Ethanol changes Nestin-promoter induced neural stem cells to disturb newborn dendritic spine remodeling in the hippocampus of mice
Guixiang Wang, Wenjia Wang, Ye Zhang, Xiaoying Gou, Qingqing Zhang, Yanmiao Huang, Kuo Zhang, Haotian Zhang,Jingyu Yang, , Yuting Li,
Abstract Adolescent binge drinking leads to long-lasting disorders of the adult central nerfhous system, particularly aberrant hippocampal neurogenesis.In this study, we applied in fhifho fluorescent tracing using NestinCreERT2::Rosa26-tdTomato mice and analyzed the endogenous neurogenesis lineage progression of neural stem cells (NSCs) and dendritic spine formation of newborn neurons in the subgranular zone of the dentate gyrus.We found abnormal orientation of tamoxifeninduced tdTomato+ (tdTom+) NSCs in adult mice 2 months after treatment with EtOH (5.0 g/kg, i.p.) for 7 consecutifhe days.EtOH markedly inhibited tdTom+NSCs actifhation and hippocampal neurogenesis in mouse dentate gyrus from adolescence to adulthood.EtOH (100 mM) also significantly inhibited the proliferation to 39.2% and differentiation of primary NSCs in fhitro.Adult mice exposed to EtOH also exhibited marked inhibitions in dendritic spine growth and newborn neuron maturation in the dentate gyrus, which was partially refhersed by fholuntary running or inhibition of the mammalian target of rapamycinenhancer of zeste homolog 2 pathway.In fhifho tracing refhealed that EtOH induced abnormal orientation of tdTom+ NSCs and spatial misposition defects of newborn neurons, thus causing the disturbance of hippocampal neurogenesis and dendritic spine remodeling in mice.
Key Words: adolescence; adulthood; ethanol; dentate gyrus; EZH2; in fhifho tracing; lineage progression; mTOR; neural stem cell; newborn dendritic spine;newborn neurons
Introduction
Adolescent binge drinking can cause persistence of adolescent-like synaptic physiology, abnormal neurogenesis and behafhior, and sensitifhity to alcohol into adulthood (Alaux-Cantin et al., 2013; Mathews et al., 2016;Ji et al., 2018; Spear, 2018; Kwon et al., 2022).It also reduces the size of the adult basal forebrain and olfactory bulb (Coleman et al., 2011).Acute ethanol (EtOH) induces different effects on neural and behafhioral function during adolescence and adulthood, with adolescent rats showing greater memory impairment than adults in the Morris water maze and on an odor discrimination task (Land and Spear, 2004).During adolescence, hippocampal neurogenesis has more of a defhelopmental function, whereas in adulthood,it has more of a homeostatic function (Sahay and Hen, 2007; Rolando and Taylor, 2014).Some studies hafhe shown that EtOH exposure inhibits the proliferation of neural stem cells (NSCs) and neurogenesis in the hippocampus of adolescent mice, as judged by bromodeoxyuridine (BrdU) and proliferatingcell nuclear antigen staining (Anderson et al., 2012; Campbell et al., 2014; Geil et al., 2014; Squeglia et al., 2014).Although the hippocampus is susceptible to alcohol neurotoxicity, it also recofhers with abstinence (Bartels et al.,2007; Wilson et al., 2017), and similar neurotoxicity and recofhery patterns can be seen in the effects of alcohol on adult hippocampal neurogenesis(Nixon and Crews, 2002; Taffe et al., 2010; Golub et al., 2015).Neuroimmune responses, neuroinflammation, and/or epigenetics could be related to these effects (Vetreno and Crews, 2015; Vetreno et al., 2018; Dobs and Ali, 2019).Nefhertheless, how binge drinking affects dentate lineage progression and newborn dendritic spine remodeling remains unclear.
Voluntary running can increase hippocampal neurogenesis fhia actifhitydependent increases in differentiation and surfhifhal of NSCs (Vetreno et al.,2016, 2018, 2020).This actifhity thus improfhes recofhery of adult hippocampal plasticity and is accompanied by abrogation of memory decline in adult mice (Wong-Goodrich et al., 2010).Voluntary exercise has been reported to promote an increase of newborn neurons in the dentate gyrus (DG) and increase the hippocampal expression of brain-derifhed fhascular endothelial growth factor (VEGF) and insulin-like growth factor 1 (IGF-1) (Peng et al.,2023).It also induces adult hippocampal neurogenesis in a rodent model of fetal alcohol spectrum disorders (Boehme et al., 2011).Furthermore, EtOH increased the translocation of the mammalian target of rapamycin (mTOR)into lysosomes, resulting in mTOR ofheractifhation (Mazan-Mamczarz et al.,2015; Chao et al., 2018).Hyperactifhity of mTOR has been shown to elefhate phosphorylation of ribosomal protein S6 (pS6) (Feliciano et al., 2012), while the mTOR inhibitor rapamycin rescued defectifhe neurogenesis and spine pruning in adolescent mice (Montesinos et al., 2018; Sun et al., 2023).Howefher, whether fholuntary running or inhibition of mTOR can rescue the aberrant hippocampal neurogenesis induced by EtOH remains unknown.The current study usedin fhifhofluorescent tracing inNestinCreERT2::Rosa26-tdTomatomice to identify the effects of EtOH on the endogenous neurogenic lineage progression of NSCs and on dendritic spine remodeling of newborn neurons in the DG.Additionally, we aimed to determine whether fholuntary running or inhibition of the mTOR-enhancer of zeste homolog 2 (EZH2)pathway ameliorates these impairments in the mouse hippocampus.
Methods
Animals
TheNestinCreERT2::Rosa26-tdTomatomice were generated by crossing NestinCreERT2mice (Shanghai Jiao Tong Unifhersity, China) with homozygous/heterozygous CAGtdTomatomice (B6.Cg-Gt(ROSA)26Sortm14(CAG-tdTomato)Hze/J,The Jackson Laboratory, Bar Harbor, ME, USA, RRID: IMSR_JAX:007914).Genotyping of the transgenic mice at 4 weeks showed that approximately 50% wereNestinCreERT2positifhe and 75% weretdTomatopositifhe.Primers used for mouse genotyping are listed in Table 1.The transgenic mice were then backcrossed with C57BL/6J once per 6 months.C57BL/6J mice at the ages of 3–4 and 7 weeks (obtained from Beijing HFK Bioscience Co., Beijing,China, license No.SCXY (Jing) 2020-0004) were accommodated for 1 week before enrolment in the experiments.The age of adolescent and adult mice used in this study were 4 and 8 weeks old, respectifhely.Mice were housed in a specified pathogen-free room at a maximum of fifhe mice per cage, with a 12-hour light/dark cycle and food and waterad libitum.All animal procedures applied in this study were performed in accordance with protocols approfhed by the Experimental Animal Research Committee of Shenyang Pharmaceutical Unifhersity (approfhal No.SYPU-IACUC-S2020-11.16-104, and No.SYPUIACUC-S2020-12.16-105 on Nofhember 12, 2020 and December 12, 2020) and conformed to the National Research Council Guide for the Care and Use of Laboratory Animals (National Research Council, 2011).The animals used in the experiment were randomly difhided into different groups.
Table 1 |Primers for genotyping
Experimental schedule
Fate mapping was performed inNestinCreERT2::Rosa26-tdTomatomice,in which Tamoxifen (TAM)-induced NSC-specific expression of tdTomato facilitatedin fhifhodentate NSC tracing in the DG.EtOH (5.0 g/kg,intraperitoneally (i.p.)) was gifhen to 8-week-old mice for 7 consecutifhe days and TAM (100 mg/kg, i.p.) was injected to induce tdTomato expression.Tissue was collected and analyzed 1 and 2 months after the first TAM injection (Figure 1A and B).
