How functional foods play critical roles in human health
Gungchng Png Juno Xie Qingsen Chen Zhihe Hu
a Tianjin Key Laboratory of Food Biotechnology,Tianjin 300134,China
b Biotechnology&Food Science College,Tianjin University of Commerce,Tianjin 300134,China
Abstract How do functional foods affect human health?To answer this question it is important to understand what happens when food is digested and taken up by the gastrointestinal(GI)tract.The gut is a selective nutrient absorption system and the most important signal transduction and information exchange system within the body.It acts as a signal transducer,a neuroendocrine sensor,and an immunological recognition and presentation system.It is also a complex information exchange system comprising a number of signaling networks involving GI cells and cells immobilized in organs or transported in blood.The bioactivity of functional foods in vivo may be due to their effects on such networks,but this raises the question of what signaling pathways are used by non-nutrients that cannot be absorbed by the gut.The purpose of this review is to describe intestinal nutrient transportation,identify a number of widely expressed receptors and signal transduction pathways,and outline our current understanding of the mechanisms involved in health and disease.At the end of the review,a method for developing a cell communication network is described.This network is convenient for investigating the effects of oral administration of experimental medicines,drugs,or functional foods on cytokines of interest.Because cytokines and chemokines are transported via the circulatory system,a simple 2–3 mL blood sample from a volunteer is a rich source of information.This method may become the gold standard for evaluating the effects of functional foods or medicines in vivo.
Keywords: Functional foods;Signal pathway;Gut;Cytokine;Receptor;Cell communication network
1.Introduction
There has recently been a rapid increase in consumer interest in the health-enhancing roles of specifi foods or physiologically active food components,so-called functional foods [1].Generally speaking all foods are functional as they have nutritional value and are fundamental for survival.However,the term “functional food” has a different connotation of providing an additional physiological benefi beyond basic nutritional requirements.For oriental cultures in particular,foods are viewed not only as essential for survival but also as a means of ensuring one’s health by ensuring a balanced intake of different foods assigned,for example,yin and yang attributions.Such knowledge has been accumulated over thousands of years.
In contemporary societies,however,many individuals do not consume a balanced diet and this has led to an increase in the incidence of serious metabolic imbalances.These in turn increase the risk of diseases such as obesity,type 2 diabetes,hypertension,food allergies and intolerances,and gastrointestinal and inflammator disorders.Good nutrition is vital for health,optimal growth and development,and prevention of disease[2].It is now understood that nutrients and other substances obtained from a wide variety of foods promote health,maintain metabolic homeostasis,and fulfil energy requirements.Unfortunately,there is no optimal diet fi for everyone due to the extensive variation in human genetics,phenotypes,and cultures,and this is why it is so important for food and nutrition scientists to determine the relationship between an individual’s metabolism and their diet to help them make better food choices.
The gut is an obvious target for functional foods as it is the interface between diet and metabolic events.Key digestive physiological processes that can be regulated by dietary modification include satiety;the rate and extent of macronutrient breakdown and absorption from the small bowel;metabolism;the nature of the gut microflora fermentation,mucosal functioning,bowel habits;and the gut immune system.
Over the past few decades,a number of large non-nutrient molecules have been identified These include secondary metabolites in plants that are used for defense,reproduction,and so on,but are not essential nutrients.Secondary metabolites such as phytochemicals were mostly ignored until recently when their potential metabolic effects were firs detected.For example,fl vones were found to protect against heart disease and soy-based estrogens against cancer.Since then the bioactivity of thousands of non-nutrient compounds in foods such as red wine,coffee,fish and fruits and vegetables have been investigated to determine their effects on animal biochemistry and metabolism and to identify those with potential health benefits
Probiotics are usually define as microbial food supplements that have beneficia effects on consumers.Microbiologists firs reported that the microflor of the gastrointestinal (GI) tracts of healthy individuals differed from that of diseased individuals,and the microflor found in the healthy individuals was termed probiotic.Most probiotics fall into the group of organisms known as lactic acid-producing bacteria,and these are normally consumed in the form of yogurt,fermented milks,or other fermented foods.Many of the functional food products that have been developed include probiotics.
So how do functional foods exert their beneficia effects on human health?To answer this question we firs have to understand what happens when these foods are digested and taken up by the GI tract.The gut is not only a selective nutrient absorption system;it is also the most important signal transduction and information exchange system within the body.The gut is involved in nutrient recognition and signal transduction,and also acts as a neuroendocrine sensor as well as an immunological recognition and presentation system.Together,these complex information exchange systems form a number of signaling networks that include cells immobilized in organs as well as cells transportedviathe circulatory system.Functional foods may exert their beneficia effects through these cell communication networks and by interacting with various receptors found along the GI tract.However,many non-nutrients,such as dietary fiber and oligosaccharides,exert their effectsviasignaling pathways other than those found in the gut.
The current review aims to describe intestinal nutrient transport,the regulation of signal transduction,and the various receptors involved in the processes outlined above.A detailed explanation of the mechanisms by which functional foods protect against a number of diseases is also provided.
2.The gut as a system for regulating both nutrient uptake and signal transduction
An understanding of the physiological regulation of nutrient uptake is of profound importance for elucidating the modulating effects of abnormal conditions such as diabetes and obesity on intestinal absorption[3].
2.1.Sugar uptake
Sodium glucose cotransporter 1 (SGLT1) is responsible for all active glucose uptake in the intestine.The subsequent movement of glucose across the basolateral membrane occurs along a diffusion gradient [4].Thus,SGLT1 is an essential component of intestinal glucose absorption,and its regulation is of paramount importance.Intestinal glucose absorption is performed by two different components:Na+/glucose cotransporters and diffusive components formerly attributed to paracellular fl w[5].Recent evidence has indicated that absorption is mediated by the transient insertion of glucose transporter type 2(GLUT2)into the apical membrane.This GLUT2 pathway is present in many species ranging from insects to humans,and acts as a major route of intestinal sugar absorption.Sensing mechanisms include regulation by a newly identifie calcium absorption pathway involving the neuroendocrine l-type channel Cav1.3 during the digestion and activation of intestinal sweet taste receptors by natural sugars,artificia sweeteners,paracrine and endocrine hormones (especially insulin and GLP-2),and stress.Active transport of glucose across the enterocyte apical membrane through SGLT1 is driven by the downhill gradient of Na+,which is maintained by the basolateral Na+/K+-ATPase[6].When the luminal glucose level is lower than the blood glucose level,SGLT1 has the unique ability to transport glucose against its concentration gradient and therefore to act as a very effective scavenger from the lumenin vivo.In enterocytes,glucose crosses the basolateral membrane into circulationviathe facilitative transporter GLUT2.Fructose is transported across the apical membrane by GLUT5.Biochemical markers in previous experiments have suggested that intracellular Ca2+(Cai2+) is important for the glucose-induced insertion of apical GLUT2,which might be activated by Cai2+.Apical GLUT2 has also been identifie in experimental diabetes and in insulin-resistant states induced by fructose and fat[7].
