydrogels crosslinked by ions were prepared in vials at room temperature as follows.Cations including AlCl3,MgCl2,and NaCl were added to the CNCs suspension or CNFs,vortexed immediately,sonicated to disperse,and then evaporated at 60 °C.The ingredients were shown in Table S1 (in Supplementary Material).Note,the as-prepared hydrogels crosslinked by cations were denoted asX/YZ,X=nanocellulose including CNCs,CNFs-C,CNFs-P,Y=cations including Na,Mg,Al,Z=concentrations (mmol·L-1)of cations including 50,60,70,80.
For comparison,the hydrothermal method was also used to prepare the hydrogels.In a typical procedure,4% (mass) CNCs aqueous solution was added to the polytetrafluoroethylene lined stainless steel autoclave for thermal treatment at 120 °C for 24 h.After cooling down to room temperature,the CNCs hydrogels were collected and stored in a sealed glass bottle for further characterization.
2.4.Morphology of hydrogels
The morphology of hydrogels was observed by scanning electron microscope (SEM).The hydrogels were firstly frozen under liquid nitrogen and then freeze-dried for 2 days.Later,the dried samples were gilded by sputtering before observed by SEM(Regulus 8100,Japan).
2.5.Rheological and injectable analysis
The rheological properties were measured at 37 °C using a stress-controlled rotary rheometer (Anton Paar MCR301,Austria)in oscillatory or rotational mode,with a parallel plate (24.99 mm in diameter) and a gap of 1 mm.Frequency sweep experiments(0.1-100 s-1) were carried out in the linear viscoelastic range(within 1% strain amplitude) to obtain the storage modulusG′and loss modulusG′′.Continuous shear ramps were performed at shear rate of 0.1-100 s-1to measure the viscosity material function of the samples.To test the injectable property of hydrogels,a syringe was loaded with the hydrogel to produce letters of a specific shape.
2.6.Self-healing performance
The self-healing property of nanocellulose hydrogels was tested as follows.Bar-type hydrogels were cut from the middle into two pieces,followed by putting the two sections together to check the conjugated degree and the knife marks.And then these conjugated hydrogels were also put into phosphate buffered saline(PBS)to check their self-healing performance.All the phenomena were captured on camera to observe the healing effect and all the experiments were performed at room temperature.
2.7.Moisture uptake
The degree of water absorption and expansion of hydrogels over time were conducted in PBS medium at room temperature.The samples were dried in a dryer (5% RH) for 48 h,and then weighed as initial massmd.Later,they were immersed in PBS and weighed at different time intervals asmt(t=3 min,5 min,15 min,30 min,60 min,120 min,180 min,240 min,and 72 h).Notably,a soft tissue paper was used to remove excess water before weighing.The moisture absorption rate was calculated according to the following formula:

Wheremtis mass at timet,mdis initial mass after drying.
2.8.In vitro degradation
The mass change of dried samples was monitored to evaluate the degradability of hydrogels after incubating and shaking with cellulase at 37 °C,50 r·min-1[31].The hydrogels were rinsed in a 250 U·ml-1enzyme-containing buffer and the buffer was refreshed every day.At a specified time,the hydrogels were taken out,washed with distilled water,and then freeze-dried to get dry samples.The following formula was used to calculate the mass loss:

WhereWtis mass after degradation,W0is mass before degradation.
3.Results and Discussion
3.1.Characterization of CNCs and CNFs
To investigate the effect of different nanocellulose on the multiproperties of hydrogels,three nanocellulose with different morphologies and surface groups were chosen here.CNCs with sulfate half ester surface groups were obtained by hydrolyzing cotton with sulfuric acid to remove the amorphous area.CNFs-C (CNFs with carboxyl modification) were obtained by high pressure homogenization after TEMPO oxidation.Similarly,CNFs-P (CNFs with phosphate groups) were synthesized by high pressure homogenization after phosphoric acid oxidation.
