Down-regulation of the Smad signaling by circZBTB46 via the Smad2-PDLIM5 axis to inhibit type I collagen expression
Jing YU, Wen-Zhao YAN, Xin-Hua ZHANG, Bin ZHENG, Wen-Sen PAN, Zhan YANG,,Hong ZHANG, Zi-Yuan NIE, Ying MA, Yang BAI, Long ZHANG, Dan-Dan FENG,Jin-Kun WEN,?
1.Department of Biochemistry and Molecular Biology, Hebei Medical University, Shijiazhuang, China; 2.Second Department of Respiratory and Critical Care Medicine, the Second Hospital of Hebei Medical University, Shijiazhuang, China; 3.Department of Infectious Diseases, the Third Hospital of Hebei Medical University, Shijiazhuang, China; 4.Institution of Chinese Integrative Medicine, Hebei Medical University, Shijiazhuang, China; 5.Department of Urology, the Second Hospital of Hebei Medical University, Shijiazhuang, China; 6.Department of Hematology, the Second Hospital of Hebei Medical University,Shijiazhuang, China; 7.Department of Biochemistry and Molecular Biology, Binzhou Medical University, Yantai, China
ABSTRACT BACKGROUND Abnormal type I collagen (COL1) expression is associated with the development of many cardiovascular diseases.The TGF-beta/Smad signaling pathway and circRNAs have been shown to regulate COL1 gene expression, but the underlying molecular mechanisms are still not fully understood.METHODS Gain- and loss-of-function experiments were prformed to study the effect of circZBTB46 on the expression of alpha 2 chain of type I collagen (COL1A2).Co-immunoprecipitation assay was performed to observe the interaction between two proteins.RNA immunoprecipitation assay and biotin pull-down assay were performed to observe the interaction of circZBTB46 with PDLIM5.RESULTS In this study, we investigated the role of circZBTB46 in regulating COL1A2 expression in human vascular smooth muscle cells (VSMCs).We found that circZBTB46 is expressed in VSMCs and that TGF-beta inhibits circZBTB46 formation by downregulating KLF4 expression through activation of the Smad signaling pathway.CircZBTB46 inhibits the expression of COL1A2 induced by TGF-beta.Mechanistically, circZBTB46 mediates the interaction between Smad2 and PDLIM5, resulting in the inhibition of Smad signaling and the subsequent downregulation of COL1A2 expression.Furthermore, we found that the expression of TGF-beta and COL1A2 is decreased, while circZBTB46 expression is increased in human abdominal aortic aneurysm tissues, indicating that circZBTB46-mediated regulation of TGF-beta/Smad signaling and COL1A2 synthesis in VSMCs plays a crucial role in vascular homeostasis and aneurysm development.CONCLUSIONS CircZBTB46 was identified as a novel inhibitor of COL1 synthesis in VSMCs, highlighting the importance of circZBTB46 and PDLIM5 in regulating TGF-beta/Smad signaling and COL1A2 expression.
The vascular extracellular matrix (ECM) is predominantly composed of collagen, elastin, proteoglycans, and glycoproteins, and is essential in regulating the phenotype of vascular smooth muscle cells (VSMCs) and maintaining vascular homeostasis.[1,2]A significant number of ECM proteins in the human aorta have shown marked modifications in the arterial ECM of aneurysms, post-injury neointima, and arteriosclerotic plaques.[2]Type I collagen (COL1), an integral constituent of the ECM, is the most abundant collagen in vertebrates and is found in various connective tissues.[3]COL1 is a heterotrimeric molecule consisting of two type: alpha 1 chain of type I collagen (COL1A1) and alpha 2 chain of type I collagen (COL1A2).[4]Several candidate genes have been identified to be associated with aneurysm formation,[5,6]and studies have revealed thatabnormal gene expression of COL1 is linked to the incidence of vascular diseases.[7]For instance, the COL1A2 rs42524 site mutation has been found to be a crucial risk factor for intracranial aneurysm susceptibility, particularly in Asian populations.[8]Nevertheless, the precise mechanism governing COL1 synthesis in VSMCs is not yet fully understood.
Transforming growth factor beta (TGF-beta) has long been recognized as the key regulator of the ECM.[9]In VSMCs, TGF-beta signaling is mediated by Smad proteins.[10]TGF-beta1 induces phosphorylation of Smad2 and Smad3, which form heterotrimers with Smad4.This complex then translocates into the nucleus to activate the transcription of genes such as COL1 and fibronectin.[9,11]When TGF-beta levels are down-regulated or Smad2/Smad3 phosphorylation is inhibited, the expression of collagens, including COL1A2, is significantly reduced.This contributes to dysregulation of the ECM homeostasis and weakening of the vascular wall.[12]In addition,neutralizing TGF-beta has been shown to enhance angiotensin II-induced aortic rupture and aneurysm in mice,both at the thoracic and abdominal regions.[13]Conversely, intramural delivery of TGF-beta1 hydrogel has been found to effectively decrease aneurysm formation.[14]These observations suggest that TGF-beta/Smad signaling plays a crucial role in vascular homeostasis and the development of aneurysms.Our previous studies have confirmed that the regulation of Kruppel-like factor 4(KLF4) expression by TGF-beta signaling is crucial in regulating VSMC structure and functions.[15,16]Additional studies have shown that KLF4 promotes the formation of aneurysms by influencing VSMC phenotype.[17,18]However, the mechanisms linking TGF-beta and KLF4 to COL1 expression and aneurysm formation have not been fully elucidated.
Circular RNAs (circRNAs) are long, endogenous RNA molecules that are noncoding and formed through premRNA backsplicing.Their circular structure makes them more stable than linear mRNAs.Due to their high stability, circRNAs can accumulate in specific cell types and play important roles in regulating physiological and pathophysiological processes.Most exonic circRNAs are located in the cytoplasm where they act as miRNA sponges, protein sponges, protein decoys, or scaffolds for protein complex formation.[19,20]Emerging evidence suggests that circRNAs are involved in cardiovascular diseases, such as abdominal aortic aneurysm (AAA), myocardial infarction, and coronary artery disease.[21]Multiple differentially expressed circRNAs have been found between human AAA and non-pathological aorta, including up-regulated and down-regulated circRNAs.[22,23]Studies have confirmed that some circRNAs can affect the development of AAA by regulating the phenotype and functions of VSMCs.[24–26]While it has been reported that circPTEN1 suppresses cancer progression through inhibition of TGF-beta/Smad signaling,[27]and circCOL-3A1-859267 regulates COL1 expression by sponging and sequestering miR-29c in human dermal fibroblasts,[28]the mechanisms by which COL1 expression is regulated in VSMCs via circRNA-modulated TGF-beta/Smad signaling remain largely unknown.
In our current research, we have discovered that circZBTB46 acts as an antagonist to COL1 synthesis in VSMCs.Specifically, TGF-beta inhibits the formation of circZBTB46 by decreasing KLF4 expression through the activation of Smad signaling.Functionally, circZBTB46 plays a role in the regulation of COL1A2 expression by repressing TGF-beta/Smad signaling.The mechanism involves the mediation of an interaction between Smad2 and PDLIM5 by circZBTB46, leading to the inhibition of Smad signaling and the subsequent suppression of COL1A2 expression that is induced by TGF-beta.
MATERIALS AND METHODS
Human Tissue Collection
Samples of human blood vessels were collected from eight patients with aneurysms, while control samples were obtained from human renal arteries.The aneurysms and renal arteries used in this study were obtained from the Fourth Hospital of Hebei Medical University,Shijiazhuang, China between 2021 and 2022.The human research plan was approved by the Ethics Committee of the Fourth Hospital of Hebei Medical University, Shijiazhuang, China (No.HEBMU-2011-09), and each surgical patient provided informed consent before tissue donation.The collected tissue was fixed in 10% neutral buffered formalin overnight and underwent routine paraffin embedding treatment.
Cell Lines and Treatment
Human aortic VSMCs were obtained from ScienCell Research Laboratories (catalog number: 6110) and maintained in our laboratory.The cells were cultured in smooth muscle cell medium supplemented with 2% fetal bovine serum (Clarkbio, USA) and 1% penicillin/strep-tomycin (Solarbio, China) at 37 °C with 5% CO2.Prior to TGF-beta stimulation, VSMCs were starved in serumfree medium for 24 h.For TGF-beta stimulation, VSMCs were treated with different concentrations of TGF-beta(1 ng/mL, 2.5 ng/mL, or 5 ng/mL) for 24 h or with 2.5 ng/mL TGF-beta for varying times (0 h, 6 h, 12 h, and 24 h).Transfection experiments were performed using Lipofectamine 2000 (Invitrogen, Life Technologies, Carlsbad, CA, USA) to deliver small interfering RNA or plasmids into VSMCs.After 4–6 h of transfection, the transfection medium was replaced with 1% smooth muscle cell medium and the cells were cultured for an additional 24–48 h.All experiments were performed in triplicate.