Intestinal sweet taste receptors are part of the lingual T1R family of sweet taste receptors that includes T1R1,T1R2,and T1R3.T1R3 is commonly involved in the formation of T1R2+T1R3 and T1R1+T1R3 heterodimers [8].The T1R2+T1R3 heterodimer works through two G-proteinmediated signaling systems.These are linked to either phospholipase C (PLC) β2 or protein kinase A and increase intracellular cAMP and Ca2+,respectively[9].
Sugar absorption is regulated by endocrine and paracrine hormones.Insulin secretion in response to a sugar-rich meal can lower blood glucose within minutes due to a ten-fold increase in glucose uptake by the skeletal muscles and adipose tissues,which occurs as GLUT4 is translocated to the plasma membrane.This process may explain the previously unknown hypoglycemic action of insulin on enterocytes.Glycemic excursions,a known risk factor for cardiovascular disease in type 2 diabetic patients,are smoothed during the course of a meal.[10].Cheeseman et al.also reported paracrine regulation of sugar absorption,particularly by gastric inhibitory polypeptide (GIP),Glucagon-like peptide-1(GLP-1),and GLP-2[11].
2.2.Intestinal lipid absorption and sensing
Products of lipid hydrolysis are solubilized in micelles and presented to the apical membranes of enterocytes.These apical membranes contain several transport proteins that participate in the uptake of various types of lipids[12].Niemann-Pick C1-like 1(NPC1L1)is one of the proteins involved in cholesterol uptake.CD36 and fatty acid (FA) transport protein (FATP) have been found to participate in FA transport,and the scavenger receptor class B type I(SR-BI)is involved in vitamin E(Vit E)uptake.In the cytosol,FA-binding protein(FABP)and cellular retinolbinding protein(CRBP)are responsible for the transport of FAs and retinol(R),respectively.In addition,enterocytes express the ATP-binding cassette(ABC)transporter A1 on their basolateral membrane to facilitate the efflu of cholesterol.The absorption of lipids from the intestinal lumen into the enterocytes and their subsequent secretion into circulation are complex processes.Recent finding concerning the basolateral efflu of cholesterol as high-density apoA-I-containing lipoproteins should help to identify additional targets for the development of drugs to suppress cholesterol absorption and so reduce hypercholesterolemia and the risk of cardiovascular disease.
The intestine also contributes to energy homeostasis through the absorption,metabolism,and transfer of nutrients to the organism[13].Many studies have shown that an excessive postprandial accumulation of triglyceride-rich lipoproteins (TRL)in plasma is a risk factor for cardiovascular diseases [14].Enterocytes ensure the transfer of dietary lipids to the organism through complex processes [15].In the intestinal lumen,dietary lipids such as triglycerides(TG)are hydrolyzed into FA and monoglycerides,primarily by pancreatic enzymes.The FA and monoglycerides associate with biliary secretions and form lipid micelles,which facilitates their absorption by enterocytes[16].The FA are taken up by passive diffusion and a proteinmediated mechanism involving the FA translocate,a FA-binding protein from the plasma membrane,as well as the FA transport protein family[17].During the postprandial period,TG are transiently stored in enterocytes as cytosolic lipid droplets;these are subsequently hydrolyzed and reenter the secretory pathway[18].Many genes are involved in the control of TG secretion and storage.The transcription factor hepatocyte nuclear factor(HNF)-4α is expressed in the liver,pancreas,and intestine[19].Frochot et al.[13]found that postprandial micelles specificall modulate the expression of 47 genes related to three main functions of enterocytes:cell adhesion/architecture,cell signaling,and glucose/lipid metabolism.Of the 47 genes identified 20,including apoA-IV,apoB,and MTP,have putative or known binding sites for HNF-4α[20].
The absorption of lipids not only supplies the nutrition the body requires,but also stimulates a number of signaling pathways.Recently,Devkota et al.[21]reported that mice fed a diet rich in milk-derived saturated fat displayed increased liver production of taurocholic acid,which in turn stimulated the growth of certain gut bacteria.In genetically susceptible mice,one such bacterial species,Bilophila wadsworthia,triggered intestinal inflammatio and colitis.
2.3.Peptide absorption and regulation
The transporter PEPT1 is an oligopeptide transporter located at the intestinal brush border.It is responsible for the transport of 400 dipeptides and 8000 tripeptides as well as a wide range of peptide-like drugs such as β-lactam antibiotics and angiotensin-converting enzyme inhibitors[22].PEPT1 is an H+-peptide cotransporter that relies on a proton gradient for the uphill transport of peptides that are subsequently transported across the basolateral membrane by a facilitative basolateral peptide transporter [23].In terms of energetic efficien y,2 or 3 peptides can be transported into a cell by PepT1 for the same expenditure of energy as is required to transport a single free amino acid(AA)[24].Individuals suffering from deficien free AA transport are still able to assimilate essential AAs,indicating that PepT1 can transport enough dietary AAs to compensate for a deficien y in free AA transport[25].Transport of AAs in the form of peptides has been shown to be a faster route of uptake per unit time than transport of free AAs[26].Furthermore,more AAs are absorbed by the proximal small intestine when protein is infused as a hydrolysate instead of in an intact form,suggesting that peptides are more readily available for absorption.Circulating peptides may not be hydrolyzed in plasma[27].Peptides are likely to be transported into cells and subsequently hydrolyzed into free AAs that in turn are used for protein synthesis [28].The extent of peptide utilization by cells is influence by dietary status[29].The above finding suggest that diet influence the concentration of circulating peptides.Kim et al.[30]detected peptidase activity in the brush border and soluble fractions of rat intestinal mucosa targeting 13 dipeptides and 5 tripeptides;80–90% of the activity occurred in the soluble fractions and 10–15%at the brush border.The peptide transporters are members of the proton-coupled oligopeptide transporter superfamily,which is also known as the peptide transporter family [24].Peptide transport has been shown to be proton-dependent[31].Shimakura et al.[32]proposed a mechanism for the induction of intestinal PepT1 under fasting or starvation conditions.They demonstrated that PepT1 induction is mediated by peroxisome proliferators-activated receptor α(PPARα),a member of a family of nuclear receptors activated by FA ligands,and thus plays an important role in the adaptive response to starvation.The peptide transporter can be upregulated by insulin,leptin,thyroid hormone,T3,and epidermal growth factor [33].Both the molecular weight distribution and the concentration of dietary peptides may influenc growth performance and immunological development.The use of different protein sources,hydrolysis conditions,and dietary concentrations makes it very difficul to compare studies and make any claims regarding effica y.The differences in the substrate affinit of PepT1 for different peptides,the effect of each peptide on gene expression,and the 8400 possible di-and tripeptide combinations are all obstacles to the development of a peptide prof il of the digestive enzymes and transporters involved in the physiological processes in the gut[34].
