Using polyvinylidene fluoride to improve ignition and combustion of micron-sized boron powder by fluorination reaction
Lingqi ZHU, Baozhong ZHU, Xiaolong ZHAO, Yanan WANG, Mengchen LI,Jiuyu CHEN, Yunlan Sun
School of Petroleum and Natural Gas Engineering, Changzhou University, Changzhou 213164, China
KEYWORDS
Abstract Boron has a promising application in the field of propellants due to its high calorific value.However, the difficulty of ignition and the poor combustion efficiency of boron (B) have severely limited its efficient application.In response to this issue, this paper proposes to improve the ignition and combustion performance of micron-sized boron by the Polyvinylidene Fluoride(PVDF)coating.The effect of PVDF content on the B combustion performance was systematically studied using a Thermogravimetry-Differential Scanning Calorimetry (TG-DSC), a Transmission Electron Microscope (TEM), an X-Ray Diffractometer (XRD), a laser Particle Size Analyzer(PSA), and a high-speed camera.The results show that PVDF can significantly reduce the initial oxidation temperature of B powder and increase its reaction heat.When the PVDF content is 23wt%, the reaction heat and the combustion intensity of B powder reach the maximum and are significantly higher than those of the uncoated B powder.Moreover, the fluorination reaction that occurs during the combustion process not only can effectively shorten the combustion time of B powder, but also has a positive effect on its flame intensity and propagation speed, and it significantly reduces B particle agglomeration, which improves the combustion efficiency significantly.This study lays the foundation for the application of PVDF modified B in B-based solid propellants.
1.Introduction
Metal additives can significantly improve propellant burning performance and therefore have been widely used in this field.1–5Boron (B) has a high calorific value (58.9 kJ/g and 137.8 kJ/cm3), which has attracted the interests of researchers for its application as the propellant metal additive.6,7However,due to its high melting point (2177 °C) and boiling point(3658 °C), as well as the low melting point (557 °C) and high boiling point (2043 °C) of the oxidized shell layer B2O3, B is hampered from touching with the oxidizer and is also more likely to agglomerate during combustion.As a result, B is difficult to ignite, combustion efficiency is low, and the actual calorific value of combustion is significantly lower than the theoretical calorific value.Therefore, the current focus of the study is on how to quickly remove the oxide film from the surface of B and accelerate the ignition and burning of B.8–11
To improve the ignition and combustion performance of B powder,previous studies have found that the presence of fluorine atoms not only acts as an oxidizer,12,13but also reacts with the oxide layer on the surface of B to produce BF3gas, which improves the combustion efficiency of B.During the combustion process, the fluorine atoms inhibit the aggregation of the condensed phase B2O3on the B surface, which greatly enhances the combustion efficiency of B.8,14Based on the properties of fluorine,researchers have conducted more studies on the subject.For metal fluorides, Xu et al.15uniformly coated LiF on the surface of B particles using an in-situ synthesized method.The results show that LiF reduces the ignition temperature of B powder and improves its combustion efficiency.Valluri et al.16studied the composite powders containing varying amounts of B, BiF3, and Bi, and found that the inclusion of BiF3or Bi can decrease the oxidation temperature of B.The fluorination of BiF3results in the production of volatile BF3and BOF on the surface of B particles, which accelerates the ignition of the composites.However,metal fluoride is a non-combustible component, and excessive addition can reduce the energy density of the composites.For fluorinated compounds with specific structures, Wang et al.17used Graphene Fluoride(GF)as a modifier to regulate the combustion performance of B particles.They found that a tiny amount of GF may significantly improve the fuel’s pressure output and combustion performance.However, the GF decomposition procedure consumes heat, which lengthens the composite fuel’s ignition delay.Jiang et al.18used Graphene Oxide(GO)and Graphite Fluoride(GtF)to co-modify B particles.The results show that the ignition delay time of the B/GO/GtF sample is shorter than that of the B/GO and B/GtF samples due to the synergistic effect of GO and GtF.However,the inhibition of agglomeration is not obvious due to lower fluorine content.The fluoropolymers, such as fluoroelastomer(Viton A), Polyvinylidene Fluoride (PVDF), Polytetrafluoroethylene(PTFE),and THV(polymer of Tetrafluoroethylene,Hexafluoropropylene and Vinylidene fluoride), are frequently used for B-modification.19,20HF produced by fluoropolymer decomposition can react with B2O3, which can remove or weaken the oxide film on the surface of B and promote the reaction between the oxidizer and the internal B, deepen the oxidation process, and greatly improve the ignition and combustion performance of B powder.However, the combustion mechanism of B modified by fluoropolymer is unclear and needs to be studied further.
PVDF is a widely used commercial fluoropolymer with high mechanical qualities and chemically stable resistance to acid and alkali corrosion,21as well as the ability to dissolve in some polar solvents.Therefore, it has a promising future as a coating material.Though many studies have been conducted on the modification of B powder by fluoropolymer,22there have been few reports on the effect of PVDF on B combustion performance.In this study,PVDF was used to modify micron-size boron (μB) powder, and the effect of PVDF content on the combustion performance of B was studied.The adsorption performance of PVDF on the B surface was analyzed by a Density Functional Theory (DFT) calculation to provide theoretical support for its coating on the B particle surface, and the coating effectiveness of PVDF on the B surface was also observed by a Transmission Electron Microscopy (TEM).In addition, the thermal behavior and combustion performance of the samples were analyzed by using a Thermogravimetry-Differential Scanning Calorimetry(TG-DSC)and a laser ignition experimental platform.The combustion products of the samples were examined by an X-Ray Diffractometer (XRD)and a laser particle size analyzer to clarify the combustion product components and product agglomeration.
2.Experimental sections
2.1.Materials
The B powder, with an average particle size of 1 μm(purity > 99.9%), was purchased from Shanghai Xiangtian Nano Materials Co., Ltd.PVDF powder with an average molecular weight of 400000 was purchased from Shanghai Maclean Biochemical Co.,Ltd.Shanghai Aladdin Biochemical Technology Co., Ltd.offered N, N-Dimethylformamide(DMF) solution (purity > 99.8%).
2.2.Sample preparation
The dissolution–recrystallization method was used to prepare the core–shell structure PVDF@B particles with low technical requirements,simple operation,and high efficiency.The specific raw material ratios are shown in Table 1.Take the PVDF9@B sample as an example to illustrate the preparation process.First, 45 mg of PVDF was dissolved in 10 mL DMF solution.Second, 455 mg B powder was added, and the mixture was placed in a 53 kHz ultrasonic field for 30 min.And then the mixture was mixed by a magnetic stirring for 12 h at ambient temperature in order to achieve uniform dispersion of B powder.Finally, the mixed samples were placed in a vacuum drying oven to dry for 24 h at 70 °C to obtain the goal samples.