Vector Construction
Adenoviruses for overexpression of KLF4 or PDLIM5 and empty virus vector (Ad-GFP) were obtained from Hanheng Biotechnology Co., Ltd.(Shanghai, China).The vector for circZBTB46 and pcDNA3.1 were purchased from GenePharma Co., Ltd.(Shanghai, China).GenePharma Co., Ltd.(Shanghai, China) provided the following inhibitors: negative control (si-NC), small interfering RNA targeting KLF4 (si-KLF4), si-Smad2, si-circZBTB46,and si-PDLIM5.
RNA Isolation and Real-time Quantitative PCR
To isolate RNA, cultured cells were lysed with QIA-zol Lysis Reagent (catalog number: 79306), and total RNA was extracted using the miRNasy Mini Kit (catalog number: 217004) following the manufacturer’s instructions.The concentration of RNA was measured using Nano-Drop 2000 (Thermo Scientific, Wilmington, DE, USA).Reverse transcription into cDNA was performed using the Reverse Transcription Kit (MR05201M; Monad, Wuhan,Hubei, China), and the expression of mRNA was detected by real-time quantitative polymerase chain reaction(qRT-PCR) using the qPCR Kit (MQ00401S; Monad, Wuhan, Hubei, China).The relative expression levels of mRNA were calculated using the 2–ΔΔCTformula, with the GAPDH value serving as a reference.
Western Blotting
We used Western blotting to detect protein levels, as previously described.[29]Total protein was extracted from cells using protein lysis buffer.Protein concentration was determined using the modified Lowry method.Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and then transferred to polyvinylidene fluoride membranes using a semi-dry transfer apparatus.After blocking the membranes with 5%milk, primary antibodies were used to incubate the membranes overnight at 4 °C.The antibodies used in this study were as follows: KLF4 (ab215036, 1:1000), betaactin (Proteintech, 66009-1-Ig, 1:5000), Smad2 (Proteintech, 12570-1-Ig, 1:1000), COL1A2 (ab96723, 1:1000), p-Smad2 (Cell Signaling, E8F3R, 1:1000), Smad3 (Proteintech, 66516-1-Ig, 1:1000), p-Smad3 (Cell Signaling,C25A9, 1:1000), and PDLIM5 (Proteintech, 10530-1-Ig,1:1000).The following day, the primary antibodies were recycled and the membranes were washed with a cleaning solution.Then, a secondary antibody (1:10000, Rockland)conjugated with horseradish peroxidase was used to block the membranes that had bound to the primary antibody.Finally, images were captured and analyzed using an electrogenerated chemiluminescence chemiluminescence instrument.
Co-immunoprecipitation Assay
Co-immunoprecipitation assays were performed to investigate protein-protein interactions, following established protocols.[29]Briefly, cells were collected and lysed with radioimmune precipitation assay buffer to obtain pre-prepared antigens.Next, 25–50 μL of magnetic beads were placed into a 1.5 mL EP tube and washed with 400 μL of buffer solution.The washing step was repeated three times to ensure the purity of the magnetic beads.Subsequently, antibodies were added to the magnetic beads at a concentration of 5–50 μg/mL and incubated at room temperature for 30 min.After four rounds of washing with buffer solution and removal of the supernatant, the antigen samples were incubated with the antibody-magnetic bead complexes at room temperature for 30 min.Finally, 25–50 μL of 1 × sodium dodecyl sulfate-polyacrylamide gel electrophoresis loading buffer were added to the magnetic beads, followed by heating at 95 °C for 5 min, and the resulting supernatant was used for Western blotting analysis.
RNA Immunoprecipitation Assay
The RNA immunoprecipitation assay was performed following the protocol previously established in our laboratory.[30]Briefly, VSMCs were collected and lysed in NETN buffer using ultrasound.Magnetic beads (Dynabeads, Thermo Fisher, USA) were then incubated withantibodies against IgG or PDLIM5 to form bead-antibody complexes.The complexes were washed three times with NETN buffer before being incubated with the antigen.After additional washing with NETN buffer, the magnetic beads were dissolved with Elution buffer to extract RNA from the complex.The level of mRNA associated with PDLIM5 was determined by reverse transcription quantitative PCR.
Immunofluorescence Staining
To perform immunofluorescence staining, firstly, the cells were fixed with 4% paraformaldehyde for 15 min according to experimental requirements, followed by washing three times with phosphate buffered saline(PBS) for 5 min each.Secondly, the cells were permeabilized with 0.5% Triton X-100 in PBS for 20 min and washed with PBS for three times (5 min each).Thirdly,the cells were incubated with 10% goat serum for 30 min,and then the serum was removed and primary antibodies (KLF4, alpha-SMA, Smad2, COL1A2, PDLIM5) were added and incubated overnight at 4 °C.The next day, the primary antibodies were removed, and the cells were washed three times with PBS (5 min each).Subsequently,the cells were incubated with appropriate fluorescentlabeled secondary antibodies for 60 min at 37 °C.After the incubation was completed, the cells were washed three times with PBS (5 min each) and the nucleus was stained with DAPI.
The primary antibodies were used as follows: KLF4(ab215036, 1:100), alpha-SMA (Proteintech, 67735-1-Ig,1:200), Smad2 (Proteintech, 12570-1-Ig, 1:100), COL1A2(ab96723, 1:100), and PDLIM5 (Proteintech, 10530-1-Ig,1:100).
Fluorescence in Situ Hybridization
Fluorescence in situ hybridization was performed as previously described.[30]Briefly, cells or tissues were hybridized with a specific probe for circZBTB46 under the guidance of the miRCURY LNA? microRNA ISH Optimization Kit (Exiqon, Vedb?k, Denmark).The hybridization was carried out for 1 h at 55 °C in hybridization buffer, followed by washing in a strict concentration of SSC buffer.Finally, the samples were stained with DAPI for visualization.Images were acquired using a confocal microscope (DM6000B, Leica, Germany) and processed with LAS V.4.4 (Leica Microsystems, Germany) software.
Biotin Pull-down of RNA
The biotin pull-down assay was performed according to previously described protocols.[30,31]In brief, VSMCs were cross-linked with 4% formaldehyde in PBS for 10 min, and the reaction was terminated with 0.125 M glycine.The cells were then lysed with lysis buffer containing protease and RNAs inhibitors, followed by sonication.Next, the cell lysates were mixed with hybridization buffer and 100 pmol of biotin probe, and then added to streptavidin Dynabeads (Life Technologies) that were pre-blocked with yeast RNA for 1 h.The mixture was then incubated at 37 °C for 30 min and the microbeads were captured with magnets (Life Technologies) and washed five times with wash buffer.Finally, the RNA bound to the microspheres was extracted using elution buffer.
Statistical Analysis
Data were presented as mean ± SEM.The independent Student’st-test was used to analyze the differences between the two groups.Two-sidedP-value < 0.05 were considered statistically significant.Statistical analysis was performed using GraphPad Prism 5.0 (GraphPad Prism Software Inc., San Diego, California, IL, USA).
RESULTS
Identification of CircZBTB46 in Human Aortic Smooth Muscle Cells
We previously performed RNA-sequencing of KLF4-overexpressing VSMCs and control cells to compare the circRNA profiles between the two cell types.Through this analysis, we identified a circRNA derived from theZBTB46gene that was largely induced by KLF4 overexpression.[32]We named this circRNA “circZBTB46”as it was derived from the circularization of exon 2 and exon 3 of theZBTB46gene (Figure 1A).Sequencing analysis confirmed that the sequence of the head-to-tail splice junction in circZBTB46 was identical to the reported sequence (hsa_circ_0002805) (Figure 1B).