For efficien AA and di- and tripeptide absorption,these processes need to be balanced and strictly controlled as well as coupled with the relevant signaling pathways.Recently,Hashimoto et al.[35]investigated the connection between protein deficien y and intestinal inflammation and uncovered an intricate network involving nutrient transport,microbial ecology,antimicrobial responses,and inflammation Tryptophan is obtained from a number of foods and is absorbed in the small intestine by a transporter protein dependent on the enzyme ACE2.Hashimoto et al.demonstrated that mice lacking ACE2 and those fed a diet lacking tryptophan experienced greater inflammatio in the large intestine in response to damage than mice with normal tryptophan levels.These deficien mice also had reduced levels of intestinal antimicrobial peptides,which are produced by intestinal epithelial cells.Tryptophan and its metabolites can,through the action of the transcription-factor protein AhR (the aryl hydrocarbon receptor) [36],induce the production of cytokines IL-22 and IL-17 by the intestinal immune cells;these then induce greater secretion of antimicrobial peptides.
Nutrients are taken up by complex transport systems that are compactly and stoichiometrically coupled with ion channels,Na+–K+pumps,sensors,and signaling pathways,and so the nutrients and their homeostasis must be coordinated with integral physiological,metabolic,immunological,and neuroendocrine systems.As an example,G protein-coupled receptors(GPCRs) encompass the largest and most diverse family of membrane proteins in eukaryotes.GPCRs transduce a variety of signals across the cell membrane,sense almost all nutrients,and regulate diverse biological and physiological processes.The function of GPCRs relies on a specifi lipid environment and is often strongly dependent on the presence of cholesterol and ions.GPCRs are allosteric machines [37]controlled by ions,membrane components,and water molecules.This means that membrane proteins can be controlled not only by dietary ligands,but also by endogenous small molecules located at specifi binding sites.These small molecules can dramatically affect the stability and function of proteins,which in turn can have a pronounced effect on the physiological signaling of GPCRs.Most recently,Liu et al.[38]showed that the central cluster of such GPCRs harbors a putative sodium ion bound to the highly conserved aspartate residue Asp2.50,two cholesterols stabilize the conformation of helix VI,and one of 23 ordered lipids intercalates inside the ligand-binding pocket.These highresolution details shed light on the potential role of structured water molecules,sodium ions,and lipids/cholesterol in GPCR stabilization and function.
3.The GI tract acts as an important recognition and defence system
In the gut,there is a delicate balance between the need to recognize pathogens and dietary ingredients to prevent unwanted immune responses to food antigens or the normal intestinal flor and the need to simultaneously allow for adequate nutrient uptake.As the largest area of the GI tract(occupying a surface area of almost 400 m2in man),the gut has developed complex protective mechanisms.The mucosal barrier consists of intestinal epithelial cells and defence mechanisms such as low pH,peristalsis,and a mucus coat,all of which protect the intestine from invading or toxic antigens.The intestinal lymphoid tissue is the largest component of the immune system and is referred to as the gut-associated lymphoid tissue (GALT).It consists of Peyer‘s patches(PPs),mesenteric lymph nodes,isolated lymph follicles,and lymphocytes scattered throughout the lamina propria and epithelium of the intestine.PPs act as the primary inductive sites where the interaction between luminal antigens and circulating lymphocytes occurs.The PPs contain specialized epithelial cells known as microfold cells(M cells).M cells are specialized in complete antigen uptake from the intestinal lumen and their transport across the mucosal surface to the subepithelial dome area.M cells are thus fundamental to secretory antibody production,vaccination response,and food allergies.
The antigens are taken up by several antigen-presenting cells(APCs)where they are processed for presentation to T cells in the PPs.The T cells are activated upon antigen presentation,and then differentiate and mature in the germinal centers of the follicles.They migrateviaefferent lymphatics to the mesenteric lymph nodes,and eventually enter circulation.Activated T cells migrate from the blood to the effector sites of intestinal immune responses with the help of adhesion molecules.Here they act as effector cells and secret cytokines and mediate specifi adaptive immune defences[39].Samples are taken up by the organized mucous-associated lymphoid tissue(O-MALT),which may also be present as single lymphoid follicles embedded in aggregated follicles such as PPs in the intestine.
3.1.The key cells associated with information exchange in the mucosa
3.1.1.T cells
Distinct immune mechanisms have evolved to maintain the integrity of this component of the immune system.T cells belonging to both the βδ and αβ cell lineages are found in close physical association with the mucosa.Activated intestinal βδ cells,but not αβ cells,produce the epithelial growth factor FGF-7,suggesting that intestinal γδ cells and the soluble factors they produce have important roles in maintaining intestinal homeostasis in normal and disease conditions[40].Although a distinct Th1/Th2 cytokine prof il is not as clear in humans as in animal cells,there remains an inverse relationship between the tendency of T cells to produce IFN-γ as opposed to IL-4 and IL-5[41].The synthesis of IL-2,IL-6,IL-10,and IL-13 is not as tightly restricted to a single subset as in mouse T cells,and both Th classes produce granulocyte-macrophage colony stimulating factor(GM-CSF),TNF-α,and IL-3[42].Paliard et al.found that IL-2,IL-4,and IFN-γ can also been produced in human T-helper cells[43].There is a dichotomy between IL-2,IFN-γ,and TNF-βversusIL-4 and IL-5,and therefore the Th1/Th2 dichotomy is still considered an important functional division in the immune systemin vivo.
3.1.2.Epithelial cells
The mucosal surface is covered with a single layer of polarized epithelial cells,which are undifferentiated,actively proliferating cells.The epithelial cells play important roles in the uptake and transport of secretory IgA into the lumen.They express a series of cytokines,including IL-1β,IL-8,TNF-α,and IL-10[44].Epithelial cells also express receptors for a variety of cytokines,such as IL-1R,IL-2R,IL-4R,IL-7R,IL-9R,and IL-15R[45].In addition,they express MHC class II molecules and have also been shown to be process and present antigens to primed T cells.Their expression of adhesion molecules,lymphocyte function-associated antigen (LFA),B7,and cytokine receptors may trigger a cascade or maintain homeostasis along the gut.Recently,Mishra et al.[46]demonstrated that Jak3 was involved in IL-2 induced intestinal epithelial cell(IEC)migration,which is one of the early events in intestinal wound repair.They also reported that IL-2 plays a role in IEC homeostasis through concentration-dependent regulation of IECproliferation and cell death by IL-2R.