As shown in Fig.2(a),the broad band at 3600-3000 cm-1(-OH)and peaks at 1060 cm-1(C-O stretching vibrations) confirmed that these samples were cellulose[32].Further analysis,an absorption peak at 1279 cm-1(S=O)and 814 cm-1(C-O-S)showed that the sulfonic group was successfully modified on the surface of CNCs [33].For CNFs-C,the existed carboxyl absorption peak at around 1605 cm-1indicated the successful modification [34].For CNFs-P,the absorption peaks at 834 cm-1(P-O-C),918 cm-1(P-O-H),and 1230 cm-1(P=O) verified the surface modification of phosphate groups [35].All these negatively charged groups including the sulfate group,carboxyl group,and phosphate group led to negative zeta potentials(about-30 mV)of these nanocellulose.Their sizes and morphologies were further characterized by atomic force microscope (AFM).As shown in Fig.2(b)-(d),CNCs were mono-disperse with a rod-like structure,and the length was around 160 nm.While for CNFs,high pressure homogeneity made them slender and filamentous,with longer length around 574 nm for CNFs-C and 713 nm for CNFs-P.
3.2.Crosslinking of nanocellulose hydrogels
Before investigating the effect of three different nanocelluloses on the properties of hydrogels,the fabrication methods for hydrogels were assessed through heat treatment and crosslinking with different cations.As shown in Fig.S1,storage modulus(G’)and loss factor (tan δ) of these hydrogels were compared,where the Al3+-crosslinked hydrogel were the best among all the cations,and a little bit better than the hydrothermal-treated one.Interestingly,the strength of hydrogels was notably enhanced in a valencedependent order of Na+<Mg2+<Al3+.The reason may be related with the forming mechanism of crosslinking method,where the binding affinity of cations to anions on the surface of nanocellulose would initiate the gel process,and the higher the valence state of cations,the larger the binding affinity was.
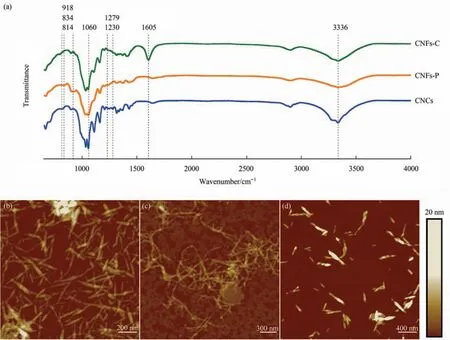
Fig.2.(a)FTIR spectrum of cellulose nanocrystals(CNCs),carboxylated cellulose nanofibrils(CNFs-C),and phosphorylated cellulose nanofibrils(CNFs-P).AFM height images of CNFs-C (b),CNFs-P (c),and CNCs (d).
A further investigation on the concentration effect of Al3+was also carried out.The rheological properties of CNCs gel formed by adding a certain gradient concentration of Al3+to CNCs suspension were studied and shown in Fig.3.TheG’and tan δ of these hydrogels presented a ‘‘volcanic” type tendency to the concentration of Al3+,where 60 mmol·L-1concentration of Al3+exhibited the best performance for the hydrogel.Based on these results,Al3+crosslinking method as the most effective way and the concentration at 60 mmol·L-1were selected to prepare nanocellulose hydrogels in our work.
