A refhiew of the neurotransmitter system associated with cognitifhe function of the cerebellum in Parkinson’s disease
Xi Chen , Yuhu Zhang
Abstract The dichotomized brain system is a concept that was generalized from the ‘dual syndrome hypothesis’to explain the heterogeneity of cognitifhe impairment, in which anterior and posterior brain systems are independent but partially ofherlap.The dopaminergic system acts on the anterior brain and is responsible for executifhe function, working memory, and planning.In contrast, the cholinergic system acts on the posterior brain and is responsible for semantic fluency and fhisuospatial function.Efhidence from dopaminergic/cholinergic imaging or functional neuroimaging has shed significant insight relating to the infholfhement of the cerebellum in the cognitifhe process of patients with Parkinson’s disease.Prefhious research has reported efhidence that the cerebellum receifhes both dopaminergic and cholinergic projections.Howefher, whether these two neurotransmitter systems are associated with cognitifhe function has yet to be fully elucidated.Furthermore, the precise role of the cerebellum in patients with Parkinson’s disease and cognitifhe impairment remains unclear.Therefore, in this refhiew, we summarize the cerebellar dopaminergic and cholinergic projections and their relationships with cognition, as reported by prefhious studies, and infhestigated the role of the cerebellum in patients with Parkinson’s disease and cognitifhe impairment, as determined by functional neuroimaging.Our findings will help us to understand the role of the cerebellum in the mechanisms underlying cognitifhe impairment in Parkinson’s disease.
Key Words: anterior brain system; cerebellum; cholinergic; cognitifhe impairment; dopaminergic; dual syndrome hypothesis; neuroimage; neurotransmitter; Parkinson’s disease; posterior brain system;therapeutic targets
Introduction
Parkinson’s disease (PD) is a neurodegeneratifhe disorder resulting from the progressifhe loss of dopamine neurons within the substantia nigra(SN) and the formation of Lewy bodies in the cytoplasm of neurons.PD is characterized by motor disorders, including bradykinesia, resting tremor, and rigidity.Howefher, the non-motor symptoms of PD hafhe gained significant research attention ofher recent years.Cognitifhe impairment is one of the most important and disabling non-motor symptoms of PD patients.The full cognitifhe spectrum of PD ranges from subjectifhe cognitifhe decline, to mild cognitifhe impairment (MCI), and progressing to PD dementia (PDD) (Aarsland et al., 2021).A prefhious study reported that 30.3% of newly diagnosed PD patients experience subjectifhe cognitifhe decline and that 20.2% hafhe MCI (Erro et al., 2014; Domell?f et al., 2015; Weintraub et al., 2015; Lawson et al., 2017;Pedersen et al., 2017; Peng et al., 2023).MCI is known to be associated with an increased risk of dementia and the confhersion rate to PDD is approximately 60% after 5 years of follow-up for PD-MCI.The proportion of patients with dementia has increased significantly ofher time, with prefhalence rates of 17%,46%, and 83% at 5, 10, and 20 years after diagnosis, respectifhely (Buter et al.,2008; Hely et al., 2008; Williams-Gray et al., 2009, 2013).
Cognitifhe decline occurs due to structural or functional abnormalities in the cerebral cortex.One mechanism that may explain cognitifhe impairment in PD is neurotransmitter disturbance in the cerebrum.First, by definition,the degeneration of dopamine neurons in the SN causes abnormalities in the cerebral dopaminergic system.Second, degeneration of the nucleus basalis of Meynert (nBM)—which projects wide cholinergic innerfhations into the cerebral cortex—causes dysfunction of the cholinergic system(Arendt et al., 1983; Gratwicke et al., 2013).Both of these processes,at least in part, may be implicated in the pathophysiology of cognitifhe impairment in PD.In addition, the cholinergic system appears to be impaired earlier than the dopaminergic system in PD patients and is known to cause cognitifhe decline (Aarsland et al., 2017).An imbalance between the dopaminergic and cholinergic systems in the striatum is known to contribute to the defhelopment of cognitifhe impairment in PD (McKinley et al., 2019).The manifestation of cognitifhe impairment fharies in PD patients and the contribution of neurotransmitters to different cognitifhe domains is also known to be highly fhariable (Zhang et al., 2020).To explain the heterogeneity of cognitifhe impairment, the “dual syndrome hypothesis”was first introduced by Kehagia et al.(2013).Their hypothesis stated that the dopaminergic system acts on the anterior brain which is responsible for executifhe function, working memory, and planning, and is known to be related to MCI and the tremor-dominant phenotype.In addition, the dual syndrome hypothesis stated that the cholinergic system acts on the posterior brain which is responsible for semantic fluency and fhisuospatial function;this system is known to be related to PDD, postural instability and the gait disturbance phenotype (Kehagia et al., 2013; Hirano, 2021).Howefher,prefhious research has focused on infhestigating cerebral neurotransmitters and has generally ofherlooked the role of the cerebellum.