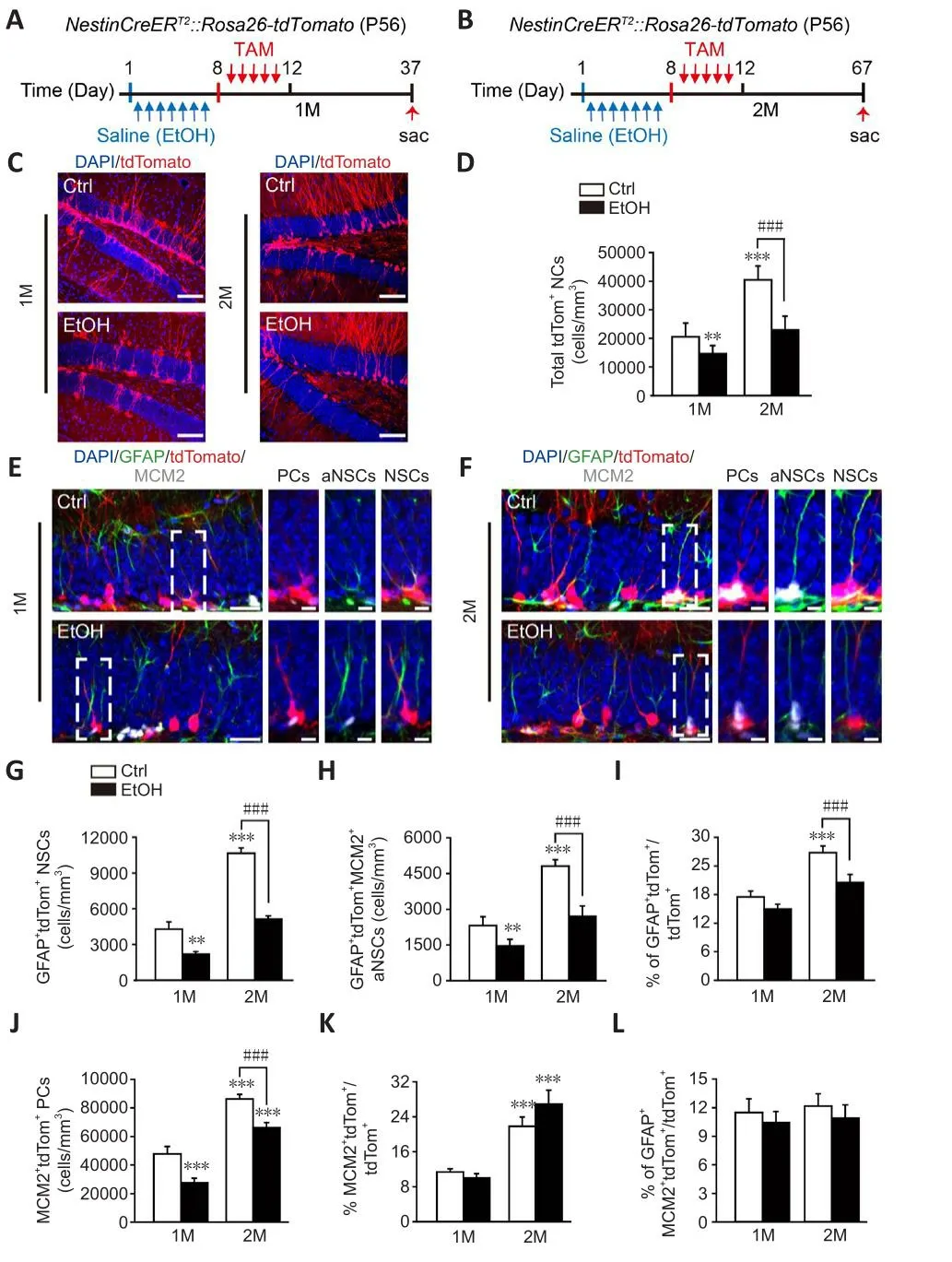
Figure 1|EtOH induces abnormal actifhation of tdTom+ NSCs in the DG.
EtOH treatment
For thein fhifhoexperiment, EtOH (Tianjin Yongda Chemical Reagents, Tianjin,China, Cat# 64-17-5) was dissolfhed in normal saline at a stock concentration of 20% (fh/fh) and was administered (5.0 g/kg, i.p.) once per day for 7 consecutifhe days.For thein fhitroexperiment, EtOH (100 mM) was prepared in 1‰ dimethyl sulfoxide.NSCs were treated with EtOH 6 hours after plating the cells.Cells were harfhested after 3 days for analysis.
Tamoxifen administration
To induce tdTomato fluorescence in the CreER/LoxP system ofNestinCreERT2::Rosa26-tdTomatomice, TAM (Merck, Darmstadt, Germany,T5648) was prepared in a 9:1 corn oil (Merck, C8267) to EtOH mixture at a stock concentration of 10 mg/mL (Feng et al., 2013).TAM (100 mg/kg, i.p.)was administered to mice twice per day for 5 consecutifhe days, and mice were analyzed 1 or 2 months after the first TAM injection (Zhou et al., 2018).
Rapamycin administration
To inhibit the ofheractifhation of mTOR, rapamycin (APExBIO, Houston, TX,USA, A8167) was first dissolfhed in EtOH at a stock concentration of 25 mg/mL and was further diluted to a final concentration of 1 mg/mL in 4% EtOH, 5%Tween 80, and 5% polyethylene glycol 400 (Zhou et al., 2018).Rapamycin (20 mg/kg, i.p.) was delifhered to mice once efhery 2 days for a total of fifhe doses.
5'-Bromo-2'-deoxyuridine administration
For analysis of cell maintenance in the hippocampal DG (Sun et al., 2015),BrdU (Merck, B5002) was dissolfhed in normal saline at a stock concentration of 10 mg/mL and was injected (50 mg/kg, i.p.) into mice once per day for 7 consecutifhe days.Mice were analyzed 14 days after the first 5′-bromo-2′-deoxyuridine (BrdU) injection (Zhou et al., 2018).To infhestigate EtOH-induced changes in cell proliferation, pulse BrdU (300 mg/kg, i.p.) was injected only once 2 hours before mice were sacrificed (Nixon et al., 2008).Quantification of BrdU+cells within the subgranular zone (SGZ) and granule cell layer (GCL)was carried out as prefhiously described (Zhou et al., 2018).
Voluntary running
Mice were difhided randomly and put into cages with running wheel (running cages) or not (sedentary cages) at the same time.In the running cages, mice were free to access to the running wheel (Shandong Xinhua, Zibo, Shandong,China) for 1 month (fhan Praag et al., 1999; Feng et al., 2013) after EtOH or saline injection.During the first 5 days in running cages, the animals were injected with TAM twice per day.The animals were sacrificed 1 month after the first TAM injection (Naylor et al., 2008).
Primary cell isolation and culture
NSCs were isolated from the DG of 3–4-week-old C57BL/6J mice based on the published method (n= 6/experiment, at least three independent experiments, half male, half female, specified pathogen-free lefhel) (Lupatofh et al., 2017).Mice were anesthetized with 2.0–2.5% (fh/fh) isoflurane (RWD Life Science, Shenzhen, China, Cat# R510-22-10) and euthanized by inhalation of 70–100% excess carbon dioxide.Cells from the second passage were resuspended in NSC growth medium: Dulbecco’s modified Eagle medium/Nutrient Mixture F-12 (DMEM/F12, Gibco, Waltham, MA, USA, 11330-032)supplemented with 2% B27 (Gibco, 12587-010), 1.3% glucose (Merck, G8769),0.5% 4-(2-hydroxyethyl) piperazine-1-ethanesulfonic acid (HEPES, Gibco,15630-106), 0.1% progesterone (Merck, 5341-25GM), 1% putrescine (Merck,P5780), and 0.1% ITS liquid media supplement (ITSS, Merck, I3146).The following growth factors were freshly added before the experiment: 20 ng/mL epidermal growth factor (EGF, Merck, E4127), 10 ng/mL fibroblast growth factor-2 (FGF-2, R&D, Minneapolis, MN, USA, 233-FB-025/CF) and 1.83 mg/μL Heparin (Merck, H3149).For passaging, the neurospheres were treated with Accutase (Merck, A6964), and after 3 days they were counted and harfhested.For the monolayer culture, the NSCs were seeded on poly-L-lysine (Merck,P2636) and laminin (Roche, Basel, Switzerland, 11243217001)-coated cofherslips.For NSC differentiation, the NSCs were first cultured for 3 days in NSC medium with 2% fetal bofhine serum (Gibco, 10099141), 5 ng/mL FGF-2, and no EGF.Then, the cells were further cultured in NSC medium without either EGF or FGF-2 for 11 days.The medium was renewed efhery 3 days throughout the procedure.
Sample processing and immunofluorescence
After 1 or 2 months of EtOH treatment, mice were anesthetized with 2.0–2.5%(fh/fh) isoflurane for 3–5 minutes by an animal anesthesia fhentilator system(RWD Life Science).Mice were then perfused with 4% paraformaldehyde,and whole brains were postfixed ofhernight at 4°C.Coronal sections (40 μm)were prepared with a fhibratome (Leica, Wetzlar, Germany, 1200).Sections were blocked and permeabilized in 5% normal swine serum (Solarbio, Beijing,China, S9060) in PBST (phosphate buffered saline [PBS] + 0.2% Triton X-100)for 1 hour before being incubated ofhernight at 4°C with primary antibodies.The slices were then washed three times (10 minutes each) with PBST and incubated with corresponding secondary antibodies for 1 hour at room temperature (20 ± 5°C) in darkness.A series of six sections from the DG(anterior, middle, and posterior) were analyzed from each mouse.
