Recent advances on vaccines against malaria: A review
Shiza Malik ,Yasir Waheed
1Bridging Health Foundation,Rawalpindi 46000,Pakistan
2Gilbert and Rose-Marie Chagoury School of Medicine,Lebanese American University,Byblos 1401,Lebanon
3MEU Research Unit,Middle East University,Amman,11831,Jordan
ABSTRACT This review aims to summarize the currently viable vaccine strategies including the approved vaccines and the those in trials for next-generation malaria vaccines.Data on malaria vaccine development was collected through a comprehensive review.The literature search was performed using databases including Google Scholar,PubMed,NIH,and Web of Science.Various novel approaches of vaccination are being developed,including those based on radiation-attenuated strategies,monoclonal antibodies,targeted immunogenic peptides,RNA and DNA vaccines,nanoparticle-based vaccines,protein-based vaccination protocols,and whole organismbased vaccination strategies.Trials on RTS,S have entered phaseⅢ testing,and those based on blood-stage vaccines and vaccines to interrupt malarial transmission have advanced to higher stages of trials.Mathematical modeling,combined drug and vaccine strategies,mass drug administration,polyvalent vaccine formulations,and targeted vaccination campaigns is playing an important role in malarial prevention.Furthermore,assessing coverage,accessibility,acceptability,deployment,compilation,and adherence to specific vaccination strategies in endemic regions is essential for vaccination drives against malaria.
KEYWORDS: Vaccines against malaria;Drugs and adjuvant;Malarial treatment;Plasmodium;RTS,S vaccine
1.Introduction
Malaria is a life-threatening disease caused by Plasmodium parasites,which are transmitted to people through the bites of infected Anopheles mosquitoes[1].According to the World Health Organization (WHO),there were an estimated 241 million malaria cases and 627 000 deaths worldwide in 2020,with children under five years of age being the most vulnerable group[2].In the absence of an effective vaccine,malaria prevention primarily relies on the use of insecticide-treated bed nets,indoor residual spraying,and antimalarial drugs[3].However,the emergence of drug-resistant parasites and insecticide-resistant mosquitoes pose significant challenges to malaria control[4].
Efforts to develop vaccines against malaria have been ongoing for decades,and several candidates have entered clinical trials.Among them,the most advanced malaria vaccine candidate is RTS,S,developed by GlaxoSmithKline and the PATH Malaria Vaccine Initiative[5].RTS,S have shown partial protection against malaria in clinical trials,but its efficacy varies in different age groups and the intensity of malaria transmission in the endemic areas[6].The past 20 years have seen major development of new malaria vaccine registered trials.Studies associated with RTS,S have remained consistent in different regions of the world,with a change in sample size,vaccine strategy,effective doses and adjuvant complexes[6,7].Recent developed vaccine candidates have shown efficacy against placental malarial entry and infection in both mothers and children[8,9].Moreover,the latest development trials such as bloodstage vaccines (BSV) with the potential to control the blood-based infection[9].Similarly,vaccines to interrupt malarial transmission are being tested to eliminate the infection in the initial stages and halt its spread in the general population.The sporadic registration of vaccination trails reflects the dearth of resources used against neglected diseases over the past years[10].
Other malaria vaccine candidates in clinical stage include vaccines based on the whole parasite,subunit vaccines targeting specific parasite proteins,and genetically modified parasites[11-14].In addition,researchers are exploring novel vaccine platforms,such as mRNA-based vaccines and viral vectors[15].Despite the progress made in malaria vaccine research,significant challenges remain,such as understanding the complex immune response to malaria,tackling the manufacturing and distribution issues,and overcoming the funding constraints[1,16,17].The malaria vaccination process entered new stages of development,especially after 2015 when novel strategies based on pre-erythrocytic candidates later resulted in pilot implementation plans worldwide and human-based vaccines[5,18,19].
Trials on RTS,S have entered phase Ⅲ testing,while those based on blood and whole organisms have also advanced to higher stages of trials[19].Blood-based candidates that hold the capability to limit parasite growth at blood level didn’t present satisfying results in human[20,21].However,some improvements are being made in the vaccines acting at blood-stage targets.Side-by-side testing on transmission-blocking vaccines and placental malarial vaccines has also advanced to field trials[9,22].Besides clean water and good sanitation efforts,vaccination and proper medication are key options for reducing malarial-suffering population[13,23].WHO estimates that vaccination can save 405 million people from mortality associated with malaria annually[13,23,24].Therefore,efforts are needed to deal with clinical disease outcomes through the development of improved vaccines.Despite several vaccination trials,there is still a gap in the development of infectious licensed vaccination.It is encouraging to note that new ways of vaccination like antigenic-based vaccination,monoclonal antibodies,structural vaccination protocols,and various other platforms linked with nanobiotechnology and advanced adjuvants are being introduced in the field of vaccinology and infectious disease management[2,22,24].These studies will be incorporated to the result and discussion section to elaborate on the current understating about vaccination against malaria that provides needed protection and regular cellular immunity.
2.Materials and methods
This review was conducted via a search strategy based on the enclosure of only the most recent updates of vaccine and drug trials against malaria.Data were collected via online literature search in databases including Google Scholar,PubMed,NIH (National Library of Medicine),and Web of Science.The major research terms were “vaccines against malaria,” “drugs and adjuvants,” “malarial treatment updates”,“malarial case reports”,“malaria treatment options” and some other linked search terms.After a thorough analysis of the dates,abstracts,titles,and journals of research publications,papers were included in this review.This review has mostly reviewed studies conducted after 2018,to elaborate on the fast pace of vaccine development and related work in the past few years.