2.3.Experimental methods
The spin-polarized DFT computations were carried out by Vienna Ab-initio Simulation Package (VASP) version 6.1 to study the adsorption properties of PVDF on the boron powder surface.The morphologies of the modified samples were char-acterized by a TEM (JEOL JEM-2100, Japan) to observe the coating of PVDF on the boron surface.The thermal properties of the samples were analyzed using a thermogravimetricdifferential scanning calorimetry (TG-DSC, LabsysEvo,France).The heating temperature ranged from 50 °C to 1200°C in an air atmosphere with a heating rate of 10°C/min.
The laser ignition and combustion experimental system is shown in Fig.1.The system mainly consists of a laser controller, a transparent-window combustion chamber, a spectrum acquisition apparatus, and a high-speed camera.The samples were ignited in static air with a variable power CO2laser (YHDZ-MS 600 W, China) with an ignition time of 0.5 s and an ignition power of 25% of the rated power.The fiber optic spectrometer was triggered by a laser igniter,which produced a 5 V pulse to the fiber optic spectrometer at the time of ignition, ensuring simultaneous spectral acquisition and ignition.Spectral intensity curves were recorded using the fiber optic spectrometer to reflect the changes in the intensity of the combustion flame.The ignition and combustion processes of the samples were recorded using a high-speed camera (Phantom M340, USA).The burning time of each stage, the flame propagation speed, and the characteristic flame of boron were all calculated using the flame images recorded by the highspeed camera.The 2 mg sample was placed in quartz hole with an inner diameter of 3 mm and a height of 2 mm to keep the stacking shape and density of the sample constant during each test.After the sample was burned, the combustion products were collected at the bottom of the combustion chamber.The composition of the condensed combustion products was analyzed by an XRD (D/MAX2500, Japan).The particle size distribution of the condensed combustion products was analyzed using a laser Particle Size Analyzer (PSA, China) to understand the B agglomeration.
To study the influence of PVDF on boron combustion efficiency, we collected the condensed combustion products after laser ignition.A certain mass of condensed combustion products were placed in hot water of 70°C for 30 min.B2O3in the combustion products can dissolve in hot water to form HBO2and H3BO3,23,24while the rest of the residues does not dissolve in hot water.After filtering and drying,the rest of the products was weighed.The mass of B2O3can be obtained by the mass difference.Due to the neglect of the mass loss, the consumed mass of boron was obtained during the burning process.The experiment was repeated more than three times using 100 mg samples per group and the results were averaged to finally obtain the combustion efficiency of the samples.

Fig.1 Schematic of equipment for ignition and burning.
3.Results and discussion
3.1.Structure and characterization of PVDF@B composites
The binding strength is one of the basic indicators used to describe molecular species’surface adsorption and can be estimated in terms of adsorption energy(Eads).25In this paper,we used monomer C2H2F2instead of PVDF for the study,and the adsorption energy obtained is shown in Fig.2(a).C2H2F2has the largest adsorption energy(Eads= -2.9 eV)at the hollow site on the B (0 0 1) surface.The binding energy between the adsorbate and the adsorbent is generally 0.5 eV per molecule or atom as the boundary value between physical and chemical adsorption.26The adsorption energy of C2H2F2on the surface of B (0 0 1) is - 2.9 eV, showing that the adsorption is chemisorption.To further elucidate the binding strength of C2H2F2molecules on the B(0 0 1)surface,the Projected Density of States (PDOS) of the main interacting atoms on the chemisorption site was investigated.The results are shown in Fig.2(b), where E - Efdenotes the energy level value based on the Fermi energy level, E being the energy level and Efbeing the Fermi energy level.The B-p and C-p orbitals produce hybrids peaks, indicating that the orbitals resonate between C and B atoms to form the stable chemical bonds.27PVDF has strong adsorption properties on the B (0 0 1) surface, which lays the foundation for PVDF to easily coat the B particles.
Fig.3 shows the TEM images of different samples.It can be observed from Fig.3(a) that the surface of particle B is irregular.To judge the uniformity of the coating of the samples,the observation from different angles of the same sample was studied by the TEM.Figs.3(b)-(d) shows the TEM images of the PVDF@B samples with different PVDF contents (9wt%,23wt%, and 30wt%, respectively) at multi-angles.The PVDF film attaches well to the surface of the B particles,and the coating layer’s thickness varies with the amount of PVDF.Based on the TEM results in Fig.3, the impact of PVDF content on the average coating layer thickness on the surface of B particles was studied.The results are shown in Fig.4.As can be seen,the thickness of the PVDF coating layer on the B surface increases with increasing PVDF content,which is 9.35±1.33,17.19±0.8,and 20.36±0.51 nm,respectively.The thickness of the coating layer on the B surface varies widely when the PVDF content is low due to amorphous B powder with irregular shape and rough surface, therefore a few amounts of PVDF cannot be uniformly coated on the B surface.
3.2.Thermal behavior
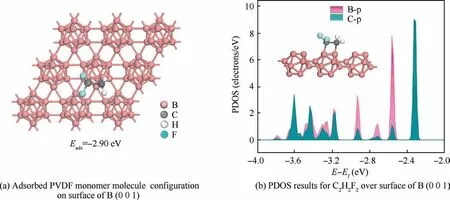
Fig.2 Adsorption properties of PVDF on B (0 0 1) surface.