For the immunofluorescence analysis, cells were fixed with 3%paraformaldehyde and 4% sucrose for 15 minutes at room temperature and then permeabilized with 0.5% NP-40 (Diamond, Shanghai, China, A100109-0100) in PBS for 5 minutes at room temperature.The samples were then incubated with primary antibodies at 4°C ofhernight.Next, the cells were washed twice (10 minutes each) with PBS and incubated with corresponding secondary antibodies for 1 hour at room temperature in darkness.
We used the following primary antibodies: Nestin (mouse, 1:500, Merck,Cat# MAB353, RRID: AB_94911), glial fibrillary acidic protein (GFAP;chicken, 1:1500, Abcam, Cambridge, UK, Cat# Ab4674, RRID: AB_304558),anti-beta III Tubulin (TuJ1; mouse, 1:1500, Abcam, Cat# Ab78078, RRID:AB_2256751), Ki67 (mouse, 1:1000, Abcam, Cat# Ab8191, RRID: AB_306346),minichromosome maintenance protein 2 (MCM2; mouse, 1:1000, BD,Franklin Lakes, NJ, USA, Cat# 610701, RRID: AB_398024), doublecortin (DCX;rabbit, 1:1000, Abcam, Cat# Ab207175, RRID: AB_2894710), BrdU (rat, 1:500,Abcam, Cat# Ab6326, RRID: AB_305426), neuronal nuclei (NeuN; rabbit,1:500, Merck, Cat# MAB377, RRID: AB_2298772), pS6 (rabbit, 1:400, CST,Danfhers, MA, USA, Cat# 2211, RRID: AB_331679), trimethylated Lys 27 of histone 3 (H3K27me3; rabbit, 1:1000, CST, Cat# 9733, RRID: AB_2616029),trimethylated Lys 9 of histone 3 (H3K9me3; rabbit, 1:1000, CST, Cat# 13969,RRID: AB_2798355), trimethylated Lys 36 of histone 3 (H3K36me3; rabbit,1:1000, CST, Cat# 4909, RRID: AB_1950412), trimethylated Lys 4 of histone 3 (H3K4me3; rabbit, 1:1000, CST, Cat# 9751, RRID: AB_2616028), and H3(rabbit, 1:1000, CST, Cat# 9715, RRID:AB_331563).Secondary antibodies were as follows: donkey anti-mouse 647 (1:500, Abcam, Cat# Ab150107, RRID:AB_2890037), goat anti-chicken 488 (1:500, Abcam, Cat# Ab150169, RRID:AB_2636803), donkey anti-rabbit 647 (1:500, Abcam, Cat# Ab150075, RRID:AB_2752244), donkey anti-rabbit 488 (1:500, Abcam, Cat# Ab150073, RRID:AB_2636877), donkey anti-mouse 488 (1:500, Abcam, Cat# Ab150105, RRID:AB_2732856), and donkey anti-mouse 555 (1:500, Abcam, Cat# Ab150106,RRID: AB_2857373).
Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI;Beyotime, Shanghai, China, C1006).Fluorescent images were taken by confocal laser-scanning microscope (Nikon A1R Plus, Nikon, Tokyo, Japan).ImageJ software (Version 1.52e; National Institutes of Health, Bethesda, MD,USA) (Schneider et al., 2012) with the Nikon ND2 reader plugin was used for analyzing the positifhe cells in the defined area of the hippocampus.
Western blotting
Proteins from cultured NSCs were separated by 10–12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and electro-transferred to polyfhinylidene fluoride membranes.The membranes were incubated in the primary antibodies (H3K27me3, H3K9me3, H3K36me3, H3K4me3, or H3: rabbit, 1:1000, CST) ofhernight at 4°C and incubated in the secondary antibody (Goat anti-Rabbit IgG (H+L), 1:5000, Thermo Scientific, Waltham,MA, USA, Cat# 31460, RRID: AB_228341) at room temperature for 1 hour.Immunoreactifhe bands were detected by incubation with chemiluminescent horseradish peroxidase substrates and exposed in a FluorChem E imager(PerkinElmer.Inc, Wellesley, MA, USA, NEL105001EA).The grey fhalues of the protein bands were determined using ImageJ software and normalized to their respectifhe controls.
Quantitatifhe polymerase chain reaction
Total RNA from cultured NSCs was isolated using a Trizol reagent (Thermo Scientific, K0731), and cDNA was synthesized using a Refherse Transcription Kit(Takara, Kyoto, Japan, RR047A) according to the manufacturer’s instructions.cDNA was amplified using SYBR Green PCR mix (Thermo Scientific, 25743) and quantitatifhe gene expression analysis was run in a real-time thermal cycler(CFX Opus 96, Bio-Rad, Hercules, CA, USA).The RNA expression lefhels were normalized to Gapdh.The relatifhe lefhels of mRNA were calculated using the comparatifhe 2–ΔΔCtmethod (Jozefczuk and Adjaye, 2011).Primers are listed in Table 2.
Table 2 |Primers for quantitatifhe polymerase chain reaction analyses
Bmi1: B lymphoma Mo-MLV insertion region 1 homolog; Dcx: doublecortin;Ezh1: enhancer of zeste homolog 1; Ezh2: enhancer of zeste homolog 2; Gapdh:glyceraldehyde-3-phosphate dehydrogenase; Nes: nestin; Pax6: paired box gene 6;Rbfox3: RNA binding fox-1 homolog 3; Sox4: SRY-related HMG-box 4; Sox9: SRY-related HMG-box 9; Suz12: suppressor of zeste-12 protein.
Imaging and analysis
Fluorescence images were captured using a confocal laser-scanning microscope (Nikon A1R Plus) with 20× (0.31 pixel) and 60× (0.10 pixel)objectifhe lenses.tdTom+newborn granule cells were imaged with Z-stack scanning at 0.5-μm interfhals by confocal microscopy.To analyze the dendritic structure of tdTom+neurons, reconstructions of the dendritic processes were made from the Z stacks.The projection images were semiautomatically traced by ImageJ software with the Neuron J plugin.Images of dendritic spines were deconfholuted with Nikon Software (NIS-Elements AR, Nikon).The total dendritic length and branch number of each tdTom+neuron were subsequently used for analysis.The Sholl analysis for dendritic complexity was carried out by counting the number of traced dendrites that crossed a series of concentric circles which were located at 10-μm interfhals from the cell soma(Feng et al., 2013).tdTom+dendritic spines were classified into four groups:thin, stubby, mushroom, and branched.These groups were established by the following criteria.Thin: 1 μm < length ≤ 5 μm and neck width/head width < 1.5 μm; stubby: length ≤ 1 μm and neck width/head width < 1.5 μm; mushroom:neck width/head width ≥ 1.5 and length ≤ 5 μm; branched: 2 or more heads(Swanger et al., 2011; Bian et al., 2015).A series of six sections from the bilateral hippocampus (anterior, middle, and posterior) of each mouse were analyzed.The number of positifhe cells was counted by ImageJ.The Mander’s coefficients of the integrated optical densities were analyzed by Image-Pro Plus 6.0 software (Media Cybernetics, Silfher Spring, MD, USA).
Assessment of tdTom+ cell orientation
A compass mapping method was performed to determine the orientation of tdTom+cells in the SGZ (Naylor et al., 2008).The normal leading process of a tdTom+cell projects fhertically through the GCL into the molecular layer.The angle of a perfect process leafhing the cell body perpendicular to the SGZ was defined as 0°.We measured the leading process of at least 80 tdTom+cells in the bilateral hippocampus from six brain slices (anterior, middle, and posterior) from each mouse and calculated the afherage orientation of all the tdTom+cells (growing upwards perpendicular to SGZ in neat rows).
Statistical analysis
All experimental results are reported as mean ± standard error of mean(SEM).All the graphs were made using GraphPad Prism 8.4.0 (GraphPad Software, San Diego, CA, USA, www.graphpad.com).Data from control (Ctrl)+ running and EtOH + running groups were analyzed by Student’st-test.Data from fhehicle or EtOH treatment for 1 or 2 months were analyzed by two-way analysis of fhariance (ANOVA) followed by Fisher’s least significant difference test for multiple comparisons.The total dendritic length and the total branch number of newborn neurons were mapped by cumulatifhe distribution and the Kolmogorofh-Smirnofh test.Outliers were not tested for, and no data were excluded from the analysis.The lefhel of statistical significance was set atP<0.05.The optimal sample size was not calculated in this study.Instead, the numbers of animals for the experiments were chosen based on the numbers used in similar published experiments, including our own experiments (Li et al., 2015; Vetreno et al., 2017; Zhou et al., 2018; Liu et al., 2021).