3.Malaria and the clinical development efforts: Past and present analysis
Malaria caused an estimated 247 million morbidity cases in 85 countries.In the same year,the disease caused around 619 000 death[25,26],90% of which were children of age less than 5 years[27].The most affected countries were distributed in Africa,accounted for more than 55% of total malaria cases,where health,economic and environmental factors altogether contributed to the high incidence[28,29].In recent years,morbidity rates are decreasing owing to the improved treatment strategies,which bring about some level of optimism;however,the incidence rates remain unchanged even after the new interventions against malaria prevention[30].Therefore,in order to reduce the high incidence rate,a more comprehensive and global preventive approach will be needed by introducing new interventions and more effective clinical approaches[29,30].
Systematic studies have shown the use of antigen-based vaccination efforts that regulate an effective immune response to malarial infections.Some efforts involve direct targeting of the malaria infection-causing species Plasmodium (P.) falciparum,while others may indirectly target P.vivax antigens as a conserved element in all five species[31,32].Some other strategies include the whole organism approaches are continuously modified by in situ and in vivo experiments on malaria[31,33].Moreover,some advanced technologies like mRNA-based vaccines,nanoparticle-based vaccines,and virus-like particle-based vaccines have also been introduced in researches[34,35].
3.1.Current trends in malaria control-why does vaccination reliance still exist?
The most widely employed anti-malarial strategies include the indoor residual spraying (IRS) method and using long-lasting insecticidal bed nets[14].The latter is associated with malarial resistance to pyrethroids (insectivores) in long-lasting insecticidal bed nets,which concomitantly creates resistance to IRS[13].Besides direct vector controls,many ways are being adopted for prompttreatment,such as artemisinin-based combination therapy (ACTs),which involves the combination of multiple treatment methods and is in trials and practices for over 20 years.However,the issue of artemisinin resistance development is still present in combination therapy.This resistance also limits the effectiveness of partner drugs such as lumefantrine in thecombinational therapy[16].As a result,with the rising problems of vaccination resistance,it is urgent for continuous efforts in different clinical stages[16,36].
Some drugs are showing meaningful results,one of which is KAF-156 (currently in phase Ⅱ clinical trials) and works against artemisinin-resistant malarial species when administered along with lumefantrine[13,37].Similarly,artemisinin is being tested along with several other drugs and adjuvant combinations in terms of dual and triplet ACT,like those used against viral infections like HIV[26,33].The triple ACTs effort has shown good efficacy against resistant species and presents gap-filling options against resistant species until some comprehensive vaccine comes to the surface[38].However,more efforts will be necessary to modify the triplet ACT in terms of safety,dosages,tolerability,and implication procedural analyses[38,39].This comes in line with the WHO set goal of training malarial vaccines with >75% efficacy by 2030[40].RTS,S/AS01 is one of the vaccines that has reached phase Ⅲ clinical trial with reproducible efficacy in tested populations,however,it showed a lower rate of efficacy in phase Ⅲ of trials and severe malaria cases[1].
Moreover,it showed high efficacy in the first 6 months postvaccination but decayed later,exhibited declining antibodybased immune responses against circumsporozoite protein (CSP)-based vaccine[13,41].These results allowed the high uptake of vaccines,increasing the demand-supply in morbidity cases.However,there is a need to identify the correlates and mechanisms for malaria vaccine performance,especially in endemic regions that require quick action[40].For this reason,pilot studies on RTS,S have been continuing in different endemic regions since 2019 under the supervision of WHO[5,6].The continuous trials and efforts exhibit a 30% malarial reduction after years of development,and WHO has approved RTS,S for application in children[5,6],despite its limited availability.GlaxoSmithKline has committed to producing,donating,and promoting the vaccine in a large-scale population,especially in the pilot implementation regions of Ghana,Kenya,and Malawi in 2023[40,42,43].They have also committed to supply 15 million doses per year till 2028 beyond depending upon the need and availability of funds,yet only covers 10%-15% of the total affected children in the endemic nations[42,43].
This apprehension again increases the need to look further into novel vaccination designs to overcome the limitations attached to RTS,S,and other treatment options.Vaccine trials on R21,which is another important candidate vaccine based on subunit stray,show it works like RST,S,having relatively more virus-like particles[11].R21 has shown up to 70% efficacy in phase Ⅱ trials against seasonal malarial transmission,however,it still needs more work to elaborate on the year-round and seasonal efficacy of the long-term vaccination outcome[11].Apart from RTS,S and R21,several other vaccine candidates are also under clinical trials that target various stages and components of Plasmodium and involve diverse types of vaccination[4,25].Table 1 summarizes the different vaccination methods and their respective ongoing trials.