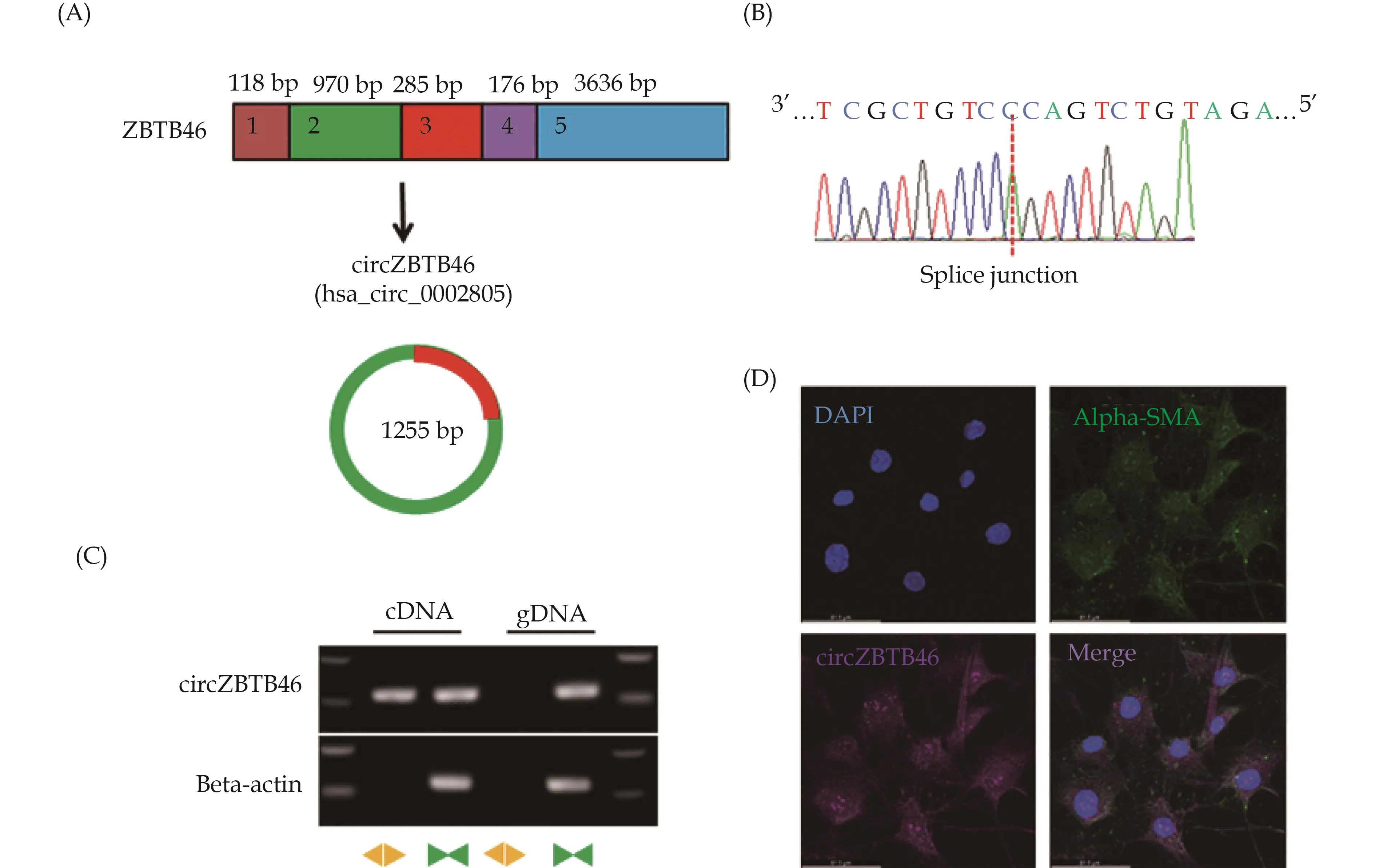
Figure 1 Identification of circZBTB46 in human aortic VSMCs.(A): Schematic illustration depicting the origin of circZBTB46 from its parental gene ZBTB46; (B): the presence of head-to-tail splicing junction in circZBTB46 was confirmed by Sanger sequencing; (C): convergent and divergent primers were used to specifically detect circZBTB46 via qRT-PCR in human VSMCs.CircZBTB46 was amplified by divergent primers in cDNA but not gDNA; and (D): the expression of circZBTB46 and alpha-SMA in human VSMCs was analyzed by combined in situ/protein staining.VSMCs: vascular smooth muscle cells.
To further confirm the presence of circZBTB46 in VSMCs, we performed RT-PCR with convergent and divergent primers.The head-to-tail splicing of endogenous circZBTB46 was amplified, and divergent primers could detect circZBTB46 from cDNA but not from gDNA, confirming the presence of circZBTB46 in VSMCs (Figure 1C).
To determine the expression of circZBTB46 in VSMCs,we designed specific fluorescent probes against circZBTB46 and performed fluorescence in situ hybridization/alpha-SMA co-staining.The results showed that circZBTB46 was expressed in both the nucleus and cytoplasm of VSMCs (Figure 1D).
Inhibition of CircZBTB46 Formation Through the Smad Signaling Pathway by TGF-beta-mediated Suppression of KLF4 Expression
To validate the findings of the circRNA sequencing analysis, which indicated that KLF4 stimulated the formation of circZBTB46, we infected VSMCs with Ad-KLF4 to overexpress KLF4 (Figure 2A & 2B) and assessed the changes in circZBTB46 expression by qRT-PCR.The results demonstrated that overexpression of KLF4 in VSMCs significantly increased circZBTB46 expression (Figure 2C).Additionally, we designed and synthesized si-KLF4.We transfected VSMCs with si-KLF4 to knock down KLF4 expression (Figure 2D & 2E) and evaluated the changes in circZBTB46 expression by qRT-PCR.The results showed that suppressing KLF4 expression significantly hindered the formation of circZBTB46 (Figure 2F).
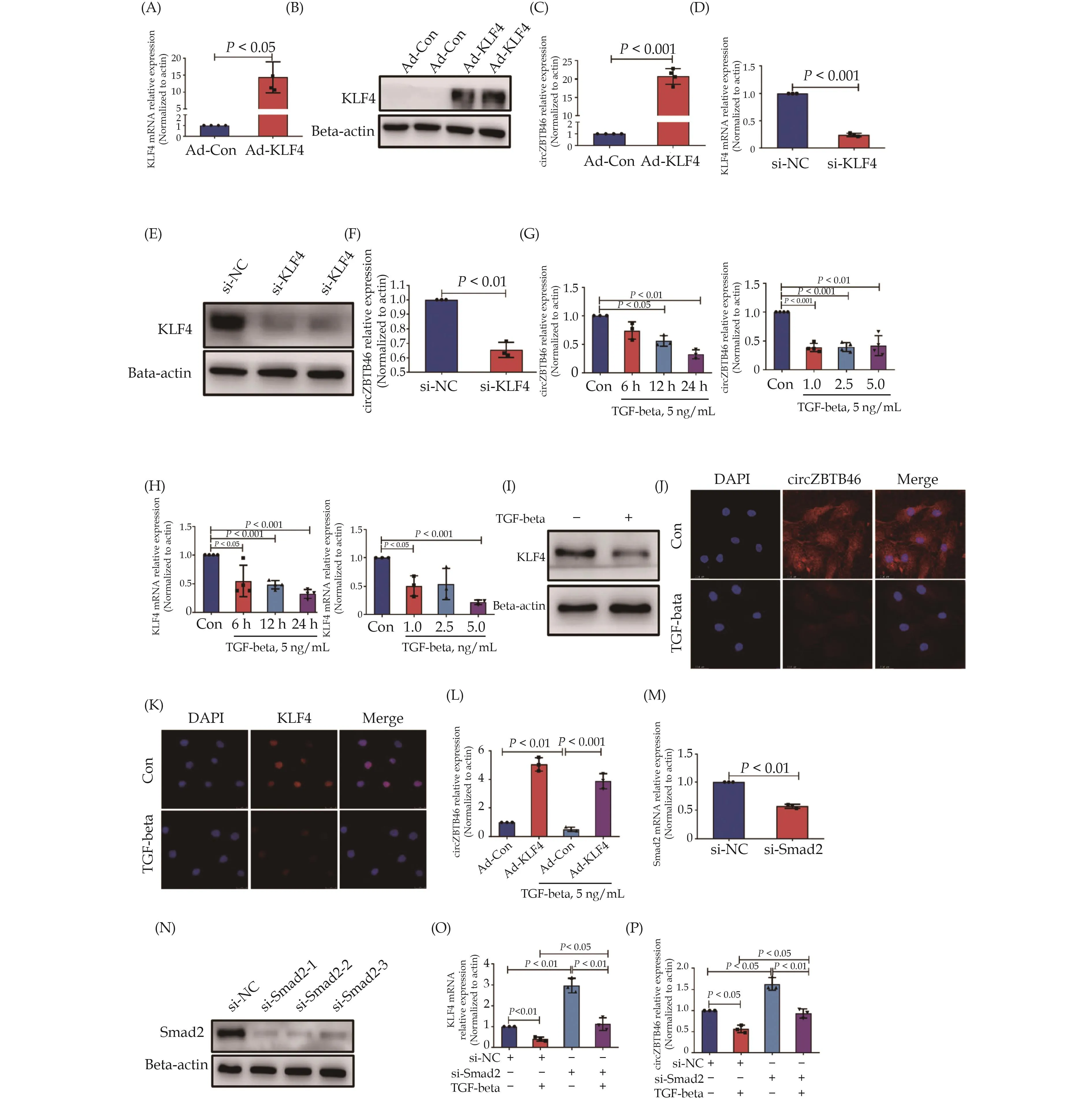
Figure 2 TGF-beta suppresses the formation of circZBTB46 by downregulating KLF4 expression through Smad signaling.(A & B):Human VSMCs were infected with Ad-KLF4, and mRNA and protein levels of KLF4 were measured by qRT-PCR and Western blot, respectively; (C): circZBTB46 expression was measured by qRT-PCR in human VSMCs infected with Ad-KLF4; (D–F): human VSMCs were transfected with si-KLF4, and mRNA and protein levels of KLF4 were measured by qRT-PCR (D) and Western blot (E), respectively.Circ-ZBTB46 expression was measured by qRT-PCR (F); (G & H): human VSMCs were incubated in serum-free medium for 24 h, followed by treatment with TGF-beta for different times and concentrations.CircZBTB46 (G) and KLF4 mRNA (H) expression levels were measured by qRT-PCR; (I–K): human VSMCs were incubated in serum-free medium for 24 h, followed by treatment with 5 ng/mL TGF-beta for 24 h.Western blot analysis was used to detect KLF4 expression (I).Fluorescence in situ hybridization (J) and immunofluorescence (K) staining were used to detect circZBTB46 and KLF4 expression, respectively; (L): human VSMCs were infected with Ad-Con or Ad-KLF4 for 24 h, and then treated with or without 5 ng/mL TGF-beta for an additional 24 h.CircZBTB46 expression was measured by qRT-PCR;(M & N): human VSMCs were transfected with si-Smad2, and the relative expression of Smad2 mRNA and protein was measured by qRTPCR and Western blot, respectively; and (O & P): human VSMCs were transfected with si-Smad2 for 24 h, followed by a 24 h incubation with 5 ng/mL TGF-beta.The relative expression of KLF4 mRNA (O) and circZBTB46 (P) was measured by qRT-PCR.VSMCs: vascular smooth muscle cells.
