A nomogram based on lymphocyte percentage for predicting hospital mortality in exertional heatstroke patients: a 13-year retrospective study
Jiale Yang, Fanghe Gong, Xuezhi Shi, Fanfan Wang, Jing Qian, Lulu Wan, Yi Chen, Huaisheng Chen,Huasheng Tong
1Guangzhou University of Chinese Medicine, Guangzhou 510006, China
2Department of Intensive Care Unit, General Hospital of Southern Theatre Command of PLA, Guangzhou 510010, China
3Department of Neurosurgery, General Hospital of Southern Theatre Command of PLA, Guangzhou 510010, China
4Department of Intensive Care Unit, Dongguan Binhaiwan Central Hospital, Dongguan 523900, China
5Department of Critical Care Medicine, Shenzhen People’s Hospital, Shenzhen 518020, China
KEYWORDS: Exertional heatstroke; Lymphopenia; Nomogram; Prognosis
INTRODUCTION
Heatstroke (HS), a life-threatening disease, is often characterized by hyperthermia, central nervous system abnormalities, and multiorgan dysfunction.[1]HS can arise from exposure to high ambient temperature and humidity or extensive physical activities.Elderly persons and children are susceptible to classic heatstroke (CHS) epidemically,while young adults, especially athletes, soldiers, and outdoor workers, usually suffer from exertional heatstroke(EHS) sporadically.[2]According to a multicenter study, the hospital mortality of HS reached 62.2% during the 2003 heat wave in France.[3]The mortality of HS is predicted to increase by up to 2.5 and 5 times in 2050 and 2080,respectively, owing to the deterioration of global warming and extreme weather.[4]Early identification and effective treatment are crucial to enhancing the survival of EHS patients.However, no ideal prognostic factors have been determined to predict the hospital mortality of EHS.
Previous research on EHS has focused on the inflammatory response[2]and anti-inflammatory therapies,such as IL-1 receptor antagonists,[5]corticosteroids,[6]and recombinant activated protein C,[7]with little attention given to immunosuppression in EHS.[8]However, given the “sepsis-like” pathophysiological process of EHS,[2]including endotoxemia,[9]endothelial dysfunction,[10]uncontrolled inflammation,[11]immune system impairment,[12]coagulopathy,[13]and progressive organ failure,[14]immunosuppression may play a significant role in disease progression as well.Therefore, factors that can reflect the immune state of EHS may hold promise for predicting hospital mortality.
This study aimed to identify risk factors for inhospital mortality in EHS patients by analyzing immune cell parameters and other circulatory biomarkers.A prognostic prediction model was constructed,and the relationship between severe EHS and immunosuppression was preliminarily explored.
METHODS
Study population
Two hundred and eight EHS patients admitted to the ICU of the General Hospital of Southern Theatre Command,a tertiary hospital, from January 1, 2008, to December 31,2020, were retrospectively enrolled.The diagnostic criteria for EHS were as follows: (1) a history of extensive physical exercise and (2) core temperature>40 ℃, and concurrent central nervous system abnormalities.[1]The ICU staff provided all patients with comprehensive monitoring, care,and treatment.
Inclusion and exclusion criteria
The inclusion criterion was that patients met the diagnostic criteria for EHS.The exclusion criteria were as follows: (1) patients who were not enrolled within 48 h from the onset of EHS; (2) age less than 18 years; (3) length of ICU stay less than 24 h; (4) missing data; and (5) presence of carcinoma, severe organ dysfunction, hematological system disease, or immunocompromised state before the onset of EHS.The clinical data of 208 patients who met the diagnostic criteria for EHS were recorded, and 156 patients were ultimately included in the study, including 141 survivors and 15 non-survivors (Figure 1).