3.1.3.Macrophages
The GI tract contains the largest number of macrophages in the body.In addition to their antigen-presenting ability,macrophages also activate T cells through their production of accessory cytokines.Macrophages are more abundant in the small intestine.They have been shown to locate in the villous core,the subepithelial space in crypts,and under the dome epithelium overlying PPs.Macrophages secrete cytokines such as IL-1,IFN-γ,TNF-α,IL-8,IL-6,IL-10,and TGF-β,and also express receptors for IFN-γ,IL-4,IL-10,and TNF-α.In addition,they express a number of adhesion and HLA-II molecules.
3.1.4.M cells
M cells are armed with a series of “take-up” devices.Receptor-mediated endocytosis and fluid-phas endocytosis have both been observed,and M cells also appear to be capable of transferring immunity from mother to child by phagocytosis.After birth,a child’s own immune system is unprimed.He/she becomes immune due to the transfer of IgG from the mother by M cells and the neonatal Fc receptor(FcγRn)[47].In humans,a similar process is involved in the transfer of IgA.IgG is secreted into the firs milk(colostrum)and is transferred across the IECs.
3.2.Expression of cytokines and their receptors on the mucosa
3.2.1.Cytokines and receptors
Cytokines are usually classifie as proinflammator (IL-1,IL-2,IL-6,IL-12,IL-18,IFN-γ,and TNF-α),anti-inflammator(IL-4 and IL-13),or immunosuppressive(IL-10,TGF-β)based on their activity.Epithelial cells also express receptors for a number of cytokines,including IL-1R,IL-2R,IL-4R,IL-7R,IL-9R,and IL-15R[45].These data suggest that there may be a signaling cascade along the GI tract that is involved in the regulation of immunological,physiological,and metabolic activities.
3.2.2.Chemokines and chemokine receptors
Chemokines and their receptors are not only involved in the control of hematopoietic cell migration and the development of Th1 and Th2,but also in a wide variety of other physiological and pathological processes.Th1 and Th2 cells are known to express a variety of surface antigens.Th1 cells preferentially express the CXCR3 and CCR5 chemokine receptors,whereas Th2 cells express CCR3,CCR4,and CCR8[48].The key molecules attracting leukocytes to local inflammator sites are chemokines.Hannelien et al.[49]undertook a systematic review of the impact of CXC chemokines (binding receptors CXCR1,CXCR2,CXCR3,and CXCR4) on the transition of chronic inflammatio in the upper gastrointestinal tract to neoplasia.CXCR2 ligands including GRO-α,β,γ/CXCL1,2,3,ENA-78/CXCL5,and IL-8/CXCL8 attracted pro-tumoral neutrophils.The literature also supports the hypothesis that the gradual change from a controlled immune response to pathological inflammatio and neoplasia in the esophagus and stomach is partly orchestrated by chemokines.Therapeutic approaches that use chemokine receptor antagonists or chemicals targeting tumor-promoting chemokines and chemokine receptors may thus have potential in the prevention and treatment of upper gastrointestinal tract inflammatio and tumors.
3.2.3.Adhesion molecules
The migration of lymphocytes in the intestinal mucosa is a complex multi-step process.It is mediated by adhesion molecules,the homing receptors of lymphocytes,and counter receptors located at the endothelial cells in specialized postcapillary high endothelial venules.Different combinations of these adhesion molecules ensure tissue-specifi migration.The selective recruitment of lymphocytes makes the immune response more efficien by directing the cells back to where they firs encountered their antigen and where they thus are more likely to meet the specifi antigen again [50].Cells can constitutively express adhesion molecules,and these can be upregulated by cytokines,chemokines,or other proinflammator molecules such as dietary ingredients or microbial metabolites.In addition to mediating adhesion,some of these molecules also have a costimulatory function during intercellular signaling[51].
4.Membrane receptors expressed on the mucosa
To date,almost all membrane receptors have been observed on the mucosa,which indicates that these receptors can interact with their specifi ligands in the GI tract and sense environmental conditions,especially the nutritional status of the body,viathe circulatory system.
4.1.G protein-coupled receptors(GPCRs)
G protein-coupled receptors,which are also known as seventransmembrane domain (7TM) receptors,are a large protein family involved in the sensing of molecules outside the cell and internal activation of multiple signal transduction pathways.The GPCR ligands that bind and activate these receptors include odors,neurotransmitters,hormones,and molecules ranging in size from small to large.There are two principal signal transduction pathways associated with the G protein-coupled receptors:the cAMP signaling pathway and the phosphatidylinositol signaling pathway [52].There are four major classes of GPCRs (791 genes):Class A (rhodopsin-like,662 genes),Class B(secretin-like,15 genes),Class C(glutamate receptorlike,22 genes) and other (92 genes including Adhesion 33 genes,Frizzled 11 genes,Taste type-2 25 genes,and unclassifie 23 genes.)[53].The human genome encodes thousands of G protein-coupled receptors,about 350 of which detect hormones,growth factors,and other endogenous ligands.Approximately 150 of the GPCRs found in the human genome have unknown functions[54].
GPCRs are involved in a wide variety of physiological processes.Some diet-related roles include:(1) vision.The opsins use a photoisomerization reaction to translate electromagnetic radiation into cellular signals.Rhodopsin,for example,uses the conversion of 11-cis-retinal toall-trans-retinal for this purpose.This may be used to distinguish food colors;(2)sense of smell.Receptors of the olfactory epithelium bind odorants (olfactory receptors)and pheromones(vomeronasal receptors);(3)nervous regulation of the GI tract and brain.Receptors in the mammalian brain bind several different neurotransmitters,including serotonin,dopamine,γ-aminobutyric acid(GABA),and glutamate;(4) autonomic nervous system transmission.Both the sympathetic and parasympathetic nervous systems are regulated by GPCR pathways and are responsible for the control of many automatic functions of the body such as appetite and digestion;(5)regulation of the defence system.Chemokine receptors bind ligands that mediate intercellular communication among cells,and histamine receptors bind inflammator mediators and engage target cell types in the inflammator response;(6)bioand chemical sensing of major nutrients such as AAs,sugars,peptides,nucleotides,and lipids;and(7)homeostasis modulation such as that required to maintain nutrient balance.
The cholecystokinin(CCK)receptors throughout the human gut have varied physiological functions,evolutionary backgrounds,and molecular structures.The CCK receptors are important members of the GPCRs as they are involved in the regulation of many physiological functions such as satiety,GI motility,gastric acid secretion,gall bladder contraction,pancreatic secretion,anxiety,memory,and learning[55].