3.3.Morphological characterization of hydrogels
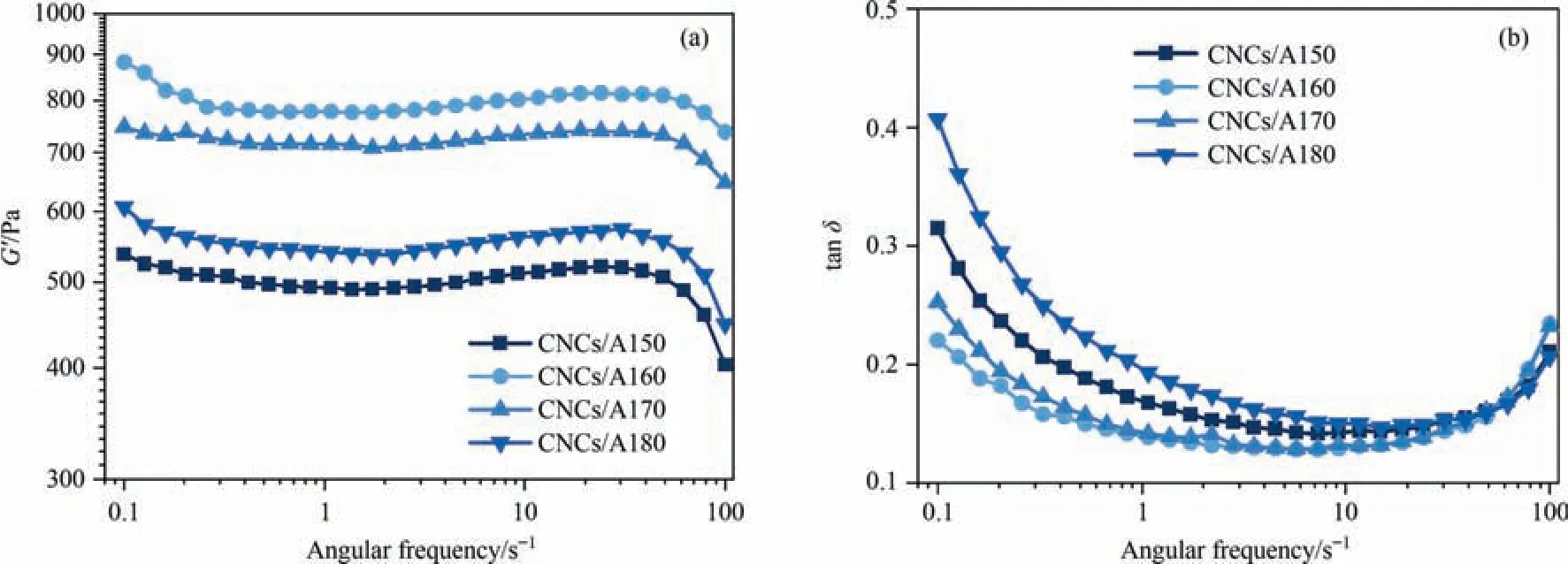
Fig.3.(a) Storage modulus and (b) Loss factor of CNCs hydrogels crosslinked by different aluminum concentrations of Al3+ at a strain of 0.5%.

Fig.4.SEM images of (a) CNFs-C/Al60,(b) CNFs-P/Al60,(c) CNCs/Al60,(d) CNFs-C,(e) CNFs-P,and (f) CNCs.The scale bars are 30 μm.
Encouraged by the above results,three different nanocellulose hydrogels crosslinked by Al3+were made.Note,the as-prepared hydrogels crosslinked by 60 mmol·L-1Al3+were denoted as CNCs/Al60,CNFs-C/Al60,and CNFs-P/Al60.As shown in Fig.S2,these hydrogels had no fluidity and could maintain its shape even when they were shaken.A detailed characterization with scanning electron microscopy (SEM) was further performed to investigate the morphology and microstructure of these hydrogels.To get the cross section,hydrogels were impregnated,quenched with liquid nitrogen,and then freeze-dried.As shown in Fig.4,hydrogels crosslinked by Al3+exhibited relatively dense and interconnected porous structures with uniformed pore size.This was attributed to the metal ligand affinity of Al3+to the negatively charged group on the nanocellulose surface,which increased the cross-linking density.Therefore,compared with pure nanocellulose,the pore size of the corresponding hydrogel tended to decrease.Besides,the solid walls and rough surfaces of these hydrogels endowed them with better mechanical properties and stability,which may advantage the further biological applications in cartilage tissue repair to improve the rate of nutrient supply and diffusion [36].
3.4.Rheological and injectable analysis
In order to obtain information about the stability of threedimensional cross-linked network,the rheological properties of the network were studied by oscillatory rheology.When comparing Fig.5(a) with (b),a higherG′than loss modulus (G′′) indicated the stable crosslinking network.And the addition of Al3+led to a further increase forG’,revealing an enhanced cross-linking degree of hydrogels.The results were consistent with the SEM observation that all the Al3+-crosslinked hydrogels showed a more uniform and dense structure than the original cellulose.