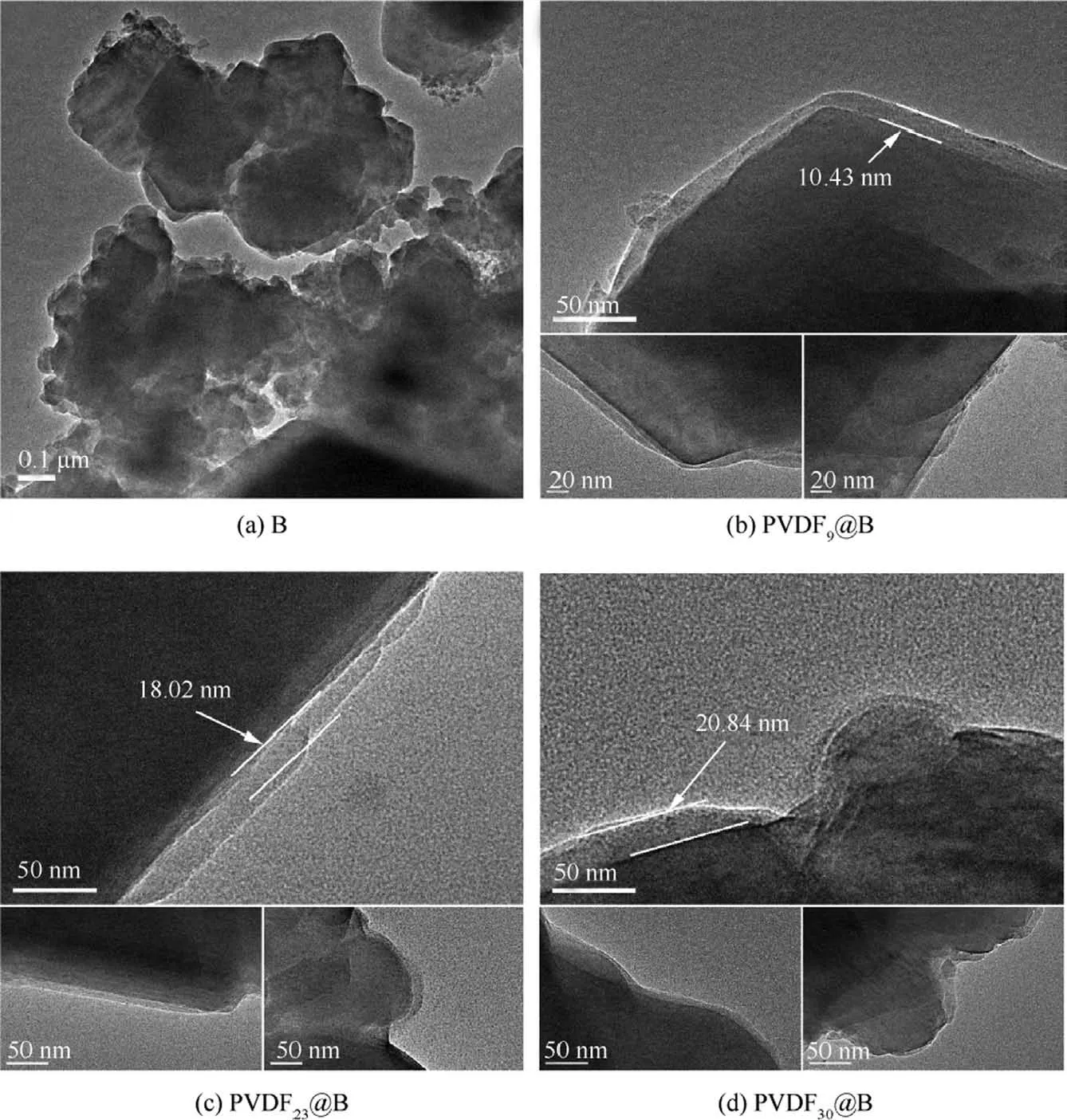
Fig.3 TEM images of different samples.
Fig.5(a)shows the TG curves corresponding to different samples, and the obtained TG-DTG relevant data are listed in Table 2.For the uncoated B samples, there is an oxidative weight gain process (725–863 °C).When the temperature exceeds 863 °C, most of B have been oxidized to B2O3, so the TG curve tends to be stable.Under high-temperature conditions,the B2O3produced by the reaction will liquefy to cover the unreacted B particles’surfaces,preventing them from coming into contact with oxygen and slowing the reaction.The final B weight is increased by 100.5wt%, which is significantly less than the theoretical weight gain of 222.0wt% when B is completely oxidized to B2O3.As a result,the oxide film’s presence on the particle’s surface hinders the oxidation process.There are two stages of weight loss when PVDF is heated in air, which are the decomposition of PVDF (425–495 °C) and the oxidation of carbon (495–600 °C) processes.28When the temperature reaches 600 °C, the weight loss reaches 100%,showing that all of the PVDF have been pyrolyzed into gaseous molecules.For four groups of PVDF@B samples with different ratios,the TG curves show the same trend,exhibiting a distinct stage of weight loss and weight gain, corresponding to the above-mentioned pyrolysis of PVDF and oxidation process of B, respectively.The decomposition starting temperature of the PVDF@B samples is about 100 °C lower than that of the pure PVDF sample, and the actual weight loss is larger than the added PVDF content (theoretical weight loss).It is shown that B powder can catalyze the decomposition reaction of PVDF at low temperatures,which changes the thermal decomposition process of PVDF in the samples.In particular,HF produced by the decomposition of PVDF reacts with the oxide layer on the surface of B powder to produce BF3gas,increasing the sample’s weight loss.As shown in Table 2, the initial weight gain temperature of B decreases from 725 °C to 650.7 °C as the PVDF content increases.Eliminating the effect of PVDF in the PVDF@B sample, the B oxidation weight gain is increased from 100.5wt% to 114wt%.The fluorination reaction of PVDF with B2O3damages the oxidation layer on the surface of B powder in the PVDF@B composites and promotes the reaction between B powder and oxidant.It not only reduces the initial oxidation temperature of B powder, but also improves the oxidation efficiency.
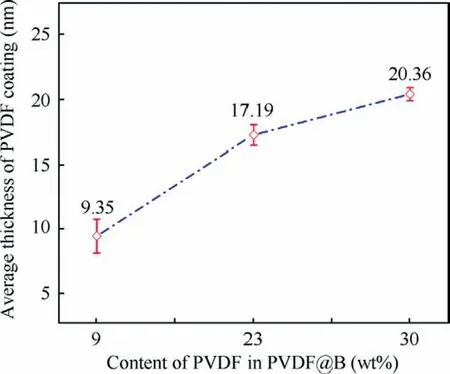
Fig.4 Effect of PVDF content on average coating layer thickness of B surface.