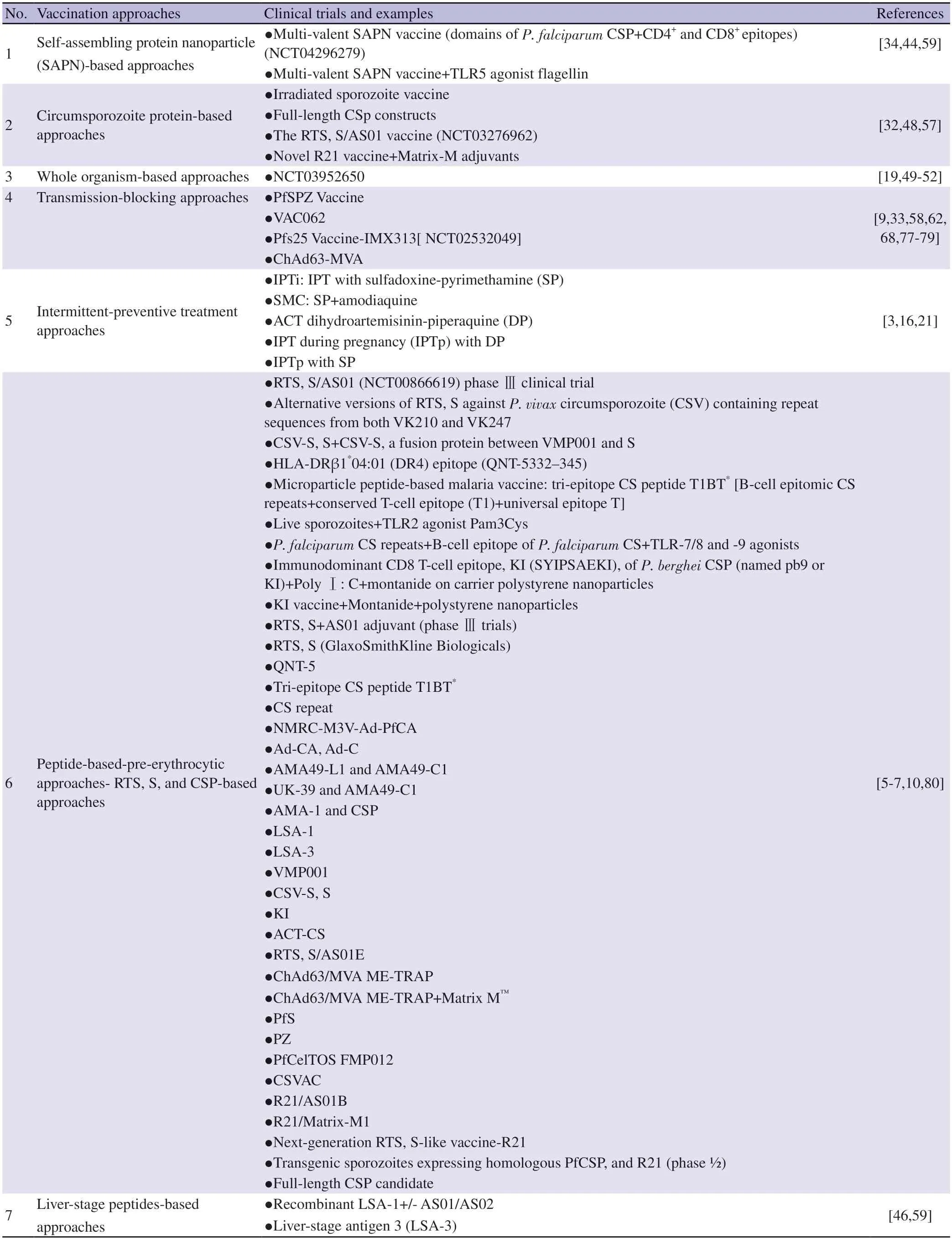
Table 1.Vaccination approaches against malaria.
3.2.Vaccination against malaria
The following section covers various vaccination efforts against malaria that have been under trial since 2000.It also includes the latest developments in particular vaccination approaches along with limitations in these approaches and future implications.
3.2.1.Self-amplifying RNA-based approaches
RNA-based vaccination methods have created successful trials in the COVID-19 pandemic and these results have helped scientists to deviate from their researches for malarial vaccination trials[34].RNA-based technologies inculcate the delivery of antimalarial antigens such as Plasmodium macrophage migration inhibitory factor (PMIF).The combination of PMIF and RNA is considered as a novel vaccination approach[34,44].Scientists are working on diverse mechanisms such as self-amplifying RNA vaccine targets against PMIF,which causes diffuse immune responses upon parasitic secretion.Studies on animal models showed that an RNA-PMIF construct enhances control against liver and blood-stage infections via increasing T-cell and B-cell based immune responses.These immunity-boosting responses have encouraged human trials in preliminary safety analyses[35].
It should be noticed that the current mRNA vaccines for COVID-19 developed by Moderna and Pfizer-BioNTech inject only limited amounts of mRNA without replicating and self-amplifying potential[45].However,self-amplifying RNA vaccines against malaria contain both replication ability and antigenic presentation ability to initiate immune responses and protein expression in hosts[15].Moreover,these mRNA-asked vaccines for malaria do not require freezing conditions for storage like that of COVID-19 vaccines,which is more suitable for storage and transportation to endemic areas[15,45].The initial supply of small doses of antigen regulates the large-scale immune responses such as postvaccination immunization.Thus,these approaches help reduce the multiple boosters and increased dosage needs,which is a great concern in resource-limited-endemic areas[12].
3.2.2.Self-assembling protein nanoparticle (SAPN)-based approaches
SAPNs are novel vaccination approaches with increasingly promising results against malaria.In this technique,scientists use peptides and proteins with a self-amplifying potential to convert them into chemically and mechanically stable particles[46].These particles undergo conformational changes and generate strong cellular and humoral immune responses with the help of coiled-coil core sequences[41].Unlike linear peptide antigens that break down rapidly,these antigens are more stable and generate long-lasting immune responses[44,46].They have the capability to induce multiple epitope targets corresponding to multi-stages of the life cycle,each able to produce targeted immune responses with appropriate conformity and functional activity against infection[44,47].Thus,these platforms are highly attractive for the vaccination formulations.
3.2.3.Circumsporozoite protein (CSP)-based approaches
CSP-based induced immune protection is a progressive vaccination strategy that has also been used to generate the currently approved RTS,S/AS01 vaccine containing central and carboxyl-terminus related to P.falciparum[48].Modifications have been made in lengths of center-,amino-,and carboxy-terminal regions in different studies.This approach has shown promise due to its capacity of incorporating other adjuvants and antipathogenic entities like monoclonal antibodies in better amino and carboxy terminals[32].The trials on RTS,S in delayed fractional boosters dosages have shown enhanced responses of 80%-90% against malaria-challenged adults and are now being tested upon children in affected areas as well[7].Similarly,trials with R21 also showed a higher effect of 77% in children over 12 months of age[11].However,these studies need further confirmation through repeated trials and follow-up mechanisms to show the long-term efficacy of vaccines.