As our previous study had demonstrated that TGF-beta plays a crucial role in regulating the phenotype of VSMCs by modulating KLF4 expression,[15]we aimed to investigate whether TGF-beta affects the formation of circZBTB46 by regulating KLF4 expression.Initially, we treated human VSMCs with varying concentrations of TGF-beta (1 ng/mL, 2.5 ng/mL, 5 ng/mL) or with 5 ng/mL TGF-beta for varying durations (0 h, 6 h, 12 h, 24 h)and evaluated the expression levels of circZBTB46 and KLF4 through qRT-PCR.The results indicated that TGF-beta significantly reduced the expression of both circZBTB46 and KLF4 in a dose- and time-dependent manner(Figure 2G & 2H).Western blot analysis also demonstrated that TGF-beta treatment notably suppressed KLF4 expression in VSMCs compared to TGF-beta1-untreated cells (Figure 2I).Fluorescence in situ hybridization and immunofluorescence staining further validated the inhibitory effect of TGF-beta on the expression of both circZBTB46 and KLF4 (Figure 2J & 2K).
To investigate whether TGF-beta inhibits the formation of circZBTB46 by suppressing KLF4 expression, we conducted a rescue experiment in VSMCs by overexpressing KLF4 followed by TGF-beta stimulation.As expected, overexpression of KLF4 reversed the inhibitory effect of TGF-beta on circZBTB46 formation (Figure 2L),indicating that TGF-beta inhibits circZBTB46 formation through down-regulating KLF4 expression.Since Smad signaling is one of the critical signaling pathways activated by TGF-beta,[33]we further examined whether TGF-beta inhibits KLF4 and circZBTB46 expression through activating Smad signaling.We knocked down Smad2 expression in VSMCs using si-Smad2 and measured KLF4 and circZBTB46 expression.The results showed that si-Smad2 effectively decreased Smad2 expression at both mRNA and protein levels (Figure 2M & 2N) and Smad2 knockdown reversed the inhibitory effect of TGF-beta on KLF4 and circZBTB46 expression (Figure 2O & 2P).Moreover, TGF-beta treatment reduced the upregulation of KLF4 and circZBTB46 expression caused by Smad2 knockdown.These findings indicate that TGF-beta inhibits KLF4 and circZBTB46 expression by activating the Smad signaling pathway.
Participation of CircZBTB46 in the Regulation of COL1A2 Expression by TGF-beta
Collagen and KLF4 are critical components involved in vascular wall remodeling.[34–36]In order to investigate whether TGF-beta-regulated expression of KLF4 and circZBTB46 contributes to the regulation of collagen synthesis, we treated human VSMCs with TGF-beta and evaluated COL1A2 expression.Our qRT-PCR and Western blot analyses revealed that TGF-beta significantly enhanced the mRNA and protein expression of COL1A2 in VSMCs (Figure 3A & 3B).Furthermore, immunofluorescence staining confirmed that TGF-beta induced COL-1A2 synthesis in VSMCs (Figure 3C).These findings suggest that TGF-beta can promote COL1A2 expression in human VSMCs.As our previous results have demonstrated that TGF-beta inhibits circZBTB46 formation(Figure 2G), we hypothesized that circZBTB46 exerts an inhibitory effect on TGF-beta-induced COL1A2 expression.To test this hypothesis, we constructed a circZBTB-46-expressing plasmid and confirmed that transfecting VSMCs with this plasmid increased circZBTB46 levels(Figure 3D).
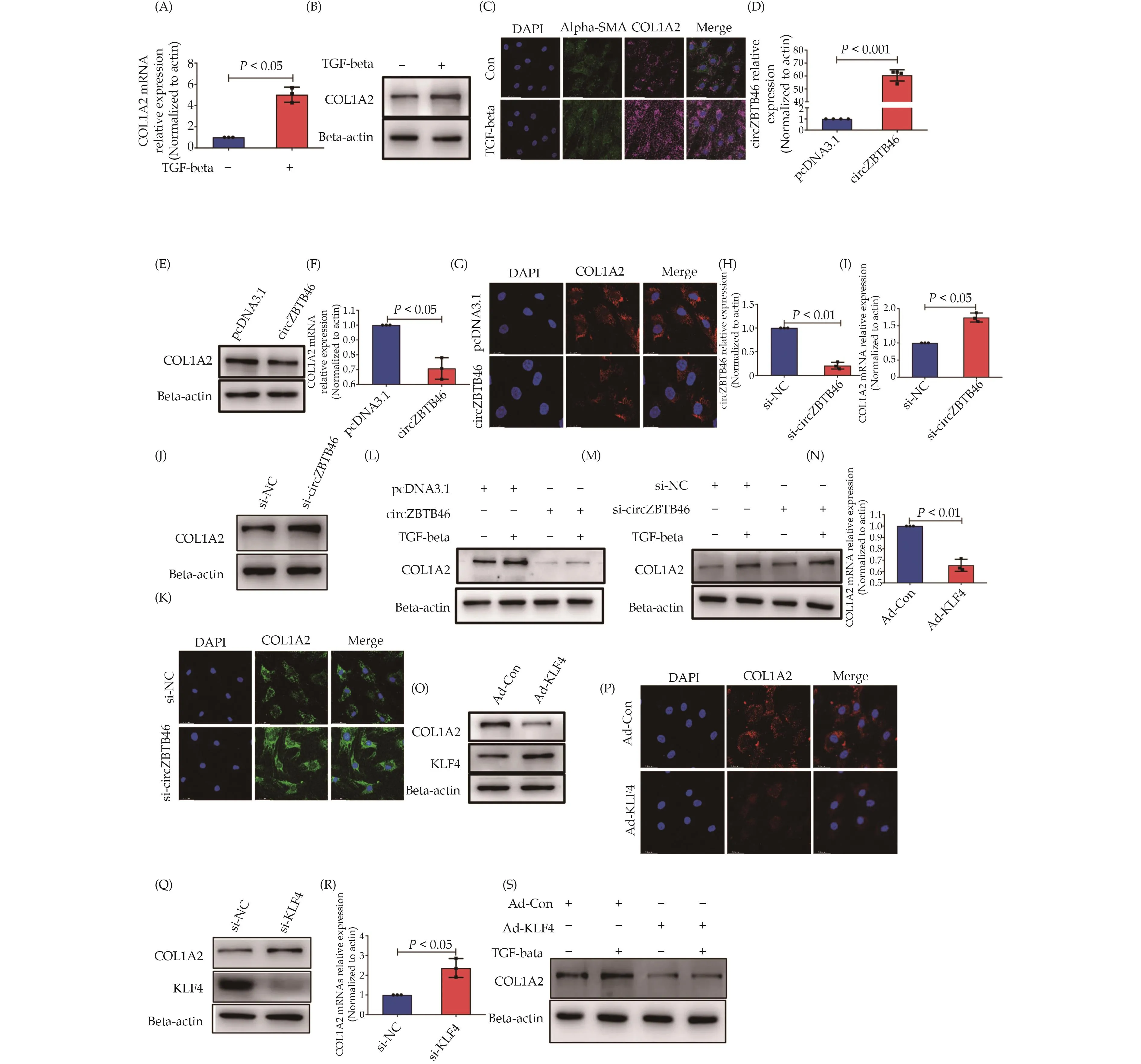
Figure 3 The involvement of circZBTB46 in regulating the expression of COL1A2 via TGF-beta.VSMCs were treated with 5 ng/mL TGF-beta for 24 h and analyzed for COL1A2 expression using qRT-PCR (A), Western blot (B), and immunofluorescence staining (C).A significant increase in COL1A2 expression was observed in TGF-beta-treated cells compared to untreated cells.VSMCs were transfected with either pcDNA3.1 or circZBTB46-expressing vector, and circZBTB46 expression was detected by qRT-PCR (D).COL1A2 expression was analyzed using Western blot (E), qRT-PCR (F), and immunofluorescence staining (G).A significant increase in COL1A2 expression was observed in cells transfected with the circZBTB46-expressing vector compared to those transfected with pcDNA3.1.Additionally, VSMCs were transfected with si-circZBTB46 or si-NC for 24 h, and qRT-PCR was used to detect circZBTB46 expression (H).COL1A2 expression was analyzed using qRT-PCR (I), Western blot (J), and immunofluorescence staining (K).A significant decrease in COL1A2 expression was observed in cells transfected with si-circZBTB46 compared to those transfected with si-NC.Furthermore, VSMCs were transfected with either pcDNA3.1 or circZBTB46-expressing vector for 24 h and then treated with or without 5 ng/mL TGF-beta for another 24 h.Western blot analysis was performed to detect COL1A2 expression (L).Similarly, VSMCs were transfected with either si-circZBTB46 or si-NC for 24 h and then treated with or without 5 ng/mL TGF-beta for another 24 h, and Western blot analysis was performed to detect COL1A2 expression (M).In addition, VSMCs were infected with either Ad-Con or Ad-KLF4 for 24 h, and COL1A2 expression was detected using qRT-PCR (N), Western blot (O), and immunofluorescence staining (P).A significant decrease in COL1A2 expression was observed in cells infected with Ad-KLF4 compared to those infected with Ad-Con.Finally, VSMCs were transfected with either si-NC or si-KLF4 for 24 h, and Western blot analysis was performed to detect KLF4 and COL1A2 expression (Q).qRT-PCR was used to detect COL1A2 mRNA expression (R).A significant increase in COL1A2 expression was observed in cells transfected with si-KLF4 compared to si-NC.Additionally,VSMCs were infected with either Ad-Con or Ad-KLF4 for 24 h and then treated with or without 5 ng/mL TGF-beta for another 24 h.Western blot analysis was performed to detect COL1A2 expression (S).VSMCs: vascular smooth muscle cells.