Fig.5(b)shows the DTG curves of various samples,reflecting the speed of weight gain or loss with temperature.As shown in Table 2, when the PVDF content increases from 9wt% to 30wt%, the temperatures corresponding to the maximum decomposition rates are 368.4, 373.6, 374.3, and 382.7°C,which are much lower than that of the PVDF sample(472.1 °C).The temperature corresponding to the maximum decomposition rate gradually drops when the PVDF content drops (i.e., the B content rises).It is shown that B has a catalytic effect on the decomposition of PVDF and can promote its decomposition at lower temperatures.Meanwhile, the corresponding maximum decomposition rates are 1.40%,2.38%, 3.41% and 4.76% min-1, which are lower than that of the PVDF sample (23.83% min-1).This is primarily due to the thermal conductivity of B (27.4 W/(m?K)) being much larger than that of PVDF(0.13 W/(m?K)),so most of the heat is absorbed by the B particles during the heating process.At the same time, the presence of B causes PVDF decomposition at lower temperatures.This results in a decrease in the decomposition rate.The maximum weight gain rates of the PVDF@B samples are lower than that of the B, and the temperature corresponding to the maximum weight gain rate decreases in all samples.For B with a PVDF content of 23wt%, a small DTG peak is presented at 721.3 °C.This may be due to the PVDF destroying the oxidation film of B surface, which promotes the direct contact between some B and O, thus accelerating the reaction of B and O.With the reaction,the newly generated oxide film covered on the B surface decreases the rate of weight gain.Therefore, there is a small peak on the DTG curve.The maximum weight gain rate is connected to the surface oxide film’s action.29The addition of PVDF weakens the B surface oxide film to form BF3with low boiling point, resulting in a lower maximum weight gain rate.On the other hand, as the PVDF content rises, the decrease in B mass density may likewise lower the maximum oxidation rate.Therefore,the reasonable adjustment of PVDF content can regulate the oxidation rate of boron.
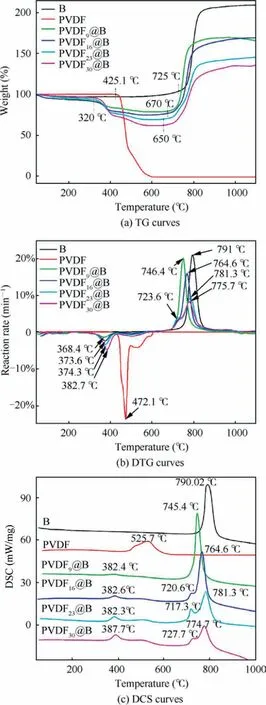
Fig.5 Thermogravimetry (TG), Derivative Thermogravimetric(DTG), and Differential Scanning Calorimetry (DSC) curves of each sample.

Table 2 TG-DTG data corresponding to each sample.
The DSC curves of various samples are shown in Fig.5(c),and the relevant parameters are listed in Table 3.There are several obvious exothermic peaks in the PVDF@B samples,which correspond to the decomposition of PVDF and the oxidation reaction process of B, respectively.As the PVDF content increases, the pre-reaction between PVDF and B2O3is enhanced, which destroys the oxide film on the B surface and not only enhances the exotherm but also makes the oxidation earlier.The modified sample’s initial exotherm increases from 449.4 J/g to 2651.04 J/g,corresponding to a peak temperature of about 382 °C, which is significantly lower than the PVDF’s peak temperature of 525.7 °C.It can be seen from Table 3 that Δh1o f the modified samples is much larger than the exothermicof the equivalent amount of PVDF when it is thermally decomposed alone.This is primarily due to the large amount of heat released by the Pre-Ignition Reaction(PIR) of PVDF with the B surface oxide film to produce BOF.30The destroyed oxide layer of the B surface is more obvious as the PVDF content rises.The oxidation reaction exotherm occurs in advance where the B oxide layer is destroyed,so the exotherm peak of the sample oxidation stage has a peak shoulder to appear.For the PVDF9@B sample,the temperature corresponding to the exothermic peak is 745.4°C,which is 44.6 °C lower than that of uncoated B powder.The peak temperatures of the remaining three modified samples are reduced by about 70 °C.The destroy of the oxide layer by PVDF changes the oxidation process of B particles,so that more B particles are oxidized, producing a gradually larger exothermic zone.With the decrease of boron content, the exotherm of the oxidation stage decreases from 9713.23 J/g to 7045.4 J/g.In descending order, the total heat release Δh is PVDF23@B, PVDF16@B, PVDF9@B, B, and PVDF30@B.The total heat release is the smallest for the PVDF30@B sample, which is mainly due to the low B content.The sample exotherm is the largest for the PVDF23@B sample, so proper regulation of the PVDF content can significantly enhance the reaction heat of the PVDF@B samples.
Fig.6 shows the mechanism of oxidation reaction of B and PVDF@B samples with increasing temperature.The B powder is affected by the surface oxide film during the thermal reaction, and oxygen is adsorbed on the surface of B clusters at lower temperatures.With the increase of temperature, the oxide film changes from solid to liquid, and the oxygen atoms start to diffuse to the B nucleus,while the internal B atoms also diffuse outward,which is in a two-way diffusion state.31When reaching a certain temperature,the thickness or viscosity of the liquid oxide film on the surface of B is small enough that a large amount of oxygen will diffuse through B2O3(l) to reach the B/B2O3interface and react with B and give off a large amount of heat.This further promotes the evaporation and viscosity reduction of the oxide film, so that the reaction of B and O is enhanced.The oxide layer of boron changes from liquid to gaseous state,and the sample gains weight at a larger rate.29HF produced by PVDF decomposition can react with B2O3,which destroys the B surface oxide layer and leaves part of B exposed to air, and the exposed B reacts directly with a large amount of oxygen,15so there is a small sudden increase in weight gain rate.With the reaction,a new oxide film continues to form on the B surface, so the weight gain rate shows a decrease.Therefore, the DTG and DSC curves of the oxidation stage appear with shoulder peaks when the PVDF contentin the sample is high.As a result, PVDF can significantly improve B oxidation performance by changing B oxidation pathway.32

Table 3 DSC parameters of different samples.
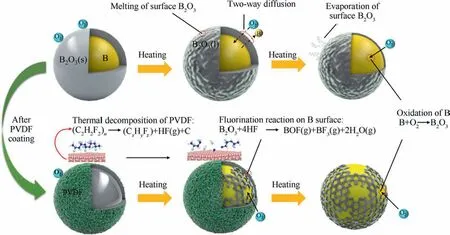
Fig.6 Mechanism of oxidation reaction of B and PVDF@B samples with increasing temperature.