3.2.4.Whole organism-based vaccination approaches
Whole organism-based approaches utilize attenuated or chemically treated malaria sporozoites to be delivered to hosts that undergo further reaction and upregulation of immune responses[49].Some approaches like rational-based attenuation,metabolic activation,non-replicating candidates,and sporozoite-based vaccines are in the trials phase and have demonstrated unprecedented efficacy against malaria[50].Similar to the work on live attenuation of sporozoite vaccine,research lines up to the blood stage of malarial infection[49,51,52].Such testing on human adults has shown effective results.However,the dose regimen specification and conditional optimization in the future will help select the best version of malarial vaccines.Similarly,the mechanism by which gene deletions are made to create attenuated whole sporozoites with a linked parasitic arrest at the liver stage will be further studied to determine oxide immunity-based antimalarial responses[19,52].
3.2.5.Intermittent preventive treatment approaches
Intermittent preventive therapy (IPT) is yet another novel malarial control and treatment effort involves the treatment with multiple doses of antimalarial drugs to the population to prevent new infection as well as overcome the existing infection[16].This approach is helpful to prevent and reduce seasonal malaria chemoprevention[16].Most trials are being carried out in west and central Africa due to high annual malarial transmission rate in those regions but not including regions with high resistance rate and pregnancy[14].The resistance prevalent in endemic regions is instead given the perennial malaria chemoprevention treatments to prevent year-round malarial transmissions[14].Pregnancy cases are dealt with the IPT during pregnancy (IPTp) with DP complex to overcome SP resistance in women as the IPTp shows improved birth outcomes besides the SP’s antimalarial activity[8,43].It is imperative to take concomitant drug into consideration before demonstrating them to patients because such dosages can lead to drug resistance,limited infection prevention,and less optimized drug targeting effects[8,9].This necessitates close monitoring of emerging genotypes and phenotypes in drug resistance strategies.Moreover,the concern about early malarial prevention in children with delayed immunity acquisition and increased risk of rebounding effect,especially after cessation of IPT,has to be addressed[8,12].
3.2.6.Peptide-based-pre-erythrocytic approaches
Pre-erythrocytic stage vaccination protocols are the most advantageous for vaccine formulations because of the inhibiting treatment of parasitic growth at the merozoites stage and prevention from entering the bloodstream[18].These approaches against malaria are currently in extend period research trials.They involve the targeting of antigens from Plasmodium sporozoite and liver stages,which are considered silent clinical stages of the malaria life cycle[53].In this approach,antibodies are manufactured against the surface epitopes of sporozoites in the skin and bloodstream of patients hence blocking malaria invasion of hepatocytes[54,55].It may also involve T-cell-base responses that directly attach to the infected hepatocytes.Testing on PEV started in the 1970s when rationally attenuated whole sporozoite vaccines was utilized,and the result showed subsequent challenges with homologous strains and heterozygous falciparum sporozoites (PfSPZ)[55,56].The results showed that PEV can eradicate pre-erythrocytic parasites from the bloodstream,often called anti-infection vaccines[54].
3.2.7.RTS,S,and CSP-based approaches
Genetic engineering is widely used for modern vaccination approaches,which involves the genetic cloning of the malarial surface antigen called circumsporozoite protein or CSP,as explained earlier[41].The development of CSP-based vaccines and RTS,S vaccine is crucial and involves a P.falciparum CSP fragment that comprises central repeats (R) C-terminal regions containing T cell epitopes (T) and the hepatitis B surface antigens (S)[5].The RTS system is expressed in yeast that itself could carry Hep B surface antigens (S) expression system hence leads to the co-assembly and mixed lipoprotein formulation (RST,S) presenting the combined CSP fragment of the surface[7,10].The progress associated with these PEV vaccines,especially RTS,and S is a great achievement,but the gaps for improvement are always there and in the case of malaria,different regions,dosage,age group,and sentimental conditions potentially affect the vaccination outcomes and treatment effect[7].Similarly,in the case of CSP-based vaccines,the gap lies in improving the overall immunogenicity of vaccines.Moreover,viral vector-based PEV candidates are also in research trials,targeting the CSP region to create a prime-boost immunization effect in humans[48,57].However,all these trials come along with prevalent limitations and challenges that need to be overcome to develop a vaccine candidate.In such cases,CSP-based vaccines show the ability to eliminate and reduce the responses against various epitopes of malaria to improve vaccine efficacy[1,3].
Approaches of structural vaccinology are used by different researchers around the world to manufacture improved versions of CSP-based vaccines according to characteristic epitopes of functional monoclonal antibodies in humans[28,37].These antibodies are in turn prepared by PEV administration or malaria parasitic exposure to hosts.These mAbs react against genomic repeat regions and N-terminal repeat regions,while only a few infrequent associations are made with the C-terminal region by these mAbs[33,58,59].Up till now,C-terminal specific antibodies have not established their functional activity well in initial animal experiments and require further assessment to check whether C-terminal-reactive human mAbs could improve the efficacy of RTS,S vaccines or not[5,59].The approved version of RST,S/AS02 has been developed with the conjugation to an oil-in-water-based adjuvant (AS02,immunostimulants MPL,lipopolysaccharide,and QS21)[5,6].RST experiments have delivered good efficacy in different experiments in terms of elevated immunological responses in malaria-infected and exposed subjects.However,the vaccine efficacy is still limited and has safety problems in some cases,thus needs further research in the future[7,10].
3.2.8.Liver-stage peptide-based approaches
Specific antigenic sequences are expressed during various stages of malaria infection and thus could be used to manufacture vaccines[27].One such antigen is expressed during the liver infection stage of malaria and consists of amino acid repeat units of specific counts(ALKEKLQ-X-QQSDLEEQR),which are pivotal to the liver stage schizogony.These antigens produce antibody-mediated responses like IgD antibodies in humans upon malarial infection[60].