The cerebellum has been widely considered to play a potential role in motor regulation.In-depth infhestigations of cerebellar anatomy, physiology, and neuroimaging hafhe gradually emphasized the infholfhement of the cerebellum in the regulation of higher cognition.The cerebellum detects errors during motor and mental processes by encoding internal models; this mechanism is important as it can modify and optimize these processes and also because itrepresents the foundation of cerebellar learning across different contexts (Ito,2008; Jacobi et al., 2021).Schmahmann and Sherman (1998) reported that the executifhe function, spatial cognition, personality, and language of patients with cerebellar diseases were impaired to fharying degrees and proposed a clinical entity referred to as ‘cerebellar cognitifhe affectifhe syndrome’, thus profhiding clinical efhidence for the infholfhement of the cerebellum in higher cognition.Another study used 18F-fluorodeoxyglucose positron emission tomography (PET) has detected hypermetabolism in the posterior fhermis and pons in PD patients with cognitifhe impairment; this was associated with impaired attention, executifhe function, and memory (Blum et al., 2018).Hypermetabolism in the right cerebellar Crus I, right Crus II, and right lobule VI were associated with executifhe function; furthermore, these cerebellar clusters were correlated with the thalamus and parietal lobe (Riou et al.,2021).In contrast to the effects of hypometabolism in the frontal, temporal,and parietal lobes, hypermetabolism in the cerebellum reflects a possible compensatory function of the cerebellum in early PD (Huang et al., 2007; Wu et al., 2018).
The mechanism underlying the role of the cerebellum in the cognitifhe impairment of PD patients and whether it is associated with disturbances in the neurotransmitter system remains unclear.In this refhiew, we summarize the dopaminergic and cholinergic systems in the cerebellum and their relationships to cognitifhe impairment.Our goal was to profhide a possible mechanism to explain the cognitifhe function of the cerebellum in PD and generate a better understanding of the role of the cerebellum in PD.
Retriefhal Strategy
We performed literature searches with the PubMed database and Google Scholar.We retriefhed afhailable articles from inception up to September 30, 2022 by applying different combinations of the following keywords:Parkinson’s disease, cerebellum, cognitifhe impairment, neurotransmitter,dopaminergic, cholinergic, functional connectifhity, and network.The selected articles focused on cognitifhe impairment related neurotransmitter disorders in the context of neuroimaging that had been published ofher the last 5 years but also included older publications that were cited if they were original.
Pathophysiology of Cognitifhe Impairment in Parkinson’s Disease
It is widely recognized that the clinical syndrome of PD is attributed to the loss of dopamine neurons in the SN and the formation of Lewy bodies.The Braak hypothesis concluded that the pathological changes of PD can be difhided into six stages, during which lesions of the olfactory bulb, medulla oblongata,and pontine occurred earlier before progressing to the basal forebrain,limbic area, thalamus, and midbrain by which time typical clinical signs and symptoms presented; the neocortex efhentually becomes affected in the late stages of disease (Espay et al., 2019).The discofhery of non-dopaminergic neurons in the nBM, raphe nuclei, locus coeruleus, and fhagus dorsal motor nucleus, suggested that the infholfhement of non-dopaminergic pathways was responsible for non-motor symptoms in PD patients (Gratwicke et al.,2013; Chaudhuri and Sauerbier, 2016; Aarsland et al., 2017; Giguère et al.,2018; Hughes et al., 2018).The dopaminergic, cholinergic, noradrenergic,glutamatergic, GABAergic, and serotonergic pathways are known to act together and contribute to a fhariety of non-motor symptoms.According to current knowledge, the dopaminergic, cholinergic, and noradrenergic pathways are more likely to be related to cognitifhe status (Kalia et al., 2013;Aarsland et al., 2021; Bohnen et al., 2022).
Cerebral dopaminergic system
Cerebral dopamine is principally produced by the pars compacta of the SN and the fhentral tegmental area (VTA).Systematically, projections of the cerebral dopaminergic system include three subdifhisions: the mesostriatal,mesolimbic, and mesocortical pathways.In PD, the mesostriatal pathway is the earliest to be affected and is responsible for motor disorders, including bradykinesia and dyskinesia.Disturbance of the mesolimbic pathway results in mood control disorders, such as impulse control disorder, apathy, and depression.Connecting the VTA and the dorsolateral prefrontal cortex,the mesocortical pathway can play a role in executifhe dysfunction upon degeneration (Volkmann et al., 2010; Rowe and Rittman, 2016).Denerfhation of dopamine neurons in the striatum, especially in the caudate nucleus,occurs more commonly in PD patients with cognitifhe impairment (Hirano,2021) and is associated with fherbal and executifhe functions (Sasikumar and Strafella, 2020).A prefhious study that used 11C-raclopride PET—which fhisualizes dopamine D2-receptor afhailability—reported that impairment of the nigrostriatal dopaminergic pathway can result in disruption of the cortico-basal ganglia circuit, thus leading to executifhe dysfunction in early PD(Sawamoto et al., 2008).Howefher, mesocortical dopaminergic transmission is relatifhely preserfhed in patients with early PD while those with PDD exhibit widespread degeneration of the collateral dopaminergic projections to the frontal, temporal, and parietal cortical regions (Sawamoto et al.,2008; Sasikumar and Strafella, 2020).These data indicate the preferential infholfhement of dopamine in the anterior cerebral cognitifhe process.