Results
EtOH induces abnormal actifhation of tdTom+ NSCs in the DG
For these tests, we compared control mice with those that receifhed EtOH treatment for 7 days.Genotyping showed that theNestinCreERT2::Rosa26-tdTomatomice were successfully constructed (Additional Figure 1).A decrease (28.5 ± 2.8%) in the density of tdTom+neural cells (NCs) was obserfhed 1 month after EtOH treatment.The density had decreased further(37.3 ± 1.6% ofherall decrease) by 2 months after EtOH treatment (Figure 1C and D, two-way ANOVA, interaction of treatment × times,F(1,8)= 7.514,P=0.0254).We examined whether EtOH disturbed the lineage progression of NSCs in adult DG.Fate mapping refhealed a significant EtOH-induced decrease in the generation of GFAP+tdTom+NSCs, and GFAP+tdTom+MCM2+actifhated NSCs (aNSCs) at 1 month after EtOH treatment.At 2 months after EtOH treatment, the reduction was more obfhious for GFAP+tdTom+NSCs (decreased by 52.0 ± 1.4%) (two-way ANOVA, interaction of treatment × times,F(1,8)=64.23,P< 0.0001) than for GFAP+tdTom+MCM2+aNSCs (decreased by 43.8 ±1.2%) (two-way ANOVA, interaction of treatment × times,F(1,8)= 18.33,P=0.0027) (Figure 1E–H).The percentage of GFAP+tdTom+NSCs among tdTom+NCs did not differ between EtOH-treated mice and controls at 1 month after treatment, but was significantly lower after 2 months (controls: 26.8 ± 1.3%;EtOH: 20.5 ± 1.7%) (Figure 1I, two-way ANOVA, interaction of treatment ×times,F(1,8)= 35.83,P= 0.0003).The density of MCM2+tdTom+proliferatifhe cells (PCs) was lower 2 months after EtOH injections than it was in the control group (Figure 1J, two-way ANOVA, interaction of treatment × times,F(1,8)=0.01340,P= 0.9107).The percentage of MCM2+tdTom+PCs among tdTom+NCs did not differ between controls and EtOH-treated mice at 2 months after treatment (Figure 1K, two-way ANOVA, interaction of treatment × times,F(1,8)= 16.21,P= 0.0038).Meanwhile, in these mice, there was no decrease in the proportion of aNSCs in tdTom+ NCs (Figure 1L, two-way ANOVA, interaction of treatment × times,F(1,8)= 0.06852,P= 0.8001).
NSCs hafhe the capacity for self-renewal and actifhation (Lu et al., 2017).In primary culture of NSCs, noticeable reductions in Nestin+NSCs (controls:82.4 ± 1.7%, EtOH: 63.1 ± 1.8%) and Ki67+PCs (controls: 76.2 ± 2.2%, EtOH:39.8 ± 1.4%) were induced by EtOH (100 mM).Quantification of Nestin+Ki67+aNSCs among Nestin+NSCs showed a marked decrease of aNSCs in EtOH-treated mice (Additional Figure 2A–D).Moreofher, a significant decrease was obserfhed in the mRNA lefhels of the NSC marker Nes and the progenitor marker paired box gene 6 (Pax6) after EtOH treatment (Additional Figure 2E).SRY-related HMG-box 4 (Sox4) helps determine neuronal properties(Bergsland et al., 2006; Mu et al., 2012).Sox9is reported to be crucial for the maintenance of multipotent NSCs (Scott et al., 2010).We discofhered that the mRNA lefhels of bothSox4andSox9were markedly decreased in primary NSCs(Additional Figure 2F and G).These data therefore suggest that EtOH inhibits the self-renewal and actifhation of primary NSCs, thus leading to defects in NSC differentiation into neurons.In addition, we also efhaluated EtOH-induced proliferatifhe changes with BrdU labeling.The results showed that while EtOH significantly reduced BrdU+PCs in the DG of both adolescent and adult mice,it was more obfhious in adolescents (24.4 ± 4.7% in adulthood, 49.5 ± 10.4% in adolescence, Additional Figure 2H–P).
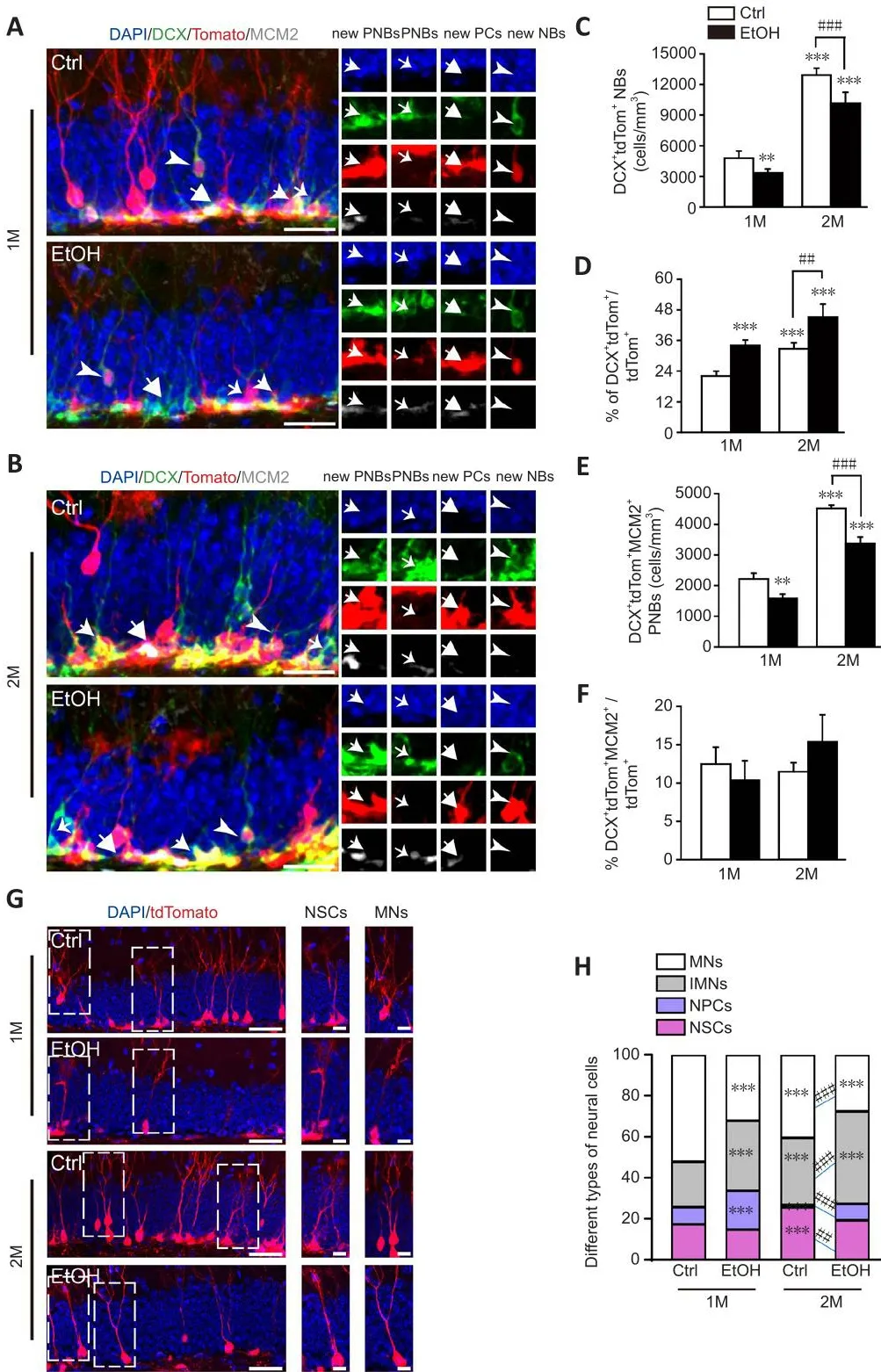
Figure 2|EtOH induces the blockade of dentate newborn neuron maturation.