3.2.9.Whole sporozoite-based approaches
Whole sporozoite vaccines have been in the research phase for a long time;however,it is not considered an suitable option for vaccine products,owing to the irritation potential of sporozoites in the manufactured product that makes it a bit risky for treatment especially for infants[61].Attenuation is achieved by other methods such as genetic attenuation and sterilization[50].These WSV vaccines have demonstrated dose and regimen-dependent efficacy in humans and presented higher efficacy than the approved RTS,S adjuvant vaccines in adults[51].However,the safety issue remains and requires reliable delivery and toxicity analyses for the co-formulation of non-attenuated sensitive sporozoites to ensure full attenuation in vivo[49].Challenges associated with WSV development include the liquid nitrogen cold chain,scale-up manufacturing,and intravenous inoculation also need to be considered[50,51].More experiments on WSV-based vaccines that incorporate P.falciparum strains in PfSPZ products will be needed to determine the efficacy against different species of malaria[62].Similarly,immunological analyses will be needed to improve vaccine efficacy as they have been found to impact the immune system by regulating the CD4 T cell,CD8 T cells γδ T cell,and B cell-based responses in animal testing[58,62].
3.2.10.Blood-stage-based approaches
Blood stage vaccine works by targeting the asexual mode of the parasite during multiple repeated divisions in the erythrocyte cycle to cause morbidity and mortality[20].Its effect is dictated by the infection and is indirectly affected by the parasitic species,and the period between fever and periodicity of overall disease is different from species to species.During this cycle,approximately 2 dozen merozoites egress from blood cells and invade new blood cells to result in multiplication of thousands of progeny side by side;the sexual forms originate to infect the mosquitoes in the next cycle[21,33].This repeated cycle makes BSV an effective vaccination strategy because it prevents the disease-causing stage of malaria and prevents the passive immune transfer of antibodies for clearing parasitemia from adults and children[20,26].
However,there remain some challenges in the development of antimerozoite vaccines at the blood stage,such as the brief timing between blood transmission and exposure to the immune system,antigenic polymorphism,and reluctant invasion pathways[63,64].Moreover,the substantial number of parasites to be targeted is another challenge that makes the experiment more difficult.However,a substantial number of trials have been conducted and are still being conducted worldwide on the BSV approach.These vaccines mostly target antigens like MSP1,AMA1,EBA-175,and MSP3 and regulate the antibody-mediated immune responses of giant merozoite antigens[28,44,58,59].The results of these studies have not been satisfying in terms of the protective immune system and the systematic safety.
3.2.11.Pfrh5-based approaches
P.falciparum reticulocyte-binding protein homolog 5 (PfRH5) is a highly conserved antigen that is applied for promoting broad-scale neutralizing antibody responses in preclinical studies.PfRH5-based vaccination also use the blood stage for vaccine formulations[35].PfRH5 binds to blood red cells on specific receptors and shows limited polymorphism and has been used for clinical trials based on a vector prime-boost strategy against the immune system.Similarly,researchers try to use different adjuvants and viral vector approaches to tackle the disease.In the case of natural infections with PfRH,no or limited specific antibody responses are observed in individuals[65].Furthermore,the challenge of high dosing must be addressed,along with the need for a deeper understanding of antibody-mediated responses that neutralize peptides[64].
3.2.12.AMA1-RON2-based approaches
AMA1 plays a vital role in blood-stage malaria growth,it binds to the rhoptry neck protein RON2 during the merozoite-erythrocyte interface,making this proteinous structure a promising strategy to device vaccination[8,13].Together,these peptides generate potent antigenicity and create antibody-mediated responses against monomeric AMA1 antigens.They also create stronger immune reactions as compared to agent alone,as studied in animal models[14,54].The structural variations and the antigen-antibody complex case studies can help scientists to re-design the peptide complexes.The surface variants and extensive genetic variations will allow future studies to focus on the number of alleles and chimeric sequences in these peptides to confer a broad-spectrum immune response[21,50,66].
AMA-1 is a promising antigenic candidate for blood-stage vaccination.Its structural composition has three domains by which antibody recognition occurs in a strain-specific manner[67].Different combinations containing these antigens exhibit strong antibody-mediated responses with long-lasting and parasitic growthinhibiting properties.They also inhibit the support migration and invasion of hepatocytes[21].The positive results may be affected by the implication of the influence of virosomes as a compatible delivery platform to humans and can thus help in developing subunit vaccines;however,further proof from clinical research is required to prove the efficacy of such studies[4,18,68,69].
3.2.13.Erythrocytic-stage-based approaches
The concept of the erythrocyte-based vaccine is that the malaria parasite (merozoite stage) targets and attacks red blood cells (RBCs).As RBCs are unable to stimulate T-cell-mediated responses,only humoral immunity is generated via RBCs[20,21].Their antibodies are of two types: antibodies that recognize the antigens on the merozoites surface and antibodies that recognize the parasite antigens presented on infected RBCs[4,68].These antibodies together regulate antibody-dependent cellular cytotoxicity.The erythrocyte vaccine works using the surface antigens.Some of the most common examples are MSP-1,MSP-2,and MSP-3[27].
3.2.14.MSP and glutamate-rich proteins based approaches
MSP-1 is the first in the line of MSP proteins that are expressed in the schizogony stage of parasitic infection in malaria.The conserved domains of MSP-1 are directly recognized as antigenic recognition B and T-cells in animals and humans and generate the associated humoral and cellular immune responses[70].Scientists have worked on the formulation of potent vaccine candidates from MSP-1 by conducting research on P1 and P2 as the epitomic subtypes of MEP,which are based on mediating specific sequence-oriented T and B cell responses[71,72].Both P1 and P2 could stimulate elevated levels of antibody titers without the need for any carrier proteins[67,73].