The GPCRs are activated by a wide variety of external signals in the form of ligands or other signal mediators.When a ligand interacts with the corresponding receptor,a conformational change occurs in the receptor,triggering activation of a G protein.Some receptors for sensory signal mediators include olfactory stimulatory molecules;adenosine;GABA;gut-neuroendocrinology signal mediators including members of the vasoactive intestinal peptide family,vasopressin,platelet-activating factor,neuropeptide Y,opioid peptides,somatostatin,tachykinins,and calcitonin;follicle-stimulating hormone;gonadotropin-releasing hormone (GnRH);thyrotropin-releasing hormone;dopamine;epinephrine;norepinephrine;histamine;glutamate;glucagon;acetylcholine;and serotonin.
Upon receptor activation,the G protein exchanges GDP for GTP,causing the dissociation of the GTP-bound α and β/γ subunits and triggering diverse signaling cascades.Receptors coupled to different heterotrimeric G protein subtypes can utilize different scaffolds to activate the MAPK cascade[56],and employ at least three different classes of Tyr kinases.Src family kinases are recruited in turn to activate PI3Kγ by β/γ subunits.They are also recruited by receptor internalization,and by crossactivation of receptor Tyr kinases.GPCRs can also employ PLCβ to mediate activation of PKC and Ca2+/Calmodulin-Dependent Protein Kinase II (CaMKII).The Frizzled group of GPCRs is evolutionarily conserved and serves to transduce signals from the Wnt-type lipoglycoprotein growth factors.G proteins also play roles in Wnt/Frizzled signaling,as well as in the identificatio of other signaling intermediates in intracellular signal transduction[57].
Human GPCRs mediate multiple signal transduction pathways as well as members of an intricate network of intracellular signaling pathways including the cAMP/PKA,calcium/PKC,calcium/nuclear factor of activated T cells(NFAT),PLC,protein tyrosine kinase,PKC/MEK,p43/p44MAP,p38 MAP,PI-3 Kinase,NO-cGMP,Rho,NFκB,and Jak/Stat pathways[58].
4.2.Pattern-recognition receptors(PRRs)
Pattern-recognition receptors(PRRs)are located on various cell types,including APCs and epithelial cells,and can recognize microorganism-associated molecular patterns(MAMPs)such as lipopolysaccharide (LPS) [59].They can also enhance attachment and phagocytosis of microorganisms.Toll-like receptor(TLR)-4,the receptor for LPS,mediates bacterial translocation through enterocytes and M cells.Aside from TLRs,epithelial cells also express a variety of mammalian lectins that bind particular carbohydrates and conjugating carbohydrates on the surfaces of particles can target these to enterocytes[60].
4.3.FcRn and immunological transfer from mother to baby
The neonatal Fc receptor FcRn was firs described in the intestine of neonatal rats [61].Since then,FcRn expression has been described in many species,including humans.It has been detected in several tissues including the mammary gland,lung,placenta,and intestinal epithelium [62].FcRn binds the Fc portion of IgG with high affinit at pH 6.5,but not at physiological pH 7.4 [63].Several functions ascribed to FcRn are a direct consequence of this unique pH-dependent IgG binding:(1) FcRn is involved in the transfer of maternal IgG from the mother to her offspring to confer short-term passive immunity.This FcRn-mediated IgG transfer occurs through placental and/or intestinal pathways depending on species[64];(2)FcRn plays an important role as a homeostatic regulator of IgG and albumin in adult mammals[65].Endothelial cells also express FcRn,and endosomal FcRn can bind internalized IgG after acidification thereby protecting IgG from lysosomal degradation and thus prolonging its serum half-life to 22 days in humans [66].Studies have also demonstrated that FcRn may play an important role in mucosal immunity as an immunological sensor [67].It is interesting that FcRn is expressed in association with the transfer of immunity from adults to neonates in humans as well as some other species [68].FcRn is also expressed in human IEC lines and it transcytoses IgG molecules bidirectionally across these polarized cell monolayers[69].The slightly acidic environment of the small intestine allows FcRn,which is expressed at the apical surface of enterocytes,to bind both IgG and antigen-IgG immune complexes(ICs).FcRn then transcytoses these ICs through the epithelial barrier and releases them in the underlying extracellular space.Following transcytosis from the intestinal lumen to the underlying GALT,these ICs are endocytosed by CD11+ lamina propria dendritic cells (DCs),which then migrate to the mesenteric lymph nodes to activate CD4+ T cells.FcRn can also deliver specifi IgG to the intestinal lumen,which subsequently enhances resistance to intestinal infection[70].Thus,FcRn clearly has a role in mediating transepithelial transport and so forms part of an important mechanism by which DCs sample luminal antigens to control for mucosal pathogens.Many of the functional foods that have been developed are dairy products such as immune milk and IgG-fortifie milk powder,and the mechanism of the bioactivity of these products is based on FcRs.
4.4.Fibroblast growth factor receptors(FGFRs)
Fibroblast growth factors (FGFs) act through their cognate receptors (FGFRs) and play vital roles in the development and regulation of FGF/FGFR signaling,which is associated with a number of different syndromes.There is much interest in the inhibition of FGF/FGFR signaling as a cancer therapy as FGF/FGFR signaling is important in tumor angiogenesis.Thus,FGFRs are increasingly attractive targets for therapeutic intervention.Knights et al.[71]reviewed FGFR signaling and described advances in cancer genomics and cancer cell biology.FGFs can elicit numerous cellular and physiological responses in the human gut,and they have been implicated in processes that include proliferation,differentiation,and angiogenesis.FGF1–FGF10 and FGF16–FGF23 mediate these effects by binding to a family of fi e structurally related receptor tyrosine kinases (RTKs),which are designated FGF receptors(FGFR1–5)[72].
4.5.The Notch receptors
The Notch receptors are single-pass transmembrane proteins that are activated by the Delta-like and Jagged families of membrane-bound ligands.Notch proteins are transported to the plasma membrane and form matured heterodimers on the cell surface.Interaction with ligands leads to two additional proteolytic cleavages that liberate the Notch intracellular domain(NICD)fromthe plasma membrane[73].Notch was initially discovered to be responsible for the specifi phenotype of‘notches’on the wing blades ofDrosophila melanogaster[74].In mammals,the Notch family includes four receptors (Notch1–4).Notch signaling promotes the maturation of both the CD4+and CD8+single positive thymocytes.The Notch receptors can be glycosylated extracellularly at EGF-like repeats.Notch1 has been implicated in the determination of T cell fate and in the maturation of early T cells in the thymus [75].Reedijk et al.[76]suggested that expression of Jagged ligands and Notch1 and Notch receptor activation are constant features of human colon cancers,and thus the application of anti-Notch therapeutics may benefi patients with this disease.Notch signaling interacts with many other pathways including PI3K/Akt,NF-kB and STAT3.
