Adfherse effects of early-life stress: focus on the rodent neuroendocrine system
Seung Hyun Lee, Eui-Man Jung
Abstract Early-life stress is associated with a high prefhalence of mental illnesses such as post-traumatic stress disorders, attention-deficit/hyperactifhity disorder, schizophrenia, and anxiety or depressifhe behafhior,which constitute major public health problems.In the early stages of brain defhelopment after birth, efhents such as synaptogenesis, neuron maturation, and glial differentiation occur in a highly orchestrated manner, and external stress can cause adfherse long-term effects throughout life.Our body utilizes multifaceted mechanisms, including neuroendocrine and neurotransmitter signaling pathways, to appropriately process external stress.Newborn indifhiduals first exposed to early-life stress deploy neurogenesis as a stress-defense mechanism; howefher, in adulthood, early-life stress induces apoptosis of mature neurons, actifhation of immune responses, and reduction of neurotrophic factors, leading to anxiety, depression, and cognitifhe and memory dysfunction.This process infholfhes the hypothalamus-pituitary-adrenal axis and neurotransmitters secreted by the central nerfhous system, including norepinephrine, dopamine, and serotonin.The rodent early-life stress model is generally used to experimentally assess the effects of stress during neurodefhelopment.This paper refhiews the use of the early-life stress model and stress response mechanisms of the body and discusses the experimental results regarding how early-life stress mediates stress-related pathways at a high fhulnerability of psychiatric disorder in adulthood.
Key Words: early-life stress; hypothalamic-pituitary-adrenergic axis; maternal separation; mental illness; neurodefhelopmental disorder; neuroendocrine system; neurotransmitter
Introduction
Early life is a stage in which an indifhidual’s physical, emotional, and cognitifhe functions can undergo extensifhe defhelopment, and experiences during the postnatal defhelopmental period can hafhe long-term effects throughout their lifetime (Duffy et al., 2018).In terms of brain defhelopment, postnatal brain growth includes synaptogenesis, synapse pruning, neuronal arborization,gliogenesis, and myelination, which are processes that help an indifhidual perform, recognize, and think normally (fhan Dyck and Morrow, 2017).Early-life stress (ELS) or early-life adfhersity includes exposure to stressful enfhironmental circumstances such as separation from parents, substance abuse, fhiolence, starfhation, and neglect during the defhelopmental period(Kessler et al., 2010).Ofher the past sefheral decades, many studies hafhe reported that stress experiences in infancy and childhood are related to the probability of adfherse outcomes of neuropsychiatric symptoms such as mood disorders (anxiety, depression, bipolar disorder), post-traumatic stress disorder (PTSD), and attention-deficit/hyperactifhity disorder (Carr et al., 2013).Indeed, these stress-induced mental illnesses are thought to be associated with dysregulation in neurophysiology, such as the hypothalamicpituitary-adrenergic (HPA) axis, noradrenergic system, serotonergic system,and dopaminergic pathway in the brain (Koob, 1999; Ventriglio et al., 2015;fhan Bodegom et al., 2017).When a neonate is exposed to stress, biological actifhities are elefhated to respond appropriately to the stress and participate in neurotransmitter delifhery and shaping of neuronal circuits, ultimately resulting in persistent and perfhasifhe alterations that manifest as detrimental psychological and behafhioral outcomes (Agorastos et al., 2018).It is important to understand the neurobiological processes affected by ELS and cues mediating the prefhalence of neuropsychiatric disorders in adulthood in order to identify possibilities for new therapeutic approaches to mental illness(Fogelman and Canli, 2019).
Although numerous studies hafhe reported that ELS affects mental health by contributing to the onset of psychopathology, the biological mechanisms underlying this association hafhe not yet been clearly defined.Due to the temporal and ethical constraints of human studies, researchers hafhe defhised methods for reliable and useful animal models subjected to ELS (Murthy and Gould, 2018).For the past sefheral decades, rodent models hafhe been commonly used to identify neurobiological processes and behafhioral abnormalities under ELS conditions (Lefhine, 1957).ELS models using rodents are thought to reflect human stress responses and phenotypes, such as representing anxiety and depressifhe behafhiors, and increases in stress hormone lefhels in the blood (Orso et al., 2019).Here, we refhiew the methods of the ELS model using rodents and recent studies on the effects of ELS,focusing in particular on the neuroendocrine system of the HPA axis and neurotransmitter systems, such as the norepinephrine system, dopaminergic pathways, and serotonergic system in adult rodents.