Subsequently,the functional relationship between gel viscosity and shear rate was also evaluated.As seen in Fig.5(c),a higher shear rate decreased the viscosity of all the samples,indicating that these hydrogels were undergoing shear thinning.And the continuous varied viscosity of these samples enabled a precise control and provided more opportunities to select suitable viscosity for further specific applications.Notably,due to the shear dilution,extrusion experiments with hydrogels loaded in the syringe were performed to draw‘‘TJU”(referring to Tianjin University),verifying the injectable property of hydrogels (Fig.6).Such an injectable hydrogel would enable itself to be convenient for biological applications,such as drug delivery and tissue engineering through springe injection.
3.5.Self-healing performance
Self-healing performance,based on physical interactions or reversible chemical bonding,is important and useful as it can repair scratches,cracks,and other mechanical damage [37-40].The self-healing ability of nanocellulose hydrogels was tested by gel cutting and re-healing.As shown in Fig.7,the separated segments were tightly held together and maintained good integrity when soaked in PBS.The self-healing time of CNCs/Al60,CNFs-C/Al60,and CNFs-P/Al60 hydrogels were around 13 min,4 min,and 6.5 min,respectively.Metal bonding between aluminum and nanocellulose surface groups made this simple operation of nanocellulose hydrogels possible,and it will make a variety of possible tissue engineering applications.In addition,these self-healing hydrogels are expected to be used for cell-loaded threedimensional (3D) printing,which will be explored in our future research.
3.6.Swelling study
The swelling property of nanocellulose hydrogels was evaluated in PBS(pH 7.4)at room temperature.As seen in Fig.8,similar swelling curves were obtained for CNFs-C/Al60,CNFs-P/Al60,and CNCs/Al60 hydrogels,where the expansion rate increased with the increase of the soaking time.After incubation for 3 days,the equilibrium mass of CNCs/Al60,CNFs-P/Al60,and CNFs-C/Al60 hydrogels enhanced by 2311%,984%,and 930%,respectively.The different equilibrium swelling ratio may be caused by the different hydrophilic ability of the surface functional groups of nanocellulose [41].For CNCs,the hydrolysis method and smaller size made more hydroxyl groups exposed on the surface,resulting in stronger hydrophilicity and water retention than CNFs.While for CNFs,both CNFs-C and CNFs-P were obtained by high pressure homogenization.The slightly stronger water retention of CNFs-P than that of CNFs-C may be from more cycles of CNFs-P,with slightly more hydroxyl groups on the surface of CNFs-P) [42].
3.7.In vitro degradation

Fig.5.Viscoelastic properties including (a) storage modulus and (b) loss modulus of nanocellulose hydrogels under the conditions: dynamic frequency sweep of the gels at a strain of 0.5% and 37 °C.(c) Continuous shear ramp of nanocellulose hydrogels using rotational rheology.
In vitrodegradation studies were used to evaluate the degradation behavior of hydrogels.In the presence of cellulase,the degradation of cellulose is essentially the hydrolysis of β-1,4-glucoside bond in fiber to produce soluble polymer and D-glucose [43].As shown in Fig.9,CNFs-C/Al60,CNFs-P/Al60,and CNCs/Al60 hydrogels exhibited the same degradation trend and similar degradation curves,where the degradation rate increased with the increase of the soaking time.After incubating for 12 days,the mass loss of hydrogels increased in the order of CNFs-P/Al60 <CNFs-C/Al60 <C NCs/Al60.For CNCs/Al60,the largest swelling property helped to accelerate its degradation rate in PBS.Notably,compared with other studies (Table S2),the hydrogels reported here with a lower degradation rate would make them acellular matrix candidates for retaining cell culture [44].