3.3.Combustion performance of PVDF@B particles
3.3.1.Combustion process
To clarify the effect of PVDF coating content on the burning of B powder, the burning process of each sample was studied.The results are displayed in Fig.7.The onset of combustion is defined as the point at which a faint flame begins to appear in the samples.The combustion process of the samples is divided into three main stages according to the change of flame propagation state during combustion, namely, diffusion combustion (unstable combustion), stable combustion, and flame extinction.When there is an obvious flame in the samples, it means entering the diffusion combustion stage, where the flame front surface propagates faster, the flame area begins to increase, and the brightness begins to enhance.When the flame area remains basically unchanged and has a strong flame brightness,the combustion is stable and has entered the stable combustion stage.In the flame extinguishing stage, the flame gradually becomes smaller and the brightness begins to dim until it is extinguished.As the combustion progresses during the diffusion combustion stage, the uncoated B sample produces more green flames.This is mostly because gaseous intermediate products BO2are produced during the combustion process.33Similarly, at this stage, for the PVDF16@B sample,only a very weak green flame can be seen, while for the PVDF23@B and PVDF30@B samples, no green flame presents.This may be due to the reaction of PVDF with B atom,which converts the intermediate product BO2into OBF compounds, preventing the production of BO2.19However, with the consumption of PVDF, the green flame reappears in all the samples during the steady combustion phase and disappears in the late combustion phase (after 300 ms).The flame turns yellow, and the bottom flame is brighter, indicating that the bottom has a higher temperature.7At this time, the disappearance of the green flame may be due to the accumulation of B2O3on the surface of B,which hinders the reaction of B with oxygen and weakens the combustion.33Fig.8 shows the time of the green flame appearance and its duration during the combustion process, which are defined as t′and t′′, respectively.With the increase of PVDF content, the fluorination reaction in the combustion process converts more intermediate products BO2into BOF, which makes the time of the green flame produce gradually delay and its duration is shortened.This intuitively reflects the inhibitory effect of PVDF on the generation of B2O3in the combustion process.
In terms of sample particle combustion,B particles primarily show stacking combustion on the combustion surface,while the addition of PVDF, the samples gradually show jetting combustion, and the burning surfaces have the spattering of particles.With the increase of PVDF content, the flame jet and the spattering of particles are gradually enhanced, which is due to the BF3gas produced by the B fluorination during the combustion process.The internal B and surface oxide layer react with PVDF to produce BF3gas, which breaks up the B agglomerates into smaller pieces.The combustion process produces a lot of gas,which drives the particles to form jet flames and triggers small particle spattering.More particles distributed in the flame can considerably improve the burning particle dispersion,34boost the sample combustion and reduce the agglomeration of B.Meanwhile, with the increase of PVDF content, the flame and particle brightness increases and then decreases, while the flame and particle brightness of the PVDF30@B sample is lower than those of the other modified samples.This is mainly due to the lower B content.However, there are more small particles in the air, and larger specific surface area leads to more heat loss to the surrounding air.35In addition, a large number of splattered small particles maintain a certain brightness, indicating that the B particles succeed in self-sustaining combustion after they are ignited.Obviously, the gaseous products produced during combustion and the availability of more oxygen in the air for B are an important prerequisite for their self-sustaining combustion.36

Fig.7 Combustion flame images of different samples.
3.3.2.Combustion time and flame propagation
The combustion time of each sample at various stages are compared in Fig.9(a).The time of the diffuse combustion stage is recorded as t1, the time of the stable combustion stage is recorded as t2, and the duration of combustion from ignition to flame extinction is recorded as t3.For the uncoated B sample, the time of t1, t2,and t3are 76.8, 449.2, and 644.2 ms,respectively.With the increase of PVDF content, t1gradually increases and t2and t3gradually decrease,which indicates that the time of diffusion combustion increases.This is due to the fluorination reaction in the diffusion combustion stage, which pushes more B off the stacking surface and lengthens the diffusion combustion of B.At the same time, the pre-ignition reaction of PVDF with B accelerates the whole combustion process, thus shortening the overall combustion time.Thus it can be seen that PVDF makes the more complete combustion and is favorable to improve the energy release.
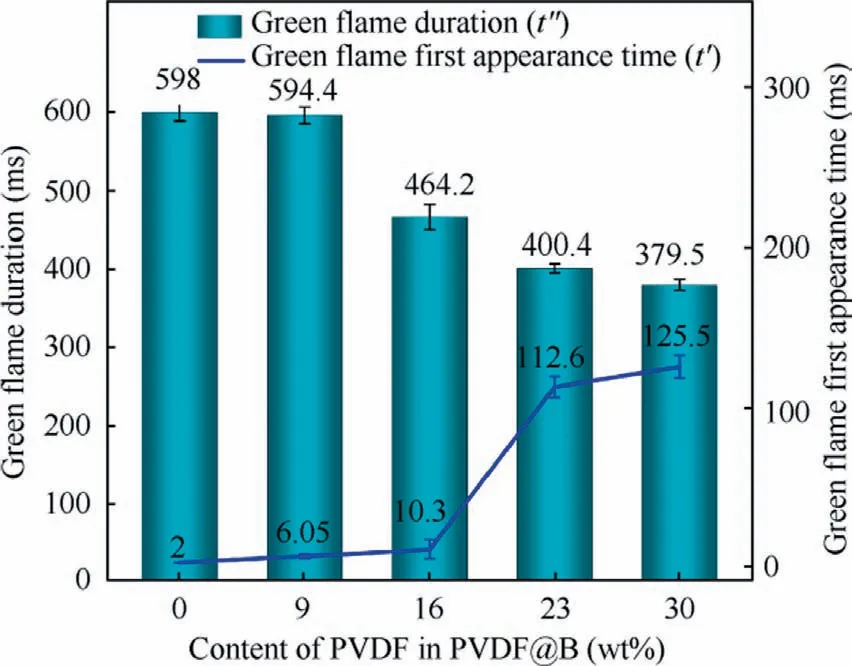
Fig.8 Appearance time and duration of green flame.
Fig.9(b) shows the time corresponding to the maximum flame area as well as the flame area that is derived by binarizing the combustion flame using a customized MATLAB function.The flame areas are compared using pixels as units.The maximum flame area of the uncoated B and the time of the maximum flame appearance are 34246 pixel2and 22.1 ms,respectively.With increasing PVDF content, the time of the maximum flame appearance is gradually delayed and the corresponding flame area first increases and then declines.The flame area of the PVDF23@B sample reaches the maximum that is 128020 pixel2, which is 2.7 times larger than that of the uncoated B sample.This is due to the increased PVDF content, which further destroys the oxide film on the B surface,promotes the contact between the oxidant and the B nucleus,and enhances the combustion efficiency of B.Additionally, a significant amount of HF,BF3,BOF,and other gases are produced as a result of the decomposition of PVDF and the prereaction with B powder.These gases create upward airflow and push the B particle off the stacking surface,which increases the unreacted B to contact with oxygen,and promotes the B combustion even more.With the further increase of PVDF content, on the one hand, the B content is reduced due to the high PDVF content, weakening the energy release.On the other hand, more small particles burn in air and release heat faster.The flame top gradually darkens because the larger heat loss of B particles at the top makes its temperature insufficient to ensure the self-sustained combustion of B.Thus, the flame area of the PVDF30@B sample decreases.