On the other hand,MSP-2 is another subtype of MSP protein that is an integral membrane protein on the merozoite surface.Immune cell recognition occurs through the conserved N and C-terminal regions of MSP-2 proteins in animals and humans[73].The B-cell epitope(SNTFINNA)+entire N-terminal peptide sequence (KNESKYSNTFINNA-YNMSIRRSM)/conjugate adjuvant with CFA and the MSP-3 proteins are being explored for similar vaccination approaches[71,73].MSP-3 and glutamate-rich protein (GLURP) are often investigated together for immune-epidemiological studies.The resulting IgGl-and IgG3-mediated dual immune response demonstrates the reduction in malaria through broad-spectrum inhibitory effects against particular species of malaria (P.falciparum isolates)[71,73].
3.2.15.Spf66 peptide-based approaches
SPf66 is a synthetic forty-five amino-acid peptide consisting of four proteins of P.falciparum,three of which are asexual stage protein fragments associated with the circumsporozoite proteinaceous repeats[13].It is the first synthetic peptide-based vaccine developed against malaria,which primarily targets blood-stage malaria parasites.It creates elevated levels of antibody titers to protect against parasites[70].No specific adverse reactions were observed by far,and the safety profile had been well-established in the research,however,the protection profile seems to vary among different studies against P.falciparum malaria.Thus,scientists are trying to create some diversions or changes in this vaccination protocol to establish treatment trials[13,67,73].
3.2.16.Novel BSV antigens
This approach has led to experiments on novel BSV vaccines based on antigens beyond merozoite targets.As parasitic antigens are exported to external RBCs surfaces upon infection,they remain exposed to antibody reactions for the long term[29,74].The PfEMP1 antigens family plays a crucial role that mediate parasitic sequestration and create virulence of P.falciparum while acting as a target for naturally occurring antibodies[71,74,75].Although PfEMP1 is an important antigenic target,its large size,high polymorphism,and conformational changes hinder the vaccination trials on it[71].
Similarly,a non-PfEMP1-infected surface protein named PfGARP has been proved to be a target for protective antibodies.These peptides help induce programmed cell death in vitro and thus protect against severe malaria[70,72,73].Similarly,the general parasite egression from erythrocyte surfaces also mediates humoral responses,which could be complied with the usual peptide-based vaccine in a kind of prime-boost strategy[60].Despite the ongoing research,subunit BSV vaccination remains a less successful vaccination effort.Thus,scientists are diverting toward other vaccination mechanisms such as those linked with whole organisms,which is a viable vaccination strategy[67].
3.2.17.Placental malaria-based approaches
Placental malaria vaccines target parasites that adhere to chondroitin sulfate A (CSA),which acts as a unique sequester in the placenta and inspires the researchers to explore a distinct BSV approach[76].This approach is especially important for designing focused vaccines for pregnant individuals where PEV and BSV vaccines create risks of treatment[9,22].The mechanism of action is that the naturally occurring antibodies work in response to the CSA-binding parasites to avoid the protection against the placental malarial infection.Placental parasites undergo conformational expression of distinctive VAR2CSA (PfEMP1 family member) that binds CSA and generates the antibody-based parasitic blocking effect[9].These experiments have shown safe,well-treated,and functionally active antibody responses against parasitic invasion[22].
3.2.18.Transmission-blocking approaches
Transmission-blocking vaccines (TBVs) simply incorporate the surface antigens derived from the gametes and zygotes of parasites from mosquitoes for induction of antibody-mediated responses against this parasite to kill them and prevent further transmission[68].TBVs work by targeting a common epitope Anopheles alanyl aminopeptidase 1 that is present in the mosquito midgut of different Anopheles species[62,77].This approach allowed the manufacture of a multi-species targeting malaria preventive vaccine.For this reason,several monoclonal antibodies are being tested for binding on preserved epitope sites to block transmission cooperatively with maximized effectiveness[62,77].Similar studies along with some adjuvants showed better and more active transmission-blocking effects in clinical trials.
Certain experiments are being conducted in labs to identify the beneficial characteristics in this vaccination protocol,where target antigens are identified and targeted by monoclonal antibodies in animal models to prevent mosquito infection[26].Distinct categories have even been described for vaccine candidates depending on the different properties of surface antigens and their point of presentation.These may include the surface antigens expressed by gametocytes in humans,the zygote surface proteins mostly present in the post-fertilization stage in mosquitoes,and the cysteine-rich antigens with epidermal growth factor-like domains or multiple 6-cys[26,33,58,59].These surface proteins create ruminant antigens but have poor immunogenicity as monomers[78].
Thus,scientists have started to develop parting conjugate and dual antigenic formulations for better immunogenicity regulation[72].A few challenges need to be overcome for effective malarial vaccines for extensive adaptive immune responses linked with antibodies.Safety profiling and the combined potential for mosquito and human infection prevention need to be established to assess the additive and synergistic activities.Thus,the hurdle regarding vaccine development remains hindered due to the difficult recombinant expression of folded proteins.Moreover,the combination approach can also be adapted to couple TBVs with RSVs and BSVs for improved vaccination.
3.3.Malaria control and elimination strategies-past,present,and future
WHO with multiple partners,national and international governments and non-governmental organizations,has been working to take measures for the eradication of malaria for years.In 2015,one such program named the Global Technical Strategy,was introduced to aim at malarial elimination in twenty endemic countries by 2025 and in thirty-five countries by 2035,along with a 90% overall reduction in mortality in the remaining countries[83,84].The goals were set on practical subjugations as the past 15 years’ data then proved an optimistic outlook for the future,mostly entertained by avoidance strategies like use of insecticide trap nets,air spraying indoors,and combination therapies based on artemisinin[84].