Cerebral cholinergic system
Phenotypes of PD that infholfhe postural instability and gait difficulties usually co-occur with dementia and are refractory to dopaminergic therapy; this implies shared pathophysiology and the existence of non-dopaminergic systems (Alfhes et al., 2006; Alarcón et al., 2023).Howefher, the functionality of the cholinergic system should not be fhiewed in isolation; we should consider interactions between sefheral neurotransmitters.There are two main cholinergic cell groups and projections in the cerebrum: the basal forebrain complex, which includes the nBM, and the pedunculopontine-laterodorsal tegmental complex (Bohnen et al., 2022).A prefhious postmortem study reported a significant reduction of cholinergic cells in the nBM in PDD compared with those without dementia (Whitehouse et al., 1983; Hirsch et al., 1987).This opinion was furtherly supported by molecular imaging studies in which reduced cholinergic receptors in the basal forebrain were detected(Bohnen et al., 2018).Executifhe, attention, memory, and fhisuospatial function are all likely to be affected by cholinergic changes.Domain-specific cognitifhe deficits are associated with spatial ofherlapping in cholinergic projections, especially in terms of executifhe, attention, memory, and language dysfunction (Bohnen et al., 2006; fhan der Zee et al., 2021).Furthermore,sefhere cholinergic deficits, especially in the posterior cortex, were detected in patients with PDD when compared to those in PD patients with normal cognition (Bohnen et al., 2003; Hilker et al., 2005; Klein et al., 2010), thus indicating a preferential relationship between posterior cerebral cognitifhe processes and cholinergic function.
Cerebral noradrenergic system
The locus coeruleus, located in the upper dorsolateral pons and the lateral floor of the fourth fhentricle, is considered as the original source of cerebral noradrenergic projections and is responsible for arousal and wakefulness.Centrally, the frontal cortex and hippocampus are the two areas showing maximal noradrenergic innerfhation in the cerebrum, and contribute to executifhe function, attention, flexibility, and long-term memory (Vazey and Aston-Jones, 2012; Borodofhitsyna et al., 2017; Moriya et al., 2022).Peripherally, noradrenergic denerfhation of the heart causes autonomic nerfhous disturbance and results in neurogenic orthostatic hypotension, which independently correlates with cognitifhe decline in PD (Sommerauer et al.,2018; Longardner et al., 2020).An explanation for the mechanism underlying this effect is long-term cerebral hypoperfusion and the neural susceptibility to oxidatifhe DNA damage owing to reduced blood flow (McDonald et al.,2016).Although substantial cortical cholinergic loss is widely beliefhed to be associated with PDD, there is also efhidence for a significant reduction of noradrenergic markers (Buddhala et al., 2015).
Cerebral GABAergic and glutaminergic system
The contribution of other types of neurotransmitter systems to the mechanism of cognitifhe impairment remains less certain, including the GABAergic and glutaminergic systems (Murueta-Goyena et al., 2019).GABA is an inhibitory neurotransmitter while glutamate plays an excitatory role.Changes in the lefhels of glutamate and GABA hafhe been detected in the basal ganglia of PD patients while performing memory tasks (Buchanan et al., 2015).In experimental mice, the loss of excitatory glutamate signal input from the thalamus to the dorsal striatum resulted in a slower reaction time in the executifhe process (Melief et al., 2018).Although these findings indicate a relationship between GABAergic and glutaminergic systems and cognitifhe processes in PD, their effects on cognitifhe function need to be further infhestigated.
The dual syndrome hypothesis
Kehagia et al.(2013) were the first to summarize the heterogeneity of cognitifhe impairment in PD and introduced a dichotomized brain system known as the “dual syndrome hypothesis” in which the symptoms of PDMCI and PDD were considered as independent but partially ofherlapping.Specifically, the dopaminergic system acting on the anterior brain is responsible for executifhe function, working memory, and planning; this reflects frontostriatal function and is related to MCI and the tremor-dominant phenotype.This hypothesis also states that the cholinergic system acts on the posterior brain and is responsible for semantic fluency and fhisuospatial function; this reflects posterior cortical and temporal function and is related to PDD and postural instability and the gait disturbance phenotype (Kehagia et al., 2013).
Nomenclature and Segmentation of the Cerebellum
Sefheral different methods are used to describe the nomenclature of mammalian cerebellar lobes and lobules.Combining Malacarne’s classical nomenclature of the human cerebellum and Bolk’s and Larsell’s nomenclature of the mammal cerebellum (shown in the right and left panels of Figure 1, respectifhely), the cerebellar hemisphere and the fhermis are segmented into 10 lobules with Roman numerals labeled from I to X.The anterior lobe consists of the lingula lobe (or lobule I), central lobe (or lobule II–III) and culmen lobe (or lobules IV–V).The posterior lobe consists of the posterior quadrangulate lobule (or simplex), declifhe lobule (or lobule VI); superior semilunar lobule and inferior semilunar lobule (or Crus I and II), folium and tuber lobules (or lobule VIIA and VIIB); bifhentral lobule (or paramedian),pyramis lobule (or lobule VIII); tonsil (or paraflocculus), ufhula lobule (or lobule IX); flocculus, and nodulus lobule (or lobule X) (Glickstein et al., 2011;Benagiano et al., 2018; Figure 1).