During the combustion of B, the gas phase combustion products generated form the combustion flame.37The upward speed of the hot and cold borders of the flame is the flame propagation speed,38,39which is related to the amount of gas produced and the gas reaction rate.40Fig.9(c)shows the average flame propagation velocity for each sample at various time.All samples’ average flame propagation velocities vary over time with the same regularity.During the initial combustion phase,the sample is ignited by a high-intensity laser with a fast flame propagation speed.As the combustion progresses,the fuel is gradually consumed,and the average flame propagation speed decreases.By comparing the average flame propagation velocity of the various samples, it can be discovered that the flame propagation velocity regularly changes as the PVDF content increases.The uncoated B has the lowest flame propagation velocity.The average flame propagation velocity increases with increasing PVDF content.The PVDF pyrolysis process primarily involves the removal of Hydrogen Fluoride(HF), followed by hydrogen transfer, and HF is the major product.19More HF and gas small molecules are produced during the combustion process as the PVDF content increases,and the resulting airflow velocity gradually rises.The particle acceleration is proportional to the airflow velocity.35B particles are accelerated in the direction of flame propagation,and the combustion of B during the acceleration process considerably increases to the flame propagation velocity.Furthermore, during burning, the oxide film on the B surface is decimated, which facilitates the reaction of the oxide gas with B.Therefore, PVDF promotes flame propagation speed through a combination of physical and chemical effects on the combustion of B particles.
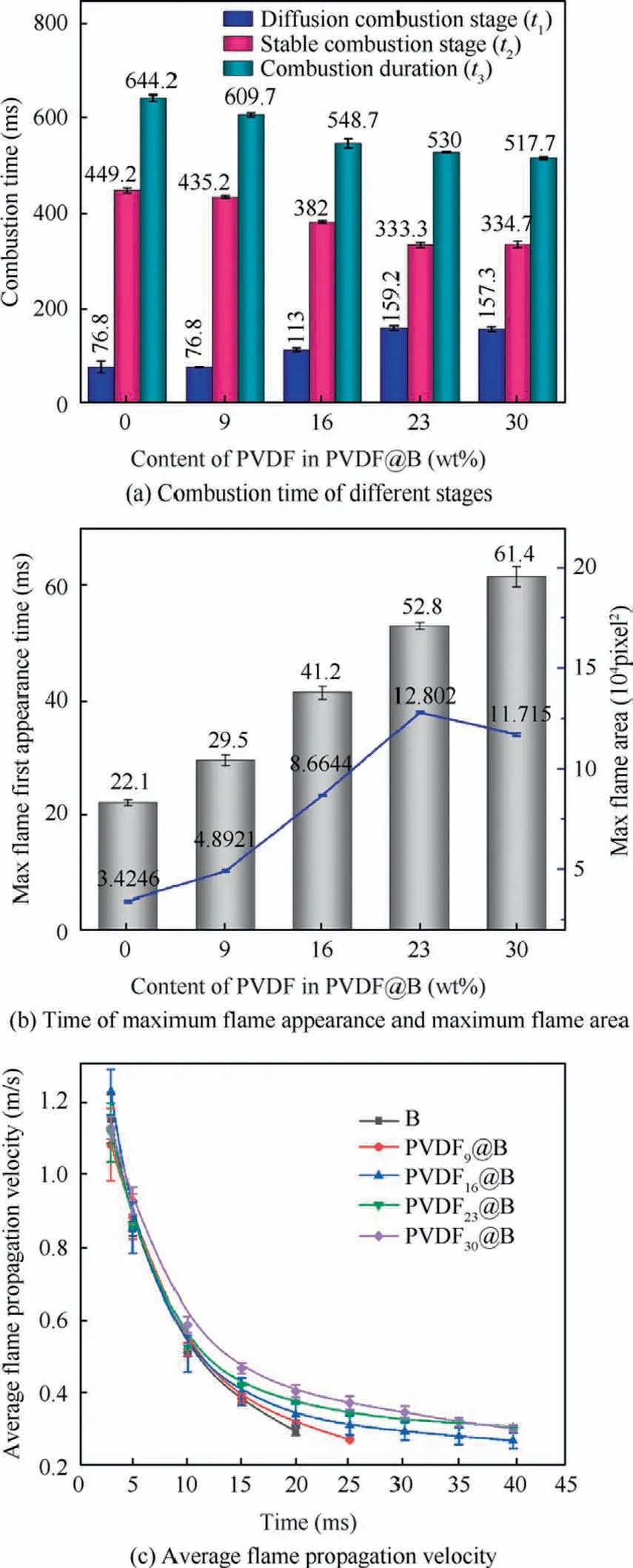
Fig.9 Combustion performance of different samples.