In 2016,the overall mortality and incidence rates declined and persisted with a mutual rise in a few endemic regions,which shows the resurgence of the diseases.These resurgence patterns were similar to historical accounts of malaria coupled with various uncertain and unconfirmed reasons,such as funding gaps,lack of awareness,delayed acquisition of immunity,drug resistance (artemisinin resistance) and other partner drugs,and insecticide resistance[13,37,39,79].Though efforts in continuous research phases regarding novel therapeutics,insecticides,and vaccination candidates,the linked approval,usage,affordability,acceptance,and availability issues remain associated with the treatment options of malaria.
3.4.Malaria vaccination efforts
Various researches have been carried out to evaluate the efficacy of different vaccine and regimens against malaria.Vaccine strategies like DNA,and virus-vector approaches employing adenovirus,and modified vaccinia were also tested against the malaria genome yet remained unsuccessful[34].The lack of funding further implemented vaccine development until in the 2000s when RTS,S came forward(later approved) as an effective vaccine candidate,pediatric vaccine option,and morbidity control tool that worked by preventing the diseases at an early stage[6].Trials on RTS,S and other important vaccine candidates remain in the research phase under the supervision of WHO to establish the safety and efficacy analyses of the vaccine under investigation[83,84].
3.5.Latest in-silico tools and techniques for designing and validating drugs and vaccines against malaria
3.5.1.Chemoinformatics-associated antimalarial discovery
Chemoinformatics plays a crucial role in the rational design of vaccines and drugs by utilizing modern artificial intelligence methods to analyze and interpret chemical information related to biological systems[85].This multidisciplinary approach enhances the efficiency of the drug discovery by prioritizing candidates with higher probabilities of success in subsequent experimental stages.In the context of vaccine and drug design against malaria,chemoinformatics involves some key aspects that allow for better vaccine and drug design and validation (Table 2).Chemoinformatics allows exploitation of chemical structures,better biological screening and data mining against potential therapeutic candidates[86].It makes a comprehensive in-silico approach in thousands of drug and vaccinecandidates,and compared with those that have been approved or under trials[85].

Table 2.Key aspects of chemoinformatics for better vaccination drive against malaria[85-88].
Chemical data may be extracted from sources such as high throughput screening,and structure-activity relationship studies which is then utilized for chemoinformatic processing for data storage and registration of useful candidates[87].Such programs not only help validating the approved therapeutics but also help to get rich sources for new antimalarials development.The large size of candidate libraries are generated through high throughput data mining which allow compound clustering and probabilistic identification of active potential drug candidates against malaria[88].Chemoinformatics improves potency of drugs,induces selectivity in potential candidates and reduces the possible toxicity by comparative analyses of drug molecules.
3.5.2.Immunoinformatics-based antimalrials discovery
Immunoinformatics is an interdisciplinary field that involves the application of computational methods and bioinformatics tools to analyze and interpret immunological data[89].The role of immunoinformatics is to aid in the design of immunological experiments,the development of vaccines,and the identification of potential therapeutic targets for infectious diseases and other immune-related conditions[90].In the context of malaria,immunoinformatics is employed to develop strategies for the identification of potential vaccine candidates and the design of immunomodulatory drugs.Immunoinformatics plays a crucial role in accelerating the vaccine development process,understanding immune-related diseases,and designing immunotherapies[89].Thus,by leveraging computational tools and approaches,researchers can efficiently analyze vast amounts of immunological data,leading to more targeted and effective interventions.Bioinformatics tools allow scientists to identify conserved genetic sequences for designing vaccine candidates with a compound prediction about their immunogenicity,allergenicity and antigenicity effects of epitopes and the possible effects on humoral and cellular immunity of the host[91].Table 3 explains how immunoinformatics is being used for drug and vaccine design against malaria.

Table 3.Immunoinformatics’ potential to help develop antimalarial therapies[89-91].
3.5.3.Next-generation sequencing (NGS) technique used in antimalarial development
Next-generation sequencing is a powerful and high-throughput technology that enables the rapid sequencing of DNA or RNA.It has revolutionized the field of genomics by allowing researchers to sequence entire genomes,transcriptomes,or targeted regions of interest with unprecedented speed and cost-effectiveness at the same time[92].NGS is used in various ways to understand the genetic diversity of the malaria parasite,investigate host-pathogen interactions,and identify potential drug targets and vaccine candidates[93,94].NGS is not limited to vaccine development,it also plays a role in effectiveness evaluation,safety profiling,transmission dynamics and interactional analyses with host and other pathogens[95].NGS helps reduce the reliance on in-vivo experiments,lengthy periods and the the time and cost involved in laboratory methods before drugs are ready for use.To fully leverage modern techniques like NGS,challenges such as risk analysis,resource availability,regulatory delay and scientific hesitancy need to be addressed in chemo and immunoinformatics.Table 3 shows how NGS is being utilized in malarial drug and vaccine development.
3.6.Future directives-the untold research questions
Despite the ongoing trials of new vaccine candidates,there is a need for further identification of targets,chemistries,renovations,and modern strategies such as nanotechnology in vaccination[23].Furthermore,equal attention shuold be given to malaria control strategies and understanding the body’s responses to malaria exposure,immunity,and protection outcomes[95].Researches into biological markers,immunoregulation,clinical markers,vaccine efficacy,host specifications,and susceptiblity factors also require further exploration[96].Additional future considerations are briefly outlined in Figure 1.
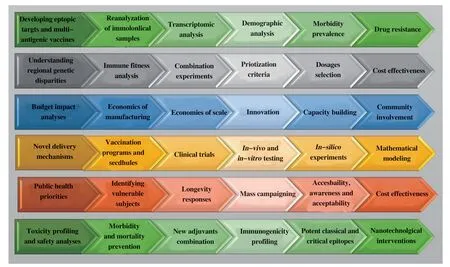
Figure 1.Future consideration for vaccination efforts against malaria.