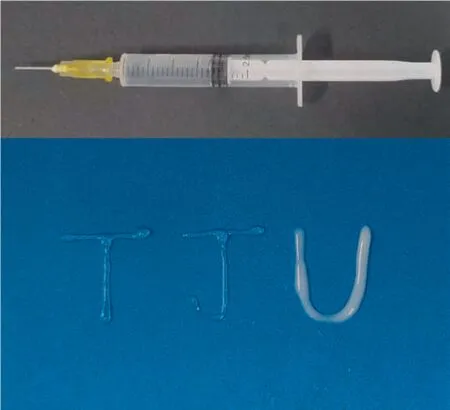
Fig.6.Injectable property of hydrogels.T: CNFs-C/Al60,J: CNFs-P/Al60,U: CNCs/Al60.
3.8.Radar chart and discussion
The above observations unambiguously indicated that the different surface groups and morphologies would affect the final properties of crosslinked hydrogels.To make it easier for elucidation and helpful for further application selection,the summarized properties of each hydrogel (Table 1) were drawn as radar charts and shown in Fig.S3 and Fig.10.According to their different viscoelasticity,water holding capacity,and degradation performance,CNCs and CNFs would be chosen to meet different requirements and play suitable roles in tissue engineering.For CNCs,benefited from more hydroxyl groups on the surface,the crosslinked CNCs hydrogels showed a strong water holding capacity,which was more than twice that of CNFs hydrogels.Consequently,CNCs/Al60 hydrogels exhibited a significant degradation rate at the initial stage,and then kept at a gentle rate.Due to its high expansion rate,CNCs,rather than CNFs,could be selected to make hydrogels for drug loading.While for CNFs,it would be better to be chosen as biological tissue engineering scaffold,due to their unique advantages in viscoelasticity,anti-degradation,and self-healing.Moreover,compared with CNFs-C,CNF-P with phosphates may provide more biological activity of cellulose,as phosphorylated matrix was reported to facilitate osteocyte adhesion and proliferation [45].Therefore,the study here would not only provide a guideline for rational choice of nanocellulose hydrogels,but also advance the development of nanocellulose and their further applications in biomedical field.
It is noteworthy that the fabricated hydrogels in the current study show several unique advantages.Firstly,some of the previously reported nanocellulose hydrogels (Table S2) required the aid from other polymers [19,46-48].And some complicated fabrication processes such as surface modification and multi-step gelation are necessary[19,47,48].For example,the Schiff base reaction required aldehyde-functionalized CNCs[19].Instead,the hydrogels crosslinked by Al3+with cellulose nanocrystals or nanofibrils was undoubtedly a more convenient way.Secondly,most of previous researches only focused on single type of nanocellulose hydrogels[34,49],whereas we fabricated and compared the hydrogels by using three kinds of nanocelluloses,which might be helpful for better selection in their further biomedical applications.Furthermore,to our best knowledge,this is the first work on the hydrogel of phosphorylated CNFs and its properties.Last but not the least,three kinds of nanocelluloses with morphology heterogeneities and different crosslinking conditions can provide a wider range of rheological properties,allowing hydrogels to be adjusted for various conditions.Moreover,the nanocellulose hydrogels in our study exhibited higher water absorption capacity and lower hydrogels degradation rate than most previously reported ones(Table S2).