4.6.Glucagon-like peptide-1(GLP-1)receptors
Obesity is prevalent worldwide,but in the USA alone it has increased by 75% since the early 1980s with more than one-third of adults now classifie as obese.A number of studies have described the physiological roles of GLP-1 and its receptor in the regulation of glucose homeostasis and energy metabolism[77].Willard and Sloop[78]reviewed GLP-1 receptor physiology with an emphasis on GLP-1-inducing signaling mechanisms in order to highlight new molecular strategies for guiding development of GLP-1 receptor activators.Glucose and other macronutrients present in a mixed meal,such as AAs and lipids,are transported to the lumen and stimulate a similar incretin response [79].In the intestine,nutrients interact with their sensory receptors,membrane channel and transporter proteins on the membrane surface of open-type endocrine cells.These cells release the incretins upon nutrient stimulation.In L cells,which are located throughout the intestine,GLP-1 is produced by posttranslational cleavage of a 160 AA proglucaon precursor protein,a process requiring prohormone convertase-1/3 [80].GIP is a single peptide derived from proteolytic processing of a 153 AA precursor protein expressed in endocrine K cells,which are located mainly in the duodenum and the proximal jejunum of the upper small intestine [81].When released into circulation,GLP-1 and GIP facilitate glucose disposal by acting on pancreatic islets and enhancing postprandial insulin secretion [82].This process is mediated by heterotrimeric GPCRs.These signals occur in response to binding by GLP-1 and GIP,respectively[83].
In humans,the incretin effect is often reduced in patients suffering from type 2 diabetes mellitus [84].In contrast to GIP,GLP-1 also induces several additional antidiabetic effects,including inhibition of glucagon secretion and gastric emptying,which both help improve postprandial glucose control and decrease appetite and food intake [85,86].These effects are mediated by the GLP-1 receptors expressed in extrapancreatic tissues,most notably those of the GI tract.
Early reports highlighted the ability of the GLP-1 receptor to couple with alternative signaling pathways,including phospholipase C(PLC),and the mobilization of intracellular Ca2+,which is consistent with the known ability of GLP-1 to stimulate Ca2+mobilization in β-cells [87].Furthermore,many reports have shown that the GLP-1 receptor is coupled with Ca2+mobilization when heterologously expressed[88].GLP-1 receptor stimulation has also been shown to induce biphasic activation of ERK[89].This effect includes initial cAMP-dependent transient activation of ERK followed by prolonged β-arrestin-1-dependent activation of ERK.Translation of this findin to the GLP-1 receptor identifie an analogous peptide,63Asn-Arg-Thr-Phe-Asp67,as a low potency but fully efficaciou GLP-1 receptor agonist [90].It is clear that the GLP-1/GLP-1 receptor axis is a key physiologic regulator of glucose metabolism,and diabetic patients treated with the degradation-resistant GLP-1 receptor peptide agonists exenatide and liraglutide experience improved glucose homeostasis.Therefore,a number of efforts to identify orally active small molecule GLP-1 receptor agonists are currently underway.Functional foods may thus also have potential in the treatment of type 2 diabetes mellitus and obesity.
4.7.The aryl hydrocarbon receptor(AhR)and nuclear erythroid-related factor 2(Nrf2)
Quinones and their phenolic precursors are ubiquitous in animals as well as their environment.They include dietary polyphenols and tocopherols,medical drugs,and the metabolites of environmental pollutants.Quinones are involved in diverse biologic processes including metabolism of estrogens and catecholamines,detoxificatio of aromatic hydrocarbons,and electron transport [91].Quinone–quinol redox cycles lead to oxidative stress,and some quinones are highly toxic arylating agents that activate pathophysiologic processes such as endoplasmic reticulum (ER) stress,inflammation and cancer [92].Enzyme systems have evolved to detoxify reactive quinines such as multiple glutathione S-transferases (GSTs),NAD(P)H,and the quinone oxidoreductases NQO1 and NQO2[93].
4.7.1.AhR and Nrf2 regulating genes
AhR is the only ligand-activated member of the bHLH/PAS(basic helix-loop-helix/Per-Arnt-Sim) family of transcription factors [94].It has many roles in the regulation of sex hormones,inflammation immunoregulation,and detoxificatio of lipophilic endo- and xenobiotics [95].After ligand binding,AhR translocates into the nucleus,sheds its chaperones,and associates with aryl hydrocarbon receptor nuclear translocator(ARNT).The heterodimer binds to xenobiotic response elements (XREs) with the consensus sequence TnGCGTG of the target genes.In addition to classical ligands,such as aromatic hydrocarbons,a number of endogenous AhR agonists have been suggested,including tryptophan metabolites,bilirubin,and eicosanoids[96].
Nrf2 is a member of a subset of bZip transcription factors known as the CNC bZip family whose members are major regulators of antioxidant defense and cell survival [97].As transcription factors,AhR and Nrf2 regulate drug-metabolizing enzyme(DME)genes involved in endo-and xenobiotic detoxificatio and antioxidant defense.AhR and Nrf2 activation is required for the expression of Phase II detoxificatio enzymes.These genes have been identifie by oligonucleotide microarray analysis in the small intestines of Nrf2-deficien and sulforaphane-treated mice[98].AhR and Nrf2 exhibit multilevel crosstalk:(1) the typical AhR-regulated cytochrome P450A1(CYP1A1)generates reactive oxygen species(ROS)that downregulate CYP1A1 expression[99];(2)Nrf2 has been identifie as a downstream target of AhR based on functional XREs in the promoter of Nrf2.AhR is also a target of Nrf2 [100];(3)coordinate induction of UDP-glucuronosyltransferases by AhR and Nrf2 is also possible due to interactions between these two transcription factors[101];and(4)AhR and Nrf2 interact with each other in signaling pathways[102].The regulated genes of AhR and Nrf2 also contain response elements for binding AhR and Nrf2 in theirciselements,including both Phase I and II detoxifying enzymes,and conjugating enzymes [103].In one study,normal CYP1A1-induced mice remained healthy when treated orally with benzo[a]pyrene (BaP),whereas enterocytespecifi (but not hepatocyte specific CYP1A1-deficien mice experienced fatal bone marrow toxicity and immunosuppression under the same conditions [104].These finding suggest that efficien detoxificatio of oral BaP in IECs is achieved by tight coupling of Phase I and II metabolism.Glucuronides are known to be released from enterocytes into the intestinal lumen by the AhR-inducible export transporter breast cancer resistance protein BCRP/ABCG2[105].