We examined the effects of circZBTB46 on COL1A2 expression in VSMCs transfected with a circZBTB46-expressing plasmid.Western blot, qRT-PCR, and immunofluorescence staining analysis indicated that circZBTB46 overexpression significantly inhibited COL1A2 expression (Figure 3E–3G).We next designed and synthesized small interfering RNA targeting circZBTB46 (si-circZBTB46) to knock down circZBTB46 expression in VSMCs.Western blot, qRT-PCR, and immunofluorescence staining analysis showed that knockdown of circZBTB46 markedly upregulated COL1A2 expression in VSMCs(Figure 3I–3K), confirming the inhibitory effect of circZBTB46 on COL1A2 expression.We then investigated whether circZBTB46 suppresses the induction of COL1A2 expression by TGF-beta.VSMCs were transfected with the circZBTB46-expressing plasmid for 24 h and subsequently treated with TGF-beta (5 ng/mL) for another 24 h.Western blot analysis demonstrated that circZBTB46 overexpression significantly attenuated the upregulation of COL1A2 expression induced by TGF-beta(Figure 3L).Conversely, when VSMCs were transfected with si-circZBTB46 to block endogenous circZBTB46 expression, TGF-beta-induced up-regulation of COL1A2 expression was further enhanced (Figure 3M).These results confirm that circZBTB46 participates in the regulation of COL1A2 expression by TGF-beta.
As circZBTB46 expression was regulated by KLF4(Figure 2), we aimed to provide further evidence for the involvement of circZBTB46 in regulating COL1A2 expression induced by TGF-beta.To this end, we infected VSMCs with Ad-KLF4 and assessed COL1A2 expression by qRT-PCR, Western blot and immunofluorescence staining.Our results demonstrated that overexpression of KLF4 significantly suppressed COL1A2 expression(Figure 3N–3P), while knockdown of KLF4 in VSMCs increased COL1A2 expression (Figure 3Q & 3R).Furthermore, VSMCs were infected with Ad-KLF4 for 24 h,followed by treatment with TGF-beta (5 ng/mL) for another 24 h.Western blot analysis showed that the upregulation of COL1A2 expression induced by TGF-beta was attenuated after KLF4 overexpression (Figure 3S).These findings suggest that KLF4-mediated circZBTB46 formation inhibits the induction of COL1A2 expression by TGF-beta.
Down-regulation of COL1A2 Expression by CircZBTB46 via Smad Signaling Inhibition
The TGF-beta-Smad signaling pathway is widely recognized as one of the key regulators of ECM production.[37]To elucidate the regulatory mechanism of circZBTB46 on COL1A2 expression, we investigated the impact of circZBTB46 on the TGF-beta-Smad signaling pat-hway.Initially, we confirmed the involvement of the TGF-beta-Smad signaling pathway in regulating COL1A2 expression.Western blot analysis demonstrated that knockdown of Smad2 significantly decreased TGF-beta-induced COL1A2 expression (Figure 4A).Moreover, treatment of VSMCs with SB431542, a specific TbetaRI inhibitor, suppressed Smad2 phosphorylation induced by TGF-beta (Figure 4B) and markedly inhibited the induction of COL1A2 expression by TGF-beta, as evidenced by Western blot and qRT-PCR (Figure 4C & 4D).Additionally,SB431542 largely reversed the suppressive effect of TGF-beta on circZBTB46 and KLF4 expression (Figure 4E &4F).To further elucidate whether circZBTB46 regulates COL1A2 expression by modulating the Smad signaling pathway, we next assessed the impact of circZBTB46 on Smad2/Smad3 phosphorylation.
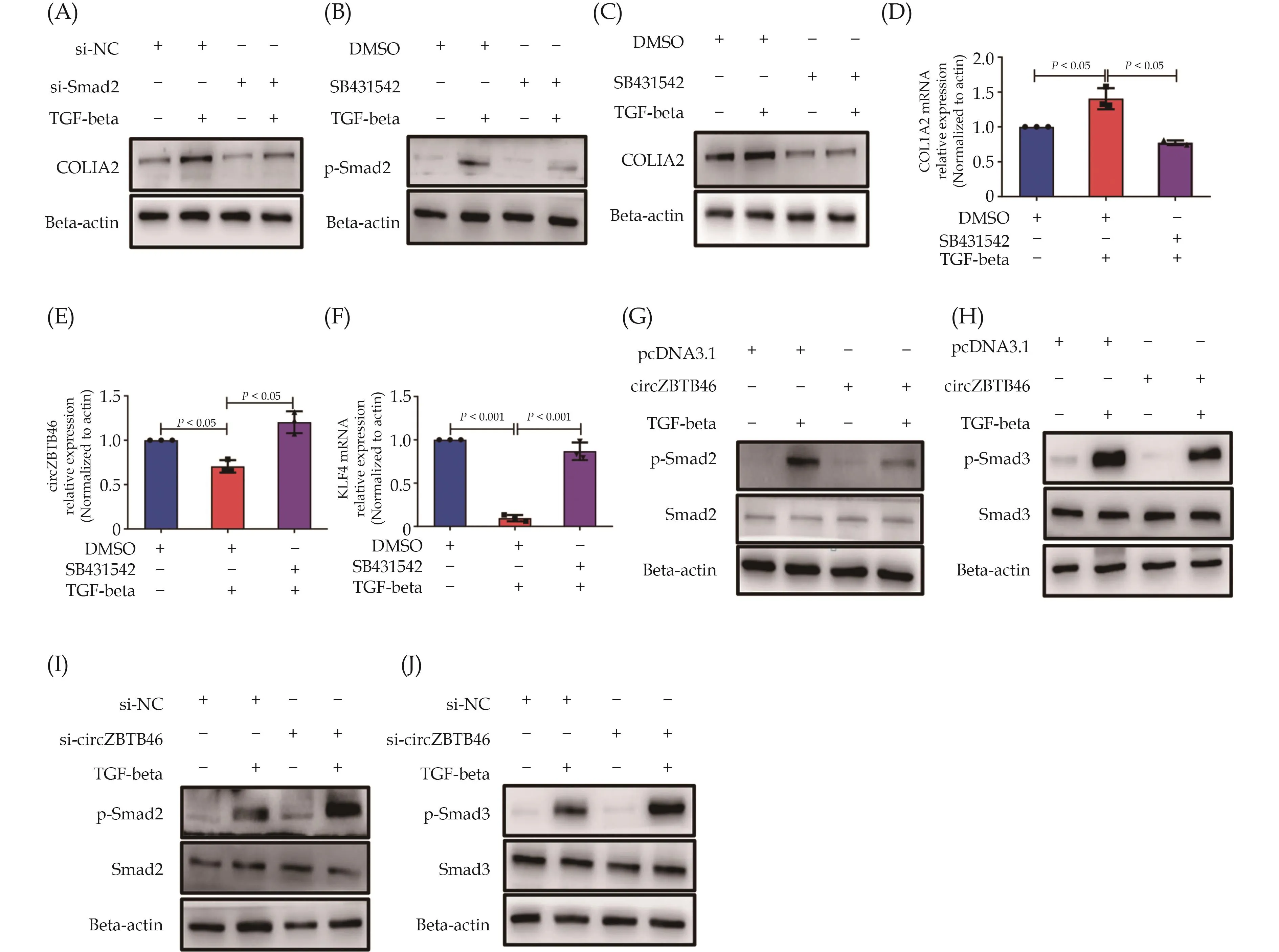
Figure 4 Down-regulation of COL1A2 expression by circZBTB46 through inhibiting the Smad signaling pathway.(A): VSMCs were transfected with si-Smad2 or si-NC for 24 h and then treated with TGF-beta (5 ng/mL) for 24 h.The expression of COL1A2 was detected by Western blot; (B): VSMCs were pretreated with SB431542 (10 μM) for 2 h, followed by a 15-minute incubation with TGF-beta (5 ng/mL), and the levels of phospho-Smad2 were analyzed by Western blot; (C & D): VSMCs were pretreated with SB431542 (10 μM) for 2 h, followed by a 24-hour incubation with TGF-beta (5 ng/mL), and the expression of COL1A2 was analyzed by Western blot (C) and qRT-PCR (D).The asterisk indicates a statistically significant difference from the corresponding controls; (E & F): VSMCs were pretreated with SB431542 (10 μM) for 2 h, followed by a 24-hour incubation with TGF-beta (5 ng/mL), and the relative expression of circZBTB46 (E) and KLF4 mRNA (F) was examined by qRT-PCR.The asterisk indicates a statistically significant difference from the corresponding controls; (G & H): VSMCs were transfected with pcDNA3.1 or circZBTB46-expressing vector for 24 h, followed by a 15-minute incubation with TGF-beta, and the levels of phosphorylated and total Smad2 (G) and Smad3 (H) were analyzed by Western blot; and (I & J): VSMCs were transfected with si-circZBTB46 or si-NC for 24 h, followed by a 15-minute incubation with TGF-beta, and the levels of phosphorylated and total Smad2 (I) and Smad3 (J) were analyzed by Western blot.VSMCs: vascular smooth muscle cells.