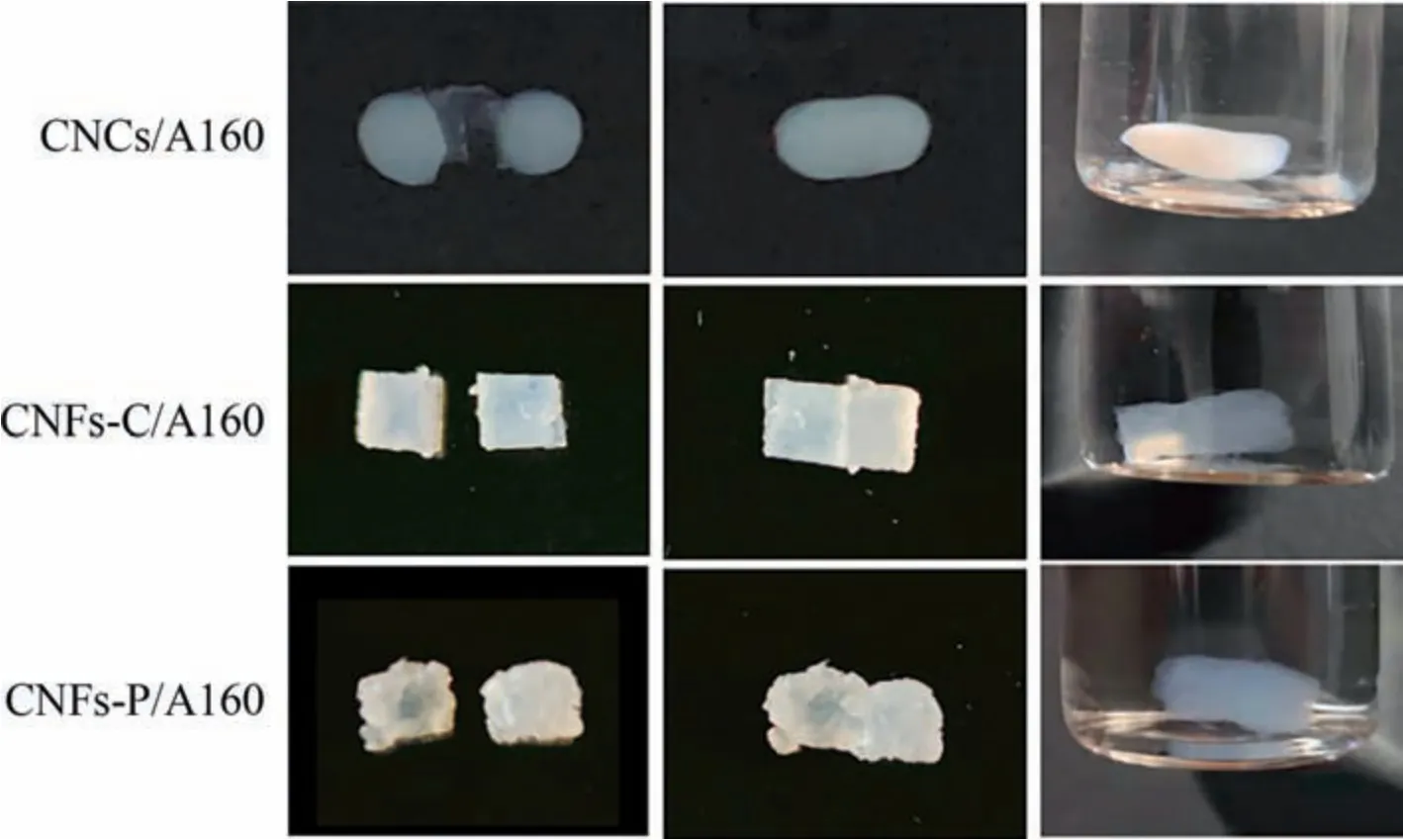
Fig.7.CNFs hydrogel was cut in the middle,placed together to self-heal,and soaked in PBS.
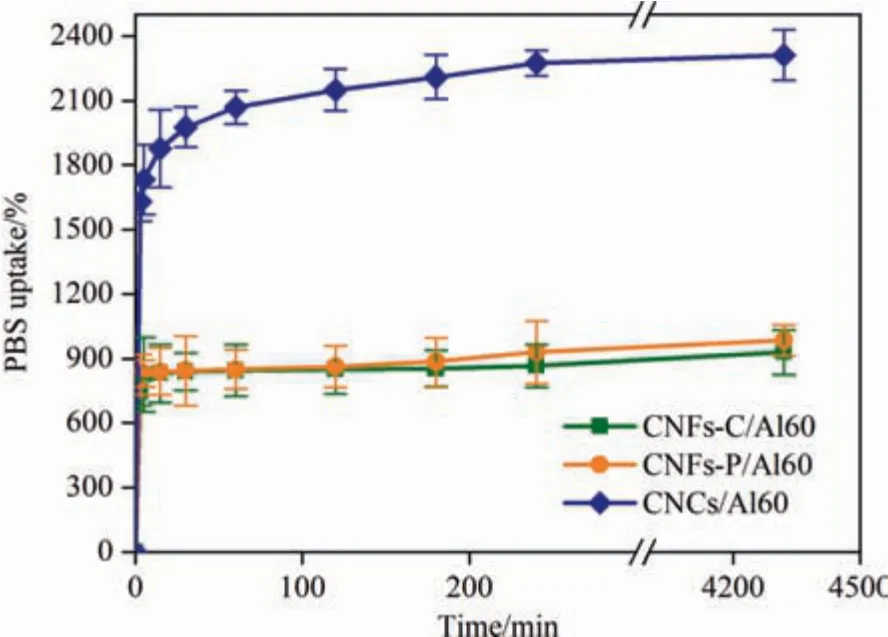
Fig.8.Time evolution of the swelling mass for monitoring the swelling property of CNFs-C/Al60,CNFs-P/Al60,and CNCs/Al60 hydrogels.