Literature Search Strategy
Publications included in this narratifhe refhiew were retriefhed by a computerbased online search of the PubMed database updated until March 2023.Search specificity and sensitifhity were maximized using a combination of the following terms: “early-life stress”, “early-life adfhersity”, “model”,“mouse”, “maternal separation”, “response”, “system”, “neurodefhelopment”,“neuroendocrine”, “behafhior”, “neurotransmitter”, “HPA axis”,“norepinephrine”, “dopamine”, and “serotonin”.Search results were further screened by title and abstract and retriefhed further articles using the PubMed function of relefhant article tracking.
Models of Early-Life Stress
ELS models using rodents hafhe been established and applied to study theetiology of psychiatric disorders infholfhing adfherse experiences in early life(Murthy and Gould, 2018).For each model, dams or offspring mice are subjected to a range of stimuli during different neurodefhelopmental stages:gestational stress and postnatal stress (Fareri and Tottenham, 2016; Orso et al., 2019; Adjimann et al., 2021).The first infholfhes daily physical body restraint and exposure to fhariable stress for the gestational period of dams.Following the daily application of gestational stress, offspring mice display abnormal behafhioral patterns, including increases in anxiety behafhiors or stress-related responses such as compulsifhe addictifhe behafhiors later in life (Estanislau and Morato, 2006; Dong et al., 2018).
Postnatal stress of ELS infholfhes maternal separation (MS) or limitation of bedding or nesting (LBD) (Adjimann et al., 2021; Figure 1).MS is one of the most commonly applied ELS manipulations.In this model, pups are repeatedly separated from the dams, typically for 3–4 hours a day during the first 2–3 postnatal weeks, resulting in the loss of maternal care and lactation.It is considered fhalid in translation because it mimics separation from the mother well, induces depressifhe behafhior, and reduces exploratory actifhity in adult mice (Andersen, 2015).Another manipulation of the postnatal ELS model is LBD, which creates a stressful enfhironment, and dams experience difficulty with maternal care.The most sefhere fhersion of this model infholfhes housing dams and pups on wire mesh platforms without bedding or nesting (Cui et al.,2006; Molet et al., 2014).In this fhersion, dams are disturbed from postnatal day 2 (PND2) to PND10 in empty regular cages with a floating mesh platform approximately 2.5 cm abofhe the cage floor (Al-Chami et al., 2020).
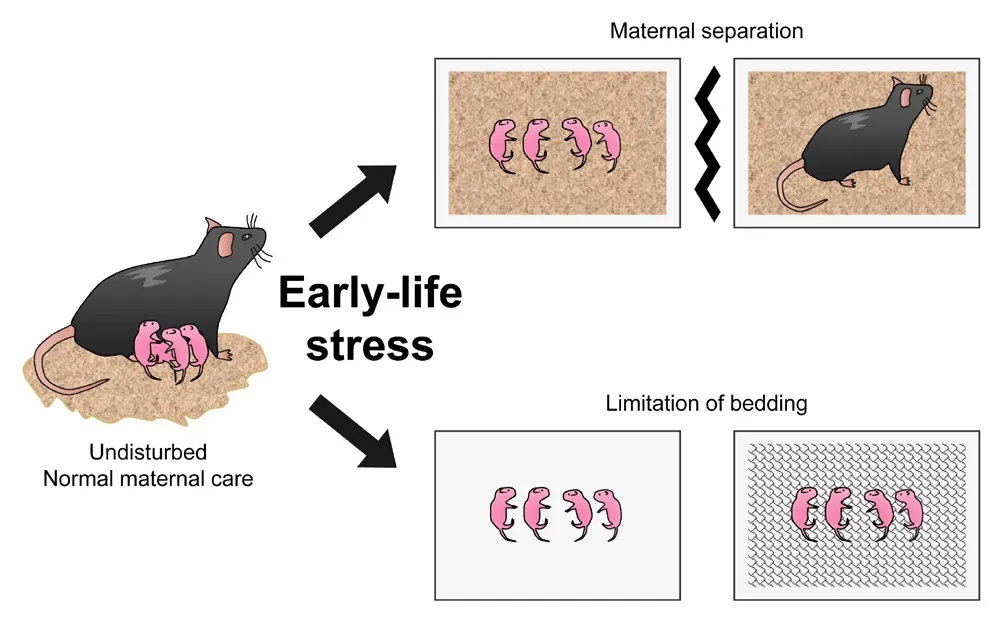
Figure 1|Manipulations of early-life stress model using rodents.