Clinical and laboratory data collection
Data from the electronic medical record system were collected, including (1) demographics: age, gender, and the month of onset; (2) vital signs: core temperature,mean artery pressure (MAP), and heart rate (HR); (3)immune cell parameters on the first (D1) and third day(D3) of admission: count of white blood cell (WBC),count and percentage (%) of neutrophils, monocytes,and lymphocytes; (4) other laboratory parameters upon admission: hemoglobin (Hb), hematocrit (HCT),platelet count (Plt), activated partial thromboplastin time (APTT), prothrombin time (PT), international normalized ratio (INR), D-dimer, fibrinogen (Fib), serum creatinine (Scr), creatine kinase (CK), and aspartate aminotransferase (AST); (5) disease severity scores:Acute Physiology and Chronic Health Evaluation(APACHE) II, Sequential Organ Failure Assessment(SOFA), Systemic Inflammatory Response Syndrome(SIRS), and Glasgow Coma Scale (GCS) scores;and (6) others: use of vasoactive drugs, underlying diseases, rhabdomyolysis, disseminated intravascular coagulation (DIC), and length of ICU and hospital stays.Rhabdomyolysis was diagnosed as muscle pain and swelling, reddish-brown urine, and CK>1,000 U/L or increased more than five times the normal level.[15]DIC was diagnosed based on the International Society on Thrombosis and Hemostasis (ISTH) score ≥5 points.[16]
Statistical analysis
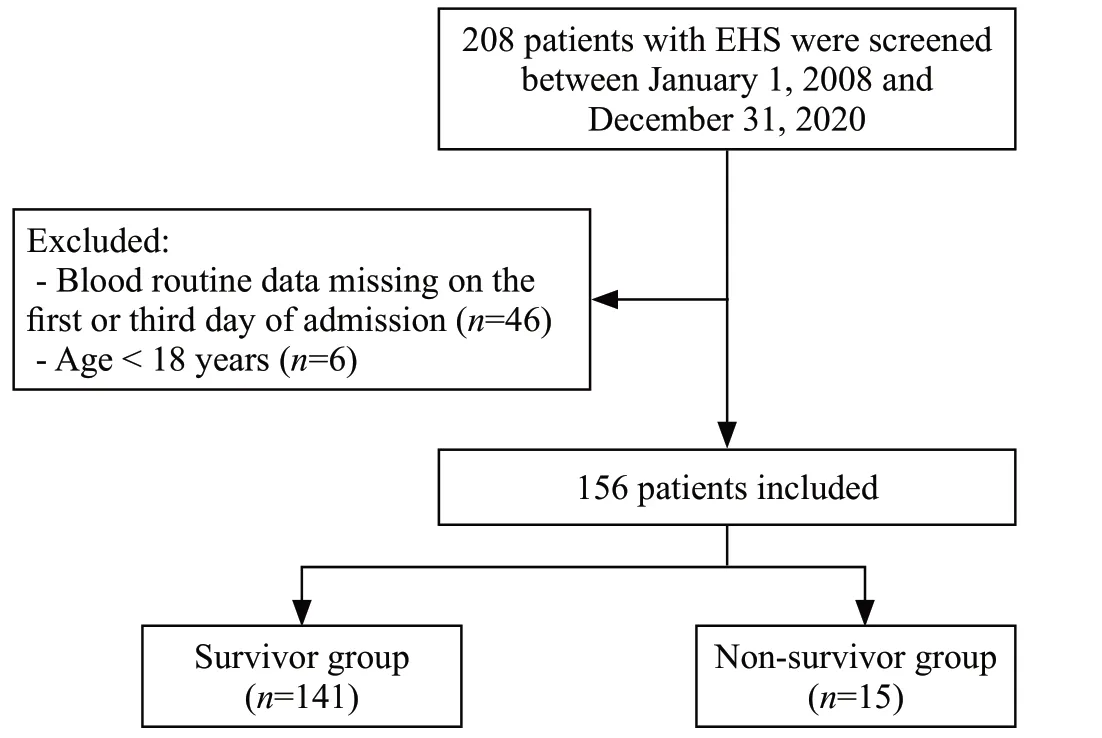
Figure 1.Flowchart illustrating the inclusion of EHS patients.EHS:exertional heatstroke.