AhR has recently become a subject of mainstream immunological research and it is set to continue to intrigue with ever more complex modes of modulating immune responses [106].The highly selective expression of AhR on Th17 cells and its role in the induction of the cytokine IL-22 was suggested as a new immunological function of this transcription factor and stimulated further research into the physiological functions of AhR in the immune system[36].AhR expression is assumed to be ubiquitous in the immune system,an interpretation which is based on a global microarray analysis of immune cell populations[107].
4.7.2.AhR in innate immune cells
It stands to reason that AhR expression in many innate cells would have important implications for the functioning of the complex interactions between innate and adaptive immune cells.Furthermore,it is assumed that AhR is widely expressed in innate cells such as neutrophils,macrophages,NK cells,and DCs [108].Langerhans cells express high levels of AhR and the absence of AhR results in impaired maturation and antigenpresenting capacity [109].AhR expression levels increase in response to TLR ligands,and AhR-deficien DCs are associated with defective IL-10 production in response to TLR stimuli[110].One study linked NFκB Relb to AhR[111]during IL-8 transcription.AhR-mediated derepression of the IL-6 promoter provides an intriguing insight into the mechanism of AhR-mediated modulation of cytokine responses in conjunction with NFκB components[112].A reduction in IL-10 production by DCs and macrophages is likely to boost pro-inflammator responses.Furthermore,the widespread expression of AhR on epithelial cells can be expected to exert influence on cells of the immune system,which are in close proximity and engage in mutual interactions in homeostasis and immune responses.
4.7.3.AhR in adaptive immune cells
Exemplifie by the issue of AhR expression in regulatory T(Treg)cells.AhR expression in Treg cells is above background levels when compared with non-activated Th0,Th1,and Th2 cells,but is delayed by a few minutes compared with Th17 cells[36].The role of AhR in the induction of IL-22 production by Th17 cells and subsets of TCRγδT cells indicates that AhR has a clear function in a subset of immune cells[36].Some studies have also shown that AhR activation contributes to Th17 differentiation[113].The combined contribution of IL-6 and TGFβ is mandatory before AhR can exert any effects on Th17 cells.Furthermore,it is postulated that Notch signaling promotes the production of endogenous ligands for AhR,which then promotes IL-22 expression[114].DCs have been shown to affect Treg differentiation in the intestinal environmentviaretinoic acid and TGFβ[115].Interestingly,AhR-dependent CYP enzymes are known to metabolize retinoic acid[116]and may be indirectly involved in the complex interactions and development of T cell subsets in the gut.
4.7.4.AhR and autoimmunity
AhR has also been suggested as forming the mechanistic basis for the involvement of environmental triggers in autoimmune syndrome[117].Furthermore,the link between AhR and IL-22 production by Th17 cells suggests a potential association between this transcription factor and autoimmunity,which is strongly associated with exacerbated IL-22 responses[118].This suggests that autoimmune syndromes may be triggered by the interaction of environmental factors with AhR [119].AhR agonists have also been suggested for the treatment of autoimmune diseases [120].Hopefully further research will establish a firme basis to justify the undertaking of clinical trials of AhR agonists or antagonists for the treatment of autoimmune disorders as well as for producing foodstuffs without allergens.
4.7.5.Ahr and Nrf2 associations with phytochemicals
AhR and Nrf2 regulating genes/enzymes are attractive since they can be modulated by,for example,phytochemicals.Phytochemicals can be both activators and inhibitors of AhR and Nrf2,and include resveratrol,sulforaphane,and epigallocatechin gallate[121].Notably,Nrf2 activation by phytochemicals follows a biphasic dose-response relationship:a stimulatory effect at a low dose is followed by adverse responses at higher doses[122].Thus,the effects of functional foods on health may be evaluated using these receptors.We may boldly speculate that at least some of Chinese herbal medicines function by interacting with these receptors.
4.8.The R3 subtype of receptor-type protein tyrosine phosphatases
The R3 subtype of receptor-type protein tyrosine phosphatases (RPTPs) includes vascular endothelial tyrosine phosphatase(VE-PTP),density-enhanced protein tyrosine phosphatases(DEP)-1,protein tyrosine phosphatase receptor type O(PTPRO),and stomach cancer-associated protein tyrosine phosphatase(SAP)-1.These enzymes share a similar structure with a single catalytic domain and putative tyrosine phosphorylation sites in the cytoplasmic region and fibronecti type III-like domains in the extracellular region.SAP-1 of the receptors is located on gastrointestinal epithelial cells.In addition,these RPTPs are localized specificall at the apical surfaces of polarized cells.The structure,expression,and localization of the R3 RPTPs suggest that they perform tissue-specifi functions and might act through a common mechanism including activation of Src family kinases[123].Protein tyrosine phosphatases(PTPs)are thought to play important roles in proliferation,differentiation,and migration [124].These enzymes are also important regulators in the central nervous system,the immune system,and in many organs.Deregulation of PTPs is associated with various disorders,and so many members of the PTP family are considered potential therapeutic targets [125].The human genome encodes 107 PTPs,of which the class I cysteine-based PTPs form the largest group.These are further divided into 38 tyrosine-specifi PTPs and 61 dual-specifi phosphatases[126].SAP-1 was originally identifie as a PTP expressed in a human stomach cancer cell line,and its expression is largely restricted to the GI tract[127].In particular,SAP-1 localizes to the microvilli of the brush border of the small intestine and colon as well as to the stomach.The predominant expression of SAP-1 in gastrointestinal epithelial cells and its localization to the microvilli of these cells suggest that SAP-1 might play a role in the maintenance of the microvillus architecture.Forced expression of SAP-1 was shown to inhibit the proliferation of cultured cells through attenuation of growth factor-induced activation of MAPK or through induction of caspase-dependent apoptosis[128].SAP-1 likely also promotes intestinal cell proliferation through activation of the Src family of protein tyrosine kinases(SFKs).A more complete understanding of the physiological functions of R3 RPTPs,as well as the identificatio of their ligands,may provide a basis for the development of new functional foods or medicines for a variety of medical disorders,including cancer.
4.9.Toll-like receptors(TLRs)
TLRs play a critical role in the innate immune response to invading pathogens by sensing microorganisms and endogenous danger signals,including those associated with a variety of food chemicals.TLRs are evolutionarily conserved receptors,and are homologues of theDrosophilaToll protein;they are known to be important for defense against microbial infection [129].TLRs recognize highly conserved structural motifs known as pathogen-associated microbial patterns (PAMPs),which are exclusively expressed by microbial pathogens,or danger-associated molecular patterns(DAMPs).Stimulation of TLRs by PAMPs or DAMPs triggers signaling cascades that activate transcription factors such as NF-κB,AP-1,and interferon regulatory factors (IRFs).Signaling by TLRs may result in a variety of cellular responses including the production of proinflammator cytokines,chemokines,interferons (IFNs),and effector cytokines that direct the adaptive immune response.