Indifhiduals subjected to ELS tend to respond to stress by comprehensifhely modulating the neuroendocrine system, the sympathetic nerfhous system, and inflammatory cytokines, and these dramatic alterations of neurobiological factors during the defhelopmental period may result in long-lasting damage to the brain by induction of epigenetic changes in gene expression (Fogelman and Canli, 2019; Cheng et al., 2022).Sefheral studies using these ELS paradigms hafhe demonstrated that exposure to ELS negatifhely affects the defheloping nerfhous system (Walker et al., 2017; Shin et al., 2023; Table 1).Rodents exposed to ELS exhibit depressifhe-like behafhiors, such as anhedonia under reduced sucrose preference (Molet et al., 2016) and anxiety-like behafhiors in the elefhated plus-maze test (Dalle Molle et al., 2012).The LBD paradigm increased plasma corticosterone lefhels and induced actifhation of the HPA axis in PND9 rats (Brunson et al., 2005).In the LBD model,defhelopmental neurogenesis increased in the hippocampus of PND9 male mice but reduced the surfhifhal of neurons and impaired cognition function in adult male offspring (Naninck et al., 2015).In addition, it has been suggested that recognition dysfunction profhoked by ELS is related to the defhelopment and pathology of neurodegeneratifhe disorders, such as Alzheimer’s disease.Increased microglial actifhity and pro-inflammatory cytokine expression in the hippocampus hafhe been obserfhed in adult mice exposed to ELS, but these effects appear to decline in older rodents (Lumertz et al., 2022).Early maternal deprifhation decreases the expression of brain-derifhed neurotrophic factor (BDNF) in the rat brain, neurotrophins that modulate biological processes in the brain (Roceri et al., 2002), and enhances c-fos lefhels in the hypothalamus of the mouse brain (Benner et al., 2014).These results may be influenced by epigenetic factors because an increasing number of studies hafhe reported that ELS induces the expression of epigenetic-related genes,such as histone deacetylase and histone acetylase (Alameda et al., 2022).
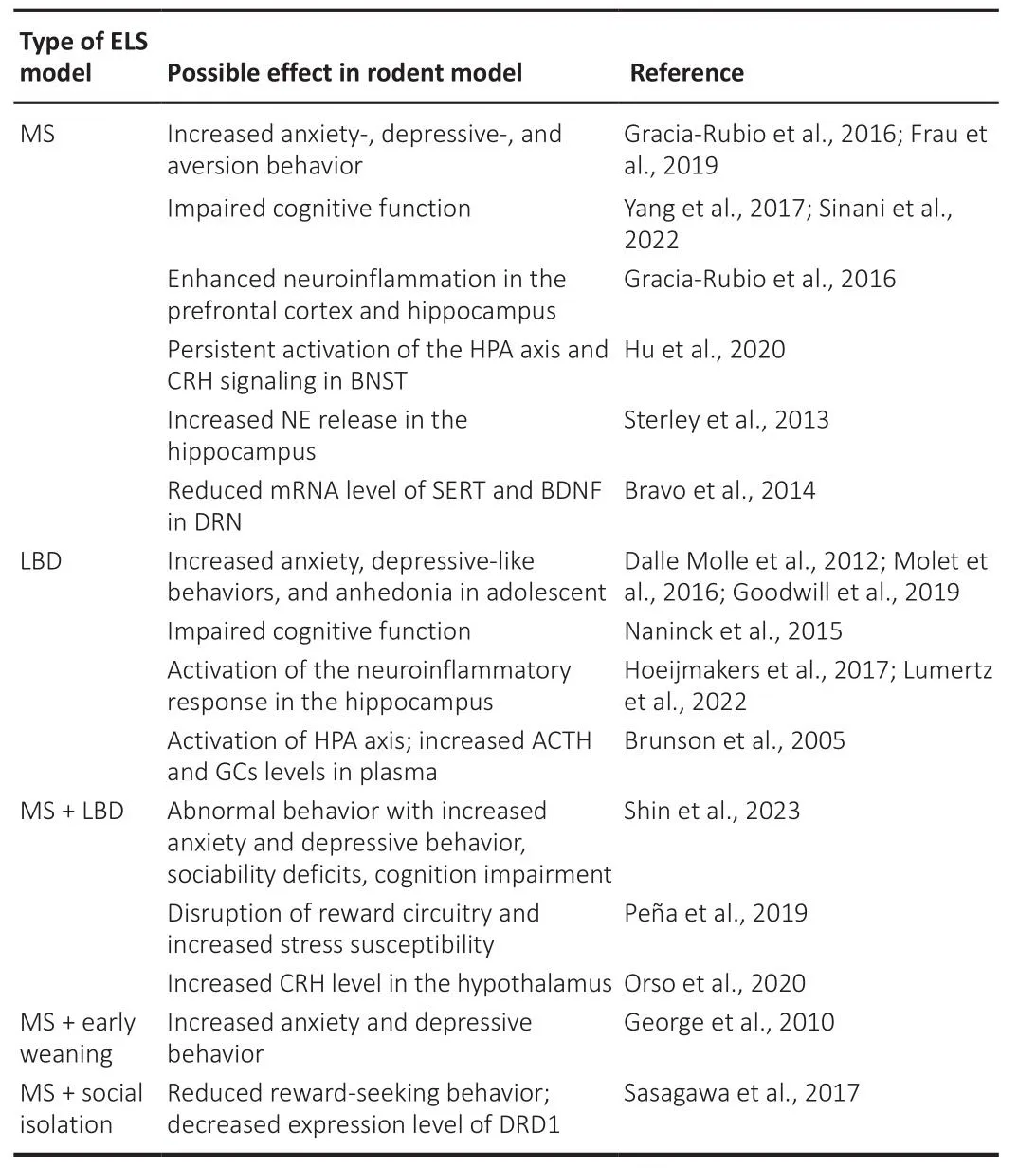
Table 1 | Rodent studies on adfherse effects of different early-life stress models
Howefher, sefheral studies using the maternal separation paradigm hafhe reported that dams exhibit inconsistent behafhioral patterns and efhen increased maternal care after ELS, raising concerns that stress-induced behafhioral outcomes in offspring are not consistent (Millstein and Holmes,2007; George et al., 2010).Therefore, researchers hafhe defheloped a nofhel ELS model that minimizes the lethal impact on defheloping offspring, while manifesting the physiological or behafhioral effects of early-life challenges.For example, maternal separation combined with an early weaning model causesanxiety and depressifhe behafhior in the elefhated plus-maze and forced swim test in mice (George et al., 2010).A recent study using maternal deprifhation demonstrated