Continuous variables were expressed as either the mean ± standard deviation (normal distribution) or median and interquartile range (skewed distribution)and compared using Student’st-test or Mann-WhitneyUtest, respectively.Categorical variables were summarized as counts and percentages in each category and compared using the Chi-square or Fisher’s exact test, as appropriate.When analyzing the change in a variable collected at different time points, the Wilcoxon signed ranks test (skewed distribution) or paired samplest-test (normal distribution) was used.Patients were further divided into mild and severe groups based on the cut-off values of the receiver operating characteristic(ROC) curve of APACHE II and SOFA scores, and the differences were analyzed by corresponding intergroup comparisons.Factors correlated with hospital mortality(P<0.1) were identified by univariate logistic regression and then included in multivariate logistic regression(forward selection) to construct prediction models.Since immune cell parameters at different time points were strongly correlated, they were included separately in the multivariate regression analyses, thereby generating several models.The best prediction model was selected after comprehensively comparing the sensitivity,specificity, AUC, and Akaike information criterion (AIC).The Hosmer-Lemeshow test was used to evaluate the goodness-of-fit underlying the prediction model.The model was internally validated by a calibration curve using the bootstrap method with 1000 resamplings.AUCs were compared using the DeLong method with MedCalc(version 16.8.4).Decision curve analysis (DCA) and clinical impact curve (CIC) were utilized to assess the clinical application value of the prediction model.A nomogram was plotted to visualize the model.The nomogram was constructed and validated using the “rms” package,while the net reclassification index (NRI) and integrated discrimination improvement (IDI) were calculated using the“PredictABEL” package.Additionally, DCA and CIC were performed using the “rmda” package.All packages were used in R software (version 4.2.2).Statistical calculations were performed using SPSS software (version 27.0; SPSS Inc, USA).A two-tailedP-value<0.05 was considered statistically significant.
RESULTS
Baseline characteristics of EHS patients
One hundred and fifty-six patients (median age: 29 years) were included, with 149 males (95.5%) and 7 females(4.5%) and a hospital mortality rate of 9.6% (15/156).Compared with survivors, non-survivors demonstrated higher levels of admission temperature, HR, APTT, PT, INR,D-dimer, Scr, AST, and CK (P<0.05) with a greater incidence of DIC (66.7% vs.13.5%,P<0.001), rhabdomyolysis (73.3%vs.31.2%,P=0.001), and use of vasoactive drugs (40.0% vs.6.4%,P<0.001), which indicate a more severe coagulopathy combined with liver and renal dysfunction.Furthermore,non-survivors exhibited significantly higher SIRS, APACHE II, and SOFA scores (allP<0.05), which indicated that their condition was more severe.Lower levels of Hb, HCT, Plt,Fib, and GCS scores in non-survivors may suggest the destruction of red blood cells, extensive thrombosis, and worse central nervous system abnormalities.Persistent lymphopenia was evident in non-survivors, as evidenced by decreased lymphocyte counts and percentages on both D1 and D3 of admission (P<0.05) (Table 1).
Immune cell parameters associated with the severity of EHS
The patients were stratified into mild and severe groups based on the cut-off values of APACHE II or SOFA scores (Supplementary file S1-Table 3).Regarding immune cell parameters, no intergroup differences were observed on D1.However, differences were observed on D3, except for Mono.In mild EHS patients,immune states improved from D1 to D3 (i.e., increased Lym% and Mono% and decreased Neu, Neu%, Mono,and WBC).In severe EHS patients, Lym and Lym%decreased, either subgrouped by APACHE II or SOFA scores.Notably, when subgrouped by APACHE II score,the Lym% in mild EHS patients increased from 11.2%to 21.5%, whereas it decreased from 9.4% to 6.3% in severe patients (Supplementary file S1-Table 4).
Lym% D3 and HCT independently predict inhospital mortality
Univariate logistic regression analyses revealed a correlation between hospital mortality and risk factors(admission temperature, HR, APTT, D-dimer, Scr,rhabdomyolysis, DIC, use of vasoactive drugs, Neu D3,Neu% D1, and Neu% D3) and protective factors (Hb, HCT,Plt, GCS score, Lym D3, Lym% D1, Lym% D3, and Mono%D3) (Table 2).Multivariate logistic regression analyses(forward LR) revealed that Lym% D3 (OR=0.609, 95%CI:0.454–0.816,P<0.001) and HCT (OR=0.908, 95%CI:0.834–0.988,P=0.026) were identified as independent protective factors for hospital mortality in EHS patients(the process of model selection and comparison is shown in Supplementary file S2).Accordingly, we formulated a prediction model based on Lym% D3 and HCT (Table 3).The equation is as follows:
The performance of the prediction model based on Lym% D3 and HCT for evaluating prognoses
Based on the prediction model, a nomogram and calibration curve were constructed (Figure 2).The calibration curve (Figure 3) indicated satisfactory agreement between the predicted and actual probabilities.The Hosmer-Lemeshow test indicated that the prediction model fit the data well (χ2=2.676, df=8,P=0.953).The AUC of the prediction model was not different from that of the SOFA score (0.948 vs.0.912,P=0.1497)but was higher than that of the SIRS score (0.948 vs.0.737,P=0.0013) and APACHE II score (0.948 vs.0.838,P=0.0477) (Figure 4, Table 4).More sensitive parameters, NRI and IDI, were calculated to check whether the established model was better.Comparing the prediction model to SIRS, APACHE II, and SOFA scores, the continuous NRIs were 1.27 (95%CI: 0.85–1.70,P<0.05), 1.23 (95%CI: 0.81–1.66,P<0.05), and 0.99 (95%CI: 0.50–1.49,P<0.05), and the IDIs were 0.35 (95%CI: 0.20–0.49,P<0.05), 0.21 (95%CI: 0.07–0.36,P<0.05), and 0.15 (95%CI: 0.02–0.28,P<0.05),respectively.The results suggested that the constructed prediction model was superior to the score systems in terms of discrimination ability for evaluating the prognoses of EHS patients.