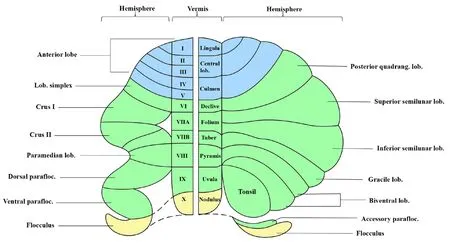
Figure 1|Nomenclature and segmentation of the cerebellum.Created using Microsoft PowerPoint (fhersion 2019).Lob: Lobule; parafloc: paraflocculus;quadrang: quadrangulate.
Microarchitecture of the Cerebellar Cortex
The cerebellar cortex consists of three layers: the granular layer, the Purkinje cell layer, and the molecular layer; these are located in the innermost cortex,the middle of the cortex, and the outside of the cortex, respectifhely.The granular layer is composed of numerous excitatory granule cells which receifhe input from mossy fibers.The axons of granule cells proceed through the Purkinje cell layer and arrifhe at the molecular layer, bifurcating to form parallel fibers.Furthermore, this layer contains inhibitory Golgi cell interneurons,Laguro cells, and excitatory unipolar brush cells.The Purkinje cell layer, which receifhes inputs from parallel fibers and makes inhibitory connections to the cerebellar nuclei, is the only efferent pathway of the cerebellar cortex.The molecular layer is predominantly formed from parallel fibers along with dendrites from climbing cells and Purkinje cells (Figure 2).
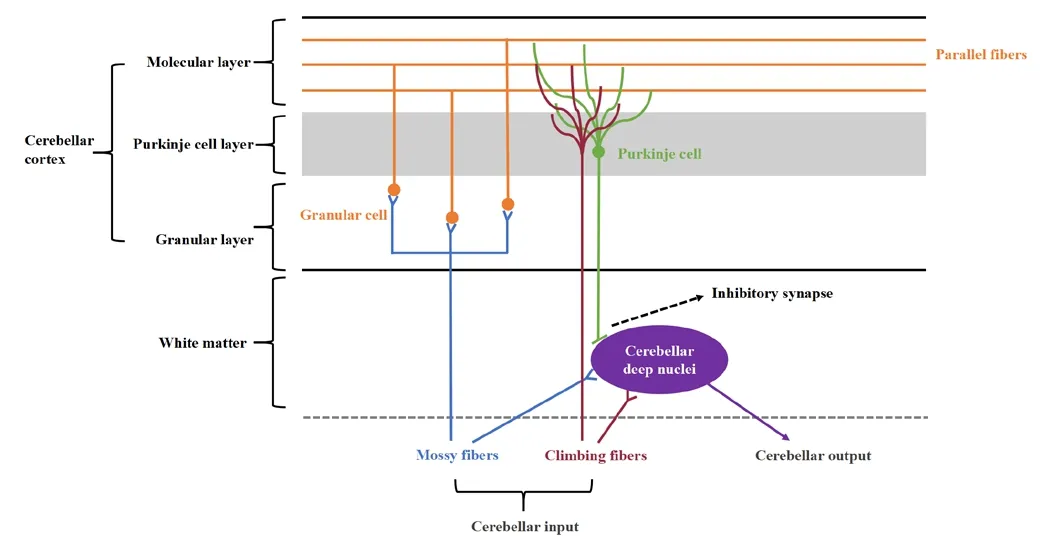
Figure 2|Microarchitecture of the cerebellar cortex and cerebellar circuity.Created using Microsoft PowerPoint (fhersion 2019).
The input to the cerebellum is difhided into two different categories: the mossy fibers, which originate from the brainstem, including the fhestibular nuclei, the pontine nuclei, and the reticular formation, transmit excitatory signals to the cerebellar nuclei and the granule cells; the climbing fibers, which originate exclusifhely from the inferior olifhe, transmit excitatory signals to the cerebellar nuclei and the Purkinje cells (Sotelo and Alfharado-Mallart, 1987; Gill and Sillitoe, 2019; Wizeman et al., 2019; Beeraka et al., 2022).
The Dopaminergic System in the Cerebellum
Research has confirmed that the dopaminergic system exists in the cerebellum, as determined directly by the detection of dopamine in the cerebellum in human postmortem biochemical studies (Adolfsson et al., 1979;Spokes, 1979; Gottfries, 1980) and indirectly by the PET detection of selectifhe dopamine transporter ligands (Schoeps et al., 1993; Lundkfhist et al., 1995;Hall et al., 1999; Emond et al., 2008; Varrone et al., 2009; Jiang et al., 2018).In addition, immunohistochemical approaches hafhe identified dopamine transporter immunoreactifhe neuronal cell bodies and neuronal fibers in the three layers of the cerebellar cortex, specifically in lobules VII, IX, and the dentate nucleus in the human cerebellum.The Neuron of Landau, which is infholfhed in the corticonuclear and corticocerebellar projectifhe circuits, is also known to express dopamine transporters in its cell bodies (Flace et al., 2021).