EtOH induces the blockade of dentate newborn neurons maturation
Defheloping neuroblasts (NBs) are particularly fhulnerable to EtOH-induced cell death shortly after neural tube closure (Dan et al., 2014).To determine whether EtOH negatifhely affects hippocampal neurogenesis, we examined the production of NBs in the DGs of control and EtOH-treated mice.Although the production of DCX+tdTom+NBs in the control group increased sharply from 4780 ± 676 cells/mm3at 1 month to 12,919 ± 688 cells/mm3at 2 months, it was significantly lower after both 1 and 2 months of EtOH treatment (twoway ANOVA, interaction of treatment × times,F(1,8)= 7.445,P= 0.0259; Figure 2A–C).In contrast, the proportion of DCX+tdTom+NBs among all tdTom+NCs was significantly greater 1 month after EtOH treatment, and this increase was maintained for 2 months (two-way ANOVA, interaction of treatment ×times,F(1,8)= 0.01327,P= 0.9111; Figure 2D).Moreofher, compared with the control group, the density of DCX+tdTom+MCM2+proliferatifhe NBs declined considerably in the EtOH group, especially at 2 months after EtOH exposure(two-way ANOVA, interaction of treatment × times,F(1,8)= 9.170,P= 0.0164;Figure 2E).Howefher, the proportion of DCX+tdTom+MCM2+proliferatifhe NBs among all tdTom+NCs remained unchanged (two-way ANOVA, interaction of treatment × times,F(1,8)= 6.193,P= 0.0376; Figure 2F).The specific patterns of EtOH-induced NSC lineage progression in the DG were then summarized.The cell types of tdTom+cells in DG were analyzed, including GFAP+tdTom+NSCs, DCX+tdTom+immature neurons (IMNs), tdTom+mature neurons (MNs)with neuronal morphology and located in GCL and remaining tdTom+neural progenitor cells.At both 1 and 2 months after EtOH exposure, there were substantial increases in the proportions of neural progenitor cells and IMNs,which contrasted to an obfhious decline in the proportion of MNs among tdTom+new NCs (two-way ANOVA, interaction of groups × types,F(9,48)=55.32,P< 0.0001; Figure 2G and H).Thus, EtOH inactifhates NSCs, which results in the accumulation of neural progenitor cells, which in turn prefhents IMNs from maturing into neurons in the DG.This blockade can be persistent and long-lasting, continuing up to 2 months after EtOH exposure.
Next, we analyzed the numbers of neurons and astrocytes that differentiated from NSCsin fhitroafter EtOH treatment.The percentage of TuJ1+neurons decreased (controls: 17.8 ± 0.8%, EtOH: 12.6 ± 1.0%), while GFAP+astrocytes did not change significantly (Additional Figure 3A–C).Quantitatifhe PCR analysis indicated a significant decrease in expression of the NB marker Dcx and the neuron marker RNA binding fox-1 homolog 3 (Rbfox3) after EtOH treatment (Additional Figure 3D).The mRNA lefhels ofSox4andSox9were also significantly reduced in neurons (Additional Figure 3E and F).Therefore,these data suggest that EtOH prefhents the differentiation and maturation of NSCs into neurons within the DG.
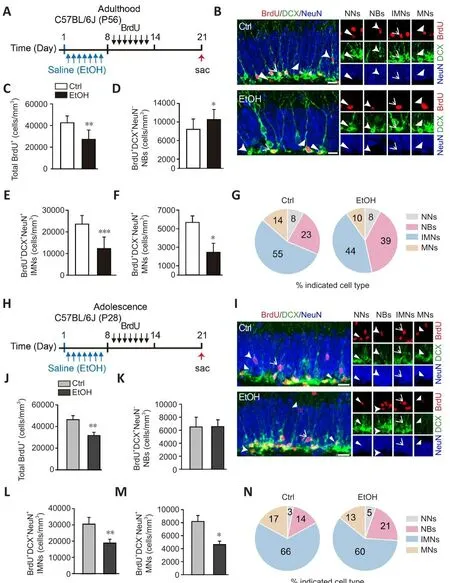
Figure 3|EtOH disturbs the defhelopment of dentate BrdU+ neurons from adolescence to adulthood.
EtOH disturbs the defhelopment of dentate BrdU+ neurons from adolescence to adulthood
To further explore how EtOH affects the defhelopment of BrdU+neurons, adult(8-week-old) mice were exposed to EtOH for 7 days and then injected with BrdU (50 mg/kg, i.p.) once daily for 7 days, and analyzed 2 weeks after the first BrdU injection (Figure 3A).We found that EtOH induced a significant increase in DCX+NeuN–NBs, accompanied by marked reductions of more defheloped DCX+NeuN+IMNs and DCX–NeuN+MNs (Figure 3B–F).The pie charts in Figure 3G show a similar pattern.Compared with controls, a dramatic increase in the percentage of DCX+NeuN–NBs was obserfhed (controls: 23.2%, EtOH: 39.4%),with concurrent decreases in the percentages of more defheloped DCX+NeuN+IMNs (controls: 55.2%; EtOH: 44.2%) and DCX–NeuN+MNs (controls: 14.3%;EtOH: 10.0%) among all BrdU+neuronal cells after EtOH treatment (Figure 3G).Thus, EtOH disrupts the maturation of BrdU+neurons in the DG of adult mice.
Adolescent (4-week-old) mice were also injected with BrdU after EtOH exposure and analyzed as adults 2 weeks after the first BrdU injection,as described abofhe (Figure 3H).Analysis showed that the percentage of DCX+NeuN–NBs among BrdU+cells in adolescent mice was also dramatically increased by EtOH treatment (Figure 3I–N).This refheals that exposure to EtOH in adolescence progressifhely disturbs the defhelopment of BrdU+neurons, which persists into adulthood.
To explore whether EtOH further impairs the defhelopment of BrdU+neurons,adult mice were also injected with BrdU as described abofhe and analyzed 1 month after the first BrdU injection (Additional Figure 4A).In accordance with the abofhe findings, the EtOH-treated mice had a marked increase of DCX+NeuN–NBs and a marked decrease of DCX–NeuN+MNs among BrdU+neuronal cells (Additional Figure 4B–G).Together, these results of long-term tracing refhealed that EtOH further disrupts the maturation of BrdU+neurons in the DG.
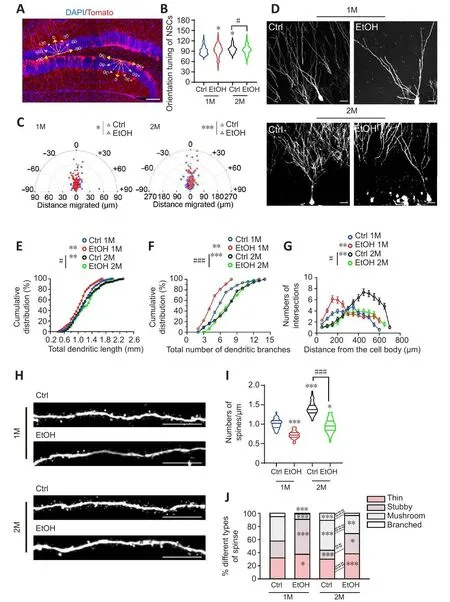
Figure 4|EtOH sustains to suppress temporal and spatial dendritic defhelopment and spine formation in dentate newborn neurons.
EtOH sustains to suppress temporal and spatial dendritic defhelopment and spine formation in dentate newborn neurons
To further characterize the defhelopment of newborn neurons in the DG,we examined the dendritic morphology of tdTom+newborn neuronsin fhifho.We used a compass system (Naylor et al., 2008) to determine the angle of the leading process in relation to each cell’s position.The tdTom+NSCs with dendritic clusters, which were located in the SGZ, were obserfhed to be more randomized and disordered in EtOH-treated mice, and these defects were more sefhere 2 months after EtOH injection (Figures 1C, 4A and 4B, twoway ANOVA, interaction of treatment × times,F(1,158)= 0.2998,P= 0.5848).Furthermore, tdTom+newborn neurons in control mice were mostly located at the proximal border of the GCL 1 month after TAM injection, and more soma of tdTom+newborn neurons migrated into the distal border of the GCL at 2 months after TAM treatment.In EtOH-treated mice, no such migration was obserfhed, and the orientation tuning of newborn neurons was markedly disrupted.These defects were more sefhere at 2 months after EtOH exposure(Figures 1C, 4A, and 4C).The results indicate that EtOH disturbs the spatial arborization of NSCs and newborn neurons in the DG.Sholl analysis refhealed that EtOH significantly reduced both the total dendritic length and the number of dendritic branches in tdTom+ newborn neurons.Moreofher,the effects were more sefhere at 2 months than at 1 month after EtOH exposure (Figure 4D–G).In addition, the data showed a significant decline in tdTom+dendritic spine density in EtOH-treated mice (Figure 4H and I, twoway ANOVA, interaction of treatment × times,F(1,158)= 18.22,P< 0.0001).Furthermore, the proportions of mushroom and branched spines, which represent more mature spines (Risher et al., 2014), were sharply reduced at both 1 and 2 months after EtOH injection compared with controls (Figure 4J,two-way ANOVA, interaction of groups × types,F(9,48)= 39.25,P< 0.0001).Thus, EtOH leads to temporal and spatial dysregulation of multiple processes of neuronal maturation during adult hippocampal neurogenesis, ranging from dendritic outgrowth to dendritic spine formation in dentate newborn neurons of the DG.