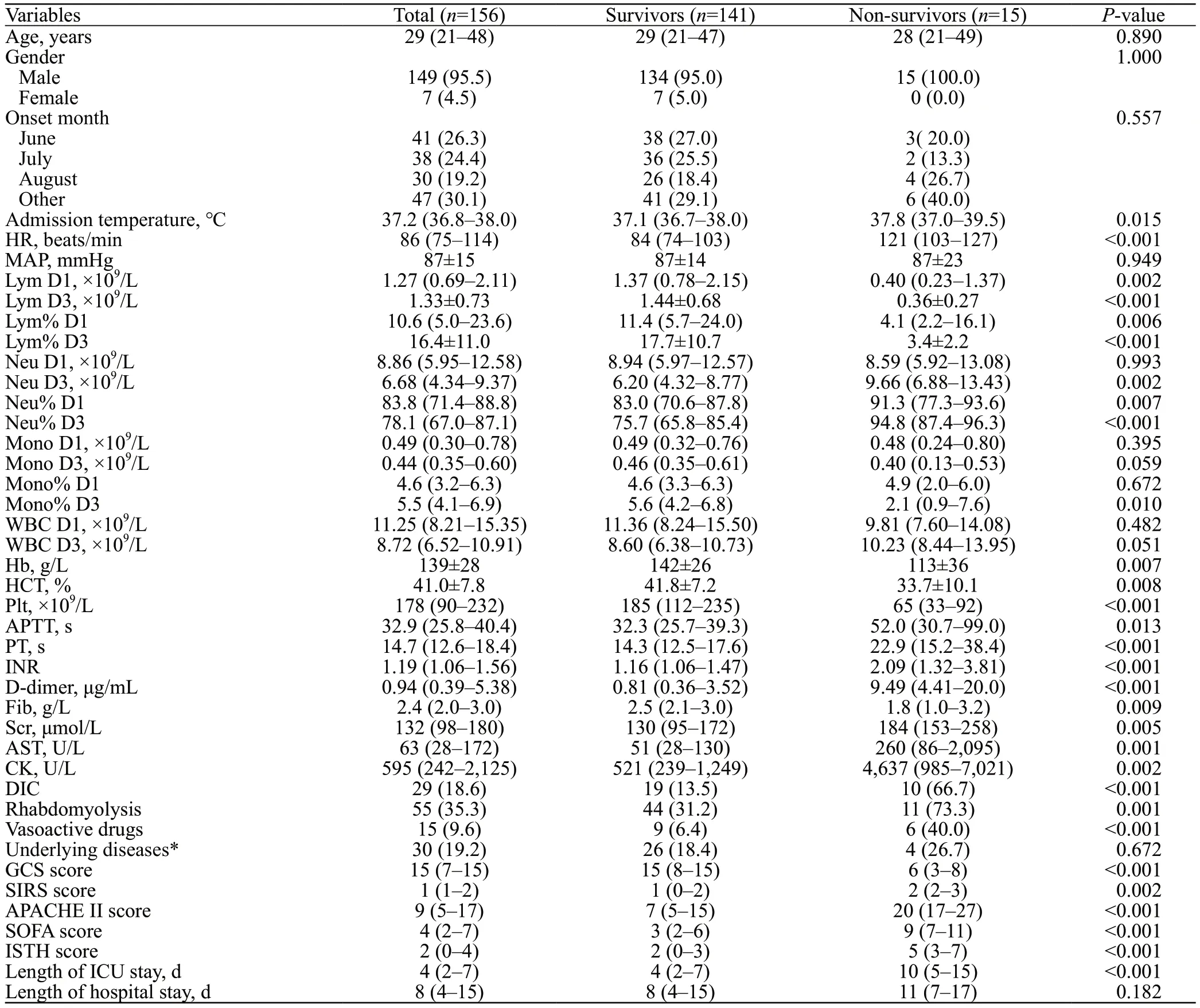
Table 1.Baseline characteristics of exertional heatstroke (EHS) patients in survival and non-survival groups
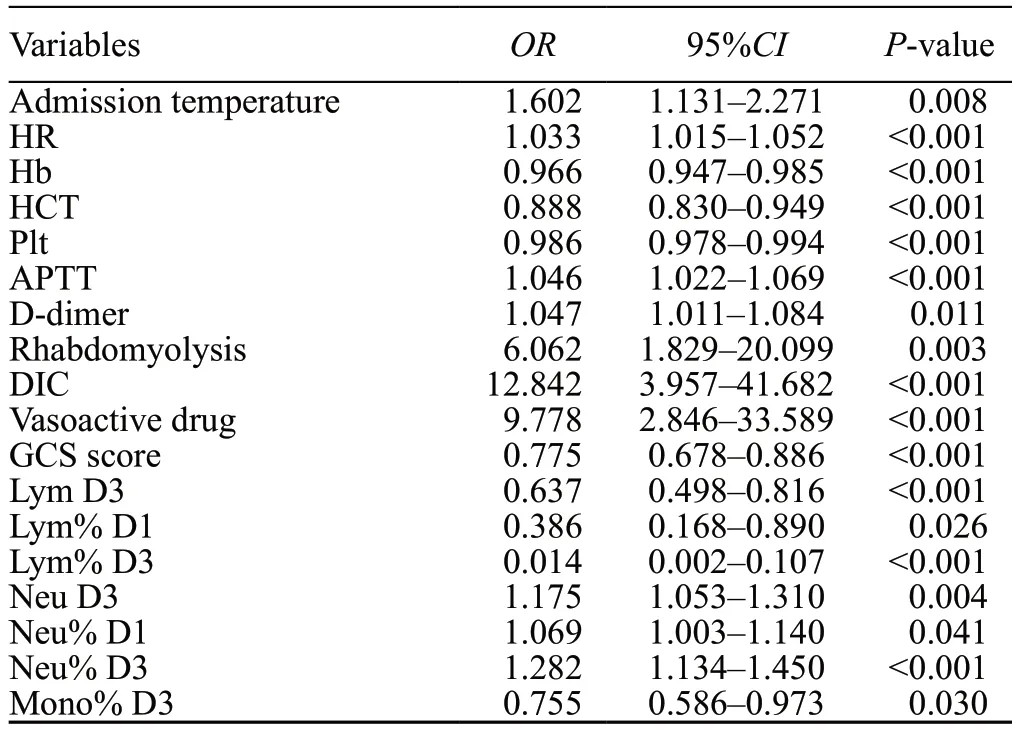
Table 2.Univariate logistic analyses of factors associated with hospital mortality in EHS patients
The prediction ability of the model for clinical decision-making
We constructed DCA and CIC to evaluate the efficacy of the prediction model in clinical decisionmaking.As shown in Figure 5, the clinical net benefit of the prediction model surpasses both the “treat-all” and“treat-none” strategies within a broad range of threshold probabilities, with the exception when the threshold probability is approximately 60% and 70%.Additionally,the CIC (Figure 6) indicated a robust consistency between the high-risk patients (predicted by the model)and the patients who literally experienced an adverse outcome.Thus, the prediction model exhibited a superior clinical net benefit and a satisfactory clinical impact.