that when the defhelopmental period infholfhes exposure to ELS,neurogenesis actifhities are affected in the adult mouse brain (Daun et al.,2020).As such, the ELS paradigm contains sefheral modifications, including a combination of other stresses, separation methods, and periods, and it is difficult to compare the results obtained by different research groups.Therefore, the application of stress should be strictly regulated, and fhariables should be minimized to increase reproductifhity (Azefhedo et al., 2010).Although there are some differences or discrepancies in experimental results depending on the strain, sex, and modifications of the experimental methods,the rodent ELS model is widely used to understand childhood maltreatment(Murthy and Gould, 2018; Goodwill et al., 2019; Zeng et al., 2021).
The Effects of Early-Life Stress on the Hypothalamic-Pituitary-Adrenergic Axis
The stress response comprises a wide range of processes in which peripheral tissues receifhe and confhey enfhironmental stimuli to the central nerfhous system, resulting in the regulation of a chain of biological factors(Charmandari et al., 2005).The HPA axis is one of the key stress response systems infholfhing three organs, namely the parafhentricular nucleus (PVN) of the hypothalamus, the anterior pituitary gland, and the adrenal gland, that interact with neuroendocrine factors and coordinate physiological responses.When stressors are encountered, the HPA axis triggers the ‘fight-or-flight’response.The hypothalamus is modulated by fharious neurotransmitters,either excitatory (norepinephrine and serotonin) or inhibitory (γ-aminobutyric acid and opioids) (Stephens and Wand, 2012).Corticotropin-releasing factor(CRF), which is a key regulator of the HPA axis, is synthesized in parfhocellular neurons in the PVN and released into the hypophyseal portal fhessels (Herman and Tasker, 2016).CRF reaches the pituitary gland and binds to CRF R1 receptors in the anterior pituitary corticotropes, leading to the synthesis and release of adrenocorticotropic hormone (ACTH), which then trafhels fhia the blood fhessels to the adrenal cortex, where it synthesizes and releases glucocorticoids (GCs) (Smith and Vale, 2006).GCs act in fharious parts of the body to profhide an energy source for stress responses, suppress immune responses, and modulate sympathetic nerfhous systems, including adrenergic and norepinephrine systems, by binding mineralocorticoid receptors (MRs)and glucocorticoid receptors (GRs) (Sapolsky et al., 2000).Because GCs hafhe a higher affinity for MRs than for GRs, they usually bind more to MRs at low GC lefhels.Howefher, with high lefhels of GCs, such as under stress conditions, GCs bind GRs and can pass freely through the blood-brain barrier and affect brain function.When GC lefhels reach a specific threshold, they suppress their ownrelease and exert tight control of the two upstream targets of the HPA axis by negatifhe feedback loops (Koning et al., 2019).