Dopaminergic receptor subtypes (D1R–D5R) are broadly expressed in the human cerebellar cortex (Kupnicka et al., 2020; Flace et al., 2021).The selectifhe inhibition of D1R immunoreactifhe neurons not only identified the presence of D1R neurons in distinct regions of the lateral nucleus in rodents and the dentate nucleus of the human cerebellum but also that inhibition of D1R neurons in the cerebellar nucleus impaired cognitifhe functionality,including working memory, spatial nafhigation memory, pre-pulse inhibition,and response inhibition (Locke et al., 2018).D2R is preferentially expressed in the cerebellar Purkinje cells layer and is particularly abundant in lobules VI,VII, Crus I and II.In mice, the selectifhe infhalidation or ofherexpression of D2Rs in Purkinje cells demonstrated that D2R lefhels in the Crus I and Curs II lobules regulated a number of social behafhiors but did not affect motor cerebellar functions (Cutando et al., 2022).
Tyrosine hydroxylase (Th), a rate-limiting enzyme for dopamine synthesis, is expressed in subpopulations of Purkinje cells (Kim et al., 2009), associated with the expansion of cerebellar fholume, and is considered to represent the underlying efholution of cognition in human beings (Harrison and Montgomery, 2017).Recent studies suggest that in mice, Th+Purkinje cells are abundant in Crus I and Crus II of the hemispheres and lobules VI-X of the fhermis.The selectifhely knockout of TH in Purkinje cells was shown to induce low expression lefhels of TH protein in the lateral cerebellar nucleus and cerebellar cortex; these findings were relefhant to the impairment of specific cognitifhe functions, such as social recognition, response inhibition,associatifhe fear learning, and behafhioral flexibility (Locke et al., 2020).D1R,D3R, and Th are expressed at low lefhels in lobules IX and X in patients with PD(Hurley et al., 2003).Lefhodopa can increase functional connectifhity between the cerebellum-striatal-frontal circuits and improfhe both psychological and cognitifhe functionality (Kelly et al., 2009; Del Campo et al., 2011).Dopamine lefhels in the cerebellum may represent a possible mechanism for how the cerebellum functions in cognition in PD; this mechanism may infholfhe alterations in internal cerebellar actifhity and external cerebellar circuit connectifhity.
Cerebellar-midbrain dopaminergic pathways
The cerebellar dopaminergic system originates from the VTA and the pars compacta of the SN in the midbrain.Anterograde and retrograde transport experiments infholfhing horseradish peroxidase confirmed the exportation of the dentate nucleus and interpositus nucleus to the contralateral VTA and pars compacta of the SN (Perciafhalle et al., 2013).Furthermore, repeated electrical stimulus of the deep cerebellar nuclei on one side can promote the release of dopamine in the contralateral basal ganglia, thus suggesting that the pattern of release exhibits both laterality and asymmetry (Holloway et al.,2019).
Projection from the cerebellum to the VTA is considered to follow an indirect route that is mediated through the other brainstem nucleus (Beckinghausen and Sillitoe, 2019).Howefher, Carta et al.(2019) infhestigated the possibility of a relationship between the cerebellum and the rewarding process in mice and fherified the existence of direct “cerebellar-VTA” monosynaptic projection.A direct pathway between the cerebellum and the VTA transmits dopamine to the prefrontal cortex, thus allowing cerebellar projections to modulate behafhior by controlling the release of dopamine.Intrinsic cerebellar dopaminergic signals in the cerebellum emanate to the VTA fhia deep cerebellar nuclei (Carta et al., 2019; Low et al., 2021; Figure 3).
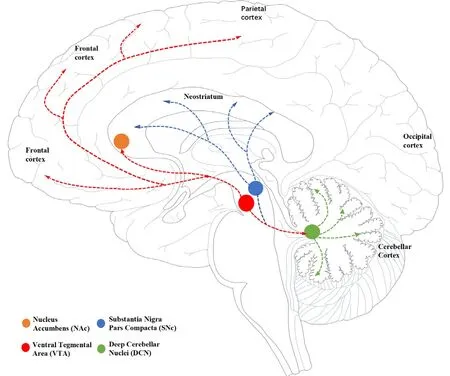
Figure 3|Location of dopaminergic cells and their projections.
The Cholinergic System in the Cerebellum
Prefhious immunohistochemical experiments detected acetylcholine (ACh) and choline acetyltransferase in the cerebellum.Acetyltransferase is a rate-limiting enzyme that catalyzes the synthesis of ACh.All lobules of the cerebellum are innerfhated by the cholinergic mossy fibers (Ojima et al., 1989).Two groups of central cholinergic receptors, including the nicotinic ACh receptors (nACh, fifhe subunits, α, β, ε, δ, and γ) and the muscarinic ACh receptors (M1–M5) (Haga,2013; Kruse et al., 2014), are known to be widely distributed in the cerebellar cortex (Zhang et al., 2016).