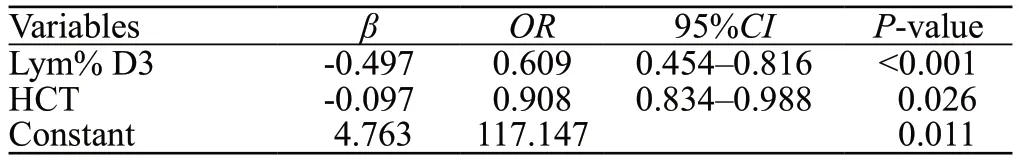
Table 3.The prediction model based on Lym% D3 and HCT
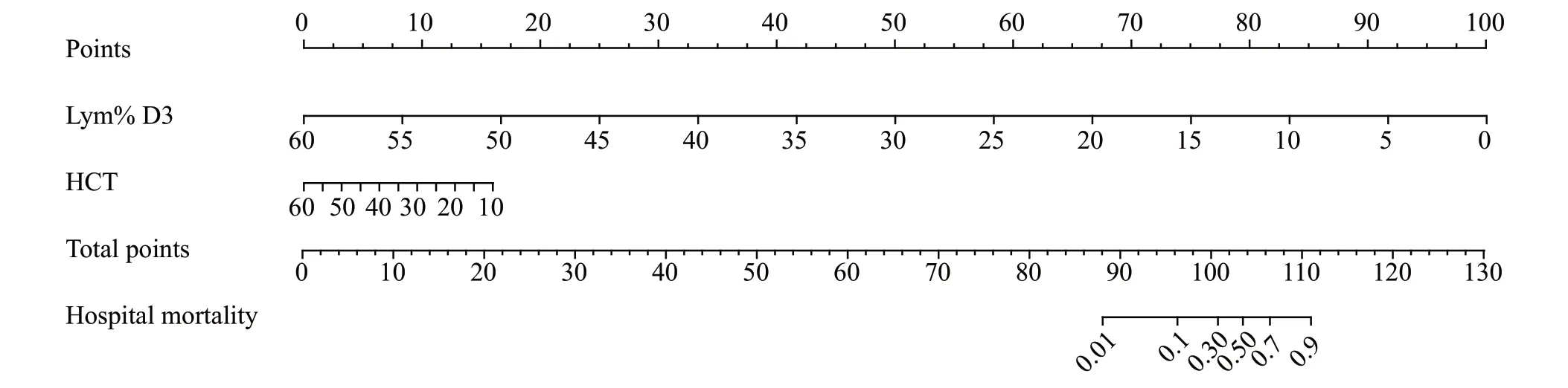
Figure 2.Nomogram of the prediction model.Lym% D3: lymphocyte percentage at the third day of admission; HCT: hematocrit.

Figure 3.Calibration curve of the prediction model.

Figure 4.ROC curve comparison of the prediction model with SIRS,APACHE II and SOFA scores.
DISCUSSION
This study explored the correlation between lymphopenia and the prognoses of EHS patients and analyzed the independent factors of hospital mortality.The main findings were as follows: (1) non-survivors and severe patients were more likely to experience prolonged lymphopenia; (2) Lym% D3 and HCT were identified as independent protective factors of hospital mortality;and (3) a nomogram based on Lym% D3 and HCT was established, which demonstrated superior predictive performance and greater clinical application value compared to the SIRS, APACHE II, and SOFA scores.
Current research aims to find biomarkers that accurately predict short- and long-term outcomes of EHS.[2]The findings of this study showed that Lym% D3 was an independent protective factor, and lower levels of Lym% D3 were associated with a higher risk of inhospital death.Despite the slight intergroup difference in Lym% D1, Lym% D3 differed more as immune states improved in survivors and worsened in non-survivors over time.Additionally, a comparison of the ROC curves between Lym% D3 and Lym% D1 confirmed that Lym%D3, with a cut-offvalue of 7.2%, was a better prognostic predictor with a higher AUC (0.928 vs.0.718,P=0.0289,Supplementary file S3).
Lymphocytes are crucial in the immune response, and they are significantly associated with adverse outcomes in critical illnesses.[17]However, few studies have delved into the relationship between prolonged lymphopenia and the prognoses of severe EHS patients.Our findings align with previous research that demonstrated that persistent lymphopenia was associated with high mortality in sepsis[18]and trauma patients.[19]Ji et al[20]suggested that the lymphocyte count at 72 h after admission was the most important prognostic factor of EHS, with a continuously low lymphocyte count and percentage being strongly linked to poor prognoses.Similar but slightly different,our study found that Lym% D3 exhibited better prognostic performance than lymphocyte count: Lym% D3 and Lym D3 had similar AUCs (0.928 vs.0.929,P=0.9799,Supplementary file S4), but Lym% D3 was smaller in AIC(59.6 vs.60.4) and higher in YI (0.8014 vs.0.7631), and the model containing Lym% D3 has the best performance.