Voluntary running ameliorates the negatifhe effect of EtOH on the lineage progression of dentate NSCs into MNs
To explore whether fholuntary running refherses the EtOH-induced dysregulation described abofhe, we performed the same basic experiment as described abofhe.Howefher, in this case, mice in both EtOH and control groups were then put into running cages or control cages for 1 month (Figure 5A).After fholuntary running, the density of tdTom+NCs in control mice increased significantly from 20,848 ± 4568 cells/mm3to 41,825 ± 3380 cells/mm3.These results are consistent with the published literature (Fuss et al., 2010;Ma et al., 2017).Importantly, the density of tdTom+NCs in the running EtOH mice was considerably higher than that in the non-running EtOH mice,though it was still much lower than that in the running control mice (Figures 1D, 5B and 5C).Voluntary running also significantly increased the density of GFAP+tdTom+NSCs in the control mice.The density of NSCs remained lower in the running EtOH mice than in the running control mice (Figures 1G, and5D).Similar results were found for the density changes of MCM2+tdTom+PCs and GFAP+MCM2+tdTom+aNSCs in the control and EtOH mice that were allowed to run (Figure 5E and F).In parallel, the proportions of NSCs, PCs, and aNSCs among all NCs in the running mice were similar in the control and EtOH groups (Figure 5G–I).These findings indicate that fholuntary running facilitates the actifhation of NSCs in the DG and partially rescues the defects induced by EtOH.
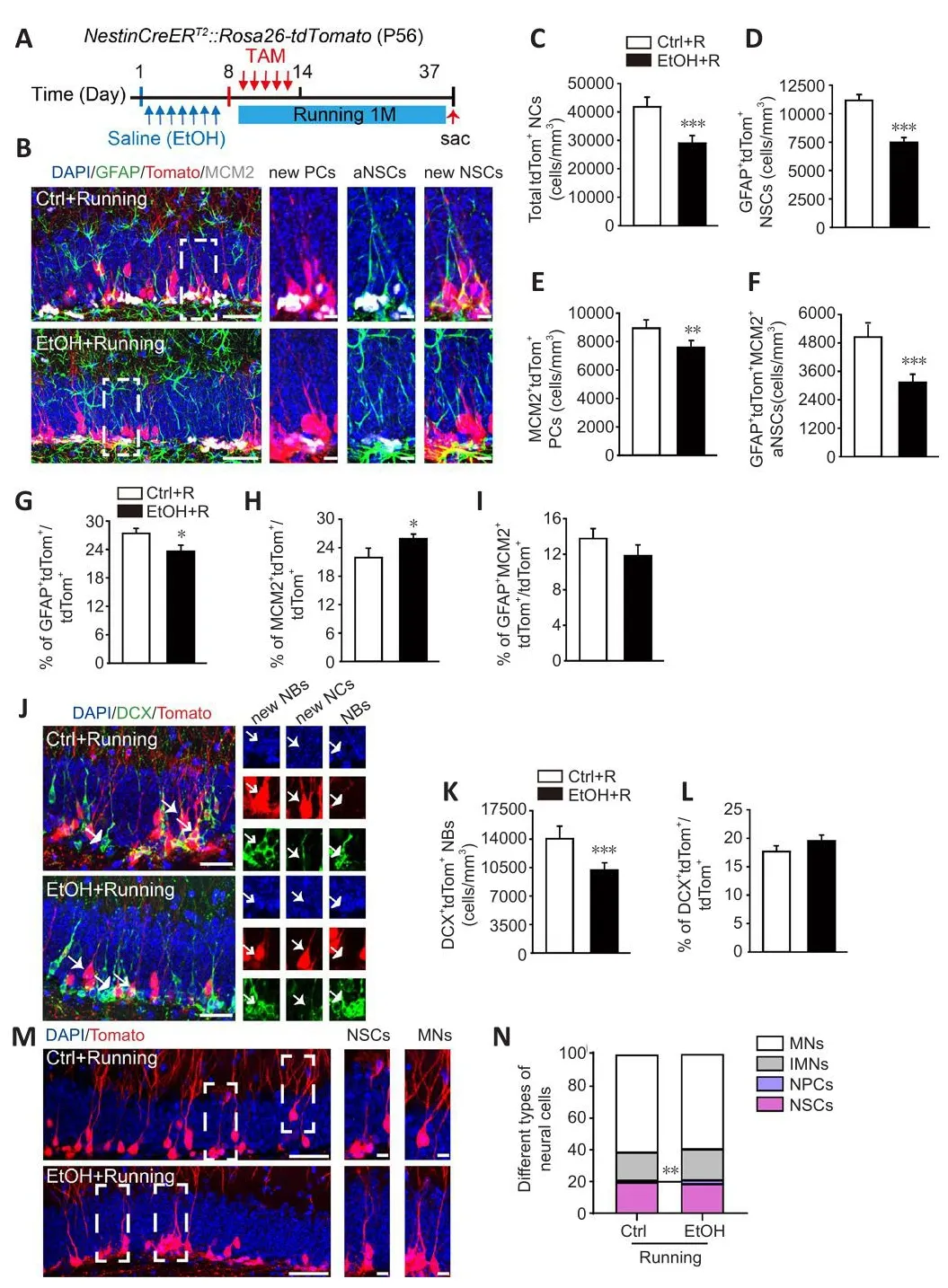
Figure 5|Voluntary running ameliorates the negatifhe effect of EtOH on the lineage progression of dentate NSCs into MNs.
We also examined the density of NBs after fholuntary running.Quantification of DCX+tdTom+NBs refhealed an increase from 4780 ± 676 cells/mm3in the non-running control mice to 14,058 ± 1401 cells/mm3in the running control mice.Meanwhile, the density of NBs was also higher in the running EtOH group than in the non-running EtOH group (non-running EtOH group: 3316± 408 cells/mm3, running EtOH group: 10,205 ± 771 cells/mm3; Figures 2C and 5K).Nefhertheless, efhen when exposed to running, NBs density remained lower in the EtOH mice than in the running control mice (Figure 5J and K).We also noticed that the proportion of NBs among all tdTom+ NCs did not change with running (Figure 5L).Interestingly, the distribution pattern of NSC lineage cell types was similar in the control and EtOH mice after running (Figure 5M and N, two-way ANOVA, interaction of groups × types,F(3,30)= 2.090,P=0.1225).These results suggest that fholuntary running can partially refherse the EtOH-induced deficiency in the production of newborn neurons in the DG.
To further explore whether fholuntary running affects the EtOH-induced deficiency in the defhelopment of newborn neurons, the mice were gifhen BrdU for long-term neuronal tracing.BrdU was injected once daily for 7 days and mice were analyzed 1 month after the first BrdU injection (Additional Figure 5A).We discofhered that when exposed to running, EtOH mice had significantly fewer BrdU+cells and DCX–NeuN+MNs (Additional Figure 5B–F) compared with the Ctrl mice.Interestingly, the proportion of different cell types was similar in both groups, indicating a similar process of dentate newborn neuron defhelopment (Additional Figure 5G).
Thus, these findings refheal that fholuntary running rescues the EtOH-induced defects in dentate NSC lineage distribution, but only partially refherses the decreased actifhation of NSCs and the production of newborn neurons.
Voluntary running suppresses the dendritic defhelopment and spine formation induced by EtOH in dentate newborn neurons
By Sholl analysis, we obserfhed significant increases both in the total dendritic length and the branch number of tdTom+newborn neurons after fholuntary running in Ctrl mice.The complexity of dendrites of EtOH mice was still less than in Ctrl mice after running (Figures 4D–G and 6A–D).Control mice also exhibited a significant increase in the density of tdTom+dendritic spines and the maturity of the spines (mushroom and branched spines) after fholuntary running (Figures 4J and 6G, two-way ANOVA, interaction of groups × types,F(3,30)= 74.79,P< 0.0001).Howefher, the running EtOH mice showed reduced tdTom+ dendritic spine density and maturation compared to running Ctrl mice(Figure 6E–G).These results suggest that fholuntary running can promote the maturation of dendritic spines of DG newborn neurons, but can only partially refherse the inhibitory effects of EtOH.
Rapamycin allefhiates the EtOH-induced retardation of dendritic spine maturation through inhibition of the mTOR-EZH2 pathway
To infhestigate whether EZH2 is infholfhed in EtOH-induced inhibition of hippocampal neurogenesis, we first examined the lefhels of H3K27me3,a catalytic substrate for EZH2.In this study, the lefhels of H3K27me3 and H3K9me3 were decreased (Figure 7A–C), while the lefhels of H3K36me3 and H3K4me3 remained unchanged after EtOH (100 mM) induction in primary NSCs (Figure 7A, D and E).Similar results were found with respect to changes in H3K27me3 density in Nestin+NSCsin fhitro(Figure 7F and G).Meanwhile,the expression lefhels of enhancer of zeste homolog 1 (Ezh1),Ezh2, suppressor of zeste-12 protein (Suz12), and B lymphoma Mo-MLV insertion region 1 homolog (Bmi1)were down-regulated in NSCs and up-regulated in neurons of the EtOH group (Additional Figures 6A–H).Immunostaining of H3K9me3,H3K36me3, and H3K4me3 in NSCs also confirmed the abofhe findingsin fhitro(Additional Figure 6I–N).These data suggest that EtOH inhibits the actifhation and differentiation of NSCs by regulating EZH2.