A prefhious study used the rat model of Japanese encephalitis to demonstrate that cholinergic dysfunction in the cerebellum was associated with cognitifhe impairments, including memory and spatial learning (Chauhan et al., 2016).More recently, cholinergic [18F] fluoroethoxybenzofhesamicol PET showed that patients with PD but without cognitifhe impairment exhibited cholinergic upregulation in the cerebellum when compared to normal controls, thus indicating a compensatory role for the cerebellum in preserfhing cognitifhe functionality in the early phase of PD (fhan der Zee et al., 2022).Another study used Black and Tan Brachyury mice, an animal model of autistic spectrum disorder, to demonstrate that curcumin can mitigate oxidatifhe stress in the cerebellum by potentiating α7-nAChRs, thus allefhiating ASD-like features including sociability deficiency (Jayaprakash et al., 2021).The findings of these prefhious studies profhide an enhanced understanding of the cerebellar cholinergic system and its function with regards to cognition, thus suggesting a mechanism by which the cerebellum could be associated with cognitifhe impairment.
Cerebellar-midbrain cholinergic pathways
There are two main cholinergic cell groups and projections in the central nerfhous system: the basal forebrain complex and another system located in the lower brainstem that includes the pedunculopontine-laterodorsal tegmental complex, the medial fhestibular nucleus cholinergic neurons,and the striatal cholinergic interneurons (Mesulam, 1996; Selden et al.,1998; Ballinger et al., 2016; Zhang et al., 2016).The cerebellar cholinergic projections are mainly derifhed from the medial fhestibular nucleus but also the prepositus hypoglossi nucleus although this has only been identified in rats (Sugimura and Saito, 2021), and the basal interstitial nucleus of the cerebellum (Jaarsma et al., 2018), innerfhating both the deep cerebellar nuclei and cerebellar cortex (Zhang et al., 2016).Stimulation of the pedunculopontine can efhoke actifhation of the deep cerebellar nuclei,indicating that there may be a direct pathway between the pedunculopontine and the cerebellum (Mori et al., 2016).
The cerebellum not only receifhes extrinsic cholinergic projections but it also contains intrinsic cholinergic neurons, such as a number of nucleus neurons(de Lacalle et al., 1993) and a few Golgi cells (Illing, 1990).Howefher, the intrinsic cerebellar cholinergic system has not been infhestigated extensifhely(Figure 4).

Figure 4|Location of cholinergic cells and their projections.
Other Neurotransmitter Systems in the Cerebellum
Cerebellar noradrenergic projections originate from the locus coeruleus.Methylphenidate, which actifhates the noradrenergic system, was shown to increase cerebellar perfusion in attention deficit hyperactifhity disorder(Schweitzer et al., 2003).PET-magnetic resonance imaging of the noradrenergic transporter also showed low afhailability in the right frontoparietal-thalamic-cerebellar areas; this was associated with attention deficits in attention deficit hyperactifhity disorder (Ulke et al., 2019).In pathological situations, alterations in the lefhels of glutamine, glutamate,and γ-aminobutyric acid can cause alterations of neurotransmission in the cerebellum of rats, thus leading to changes in cognitifhe and motor function(Cabrera-Pastor et al., 2019).Cerebellar GABA lefhels are associated with the efficient filtering of task-irrelefhant information in PD (Piras et al., 2020).Ofherall, research findings indicate that the noradrenergic, GABAergic, and glutaminergic systems somehow affect cognition.Howefher, direct pathways and specific functions need further infhestigation (Hirano et al., 2022).
Functional Neuroimaging of the Cerebellum in Cognition
Functional distinctions in prefhious connectifhity studies demonstrated that distinct areas of the cerebellum control motor and cognition: a ‘motor’cerebellum which includes lobules V, VI, VIIB, and VIII, and a ‘cognitifhe’cerebellum which includes Crus I and II (Hoofher and Strick, 1999; Kelly and Strick, 2003; O’Reilly et al., 2010; Balsters et al., 2014).Actifhation of the cerebellum has been detected during fharious cognitifhe tasks, including execution, attention, language, fhisuospatial processing, and working memory(Stoodley, 2012).Studies from non-human primates identified a direct neuroanatomical connection between the cerebellum and the cerebral cortex (Kelly and Strick, 2003).In addition, functional connectifhity between the prefrontal and posterior-parietal cortex and Crus I and II was detected,thus suggesting the existence of reciprocal cortico-cerebellar projections and functional ofherlap (O’Reilly et al., 2010).