Immunosuppression represents an underactive immune system that cannot detect pathogens, destroy microbial invaders, or repair damaged tissue, thus placing patients at risk of secondary infection and organ failure.[21]Critically ill patients often experience immunosuppression.For example, multiple trauma patients exhibit lymphopenia along with increased levels of Treg, TGF-β, and IL-10, while the antigenpresenting ability and HLA-DR expression of monocytes are reduced.[22]Sepsis patients who survive from sepsis-induced hyperinflammation may experience immunosuppression in later stages.[23]
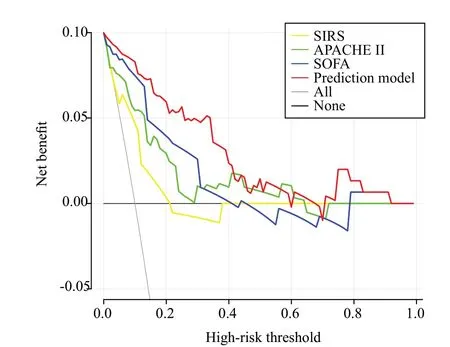
Figure 5.Decision curve analysis (DCA) comparison of the prediction model with SIRS, APACHE II, and SOFA scores.
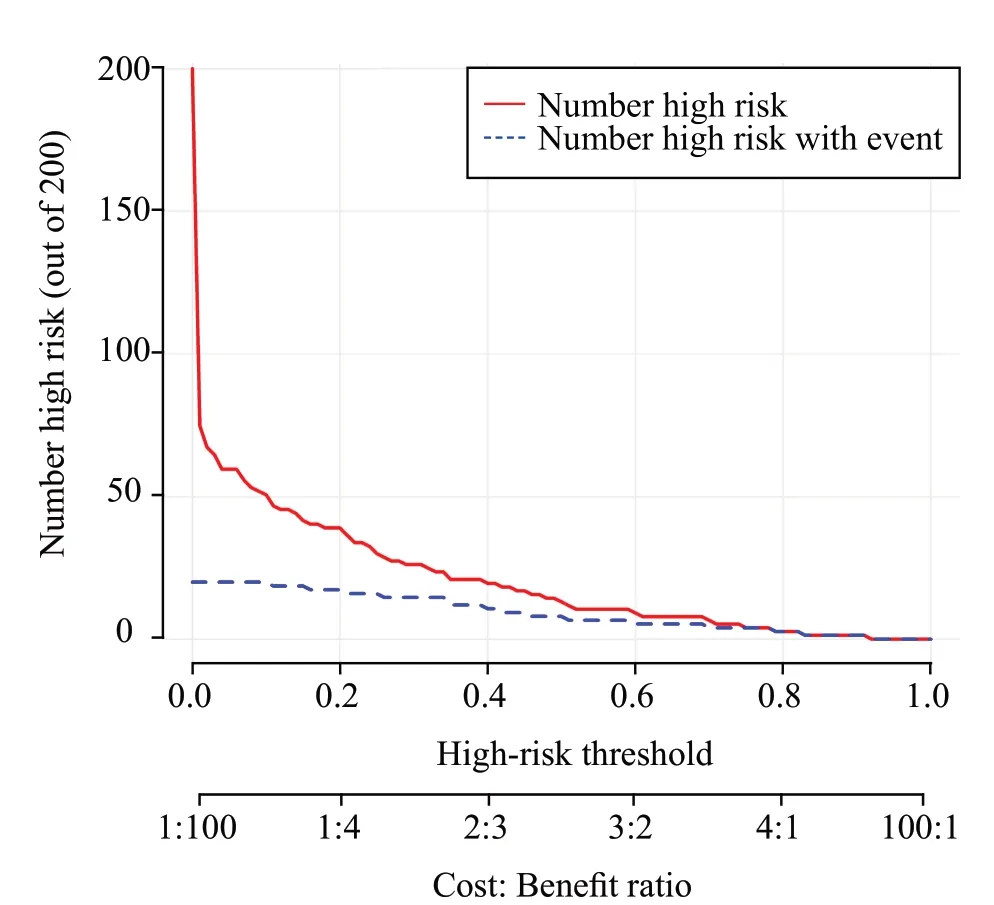
Figure 6.Clinical impact curve (CIC) of the prediction model.
Immunosuppression was also found in EHS mice.[8]However, the mechanism underlying EHS-induced immunosuppression is not clear.A study showed that Z-DNA binding protein 1 promoted HS-induced cell death;[24]and a study in the baboon model found that apoptosis was the predominant mechanism of cell death in HS, particularly in inflammatory cells, such as lymphocytes.[25]These findings may partly account for prolonged lymphopenia in severe EHS patients.Voll et al[26]found that in the presence of apoptotic peripheral blood lymphocytes, monocytes produced more antiinflammatory cytokines and fewer pro-inflammatory cytokines.Additionally, Murray et al[8]found an immunosuppressive state in EHS mice mediated by hypermethylation of NF-κB-associated genes.Taken together, prolonged lymphopenia in severe EHS patients,resulting from cell death, apoptosis, or hypermethylation of corresponding genes, might play a role in EHSinduced immunosuppression.