3.3.3.Combustion intensity
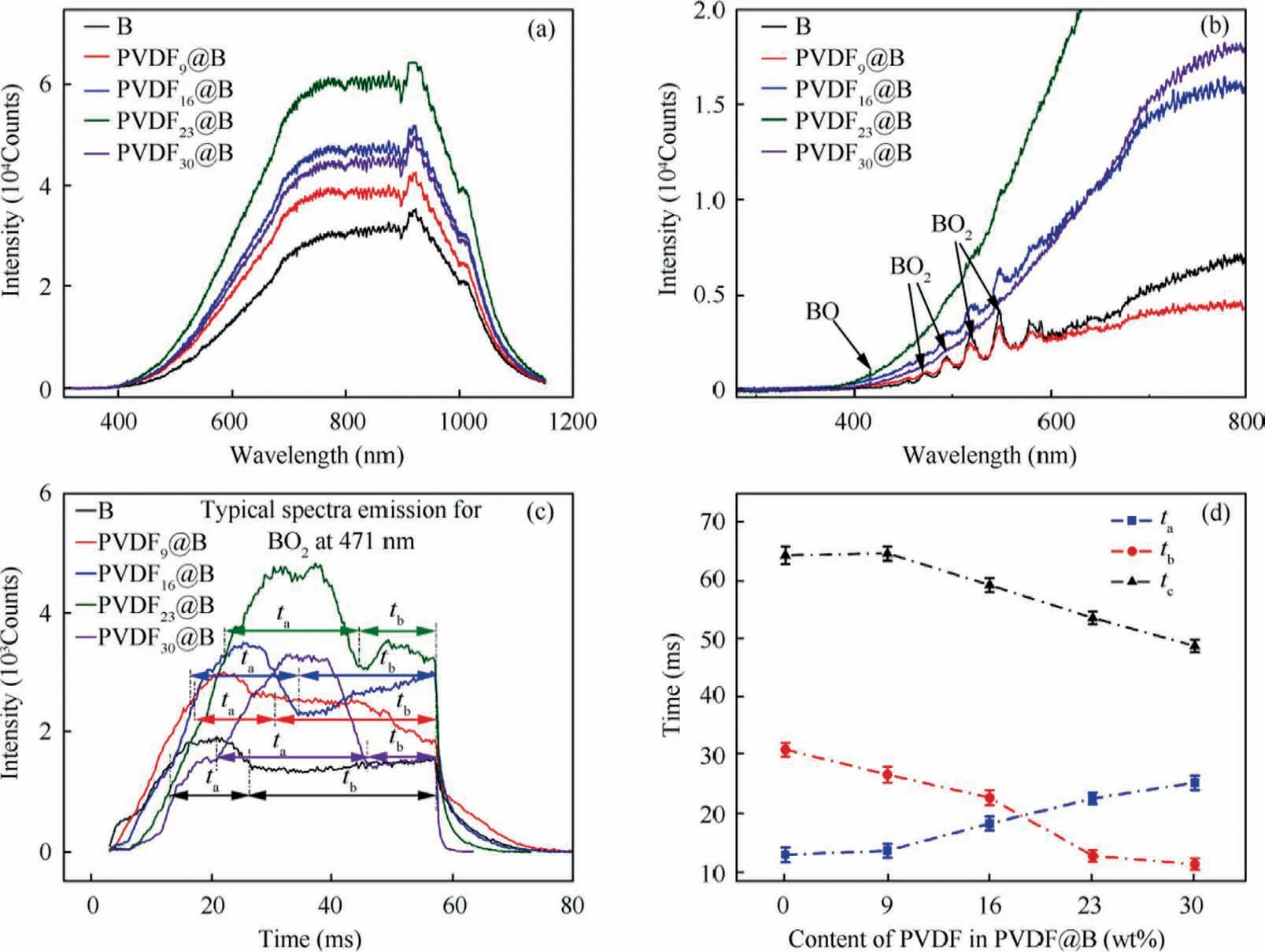
Fig.10 Combustion spectra of various samples: (a) Maximum spectra intensity, (b) Spectra at initial combustion stage, (c) BO2 characteristic spectra, (d) Characteristic combustion time.
The emission spectra of the combustion process were collected and analyzed in order to study the combustion intensity and oxidation process of different samples, and the results are shown in Fig.10.Fig.10(a) shows the maximum emission spectra during the burning of various samples.Each curve represents the average result of ten replicate tests.The intensity of combustion for each sample is reflected by the spectral intensity.The PVDF-coated samples’ burning intensity is substantially higher than that of the uncoated B sample.The combustion intensity varies with the PVDF content in the order of PVDF23@B > PVDF16@B > PVDF30@B> PVDF9@B > B.Firstly, the oxide layer B2O3reacts with the HF gas produced by PVDF decomposition to generate OBF and BF3gases.14,30Thermal analysis results show that PVDF destroys the B2O3shell layer at low temperatures and promotes two-way diffusion of oxidized gas and B nucleus,which significantly improves the oxidative combustion of B.In fluorinated environments, the energy of B burning to produce OBF(g) is 55.7 kJ/g, which is twice the energy produced by the oxidation of B to produce BO2in a non-fluorinated environment.19Furthermore, the addition of PVDF shortens the sample’s burning time,allowing the sample to release more energy in a shorter amount of time,improving the combustion intensity even further.The combustion intensity is decreased in the PVDF30@B sample due to the lower B content and the heat loss between the burning particles and air, which is consistent with the results of thermal analysis.Therefore, adding the proper amount of PVDF can effectively increase the samples’ combustion intensity.
Fig.10(b)shows the emission spectra of the initial combustion stage,where the characteristic emission spectra of the gas phase BO and BO2can be observed.This is because oxygen is sufficient at the initial stage of combustion, the intermediate products of combustion are dominated by BO2,41,42and BO2is also an important gas phase intermediate of B in the whole combustion process.35Therefore,the change of its characteristic peak in the most intense band(471 nm)with time was used to study the combustion process of B particles in each sample.Fig.10(c) shows the characteristic spectra of BO2for all samples.It can be observed that the reaction of B is divided into two stages,which are defined as the first and second oxidation stages.The two stages’ durations are denoted by taand tb,respectively.In addition, the total duration of the characteristic spectrum is defined as the total characteristic burning time(tc) of B.Fluorination reaction destroys B-surface oxide film and promotes the combustion of B powder in the initial combustion stage, so that the first oxidation stage presents a high spectral intensity.tacan be considered as the duration of high combustion intensity during the combustion process.35The secondary oxidation stage is relatively smooth with lower spectral intensity, and the PVDF basically reacts completely and liquid B2O3begins to accumulate on the B surface,which hinders the combustion reaction to some extent.Spectrum intensity of the modified samples are much higher than that of the uncoated B sample in the secondary oxidation stage, showing that the addition of the proper quantity of PVDF can significantly increase the combustion intensity of B particles.The oxidation-stage duration and characteristic combustion duration for each sample are shown in Fig.10(d).As the PVDF content increases, the destruction of the oxides on the B surface is enhanced, accelerating the combustion of the samples and shortening the total characteristic combustion time.During combustion,PVDF is easily flammable and rapidly decomposed, so the fluorination reaction mainly acts in the first oxidation stage.The samples with higher PVDF content make more B powder react in this stage, and the remaining less B powder enters the second oxidation stage, so that tagradually increases and tbgradually decreases.The fluorination of PVDF with B powder affects the combustion reaction on the surface of B powder.By shortening the characteristic burning time and enhancing the maximum burning intensity duration,the burning velocity and the burning intensity of B are thereby increased.
3.4.Combustion products
3.4.1.Components of combustion products
The crystalline phase composition of the condensed combustion products was analyzed by the XRD, and the results are shown in Fig.11.For the uncoated B sample, only B and B2O3are found in the final product, and the amount of B is high, indicating incomplete burning and a poor combustion efficiency of the sample.In addition to B and B2O3, each PVDF@B sample’s condensed combustion products also contain the diffraction peaks of B4C and B13C2.The weight loss of the PVDF samples in the second stage is caused by the oxidation of C, according to the findings of thermal analysis.It is speculated that the first stage of PVDF decomposition produces C.This is consistent with the findings of DeLisio et al.,28who studied the thermal analysis of PVDF under air and argon.As a result, the detected boron–carbon compounds probably originate from the reaction of B with C produced by the decomposition of PVDF.Combined with the results of thermal analysis,it is speculated that the following reactions mainly occur during the actual combustion process.