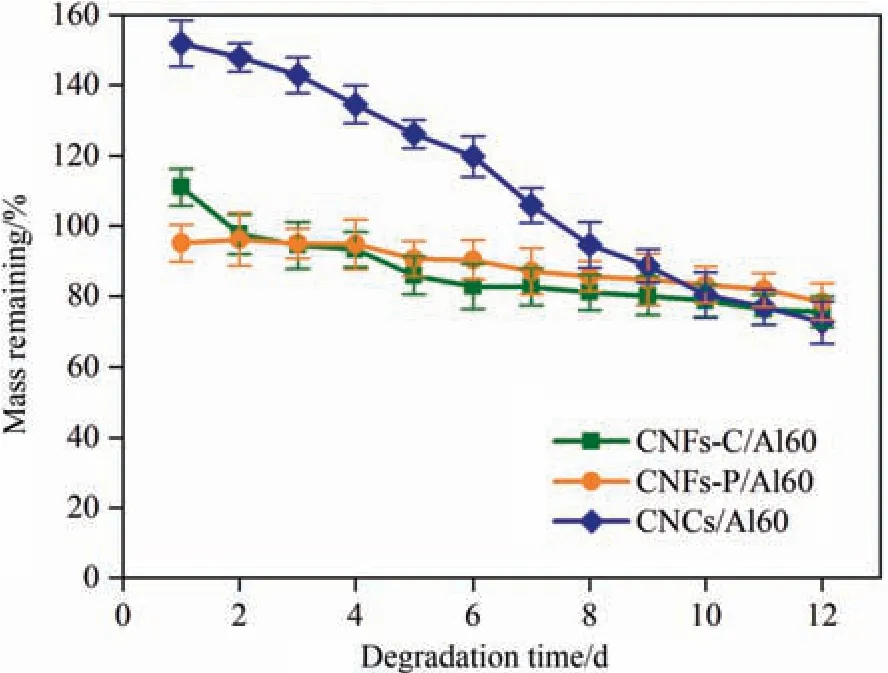
Fig.9.Degradation profiles of CNFs-C/Al60,CNFs-P/Al60,and CNCs/Al60 hydrogels in enzymatic condition for 12 days.