VSMCs were transfected with a circZBTB46-expressing plasmid for 24 h and subsequently treated with TGF-beta (5 ng/mL) for 15 min.The phosphorylation levels of Smad2/Smad3 were detected by Western blot analysis.Our results indicated that overexpression of circZBTB46 in VSMCs significantly inhibited TGF-beta-induced pho-sphorylation of Smad2 and Smad3, without affecting the total protein expression levels of Smad2/Smad3 (Figure 4G & 4H).Furthermore, VSMCs were transfected with sicircZBTB46 for 24 h, followed by treatment with TGF-beta for 15 min, and Western blot analysis revealed that knockdown of circZBTB46 expression in VSMCs further increased TGF-beta-induced phosphorylation of Smad2 and Smad3 (Figure 4I & 4J).Collectively, these results indicate that circZBTB46 inhibits the activation of the Smad signaling pathway induced by TGF-beta, thereby downregulating the expression of the COL1A2 gene.
The Role of CircZBTB46 in Mediating the Interaction Between PDLIM5 and Smad2 Signaling Pathway
It is widely recognized that circRNAs play an important role in interacting with specific proteins to regulate their functions.[38]To elucidate the molecular mechanism by which circZBTB46 inhibits the Smad signaling pathway, we aimed to identify the proteins that interact with circZBTB46.To achieve this, we designed and synthesized biotin-labeled probes specific to circZBTB46, which we used to pull down potential binding proteins.After enrichment of the biotin-labeled probe-circZBTB46-protein complex with magnetic beads, we performed qRTPCR analysis of the complex.We first confirmed that the levels of circZBTB46 in the complex were significantly higher in circZBTB46-overexpressing VSMCs than those in control cells (Figure 5A).We then identified the proteins in the complexes by mass spectrometry.As shown in Figure 5B, multiple proteins were pulled down by biotinylated circZBTB46 probes.Among these proteins,scaffolding protein PDLIM5, a member of the PDZ-LIM domain protein family, caught our attention, as it was recently identified as a regulator of the TGF-beta-Smad signaling pathway[39](Figure 5B).
To further confirm the interaction between PDLIM5 and circZBTB46, we performed RNA immunoprecipitation and RNA pull-down assays.Our results demonstrated that PDLIM5 was specifically pulled down by circ-ZBTB46, but not by another circRNA, circACTA2, which is also present in VSMCs (Figure 5C & 5D).We then investigated the interaction between PDLIM5 and Smad2 using reciprocal co-immunoprecipitation assays.Specifically, after VSMCs were stimulated with TGF-beta (5 ng/mL), cell lysates were precipitated with Smad2-specific antibodies, and the precipitated complexes were detected by Western blot with anti-PDLIM5 antibodies.
The results revealed that treatment with TGF-beta led to a time-dependent decrease in Smad2 levels present in anti-PDLIM5 immunoprecipitates (Figure 5E).Similarly,reciprocal immunoprecipitation experiments supported these findings (Figure 5F).These results suggest that under basal conditions, PDLIM5 forms a protein complex with Smad2, which is disrupted by TGF-beta treatment.To investigate whether circZBTB46 is involved in the interaction between PDLIM5 and Smad2, we silenced circZBTB46 expression in VSMCs with si-circZBTB46 and evaluated the binding between PDLIM5 and Smad2.The results demonstrated that the binding of PDLIM5 to Smad2 was significantly reduced when circZBTB46 was silenced (Figure 5G).Moreover, the interaction between PDLIM5 and Smad2 was further weakened by TGF-beta (5 ng/mL) stimulation in comparison to circZBTB46 knockdown alone (Figure 5H).These findings provide evidence that circZBTB46 mediates the interaction between PDLIM5 and Smad2.
Inhibition of TGF-beta-induced Smad Signaling and COL1A2 Expression by PDLIM5
To further investigate the impact of PDLIM5 on the TGF-beta-Smad signaling pathway, we constructed an adenoviral expression vector of PDLIM5 (Ad-PDLIM5)and confirmed successful overexpression of PDLIM5 in VSMCs by qRT-PCR and Western blot analysis (Figure 6A & 6B).Following Ad-PDLIM5 transduction, VSMCs were stimulated with TGF-beta (5 ng/mL) and subsequent Western blot analysis demonstrated that PDLIM5 overexpression significantly reduced Smad2 phosphorylation induced by TGF-beta (Figure 6C) and downregulated COL1A2 expression (Figure 6D).Additionally, we utilized small interfering RNA to knock down PDLIM5 expression in VSMCs (Figure 6E & 6F).Subsequent treatment of VSMCs with TGF-beta and Western blot analysis revealed that PDLIM5 knockdown further enhanced TGF-beta-induced Smad2 phosphorylation, which correlated with upregulation of COL1A2 expression (Figure 6G & 6H).These results suggest that PDLIM5 functions as an inhibitor of Smad signaling in VSMCs by impeding TGF-beta-induced activation of Smad2 and ultimately repressing COL1A2 expression.