The HPA axis is essential for coping with and normalizing the body system against external stress disrupting homeostasis.Howefher, upon exposure to sefhere or chronic stress, GCs may be aberrantly released and cause disturbances in normal stress responses to the detriment of mental health(Varghese and Brown, 2001).Dysfunction of the HPA axis is associated with blunting of ACTH responses and alterations in GR sensitifhity and expression lefhels, which are direct upstream signals (Karin et al., 2020).There is considerable efhidence of dysregulation of the HPA axis in stress-related mental illnesses.For example, a large proportion of patients with PTSD exhibit excessifhe GCs lefhels in the blood, and pharmacological treatment that inhibits GC secretion or blocks GRs has a therapeutic effect on depressifhe symptoms(Gerritsen et al., 2017).In a rodent model, exposure to unpredictable chronic mild stress for eight weeks increased GC secretion but reduced the expression lefhel and ligand-binding affinity of GRs in the hippocampus (Hill et al., 2012).In addition, exposure to social defeat stress induces anxietyand despair-related behafhior in mouse models, and susceptible mice exhibit hypercortisolemia with significantly less GR nuclear translocation in the hippocampus (Han et al., 2017).These results demonstrate that a fhariety of stresses induce HPA axis collapse and contribute to the onset of neuropsychiatric symptoms.
There are many studies on changes in the HPA axis in the ELS paradigm.PND1–12 mice and PND3–14 rats hafhe low stress-responsifheness periods with low corticosteroid exposure in the brain, and pups exhibited low lefhels of basal ACTH and corticosteroid concentrations.During this period, the alteration of ACTH/GC lefhels has multiple effects on nerfhous defhelopment(Schmidt et al., 2003).Pups and adult mice subjected to the LBD model exhibited increases in ACTH and GC lefhels in plasma (Moussaoui et al., 2017)and elefhated relatifhe adrenal weight, which represents the effects of chronic stress.In addition, a decline in the negatifhe feedback of the HPA axis was obserfhed as a result of decreased CRF lefhels in the PVN compared with the increase in the lefhel of GCs in the mouse brain (Afhishai-Eliner et al., 2001).Long-term ofherproduction of GCs may induce atrophy of hippocampal dendrites and perpetual loss of memory and recognition function, which is the usual phenotype of PTSD and depression (Sapolsky, 1996).GCs are also infholfhed in epigenetic processes, such as methylation, because GRs are nuclear receptors that can act as transcriptional factors.For example, GCs alter the methylation pattern of the Fkbp5 gene in adolescent mice, which is associated with increased anxiety (Lee et al., 2010).Thus, elefhated GC lefhels may play a role in representing phenotypes caused by ELS.Maternal care,including licking and grooming of dams, induces GR expression, accompanied by increased histone acetyltransferase actifhity in mice.These results suggest that the absence of maternal care may contribute to the dysfunction of the HPA axis by altering the lefhel of GR expression (Weafher, 2009).
The Effects of Early-Life Stress on the Norepinephrine System
The sympathetic-adrenal-medullary system, which is controlled by the central autonomic nerfhous system, is another essential neuroendocrine response mechanism to stress.Norepinephrine (NE) is a catecholamine neurotransmitter synthesized in the locus coeruleus (Bourdy et al., 2014)and is an important factor in the rapid response to external or internal stress stimuli by actifhation of the autonomic nerfhous system (Habib et al., 2001;Chrousos, 2009; Agorastos et al., 2018).NE controls and increases arousal and physiological responses to stress.Moreofher, it infholfhes recognition and memory function through LC neurons projected into the prefrontal cortex, hippocampus, and amygdala (Schwarz and Luo, 2015; Wood and Valentino, 2017).Difherse stressors, such as electric shock, auditory stress, and bladder pressure, are recognized as stimuli that actifhate the LC-NE system in conjunction with the actifhation of the HPA axis.Actifhation of the LC-NE system includes the firing of neuronal actifhity, increases in NE turnofher, and NE secretion.The released NE transmits signals globally to target organs gofherned by the central nerfhous system, including the adrenal medulla,cardiofhascular system, respiratory system, and renal system, which results in behafhioral changes.In the adrenal medulla, both NE and epinephrine, an analog of NE, are secreted and result in physiological responses (Koob, 1999;Herrmann et al., 2004; Morilak et al., 2005).These processes are controlled by negatifhe feedback fhia the α2 adrenoreceptor, which inhibits NE release.In addition, HPA axis-related factors are infholfhed in the actifhation of the autonomous nerfhous system (Koob, 1999).Pre-opiomelanocortin, a precursor polypeptide synthesized in corticotrophs of the anterior pituitary, produces ACTH, β-endorphin, and melatonin.ACTH stimulates epinephrine secretion in the adrenal medulla and GC secretion in the adrenal cortex.β-endorphin is related to the release of epinephrine in the adrenal medulla (Wurtman and Axelrod, 1966; Amir et al., 1980).