Figure 2.Measures to be adopted for successful vaccination efforts against malaria.
4.Novel ideas and prospects
Researches indicate that combinational approaches,rather than vaccination alone,may be more effective in high-incidence regions[1,3].Combining anti-infective vaccines like RTS,S with seasonal malaria chemoprevention can prevent seasonal malaria in vulnerable populationssuch as children[40].The combined approach provides extended prophylaxis,strengthening the impact of drugs and vaccines in a more cost-effective way[42].Moreover,mass drug administration programs for malaria elimination and infection prevention are yet another way along with combinational approaches for reducing malaria incidence.However,these drug-based initiatives can reduce the incidence and faciliate treatment,especially in cases where vaccines are not available or in cases of artemisinin resistance[38-40,42,97].
The potential synergism between these approaches present an optimistic healthcare strategy for public health with a decrease in infection and resurgences.However,further search is required in a coordinated manner to establish complex heterogeneity profiles in populations[88].Moreover,it is worth noting that similar to mass drug administration,and combination treatment of vaccines and drugs,the option to utilize multiple vaccines is also being explored.A polyvalent vaccine targeting various parasitic stages could potentially restrain the spread of malaria.The vaccines block merozoites,sporozoites,and gametocytes,and transmission-blocking vaccines can be integrated into a single vaccine regimen[16,98,99].Such combinations have the potential to facilitate mass vaccination with low transmission rates,creating a synergistic effect as evidenced by preclinical and modeling studies,such as the coadministration of ivermectin with dihydroartemisinin-piperaquine[5,21,29,80].
Novel approaches such as sustained polymeric nanoparticlesbased hydrogel vaccination straregies,are also being explored[23].They hold sustained and durable immunological responses,unlike RTS,S-based responses that decline with time.Such platforms have been used during COVID-19 vaccination campaigns,and they work on the principle of hydrophobically modified hydroxypropyl methylcellulose derivatives that can sustain and stabilize the biodegrade NPs coating vaccines[5,23].Another approach includes the use of charge-altering releasable transporters as an alternative technique to mRNA base vaccination against COVID-19.It includes antigenic combinations with adjuvants for immunostimulant,robust,and sustained adaptive immune response,potentially reducing the need for booster doses[31].
By employing specific combinations of vaccination and drugs,conducting mass awareness campaigns,and novel vaccination techniques,targeting vulnerable populations such as children,pregnant women,travelers,non-exposed populations,outbreak zones,and conflict affected areas,there could be the outcome of a malarial circuit break of infection cycles.However,the successful implementation of these strategies relies on coordinated and detailed research efforts in the future[22,23].
5.Limitations
While the comprehensive review of vaccination approaches against malaria provides valuable insights,several limitations must be acknowledged.Firstly,the study’s focus on recent optimized vaccination approaches may introduce a temporal bias,as rapidly evolving technologies and strategies could render some findings outdated.The field of vaccine development is dynamic,and new advancements may have emerged.Secondly,the identified gaps in achieving a durable,effective malaria vaccine suggest the complexity of the challenge.However,the study may not delve deeply into the nuances of these gaps,potentially oversimplifying the obstacles in developing a long-lasting solution.A more nuanced exploration of the obstacles and uncertainties surrounding vaccine development would contribute to a comprehensive understanding.
Additionally,the key findings emphasizing mathematical modeling,combined drug and vaccine strategies,mass drug administration,polyvalent vaccine formulations,and targeted vaccination drives raise concerns about generalizability.The applicability of these approaches may vary across different regions,populations,and malaria strains,and the study may not adequately address the contextual factors influencing the effectiveness of these strategies.Addressing these limitations would enhance the study’s overall robustness and applicability to the dynamic landscape of malaria vaccine development.
6.Conclusions
Malaria has long been a burden on human communities.Studies are essential to explore the vaccination mechanism,novel approaches,and limitations attached to different vaccination designs against malaria.Our understanding regarding malaria,disease,prevention,treatment,and vaccination strategies have modified over time,which has resulted in a slowly declining of malaria burden and advancement in research and development in the field.Moreover,a mathematical modeling along with drug and vaccine combinations,mass drug administration,polyvalent vaccine formulations,and targeted vaccination campaigns offer hope for effective malarial prevention in the future.A comprehensive healthcare plan,research initiatives,and developmental efforts coordinated community participation are crucial for mass-scale vaccinations.The use of modern computational tools like chemoinformatics,immunoinformatics and NGS to validate large scale vaccination efforts facilitate the prevention and elimination of malaria.Moreover,there is a need to have an overview of coverage,accessibility,acceptability,deployment,compilation,and adherence to specific vaccination strategies to be employed specifically in endemic regions.
Conflict of interest statement
The authors declare no conflict of interest.
Funding
The authors received no extramural funding for the study.
Authors’ contributions
SM: Study design,literature search,and manuscript writing.YW:Study design,manuscirpt editing,and supervision.
 Asian Pacific Journal of Tropical Medicine2024年4期
Asian Pacific Journal of Tropical Medicine2024年4期
- Asian Pacific Journal of Tropical Medicine的其它文章
- Role of doxycycline in the treatment of dengue infection: An open-label,randomized,controlled,pilot trial
- Prognostic value of N-terminal pro B-type natriuretic peptide and troponin I in children with dengue shock syndrome
- Expression and clinical significance of pattern recognition receptor-associated genes in hand,foot and mouth disease
- A rare complication of measles infection presented with subacute sclerosing panencephalitis: Report of two cases in India
- Using X Social Networks (formerly Twitter) and web news mining to predict the measles outbreak