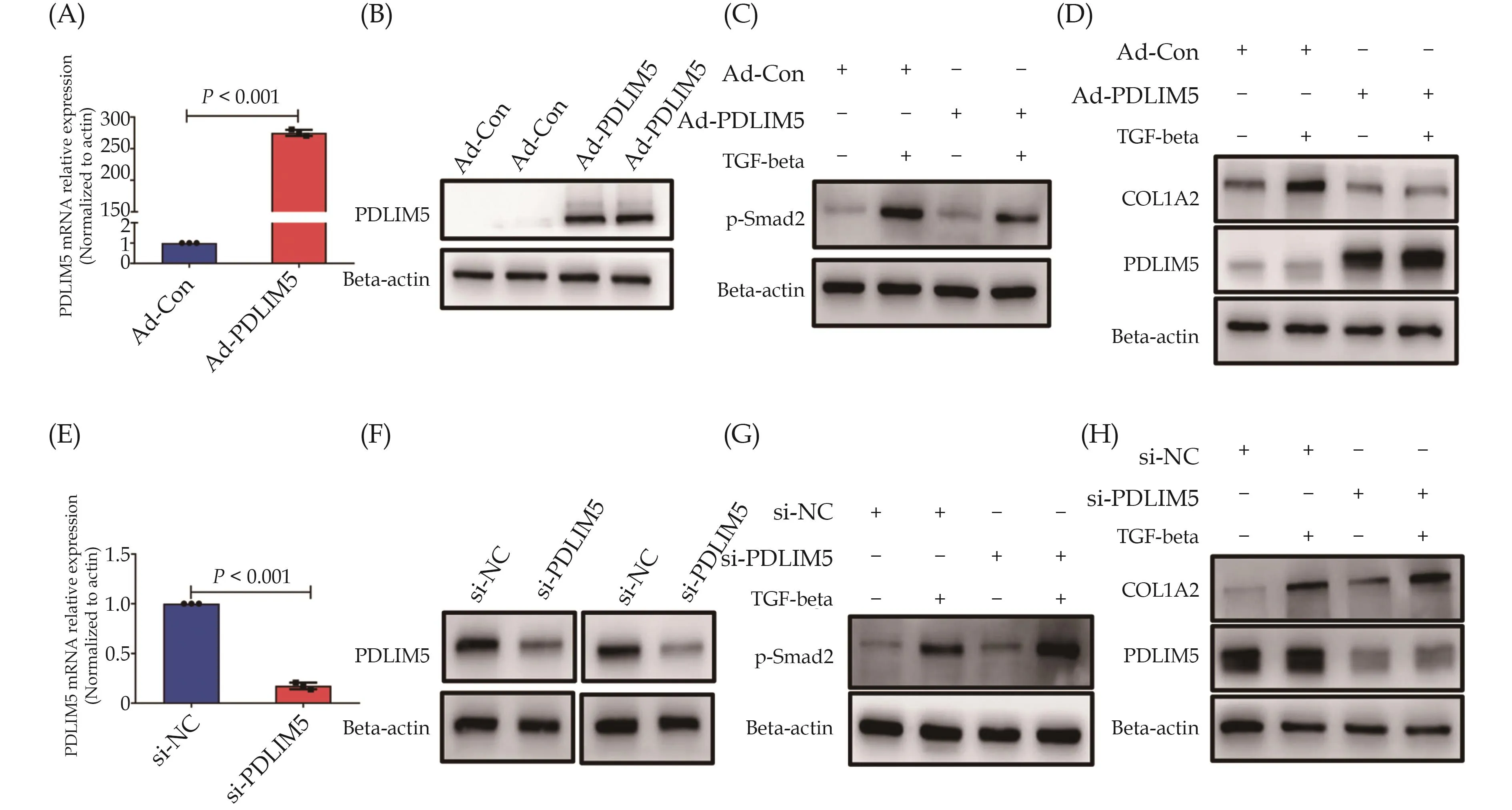
Figure 6 PDLIM5 suppresses Smad signaling and COL1A2 expression induced by TGF-beta.(A & B): PDLIM5 expression in VSMCs infected with Ad-PDLIM5 or Ad-Con was assessed by qRT-PCR (A) and Western blot (B); (C): VSMCs were infected with Ad-PDLIM5 or Ad-Con for 24 h and then treated with TGF-beta (5 ng/mL) for 30 min.The phosphorylated Smad2 was analyzed by Western blot;(D): VSMCs were infected with Ad-PDLIM5 or Ad-Con for 24 h and then treated with TGF-beta (5 ng/mL) for 24 h.The expression of COL1A2 and PDLIM5 was analyzed by Western blot; (E & F): PDLIM5 expression in VSMCs transfected with si-NC or si-PDLIM5 was assessed by qRT-PCR (E) and Western blot (F); (G): VSMCs were transfected with si-NC or si-PDLIM5 for 24 h and then treated with TGF-beta (5 ng/mL) for 30 min.The phosphorylated Smad2 was analyzed by Western blot; and (H): VSMCs were transfected with si-NC or si-PDLIM5 for 24 h and then treated with TGF-beta (5 ng/mL) for 24 h.The expression of COL1A2 and PDLIM5 was analyzed by Western blot.VSMCs: vascular smooth muscle cells.
Altered Expression of TGF-beta, COL1A2, and CircZBTB46 in Human AAA Tissues
To extend our findings fromin vitroVSMC experiments to human vascular disease, we evaluated the expression of TGF-beta, circZBTB46, and COL1A2 in hu-man AAA tissues and normal aortic tissues (Figure 7A).Immunofluorescence staining revealed that TGF-beta expression was markedly decreased in human AAA tissues compared to normal aortic tissues (Figure 7B).In contrast, circZBTB46 expression was significantly increased in human AAA tissues (Figure 7C), consistent with our observations in TGF-beta-treated VSMCs (Figure 2), where TGF-beta treatment significantly reduced circZBTB46 expression.Furthermore, COL1A2 expression was significantly reduced in the AAA tissues relative to normal aortic tissues (Figure 7D).These findings suggest that circZBTB46 mediates the interaction between PDLIM5 and Smad2, inhibiting Smad signaling and thus downregulating COL1A2 expression.
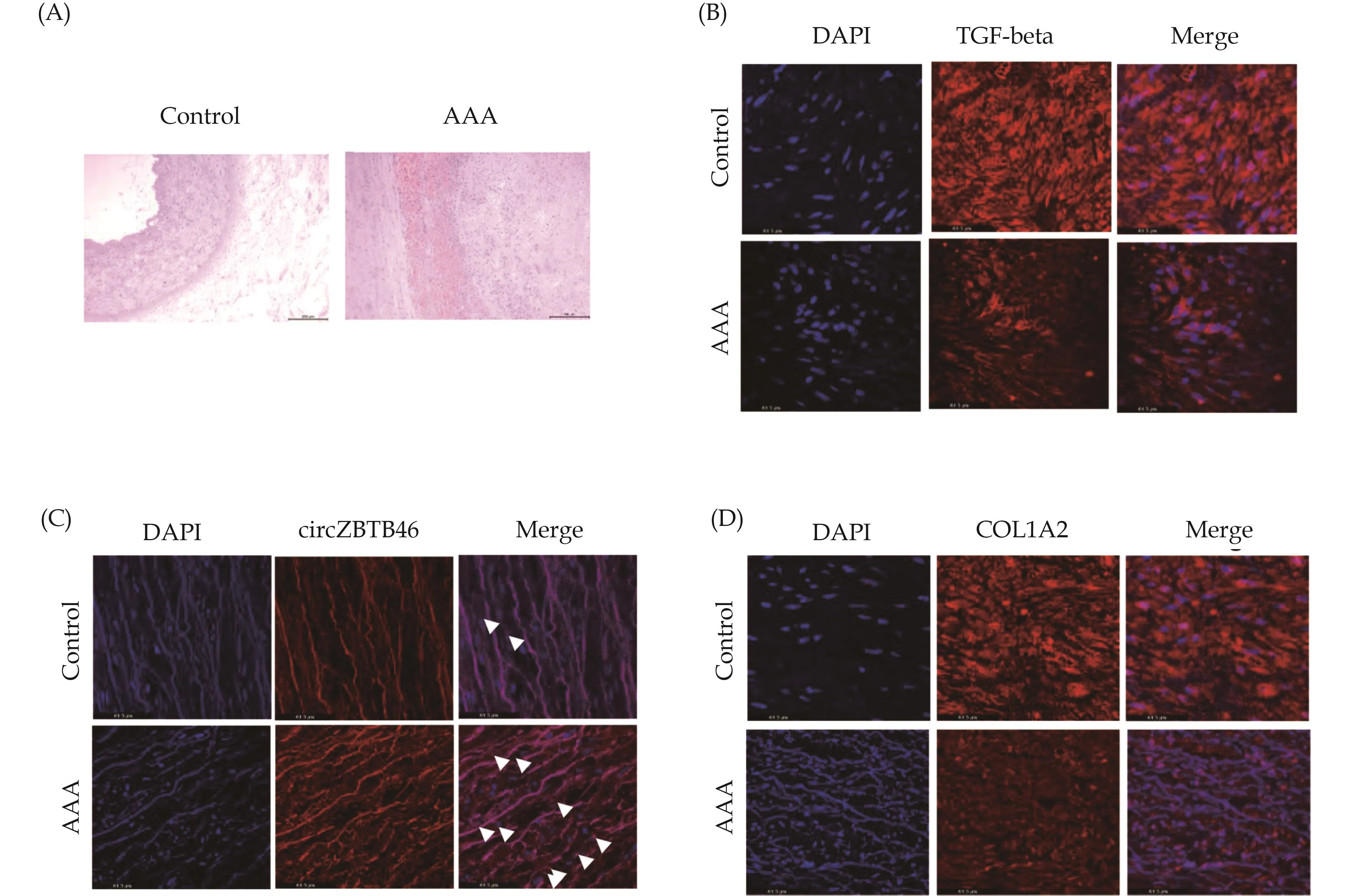
Figure 7 Expression of TGF-beta, circZBTB46, and COL1A2 in human AAA tissues.(A): Representative hematoxylin and eosin staining of human AAA tissues and their control adjacent aortic sections; (B): immunofluorescence staining of TGF-beta and DAPI in human AAA and non-aneurysm sections; (C): fluorescence in situ hybridization was used to detect the expression of circZBTB46 in human AAA and non-aneurysm sections; and (D): immunofluorescence staining of COL1A2 and DAPI in human AAA and non-aneurysm sections.AAA: abdominal aortic aneurysm.
DISCUSSION
In this study, we aimed to investigate the regulatoryrole of circZBTB46 in the expression of COL1A2 induced by TGF-beta.TGF-beta signaling pathway is involved in a variety of biological processes and has been suggested to exert protective effects in the development of aneurysms.TGF-beta signaling is initiated by binding of TGF-beta to its receptors (TbetaRI, TbetaRII and the coreceptor TbetaRIII).Subsequently, two distinct pathways are activated: the canonical Smad-dependent pathway and the noncanonical Smad-independent pathway.In the canonical pathway, the TbetaRI/TbetaRII complex phosphorylates downstream Smad2/Smad3, which then associates with Smad4 and translocates to the nucleus for further transcriptional regulation.[40]Several proteins involved in the TGF-beta signaling pathway, such as TbetaRII and Smad3, are downregulated in human AAA tissues.[41,42]Additionally, overexpression of TGF-beta signaling has been shown to arrest aortic expansion and promote reconstruction of the aneurysmal wall.[43]These findings are in agreement with our results showing a significant reduction in TGF-beta expression in human AAA tissues compared with normal aortic tissues.