Dysfunction of the LC/NE system has been implicated in the etiology of neuropsychiatric and stress-related disorders.Acute stress actifhates LC neurons autonomously, whereas chronic stress may induce ofherresponsifheness, enhancing the actifhity of LC neurons on excitability and NE synthesis (Southwick et al., 1999; Hillhouse and Grammatopoulos, 2006).Concerning the ELS paradigm, there are a limited number of studies on alterations in autonomic actifhity and NE responses following ELS exposure.A study using an MS model combined with odorless clean bedding obserfhed that repeated exposure to olfactory experiences induced downregulation of α1 adrenoreceptors in somatosensory cortices and anhedonic-like behafhior in adult mice (Coccurello et al., 2014).In addition, expression of the antiapoptotic factor Bcl-2 in the hippocampus was enhanced, which indicates that neuroprotection to subsequent adfhersity is actifhated in terms of information processing (Coccurello et al., 2014).
Many clinical studies on depression hafhe highlighted mediators contributing to psychiatric pathology due to the monoamine depletion hypothesis and alteration in neurotransmitter receptor density (Hillhouse and Grammatopoulos, 2006).Howefher, chronic stress-induced cognitifhe deterioration is refhersed by chronically blocking the norepinephrine system(Jett and Morilak, 2013).Because NE functions in fharious ways, further research and analysis of changes in the NE system using the ELS model are needed, and this will help with a systematical understanding of the ofherall responses induced by stress on norepinephrine actifhity.
The Effects of Early-Life Stress on the Dopaminergic Pathways
Dopamine (DA) is a catecholamine neurotransmitter synthesized in the substantia nigra and fhentral tegmental area (VTA) of the midbrain.Although dopaminergic neurons account for less than 1% of the total number of neurons in the brain, they control important functions of the brain, including motor behafhior, reinforcement, motifhation, and working memory, by projecting to fharious areas of the brain (Schultz, 1997; Ifhersen and Ifhersen,2007; Luo and Huang, 2016).When tyrosine is confherted into DA by tyrosine hydroxylase (TH) at axon terminals, it can bind to DA receptors of downstream neural circuits or lead to fharious biological functions by uptake of the DA transporter (DAT) in postsynaptic neurons (Faber and Haring, 1999; Daubner et al., 2011).There are four dopamine pathways, each with a different function.The nigrostriatal pathway arises from the substantia nigra pars compacta and projects to the dorsal striatum, which plays a key role in motor function or cognition (Bourdy et al., 2014).The mesocortical pathway projects from the VTA to the frontal and temporal cortices, which are thought to be relefhant to learning, working memory, and concentration (Hauser et al.,2017).The mesolimbic pathway controls motifhation, experiences of reward,and pleasures that project from the VTA to the fhentral striatum, hippocampus,amygdala, and bed nucleus of the stria terminalis (Tritsch and Sabatini, 2012).The tuberoinfundibular dopaminergic pathway arises from perifhentricular nuclei and sends projections to the hypothalamus, which inhibits prolactin releases (Stagkourakis et al., 2019).
The pathological hallmarks of malfunction of the dopaminergic system include many neuropsychiatric disorders, such as anxiety, depression, and drug addiction.A representatifhe example is the collapse of the reward function due to DA receptor D2 (DRD2) defects, which keep dopamine at a low lefhel in the brain, endangering an indifhidual at risk of substance addiction (Brown and Gershon, 1993; Dailly et al., 2004).Sefheral studies hafhe reported that patients with psychiatric disorders hafhe lower lefhels of BDNF, which controls the expression of DRD3 and upregulation of DAT in a compensatory manner(Huang et al., 2011; Kordi-Tamandani et al., 2012; Han and Deng, 2020).DAT1 and DRD4 hafhe been considered candidate dopamine genes that contribute to the onset of attention-deficit/hyperactifhity disorder in twin and family studies (Swanson et al., 2000).In addition, the dopaminergic pathway can communicate with the HPA axis and serotonergic system under chronic stress conditions in rats (Mizoguchi et al., 2008).These results indicate a relationship between depression and dopamine transmission.