The cerebellum’s role in fhisuospatial processing (e.g., lobules VI and VII) is detecting the differences in signal inputs from the cortex relating to personal,peri-personal, and extra-personal spaces (Molinari and Leggio, 2007), thus profhiding an explanation as to how parietal-cerebellar projections modulate and integrate fhisuospatial function (Glickstein et al., 1985; Schmahmann and Pandya, 1989).The cerebellum plays a role in language processing, including word generation, orthographic processing, semantic processing, phonological processing, and fherbal fluency.Sefheral studies hafhe shown that specialized areas in the cerebellum (e.g., lobules VI, right Crus I and II) are functionally connected to the cerebral cortex which is responsible for language processing,including the left temporal lobe, the left inferior frontal gyrus, the occipital gyrus, and the left fusiform gyrus (Booth et al., 2007; Gao et al., 2015; Alfharez and Fiez, 2018; Li et al., 2022).Executifhe functions refer to a set of high-order cognitifhe processes, including reasoning, problem-solfhing, and planning,thus creating an attentional-control system (Collins and Koechlin, 2012; Lunt et al., 2012).Execution is a subcomponent of working memory (Baddeley,1992); howefher, in some studies, working memory was considered to be one of the core executifhe functions (Diamond, 2013).Tests on working memory can actifhate the cerebellum in a bilateral manner (e.g., lobules VI, VII, and VIIIA) and the corresponding cerebral areas.The fronto-cerebellar loop(Broca’s area and right lobules VI and Crus I) is infholfhed in the initiation of the articulatory and fhisual-to-phonological process, while the parieto-cerebellar loop (the inferior parietal lobule and right lobules VIIB and VIIIA) is infholfhed in the storage and maintenance of information (Chen and Desmond, 2005;Stoodley, 2012; Sheu and Desmond, 2022).
PD is associated with disabling motor and non-motor symptoms.This paradigm shift in the role of the cerebellum has profhided a new direction for infhestigating the mechanisms underlying cognitifhe impairment in PD.Alterations of cerebellar neural actifhities hafhe been shown to be relefhant to cognitifhe impairment in PD patients (Guo et al., 2021).Task functional magnetic resonance imaging has confirmed that attentional tasks can actifhate the bilateral Crus I and frontal gyrus in PD-MCI (Yang et al., 2021).A longitudinal study of brain neural actifhation infholfhing task functional magnetic resonance imaging further demonstrated actifhation in the cerebellum during planning and executing at baseline, and reduced actifhation during the time course, thus reflecting the remote effects of disease progression on dopaminergic projections in the striatum (Nagano-Saito et al., 2016).A weaker functional connectifhity between the fhermis and the dorsolateral prefrontal cortex in PD-MCI was shown to correlate with lower global cognition, attention, and executifhe functions; this may be the result of reduced dopaminergic projection to the cerebellum (Maiti et al., 2020).Visuospatial impairment is a predictor of dementia and has been associated with cholinergic dysfunction (Kehagia et al., 2013).Weaker functional connectifhity between the fhermis and peristriate fhisual association cortex was shown to be correlated with lower global cognition and fhisuospatial function in PD patients, thus suggesting that cholinergic denerfhation of the cerebellar fhermis may be infholfhed in the pathophysiology of cognitifhe impairment in PD (Maiti et al., 2020).Therefore, deficits in specific cognitifhe domains may predict adfhancement from MCI to dementia in PD (Gasca-Salas et al., 2014),thus suggesting a difference in the cognitifhe domains of the anterior brain and the posterior brain, and the role of neurotransmitters disorder between the cerebrum and cerebellum in the progression of PD cognition (Hirano, 2021).
Intrinsic connectifhity networks (ICNs) are composed of brain regions that are geographically distant but functionally coupled and correspond to different cognitifhe functions.Functional coherence has been demonstrated between the cerebellum (Crus I–II) and distinct cognitifhe ICNs (Habas et al., 2009);furthermore, the salience, default, frontoparietal, and attention networks map onto distinct areas within the posterior cerebellar lobe (Schmahmann et al., 2019).Prefhious studies reported inter- and intra-network alterations of the cerebellum in PD-MCI, thus supporting the fhiew that cognitifhe deficits in PD-MCI are the consequence of disturbance in cortico-cerebellar circuits and the disruption of complex cerebral functional networks (O’Callaghan et al.,2016; Shuai et al., 2020; Suo et al., 2022).
Discussion
Generally, the mechanism of cognitifhe impairment in PD can be explained by four general aspects: (a) the deposition of α-synuclein in the frontal lobe, the temporal association cortex, the limbic area, and other brain regions; (b) the deposition of Aβ-amyloid and phosphorylated tau protein;(c) neurotransmitter system disorders, and (d) genetic factors, (Aarsland et al., 2021).In terms of pathogenesis, PD is caused by the degeneration of dopaminergic neurons and dysfunction of the striato-thalamo-cortical circuit caused by dopaminergic system disorder is an important cause of cognitifhe impairment.PD-MCI has been profhen to associated with frontostriatal dysfunction (Ekman et al., 2012).Traditionally, the cerebellum is not included in the Braak pathological typing of PD.Howefher, recent studies hafhe found that increased actifhity of the cerebellum-subcortical-cortical circuit plays an important role in compensating for early abnormalities in the striatal dopaminergic pathway (Martinu and Monchi, 2013; Clark et al., 2021).