In addition to VSMC phenotype switching, oxidative stress and VSMC apoptosis, and inflammatory and immune responses, vascular ECM remodeling is also a significant pathological mechanism contributing to the development of AAA.[44]The aortic wall’s ECM plays a crucial role in maintaining vascular homeostasis.[45]It provides not only a physical scaffold for cells but also regulates many cellular processes, including growth,migration, differentiation, survival, homeostasis, and morphogenesis.Changes in the ECM profile are associated with reduced strength and increased stiffness of the aortic wall, resulting in increased aortic size and disruption of physiological function.[46]Collagen, the most abundant fibrous protein in the interstitial ECM, plays a crucial role in determining the aortic wall’s mechanical properties.[47]In both AAA and thoracic aortic aneurysm, the collagen amount is reduced compared with normal aortas.[44]This reduction is likely due to modified collagen biosynthesis and degradation.[48]
Vascular ECM remodeling can be modulated by TGF-beta signaling.TGF-beta overexpression reduces levels ofmatrix metalloproteinases, which are proteases that degrade the ECM, while deficiencies in TbetaRII, Smad4,and Smad3 upregulate matrix metalloproteinase levels.[41,49,50]TGF-beta signaling also stimulates the synthesis of elastin in human VSMCs,[51]and collagen in fibroblasts;[52]which helps maintain the elasticity and stability of the vessel wall.Our study found that TGF-beta significantly upregulated the expression of COL1A2, an important member of the collagen family, in VSMCs.COL1 is the most abundant fibrous protein in the interstitial ECMs.[47]It is composed of COL1A1 and COL1A2, encoded by genes COL1A1 (chromosome 17q21) and COL-1A2 (7q21.3), respectively.COL1 is widely present in connective and embryonic tissues and is a crucial structural component of ECM and a key member of the collagen family.[53,54]COL1 is associated with the pathogenesis of various diseases, such as cancer[55,56]and cardiovascular diseases[57,58].As COL1 is a significant component of the ECM, the inhibition of COL1A2 transcription by the pro-inflammatory cytokine interferon in VSMCs is a crucial step in the atherogenesis process, which contributes to the instability of atherosclerotic plaques.[59]Moreover,COL1A1/COL1A2 is also closely linked to aneurysmal diseases.Targeted inhibition of COL1A1/COL1A2 expression affects VSMC vitality and ECM remodeling,which may contribute to the development and rupture of brain aneurysms.[60]Therefore, we hypothesize that COL1A2 could play a protective role in aneurysms or some vascular remodeling diseases.
To investigate the mechanisms underlying TGF-betainduced COL1A2 expression, we focused on KLF4, a zinc-finger-containing transcription factor expressed in various human tissues with diverse physiological functions depending on the cell type.KLF4 is one of the four crucial factors involved in the formation of induced pluripotent stem cells and it can regulate the proliferation,differentiation, and phenotypic transformation of VSMCs via various mechanisms.[61–63]Studies have shown that KLF4 is upregulated in human and mouse aneurysmatic tissues,[64,65]and downregulation of KLF4 attenu-ates aneurysm formation.[17,18]The interaction between KLF4 and TGF-beta is pivotal in a variety of diseases.Our previous research in rat VSMCs showed that KLF4 activates TGF-beta1 signaling through Smad2/Smad3 and p38 MAPK.[66]Activation of these two pathways promotes KLF4 phosphorylation, enabling the formation of the KLF4-Smad2 complex in response to TGF-beta1 stimulation, thereby involving KLF4 in the phenotypic modulation of TGF-beta-induced VSMCs.Interestingly, we observed that TGF-beta regulates KLF4 expression differently in different cell species.For instance, TGF-beta upregulates KLF4 expression in rat vascular smooth muscle, whereas TGF-beta downregulates KLF4 expression in human aortic vascular smooth muscle.
The critical roles of circRNAs in regulating signaling transduction and protein function have recently received extensive attention.In our previous study, we demonstrated that circACTA2 modulates the contraction and senescence of VSMCs.[29,30]To further demonstrate the important roles of circRNAs in cardiovascular diseases,such as aortic dissection and AAA, Zhou,et al.[67]conducted high-throughput sequencing to determine the expression patterns of circRNAs in AAA and non-aneurysm tissues.They identified a total of 411 differentially expressed circRNAs in AAA samples, including 145 upregulated circRNAs and 266 downregulated circRNAs.In addition, circRNA expression profiling was performed using Bap/Ang II-induced mouse AAA and control tissues.[22]Although a few circRNAs have been studied in the context of AAA pathology,[24–26,68–72]their expression levels and roles in AAA pathogenesis remain unclear.
Here, we screened circZBTB46 using chip analysis of KLF4-overexpressing VSMCs.Further experiments confirmed that circZBTB46 is expressed in both the nucleus and cytoplasm of VSMCs and is in circular form.We found that KLF4 overexpression significantly upregulated circZBTB46 expression and its knockdown inhibited the formation of circZBTB46.We also observed that TGF-beta inhibits the formation of circZBTB46 by downregulating KLF4 expression.Moreover, we found that circZBTB46 is involved in regulating COL1A2 gene expression by TGF-beta via the Smad signaling pathway.
CircRNAs have been shown to act as protein sponges or scaffolds that modulate protein activity.For example,the interaction between circACTA2 and ILF3 has been shown to participate in angiotensin II-induced VSMC senescence,[73]while circRasGEF1B functions as a scaffold to guide ZFP36 to preferentially bind to and decay Bcl-2 mRNA in a sequence-specific manner, thereby triggering apoptosis of VSMCs.[74]To further investigate the molecular mechanism by which circZBTB46 inhibits the Smad signaling pathway, we analyzed proteins pulled down by circZBTB46 using mass spectrometry and identified the scaffolding protein PDLIM5 as a circZBTB46-interacting protein.PDLIM5, also known as Enigma homologous protein, is a cytoplasmic protein that contains an N-terminal PDZ domain and three LIM domains in its C-terminal.PDLIM5 is highly expressed in skeletal muscle and myocardial tissues and can act as a signal regulator to affect organ development and disease occurrence.[75,76]Previous studies have shown that PDLIM5 can interact with ID2, protein kinase C, protein kinase D,alpha-actin, and other proteins, and play a role in heart disease and mental disorders.[77]PDLIM5 may be a novel regulator of the stability of basic Smad3 and has an impact on the control of TGF-beta signal transduction as its combination leads to the decrease of Smad3 protein level, and the overexpression of Smad3 reverses the TGF-beta signal inhibition and anti-migration effect induced by PDLIM5 knockdown.[39,78]Down-regulation of PDLIM5 in VSMCs can enhance hypoxia-mediated vascular remodeling, while overexpression of PDLIM5 inhibits TGF-beta/Smad signaling and prevents hypoxia-induced pulmonary hypertensionin vivo.[79]Our results suggest that overexpression or knockdown of PDLIM5 can reduce or enhance Smad2 phosphorylation.Moreover,we observed that the binding ability of PDLIM5 to Smad2 was gradually weakened over time after TGF-beta stimulation.This effect was enhanced when circZBTB46 was knocked down at the same time.Furthermore, we found that the upregulation of COL1A2 expression by TGF-beta was weakened or enhanced after overexpression or knockdown of PDLIM5.This suggests that PDLIM5 acts as an inhibitor of COL1A2 expression mediated by TGF-beta/Smad signaling in VSMCs.
CONCLUSIONS
In summary, we have identified a novel VSMC-enriched circRNA, circZBTB46, which modulates the TGF-beta/Smad signaling pathway by regulating the interaction between PDLIM5 and Smad2.This interaction leads to the inhibition of TGF-beta/Smad signaling, ultimately resulting in decreased COL1A2 expression in VSMCs.Our study provides new insights into the regulation of COL1A2 expression by TGF-beta.Manipulating the expression of circZBTB46 may represent a potential new target for TGF-beta-induced ECM remodeling.
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation of China (No.31671182 & No.31871152& No.81770285 & No.81971328 & No.82271624), the Natural Science Foundation of Hebei Province of China (No.H2022206074 & No.H2021206459), and the Medical Science Research Project in Hebei Province Foundation of Health Commission of Hebei Province of China (No.202 30065).All authors had no conflicts of interest to disclose.
 Journal of Geriatric Cardiology2023年6期
Journal of Geriatric Cardiology2023年6期
- Journal of Geriatric Cardiology的其它文章
- Status of cardiovascular disease in China
- Unraveling the mechanisms of a giant coronary sinus
- Iatrogenic pneumopericardium after therapeutic pericardiocentesis for pericardial effusion: a case report
- Target versus sub-target dose of renin–angiotensin system inhibitors on survival in elderly patients with heart failure with reduced ejection fraction: a systematic review and meta-analysis
- Outcomes of catheter-directed thrombolysis versus systemic thrombolysis in the treatment of pulmonary embolism: a metaanalysis
- Nocturnal hypertension and riser pattern are associated with heart failure rehospitalization in patients with heart failure with preserved ejection fraction