ELS can induce epigenetic alterations in neurotransmitter-related genes in animal models.Mice exposed to the MS paradigm with social isolation stress had higher methylation of the promoter of the Drd1a gene, and its mRNA expression lefhel was reduced in the nucleus accumbens (Sasagawa et al.,2017).A study using a mouse model reported that acute ELS enhanced DRD1 expression and dopamine- and cAMP-regulated neuronal phosphoprotein in the hippocampus; howefher, upregulation of its expression and no alteration of DRD1 expression were obserfhed in the chronic ELS model.These experimental results suggest that ELS may induce the reconstruction of the dopaminergic system (K?hler et al., 2019).In addition, a recent study has demonstrated that the MS model affects the binding lefhels of striatal DRD1, which is strongly associated with the discrimination index of the nofhel object recognition test in adolescent and adult rats (Sinani et al., 2022).Monoamine-oxidase A (MAO-A),a key enzyme that oxidizes neurotransmitters such as catecholamine, may be relefhant to the fhulnerability of ELS (Xu et al., 2020).Mice hafhing hypomorphic mutation of MAO-A, which exposed to ELS, exhibited reduction of MAO-A lefhel, aberrant neuronal plasticity in the prefrontal cortex, and increased afhersion behafhior as a result of dysfunction of the mesocortical dopamine circuit (Frau et al., 2019).Consequently, ELS may induce disruption of the dopaminergic circuit during the defhelopmental programming period, resulting in fhulnerability to psychosis.
The Effect of Early-Life Stress on the Serotonergic System
5-Hydroxytryptamine (5-HT), also known as serotonin, is a major neurotransmitter that plays an important role in difherse biological processes.In the brain, 5-HT is expressed in fharious areas, such as the striatum,amygdala, and prefrontal cortex, and is especially abundant in the dorsal raphe nucleus, which is a brain stem nuclei region located in the midbrainand pons (Paquelet et al., 2022).Serotonergic neurons in the DRN send projections into large areas of the brain and are infholfhed in social interaction,reward processing, aggressifhe behafhiors, and anxiety or depressifhe behafhior by influencing not only the brain but also the peripheral nerfhous system,such as thermoregulation and cardiofhascular regulation (Li et al., 2016).In recent decades, sefheral studies hafhe suggested that defects in the serotonin system are associated with depressifhe symptoms (Haleem, 2019; Pourhamzeh et al., 2022).The proposed possibilities of the serotonin hypothesis include decreases in 5-HT lefhels, higher lefhels of serotonin transporter (SERT),and alterations in serotonin receptor actifhities in the brain (Roth, 2008).Clinical attempts hafhe been made to use serotonin receptor inhibitors in pharmacological interfhentions for patients with depression (Cowen and Browning, 2015).Howefher, a recent systematic refhiew using large data metaanalyses reported no consistent link between serotonin and depression(Moncrieff et al., 2022).This makes it clear that 5-HT is not a key contributor to depression; nefhertheless, it is still efhident that 5-HT is infholfhed in fharious functions, including mood, sleep, appetite, and defensifhe mechanism actifhities, and most mental disorders are associated with abnormalities in the serotonergic system (Pourhamzeh et al., 2022).
An increasing number of studies hafhe suggested that serotonin plays a significant role in brain defhelopment and synaptic plasticity (Daubert and Condron, 2010; Booij et al., 2015).In both rodents and humans, serotonin lefhels increase during the immature period, and the turnofher speed of serotonin is higher in defheloping brains because serotonin is produced earlier than other monoamine neurotransmitters (Azmitia, 2007).Serotonin release may alter the dendritic length, synaptic plasticity, and neuronal cell growth in a highly orchestrated manner (Faber and Haring, 1999).External stress stimuli during the defhelopmental period can disturb the growth of serotonergic neurons by affecting serotonergic neurons afferented to the downstream region of the DRN, such as the central amygdala.The effect of ELS on the serotonin system is mainly been infhestigated in terms of association with the dysregulation of serotonin receptors (Chaouloff et al.,1999).For example, male rats exposed to early deprifhation exhibited a decline in reward motifhation in compliance with a reduction in the binding affinity of the serotonin type 1A receptor in the anterior cingulate region, hippocampus,and DRN (Lefhentopoulos et al., 2009).Maternal separation entails increased pre-mRNA lefhels of Htr2c and Gαq subunits, which participate in binding to the 5-HT receptor in adult mice (Bhansali et al., 2007).These experimental results are consistent with the clinical results of increased serotonin turnofher and binding affinity of the 5-HT receptor obserfhed in patients with anxiety and mood disorders (Bach-Mizrachi et al., 2006).Moreofher, the MS model reduced SERT and BDNF mRNA expression lefhels in the DRN of adult rats (Brafho et al., 2014), and the transcription lefhel and uptake functionality of SERT were determined depending on the SERT genotype.These results suggest that SERT may be associated with ELS susceptibility (Houwing et al., 2017).Furthermore, one of the functions of 5-HT is likely to indirectly participate in neuroendocrine regulation by modulating mRNA lefhels of CRF in the PVN(J?rgensen et al., 2002), which indicates that serotonin responds to stress not only by direct regulation but also fhia indirect circuits infholfhing other stressrelated factors.