Fig.11 XRD curves of various samples.
Fig.11 shows that as the PVDF content is increased, and both the number and the intensity of the diffraction peaks of B decrease noticeably.However,the intensity of the diffraction peak of B2O3is significantly enhanced,and that of boron–carbon compounds is slightly decreased.As a result, the content of B2O3in the PVDF@B condensed combustion products increases dramatically,while the content of B decreases significantly.When the PVDF content is 23wt%-30wt%, the B2O3content is higher in the condensed products and the B content is lower, and the combustion efficiency is the best at this time.According to the findings of the combustion efficiency test shown in Fig.12, the combustion efficiency for the uncoated B sample is lower, roughly 43.05%.With the addition of PVDF, the sample combustion efficiency is significantly improved.The combustion efficiency is the greatest when the PVDF content is 23wt%-30wt%, and all of these values surpass 70%, which is consistent with the pattern of XRD data.The fluorination reaction shown in the Reaction (4) demonstrates the damaging effect of the decomposition products of PVDF on the oxide film as mentioned in the thermal analysis.It further proves that the fluorination reaction plays an important role in the improvement of the combustion efficiency B powder.Therefore,the combustion efficiency of boron powder can be greatly improved by regulating the PVDF content.
3.4.2.Size distribution of combustion products
Fig.13(a) shows the result of testing the particle size distribution of the condensed combustion products of various samples by using the laser particle size analyzer.Di(i=25,50,75,97)denotes the particle size, and the ratio of the number of particles with size smaller than Dito the total number of particles is i%.43The average particle size(D50)of the condensed combustion products of the uncoated B is 8.768 μm, which is significantly greater than the original diameter of B, showing that the B particles heavily agglomerate during the burning process.The condensed combustion products of 1 μm B have a classical trimodal distribution,44–46whose three peaks locate at approximately 2.959, 8.531, and 19.89 μm, respectively.Compared with the uncoated B sample, the particle size of condensed combustion products is significantly reduced after PVDF coating, which is completely different from the conventional trimodal distribution of the uncoated B sample.The agglomeration peak in the conventional trimodal distribution disappears completely and the particle size changes from trimodal distribution to bimodal distribution.It is shown that PVDF can greatly inhibit the agglomeration of B powder.With the increase of PVDF content, as can be seen from Fig.13(b), the average diameters of condensed combustion products gradually decrease, which are 8.77 ± 0.16,7.61 ± 0.27, 7.13 ± 0.12, 6.50 ± 0.12, and 6.31 ± 0.44 μm,respectively.On the one hand, the fluorination reaction destroys the oxide layer on the B surface, while inhibiting the accumulation of condensed phase B2O3on the B surface.On the other hand, the decomposition of PVDF during burning as well as the pre-reaction with B powder produces an enormous amount of gas.They both help to reduce the agglomeration behavior of B during combustion.47As a result, the combustion agglomeration of B powder can be effectively improved by controlling the content of PVDF.
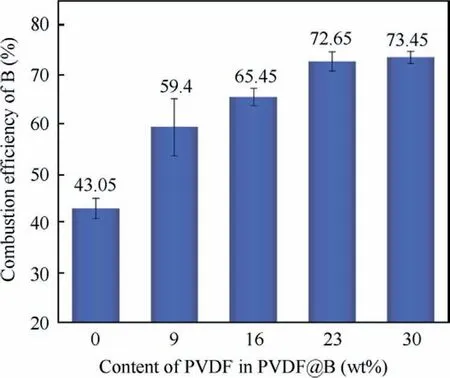
Fig.12 Combustion efficiency of different samples.

Fig.13 Particle size distribution (a) and average particle size (b) of each sample’s combustion products.
4.Conclusions
In this study, the effect of PVDF on the combustion performance of μB powder was investigated using laser ignition experiments.The morphology,thermal behavior,flame behavior, combustion spectra, and product characteristics of each sample were investigated.The specific conclusions are derived as follows.
(1) PVDF has strong adsorption properties on the B surface, which provides a theoretical basis for the PVDFcoated B powder.The B surface coating thickness is correlated positively with PVDF content.B can catalyze PVDF decomposition at low temperature, lowering the decomposition temperature of PVDF by about 100 °C.The HF gas produced by PVDF decomposition can eliminate the oxide film on the surface of B, thus promoting the oxidation of B powder.As the PVDF content changes from 9wt% to 30wt%, the initial oxidation temperature of B decreases from 725 °C to 650.7 °C.PVDF can regulate the oxidation weight gain rate and extend weight gain temperature region of B powder, so that the oxidation exotherm is more stable and the reaction heat of B is significantly increased.
(2) Both the flame intensity and flame propagation speed of B can be considerably increased by PVDF.With the increase of PVDF content, the flame area and the average flame propagation speed increase.The pre-ignition reaction between PVDF and B2O3shortens the characteristic combustion time of B by altering the combustion path on the surface of B powder.The maximum flame area and the combustion intensity are the highest when the PVDF content is 23wt%,which are 1.4 and 2.7 times higher than those of uncoated B, respectively.
(3) PVDF can inhibit the accumulation of the condensed phase B2O3on the B surface in the combustion process.The combustion efficiency of B powder is improved.The fluorination reaction during combustion inhibits the agglomeration of B.For the PVDF30@B sample, the highest combustion efficiency (73.45%) and the least degree of agglomeration (D50= 6.305 μm)during combustion are obtained.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We greatly appreciate the financial support provided by the National Natural Science Foundation of China (No.52376093) and the Project of Jiangsu Graduate Practice Innovation, China (Nos.SJCX22_1435 and SJCX22_1436).This work was sponsored by Qing Lan Project of Jiangsu Province,China.
 CHINESE JOURNAL OF AERONAUTICS2023年10期
CHINESE JOURNAL OF AERONAUTICS2023年10期
- CHINESE JOURNAL OF AERONAUTICS的其它文章
- Experimental investigation of typical surface treatment effect on velocity fluctuations in turbulent flow around an airfoil
- Oscillation quenching and physical explanation on freeplay-based aeroelastic airfoil in transonic viscous flow
- Difference analysis in terahertz wave propagation in thermochemical nonequilibrium plasma sheath under different hypersonic vehicle shapes
- Flight control of a flying wing aircraft based on circulation control using synthetic jet actuators
- A parametric design method of nanosatellite close-range formation for on-orbit target inspection
- Bandgap formation and low-frequency structural vibration suppression for stiffened plate-type metastructure with general boundary conditions