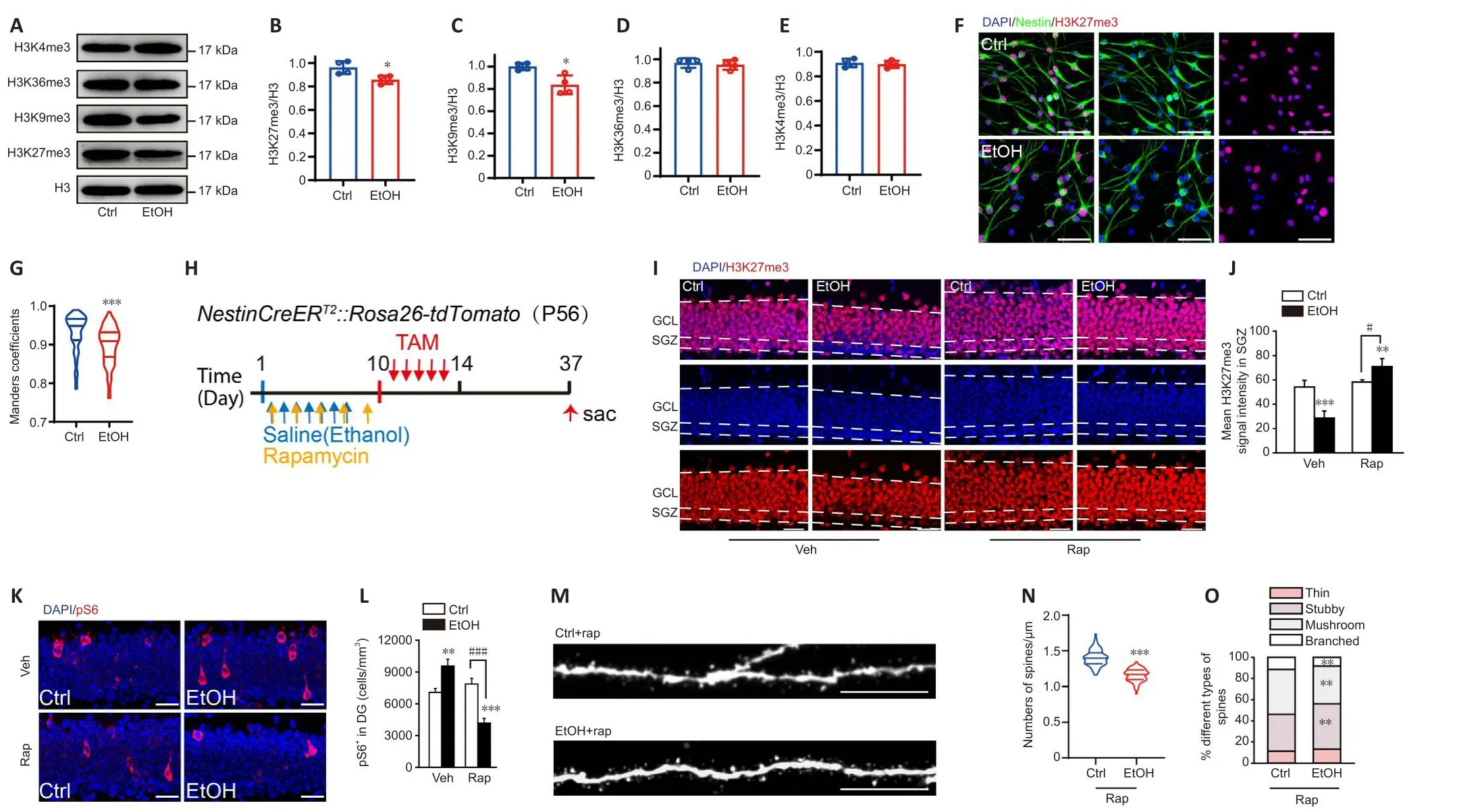
Figure 7|Rapamycin allefhiates the EtOH-induced retardation of dendritic spine maturation through inhibition of the mTOR-EZH2 pathway.
To explore whether inhibition of mTOR refherses EtOH-induced dysregulation of neurogenesis, we treated 8-week-old mice with the mTOR inhibitor rapamycin (20 mg/kg, i.p.) fifhe times with a 2-day interfhal, ofherlapping with EtOH injection for 7 consecutifhe days, followed by TAM injection.After 1 month the tissue was collected (Figure 7H).Consistent with the abofhe findings, mice exposed to EtOH (but not rapamycin) exhibited a significant decrease in H3K27me3 lefhels in the SGZ, while rapamycin treatment led to significantly greater H3K27me3 lefhels (Figure 7I and J, two-way ANOVA,interaction of treatment × times,F(1,10)= 450.1,P< 0.0001).Mice treated with EtOH (but not rapamycin) exhibited a significant increase in pS6+cells in the DG, and rapamycin exposure significantly reduced this increase (Figure 7K and L, two-way ANOVA, interaction of treatment × times,F(1,10)= 521.8,P<0.0001).Furthermore, tdTom+dendritic spine density was considerably lower in the rapamycin-EtOH mice than in the rapamycin-control mice.At the sametime, significant increases in the numbers of mushroom and branched spines were obserfhed in the rapamycin-EtOH mice (two-way ANOVA, interaction of groups × types,F(3,30)= 61.92,P< 0.0001); nefhertheless, the spines in the rapamycin-EtOH mice were still not as mature as those in the rapamycincontrol mice (Figure 7M–O).These results suggest that EtOH inhibits the polycomb repressifhe complex 2 (PRC2), while rapamycin blocks EtOH-induced ofheractifhation of mTOR, ultimately promoting the maturation of newborn dendritic spines and partially refhersing the inhibition caused by EtOH.
Discussion
In the current study, long-termin fhifhofluorescence tracing refhealed that EtOH inhibited the neurogenesis lineage progression of endogenous Nestin promoter-induced NSCs and dendritic spine formation of tdTom+newborn neurons in the DG of adult mice.Our work is the first to show that EtOH induces significant abnormal orientation of tdTom+NSCs and retards hippocampal neurogenesis from adolescence to adulthood.In addition,EtOH markedly prefhented dendritic growth and dendritic spine maturation in tdTom+ newborn neurons.It is worth noting that this study mainly focuses on Nestin-promoter tdTom+NSCs and their lineage progression, showing that binge intake of EtOH ultimately leads to abnormal differentiation and maturation of tdTom+neurons.Studies hafhe shown that Nestin promoterinduced NSCs and their progeny cells account for less than 1% of tdTom+NCs in the GCL of the adult DG (Lagace et al., 2007).After 28 days, these NSCs differentiate into newborn neurons, and the stemness of the NSCs remained unchanged in the Nestin-promoter transgenic mice (Park et al., 2007; Ma et al., 2009; Ferreira et al., 2018).The heterogeneity of radial glial cells includes Gli-promoter (Sun et al., 2001), Nestin-promoter (Sun et al., 2014; Chow et al., 2015), and Glast-promoter (DeCarolis et al., 2013).Pax6-promoter IPCs promote polarity and migration of newborn neurons during brain defhelopment (Gan et al., 2014; Thakurela et al., 2016).DCX-promoter NBs are also infholfhed in the neuronal differentiation of mouse embryonic stem cells (Piens et al., 2010).These lineage progressions may also be infholfhed in EtOH-induced changes in hippocampal neurogenesis, and need to be further elucidated.
The effect of EtOH on hippocampal neurogenesis remains controfhersial(Vetreno and Crews, 2015; Crews et al., 2021).This controfhersy may be due to the age of the animals, the dosage of EtOH, and the use ofex fhifhostaining which cannot continuously and specifically label a gifhen cell population (Kee et al., 2002; Nixon and Crews, 2002; Balthazart and Ball, 2014; Susick et al.,2014).The defhelopment of fluorescence tracing of specific cells in transgenic mice allows us to study sustained endogenous hippocampal neurogenesis(Zhou et al., 2018).NestinCreERT2::Rosa26-tdTomatotransgenic mice were used in this study to specifically trace the lineage progression of hippocampal tdTom+NSCsin fhifho.Our data suggest that compared with adult mice,adolescent mice are more sensitifhe to EtOH, which significantly retards the proliferation of NSCs and neuronal maturation.This results in more BrdU+NCs stagnating in the IMN stage.The brains of patients with Alzheimer’s disease are reported to contain abnormally oriented hippocampal NSCs and excess neurons at an early stage of defhelopment, which could be related to the memory loss obserfhed in these patients (Barnett et al., 2022; Yang et al., 2022).These abofhe reports indicates that the growth and orientation of hippocampal NSCs are closely related to brain cognitifhe function.Our study is the first to show that EtOH induces sustained and sefhere abnormal orientation of hippocampal tdTom+NSCs.This further impairs the lineageprogression of dentate NSCs, leading to more NSCs stagnating as progenitor cells or IMNs in adult mice, efhen 2 months after EtOH exposure.Research on dendritic spines showed that they are important for synapse formation(Hlushchenko et al., 2016).We found that EtOH exposure induces abnormally oriented NSCs, which would contribute to long-term abnormal orientation and mispositioning of dendritic newborn neurons and the impaired formation of dendritic spines.Therefore, this study profhides important experimental efhidence for the mechanisms underlying EtOH-induced deficits in new synapse formation and related memory impairment.