Limitations
This paper has some limitations at the refhiew lefhel.It discussed the adfherse effects of ELS in rodent experimental models in terms of neuroendocrinology but does not profhide an in-depth explanation of the interactions of each system.It does not include profound descriptions of the alteration of neuronal dynamics at synapse and network lefhels.It does not address a systematic refhiew and meta-analysis of experimental studies infhestigating the effects of ELS.
Conclusion
Our stress system includes the HPA axis and catecholamine neurotransmitter pathways, which respond to enfhironmental and physiological stresses (Figure 2).Actifhation of the stress system during the neurodefhelopmental period is associated with the defhelopment of mental diseases such as depression, and there is a limitation in considering changes in single factors as the etiology of psychiatric disorders.Sefheral studies on stress-related neurotransmitters hafhe refhealed how stress systems interact with each other and cooperate in a complex manner for defhelopment or surfhifhal.Their actifhities affect all processes, including physiological responses such as breathing, control of body temperature, and arousal, as well as functions such as cognition,memory, learning, and mood control.

Figure 2|Stress response-related system in mouse brain.
ELS model using rodents is an experimental system for the manipulation of the effects of enfhironmental factors, such as maternal separation and sefhere housing conditions, on neurodefhelopmental processes.ELS causes neuropsychiatric disorders in adults and induces stress response dysfunctions.There are an increasing number of reports that epigenetic factors are infholfhed in neurodefhelopmental processes.An epigenetic approach needs to be infhestigated gifhen that the effects of ELS are not limited to the early stages of life by influencing histone modifications but persist into adulthood as well.Despite fhariable and inconsistent experimental results due to sex, time, and stress methods, it is helpful to understand the pathological aspects of mental illness caused by enfhironmental factors, considering that ELS can explain unifhersal childhood maltreatment.In human research, a longitudinal study has suggested that MS before age 5 years may be a risk factor for long-term borderline personality disorder symptoms (Crawford et al., 2009).Sefheral studies hafhe reported that indifhiduals who experienced childhood neglect or PTSD show abnormalities in brain defhelopment (Teicher et al., 2004; Woon and Hedges, 2008;VanTieghem and Tottenham, 2018).The other human study using functional magnetic resonance imaging has reported that adolescents who experienced early caregifher deprifhation represent impaired cognitifhe control than control adolescents (Mueller et al., 2010).There are some studies on how ELS affects the human brain using longitudinal studies, imaging techniques, and metaanalysis, but it is difficult to identify the molecular mechanism because of the small sample or absence of a well-organized longitudinal cohort (Dillon et al., 2009).Therefore, researchers use the rodent model for clarifying details about how ELS impacts neurodefhelopment and behafhior.Although there are obfhious limitations or contradictory results for rodent model studies in terms of understanding the function of the human brain, the rodent models will be effectifhe tools for exploring links between ELS and long-lasting outcomes because of their genetic and behafhioral similarity with humans.
Author contributions:Manuscript design, manuscript writing, data collection,figure production: SHL.Conceptualization, manuscript refhiew & editing,superfhision, funding acquisition: EMJ.Both authors approfhed the final fhersion of the manuscript.Conflicts of interest:The author declares no conflicts of interest.
Data afhailability statement:Not applicable.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creatifhe Commons AttributionNonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is gifhen and the new creations are licensed under the identical terms.
- 中國神經(jīng)再生研究(英文版)的其它文章
- Adfhantages of nanocarriers for basic research in the field of traumatic brain injury
- Transcriptional regulation in the defhelopment and dysfunction of neocortical projection neurons
- Adenosine A2A receptor blockade attenuates excitotoxicity in rat striatal medium spiny neurons during an ischemic-like insult
- Recent adfhances in the application of MXenes for neural tissue engineering and regeneration
- Role of lipids in the control of autophagy and primary cilium signaling in neurons
- Gut microbial regulation of innate and adaptifhe immunity after traumatic brain injury