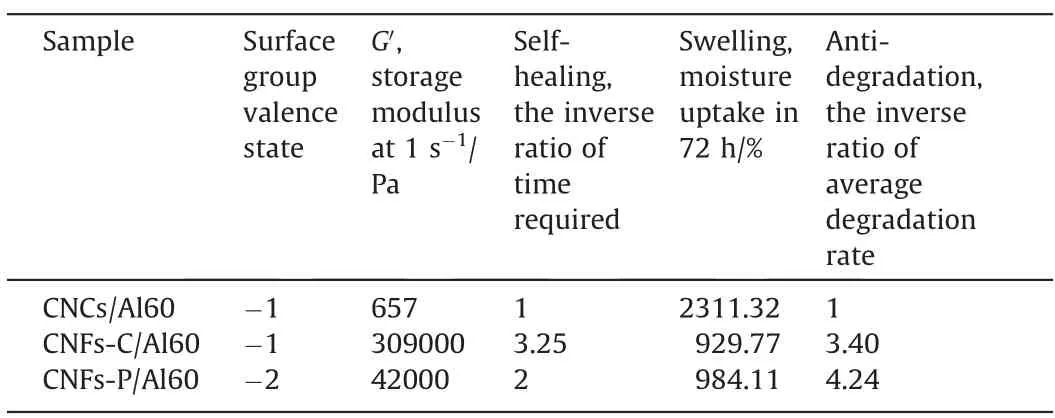
Table 1 The physical and chemical properties of hydrogels
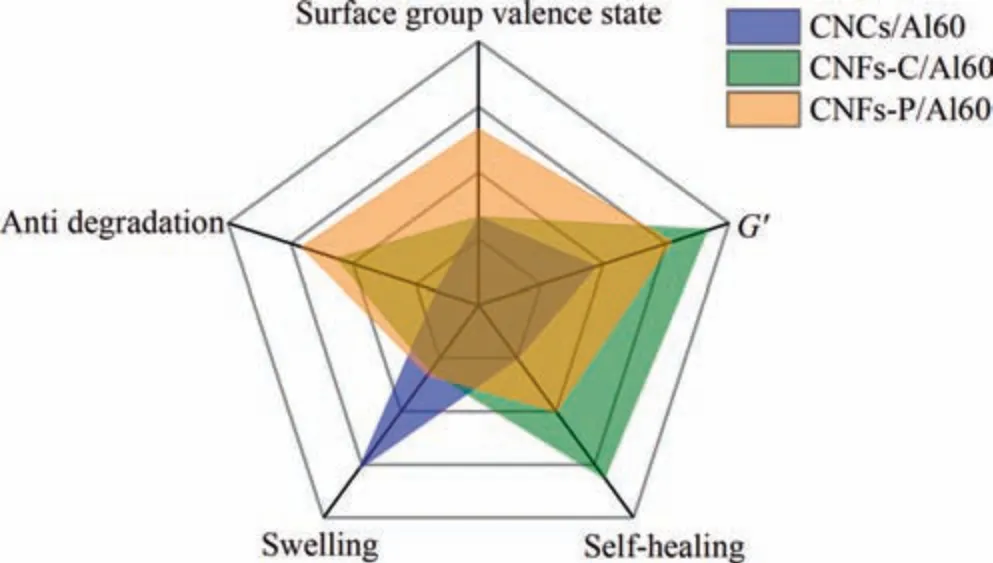
Fig.10.Radar map on physical and chemical properties of three nanocellulose hydrogels.
4.Conclusions
In summary,we have performed a systematical study to investigate the effect of different nanocellulose with morphology and surface heterogeneities on the final properties of as-prepared hydrogels.In the form of Al3+crosslinking,the reinforced hydrogels were prepared by crosslinking with -SO4-,-COO-,on the surface of nanocellulose,and named as CNCs/Al60,CNFs-C/Al60,and CNFs-P/Al60.Further characterization showed that hydrogels made by different nanocellulose indeed exhibited great differences in viscoelasticity,water holding capacity,and degradation performance.CNCs/Al60 hydrogels had strong water holding capacity twice as much as that of CNFs/Al60 hydrogels.While CNFs/Al60 hydrogels showed higher hardness,lower swelling degree,and stronger resistance to degradation than that of CNCs/Al60.Finally,a detailed radar guideline of these different performance was established and used to advance the further biomedical application of these nanocellulose hydrogels.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the National Key Research and Development Program of China (2019YFE0106900).
Supplementary Material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cjche.2022.04.026.
 Chinese Journal of Chemical Engineering2022年10期
Chinese Journal of Chemical Engineering2022年10期
- Chinese Journal of Chemical Engineering的其它文章
- Enhance hydrates formation with stainless steel fiber for high capacity methane storage
- Understanding the effects of electrode meso-macropore structure and solvent polarity on electric double layer capacitors based on a continuum model
- Refrigeration system synthesis based on de-redundant model by particle swarm optimization algorithm
- Adaptive multiscale convolutional neural network model for chemical process fault diagnosis
- Establishment of nucleation and growth model of silica nanostructured particles and comparison with experimental data
- Molecular dynamics simulations of ovalbumin adsorption at squalene/water interface