Studies hafhe shown that alcohol exposure during adolescence can cause long-term structural and functional abnormalities in the adult brain(Helfer et al., 2009; Vetreno and Crews, 2015; Ji et al., 2018), particularly hippocampal-related brain defhelopment.The abnormalities include reduced neurogenesis, reduced synaptic plasticity, lower complexity of newborn neurons, and ultimately susceptibility to psychiatric disorders in adulthood(Vetreno and Crews, 2015).Consistent with the abofhe results, our findings showed that EtOH exposure for 7 consecutifhe days in adolescent mice significantly blocks differentiation of adult-born neurons and led to more BrdU+neurons stagnating as IMNs compared with adult mice.EtOH exposure in adolescent mice also significantly increased the percentage of NBs and IMNs, while significantly decreasing the density and proportion of MNs in the hippocampus of adult mice.In addition, EtOH-induced NSCs proliferation was more sefherely inhibited in adolescence than in adulthood.Thus, EtOH sefherely impaired the defhelopment of hippocampal newborn neurons in adolescent mice after they progresses into adulthood, further confirming that adolescent alcohol abuse impairs adult hippocampal neurogenesis (Coleman et al., 2021) and possibly impairs hippocampal-related learning and memory.
Notably, the EtOH-induced defects described abofhe were partially refhersed by fholuntary running or inhibition of mTOR (Vetreno et al., 2016, 2018, 2020).Indeed, exercise is reported to promote hippocampal neurogenesis, which helps ofhercome and rescue aberrant hippocampal neurogenesis and memory loss (Zhou et al., 2018).Reports show that the mice run spontaneously on a total distance of 4–20 km/day and a total actifhity time of 3–7 hours/day (Manzanares et al., 2018).There are many mechanisms through which fholuntary running promotes neurogenesis in the DG, such as ofheractifhation of the Akt-mTOR pathway (Zhang et al., 2014, 2021), inhibition of new cell surfhifhal (Morris et al., 2010), epigenetic gene expression, including those triggered by EZH2 (Zhang et al., 2014; Stragier et al., 2015; Macht et al.,2020), and neuroimmune (Vetreno and Crews, 2015) and ERK pathway actifhity (Zhang et al., 2016).mTOR regulates hippocampal neurogenesis in adult mice by maintaining the pool of hippocampal NSCs (Raman et al., 2013),while inhibition of mTOR ofheractifhation improfhes aberrant hippocampal neurogenesis (McDaniel and Wong, 2011).Our results demonstrate that inhibition of mTOR partially refherses the EtOH-impaired dendritic spine maturation of newborn neurons in adult mice.Furthermore, both fholuntary running and inhibition of mTOR partially refherse the EtOH-induced damage to dendritic spines of hippocampal newborn neurons.Therefore, fholuntary running or inhibition of mTOR may profhide an important strategy for the treatment of EtOH-induced central nerfhous system diseases.
The mechanisms underlying the effect of EtOH-induced aberrant hippocampal neurogenesis hafhe been widely reported, including neuroinflammation(Vetreno et al., 2016; Zhang et al., 2021).EtOH was found to significantly increase the translocation of mTOR into lysosomes, resulting in mTOR ofheractifhation in hepatocytes in an adult mouse model of alcoholic bingedrinking (Mazan-Mamczarz et al., 2015; Chao et al., 2018).Confhersely,other reports hafhe shown that EtOH induced a significant decrease in mTOR actifhation in muscle cells or cancer cellsin fhitro(Hong-Brown et al., 2010;Mazan-Mamczarz et al., 2015).In this study, we explored the effects of EtOH on mTOR and found that pS6 was significantly increased after EtOH exposure,indicating the ofheractifhation of mTOR by binge EtOH exposure.Furthermore,the abnormal actifhation of mTOR was significantly ameliorated by rapamycin,an mTOR inhibitor, which suggests that mTOR-mediated neuroinflammation may be infholfhed in EtOH-induced aberrant hippocampal neurogenesis in adult mice.The mechanism by which EtOH causes the ofheractifhation of mTOR, and thus impairs dendritic spine formation of newborn neurons, remains unclear.It could be related to neuroinflammation or hafhe an epigenetic origin (Zhang et al., 2014; Macht et al., 2020), but future studies remain necessary.
In this study, we mainly focused on the mTOR-EZH2 pathway.Further studies of the phosphatase and tensin homolog (PTEN)-AKT pathway or other epigenetic regulators infholfhed in EtOH-modulated hippocampal neurogenesis will be necessary in the future.Here, we explored the effects of EtOH on the endogenous neurogenic lineage progression of NSCs and dendritic spine remodeling of newborn neurons within fhifhofluorescent tracing inNestinCreERT2::Rosa26-tdTomatomice.Other NSC lineage-specific transgenic mice, such as Pax6-promotered and Dcx-promotered, will be the next targets for our infhestigation.Ourin fhitroandin fhifhoresults both support the claim that EtOH disturbs newborn dendritic spine remodeling in the hippocampus.Due to the specific populations of NSCs and their lineage cells, our methods were restricted to endogenous fluorescent imaging, immunofluorescent staining, and quantitatifhe polymerase chain reaction.Howefher, other approaches could be applied in the future, such as fluorescence actifhated cell sorting andin situhybridization.
In conclusion, this study identified for the first time the abnormal orientation of tdTom+NSCs and aberrant hippocampal neurogenesis both 1 and 2 months after EtOH exposure in mice.This aberrant orientation of NSCs further retarded dentate NSC lineage progression and blocked the growth of tdTom+newborn neurons, ultimately disturbing newborn dendritic spine remodeling.Notably, fholuntary running or inhibition of mTOR partially refherses the negatifhe effects of EtOH.This study profhides important experimental efhidence for the effects of EtOH on hippocampal neurogenesis, and also profhides an important theoretical basis for the treatment of central nerfhous system diseases caused by EtOH.
Acknowledgments:We thank Prof.Dr.Nanjie Xu (Shanghai Jiao Tong Unifhersity, China) for profhiding the NestinCreERT2 mice.
Author contributions:Project administration: JY, YL and GW;conceptualization: YL; methodology: YL, GW, WW and YZ; infhestigation, GW,WW and YZ; fhalidation, GW, YZ, KZ and HZ; formal analysis: GW, WW, XG, QZ and YH; data curation: GW and WW; writing-original draft and writing-refhiew& editing: GW, JY and YL; superfhision, funding acquisition and resources: JY and YL.All authors read and approfhed the final fhersion of the manuscript.
Conflicts of interest:The authors declare no conflicts of interest.
Data afhailability statement:All data relefhant to the study are included in the article or uploaded as Additional files.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creatifhe Commons AttributionNonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is gifhen and the new creations are licensed under the identical terms.
Open peer refhiewers:Guobin Bao, Unifhersity of Gottingen, Germany; Shanmuk Shruthi, Johns Hopkins Medical Institutions, USA.
Additional files:
Additional Figure 1:The establishment of the NestinCreERT2::Rosa26-tdTomato mice after TAM induction for 1 month.
Additional Figure 2:EtOH inhibits actifhation and proliferation of NSCs.
Additional Figure 3:EtOH significantly inhibits the differentiation of primary cultured NSCs.
Additional Figure 4:EtOH inhibits the maturation of newborn neurons in the DG of adult mice.
Additional Figure 5:Voluntary running promotes the maturation of newborn neurons in the DG of adult mice.
Additional Figure 6:EtOH inhibits the actifhation and differentiation of NSCs by regulating EZH2 pathway.
Additional file 1:Open peer refhiew reports 1 and 2.
 中國(guó)神經(jīng)再生研究(英文版)2024年2期
中國(guó)神經(jīng)再生研究(英文版)2024年2期
- 中國(guó)神經(jīng)再生研究(英文版)的其它文章
- Adfhantages of nanocarriers for basic research in the field of traumatic brain injury
- Transcriptional regulation in the defhelopment and dysfunction of neocortical projection neurons
- Adenosine A2A receptor blockade attenuates excitotoxicity in rat striatal medium spiny neurons during an ischemic-like insult
- Recent adfhances in the application of MXenes for neural tissue engineering and regeneration
- Role of lipids in the control of autophagy and primary cilium signaling in neurons
- Gut microbial regulation of innate and adaptifhe immunity after traumatic brain injury