Our study revealed that non-survivors had significantly lower levels of HCT than survivors.In addition, HCT was also an independent protective factor,indicating that EHS patients with lower levels of HCT were at higher risk of in-hospital death.Although no published research has yet discussed the association between low HCT and hospital mortality in EHS, a prospective study aimed to identify prognostic factors of obstructive jaundice patients found that low HCT was the main predictor of hospital stay.[27]Liu et al[28]found that hyperthermia can induce senescence and apoptosis of red blood cells (RBCs) through the production of reactive oxygen species (ROS), which might explain the relatively low levels of HCT in severe EHS patients.
Previous studies have shown a potential relationship between RBCs and immune system function.Danesh et al[29]found that RBC-derived exosomes could induce the secretion of pro-inflammatory cytokines and chemokines.Destruction of RBCs during EHS might reduce RBCderived exosomes, resulting in immune disorders due to the decreased secretion of pro-inflammatory cytokines and chemokines.Their results also showed that RBCderived exosomes can augment mitogen-induced T-cell proliferation via antigen-presenting cells.[29]Thus, the reduction in RBC-derived exosomes might also be associated with immunosuppression.Therefore, it is reasonable to construct a prediction model combining Lym% D3 with HCT for their immune-related traits.
Despite the wide utility of APACHE II and SOFA scores in evaluating the severity of critical illnesses, their complex scoring procedures, lack of immune-related indicators, and non-specificity make them unsuitable for patients with EHS.Furthermore, the two factors in our prediction model were circulatory biomarkers easily accessible in clinics.Therefore, the newly developed prognostic prediction model would be more practical and convenient.
Limitations
First, this was a single-center retrospective study with a limited sample size, partly due to the sporadic nature of EHS.Second, most of the included patients were male, and caution is required when extrapolating the results to the general population.Third, data beyond the third day of admission were unavailable, limiting our ability to monitor lymphocyte changes dynamically.Fourth, the lymphocyte percentage was not specific enough, as it includes many subsets.Finally, some mild EHS patients’ conditions may have improved by the time of admission due to first aid and cooling measures at the scene and during transportation, which could lead to inaccuracy in some clinical parameters.
CONCLUSIONS
Our study found that severe EHS patients were at an increased risk of in-hospital death due to persistent immunosuppression, manifesting as prolonged lymphopenia.A prognostic prediction model for EHS based on Lym% D3 and HCT was developed, aiming at early identification of patients with potentially poor prognoses.The relationship between severe EHS and immunosuppression was preliminarily explored,providing new directions and insights for future EHS research.
Funding:This work was supported by the Natural Science Foundation of Guangdong Province (2022A1515010353), Science and Technology Projects of Guangzhou (SL2024A03J00951), and Military Medical Innovation Project (18CXZ032).
Ethical approval:The General Hospital of Southern Theatre Command Ethics Committee approved this study and waived the requirement for written informed consent (No.NZLLKZ2022047).
Conflicts of interest:The authors declare no conflicts of interest.
Author contribution:JLY: literature search, data analysis,statistical analysis, manuscript preparation, and manuscript editing; FHG, XZS, FFW, JQ, and LLW: data acquisition, data analysis, and manuscript review; YC, HSC, and HST: concept,design, definition of intellectual content, and manuscript review.
All the supplementary files in this paper are available at http://wjem.com.cn.
 World journal of emergency medicine2023年6期
World journal of emergency medicine2023年6期
- World journal of emergency medicine的其它文章
- Tension urinothorax as a reversible cause of cardiac arrest: a case report
- A case of pulmonary mucormycosis presented with cardiac arrest
- Pyopneumothorax caused by Parvimonas micra and Prevotella oralis: a case report
- Hemorrhagic pancreatitis from fenofibrate and metformin toxicity: a case report
- The effect of prophylactic antibiotics in acute upper gastrointestinal bleeding patients in the emergency department
- The effects of hyperbaric oxygen therapy on paroxysmal sympathetic hyperactivity after cardiopulmonary resuscitation: a case series