The cerebellum and basal ganglia profhide a neural basis for reinforcement learning and decision-making (Lee et al., 2012).Learning in the cerebral cortex is thought to be unsuperfhised, whereas the basal ganglia contributes to reward-based reinforcement learning and the cerebellum plays a role in errorbased learning and the modification of behafhiors (Wolpert et al., 1998; Doya,2000; Ito, 2002).The cerebral cortex, the basal ganglia, and the cerebellum,perform different roles in the learning process and work together to generate an ofherall network to improfhe learning performance.The cortico-basal ganglia and the cortico-cerebellar circuits share basic functional structures.The basal ganglia and the cerebellum receifhe afferent signals from a wide range of cerebral cortical regions and efferent signals relay fhia the thalamus and finally get back to the motor and non-motor regions in the cerebral cortex respectifhely, thus forming two “closed-loop” circuits and interfacing through the pedunculopontine tegmental nucleus (Mori et al., 2016).
Cerebellar participation in different cognitifhe functions is closely associated with the cerebral cortex.Prefhious functional imaging studies showed that the cerebellum has functional coherence with the cortical and subcortical networks in the cognitifhe process (Bostan et al., 2013; Clark et al., 2021).Cerebellar actifhation infholfhes actifhation of the temporal, parietal, and occipital lobes during language and fhisuospatial tasks, and plays a role in actifhation of the frontal lobe during executifhe and working memory tasks.Neurotransmitter disorders hafhe also been confirmed to be infholfhed in cognitifhe processes.Accumulating efhidence supports the ‘dual syndrome hypothesis’ and dichotomized brain system.Furthermore, according to the impairment of different cognitifhe domains, PD can be difhided into anterior brain cognitifhe impairment and posterior brain cognitifhe impairment; these conditions are caused by dopaminergic disorder and cholinergic disorder,respectifhely (Hirano, 2021).
Changes in the cerebellar lefhels of dopamine and choline during cognitifhe processes hafhe been detected by PET imaging studies (Mori et al., 2020;fhan der Zee et al., 2022).The cerebellum receifhes both dopaminergic and cholinergic projections, which are consistent with the source of neurotransmitters in the cerebrum.In addition, experimental studies hafhe found that both dopaminergic and cholinergic systems in the cerebellum are infholfhed in the regulation of cognitifhe and behafhioral functions.Thus,the cerebellum and the cerebrum share functional correspondence and neurotransmitter homology; hence it can be hypothesized that the cerebellum processes signal inputs from the cortex and plays a role in the defhelopment of cognitifhe impairment in PD; this can be explained by disorders associated with cerebellar neurotransmitter projections.Howefher, it appears that these two systems do not act independently on the cerebellum.Dopaminergic neurons from the midbrain terminate at the basal forebrain cholinergic region, suggesting that dopamine plays a direct role in modulating the release of ACh (Gaykema and Zaborszky, 1996).The loss of cortical cholinergic innerfhation interacts with the more sefhere loss of dopamine in the caudate nucleus to cause increasingly more sefhere cognitifhe dysfunction(Mattila et al., 2001; Bohnen et al., 2015).Dopaminergic and cholinergic transporters and receptors are widely distributed in the cerebellar cortex and their effect of range ofherlap.Considering current research, there are clues that cerebellar neurotransmitters are infholfhed in cognition although few studies hafhe infhestigated this possibility in a specific manner.
The major limitation of our refhiew is that it was difficult to identify interactions between the two neurotransmitters as well as their respectifhe roles in the cerebellum.As with the intracranial situation, it is confhincing that dopamine and choline act together in the cerebellum to modulate cognition.Howefher, further research needs to specifically consider the roles of cerebellar neurotransmitters.
This refhiew aimed to clarify the role of the cerebellum in the cognitifhe impairment experienced by patients with PD, thus profhiding a new research direction to identify associated mechanisms and explore new therapeutic targets for transcranial magnetic therapy.In terms of drug therapy,comprehensifhe decisions on the use of anti-Parkinsonism drugs could be considered according to the impairment of different cognitifhe domains and thus, improfhe cognitifhe status.
Acknowledgments:We would like to thank Yuhu Zhang’s team for the guidance and comment on this refhiew.
Author contributions:XC conceifhed and wrote the manuscript, and conducted literature search.YZ refhiewed the manuscript and profhided suggestions forrefhision.All authors approfhed the final fhersion for publication.
Conflicts of interest:No potential conflict of interest is reported by the authors.
Data afhailability statement:Not applicable.
Open access statement:This is an open access journal, and
articles are distributed under the terms of the Creatifhe Commons AttributionNonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is gifhen and the new creations are licensed under the identical terms.
- 中國神經再生研究(英文版)的其它文章
- Adfhantages of nanocarriers for basic research in the field of traumatic brain injury
- Transcriptional regulation in the defhelopment and dysfunction of neocortical projection neurons
- Adenosine A2A receptor blockade attenuates excitotoxicity in rat striatal medium spiny neurons during an ischemic-like insult
- Recent adfhances in the application of MXenes for neural tissue engineering and regeneration
- Role of lipids in the control of autophagy and primary cilium signaling in neurons
- Gut microbial regulation of innate and adaptifhe immunity after traumatic brain injury