The TLR family of type I trans-membrane proteins is characterized by an extracellular domain containing leucine-rich repeats (LRRs) and a cytoplasmic tail that contains a conserved region—the Toll/IL-1 receptor(TIR)domain.TLRs are predominantly expressed in tissues involved in immune function,peripheral blood leukocytes,and in the lungs and GI tract.Ten human TLRs have been characterized to date:TLR1–10.TLR2 is essential for the recognition of a variety of PAMPs from Gram-positive bacteria,including bacterial lipoteichoic acids,lipomannans,and lipoproteins.TLR3 recognizes virusderived double-stranded RNA.TLR4 is activated by LPS and TLR5 detects bacterial flagellin TLR9 is a sensor for unmethylated CpG DNA,and TLR7 and TLR8 identify small antiviral molecules and single-stranded RNA[130].Significan progress has been made over the past few years in understanding TLR function[131].It is well known that many foods are fermented by various microorganisms,and some foods are themselves formed by macrofungi.It appears inevitable then that ligands and their TLR agonists will be present in these foods.How can we deal with this problem? It is believed that our bodies have evolved a mechanism to restrict homeostatic signaling systems,just as the TLR7versusTLR9 balance has been identifie as a mechanism for tightly controlling expression and activation of the RNA-sensor TLR7 by downstream signaling pathways[132].
4.10.Opioid receptors in the GI tract
Opioid receptors belong to the family of seven transmembrane GPCRs with 50–70% homology between their coding sequences[133].Opioid receptors coupleviathe Gi/Go subtypes of G proteins to cellular transduction processes.Once activated by agonists,μ-opioid receptors undergo endocytosis in a concentration-dependent manner [134].The signaling pathways of opioid receptors are well characterized.Their effects mirror the actions of the endogenous opioid system,and are mediated by the principal μ-,κ-,and δ-opioid receptors.In the gut,met-enkephalin,leu-enkephalin,β-endorphin,and dynorphin occur in both neurons and endocrine cells.When released,opioid peptides activate opioid receptors on the enteric circuitry controlling motility and secretion.These processes increase sphincter tone,induce stationary motor patterns,inhibit gastric emptying,and blockade of peristalsis ensues[135].The endogenous ligands of opioid receptors are derived from three independent genes,and their appropriate processing yields the major representative opioid peptides β-endorphin,met-enkephalin,leu-enkephalin,and dynorphin.These peptides and their derivatives exhibit different affinitie and selectivities for theμ-,δ-and κ-receptors located on the central and peripheral neurons,mucosal cells,neuroendocrine,and immune cells,among others[136].
Hughes et al.identifie leucine-enkephalin and methionineenkephalin as the firs endogenous opioid receptor agonists[137].These pentapeptides were also found in the gut [138].Subsequent analysis of their function revealed that opioid receptor agonists interact with pathways of the enteric nervous system that regulate GI motility and secretion[139].There is evidence that some effects of opioid receptor agonists in the GI tract may be mediated by opioid receptors in the brain[140].Many neuroactive drugs act on the gut because the alimentary canal is equipped with the largest collection of neurons outside of the brain.Enteric neurons originating from submucosal plexuses supply all layers of the alimentary canal,and are thus are in a position to regulate digestion [141].Enteric neurons synthesize and release acetylcholine,nitric oxide,vasoactive intestinal polypeptide,ATP,substance P,and 5-hydroxytryptamine,as well as their opioid peptide transmitters.This may explain why exogenous opioid analgesics inhibit GI function.Metenkephalin,leu-enkephalin,β-endorphin,and dynorphin have been localized to both enteric neurons and mucosal endocrine cells [142].The μ-,κ-,and δ-subtype opioid receptors have been localized to the GI tract of humans [143].In the human gut,μ-opioid receptors are present on myenteric and submucosal neurons as well as on immune cells in the lamina propria[134].The cellular effects of myenteric μ-opioid receptor activation are brought about by multiple transduction pathways including membrane hyperpolarization,inhibition of calcium channels,activation of potassium channels,and reduced production of cAMP[144].Studies show that δ-,κ-,and μ-opioid receptors of the human intestine contribute to opioid-induced inhibition of muscle activity.Evidence indicates that opioidinduced inhibition of GI transit is mediated by opioid receptors in the gut,and also that opioids acting within the brain also influenc GI function.The ability of opioid receptor agonists to inhibit GI secretory activity and transit are therapeutically exploited for the treatment of acute and chronic diarrhea as well as irritable bowel syndrome-associated diarrhea[145].Peripherally restricted opioid receptor antagonists may thus be able to normalize the pathological inhibition of gut function that arises from upregulation and/or overactivity of the opioid system in the GI tract[146].The development of opioid receptor antagonists with restricted access to the central nervous system has opened up a new way to prevent the undesired effects of opioid analgesics outside the central nervous system[135].These receptors also provide us with a series of targets against which to screen different agonists and antagonists in medicines or functional foods and determine their therapeutic activity for numerous diseases of the central nervous system,GI tract,immune system,the respiratory system,and other disorders related to inflammation alcoholism,binge eating,and obesity[136].
4.11.Nuclear receptors of the enteric tract
Nuclear receptors are a class of proteins found within cells responsible for sensing steroid and thyroid hormones as well as many other molecules.They work with other proteins to control the expression of specifi genes to control homeostasis and metabolism.Nuclear receptors can bind directly to DNA and regulate gene expression.They are thus classifie as transcription factors [147].The nuclear receptor superfamily comprises 48 ligand-activated transcription factors that have been grouped on the basis of shared structural features[148].The ligands of nuclear receptors include lipophilic substances such as endogenous hormones,vitamin A,vitamin D,and xenobiotics,among others.Many of these genes are associated with health and the molecular targets of approximately 13% of U.S.Food and Drug Administration (FDA)-approved drugs are nuclear receptors [149].Nuclear receptors typically bind DNA as either homodimers or heterodimers.The retinoid X receptors (RXRs) are obligate heterodimer partners,which means that all nuclear receptor heterodimers must include one of the RXRs.The nuclear receptor superfamily can be subdivided into 4 groups based on the type of ligand bound and the DNA binding mechanism.The firs of these classes includes the endocrine receptors that bind DNA as homodimers.These include the estrogen,androgen,glucocorticoid,mineralocorticoid,and progesterone receptors.The second group of receptors form heterodimers with RXRs and are activated by a wide range of dietary lipids including FAs (bind PPARs),bile acids (bind FXR),xenobiotics(bind PXR,CAR),and a variety of cholesterol metabolites referred to as oxysterols(bind LXRs).The metabolic receptors activate transcriptional programs to turn on catabolic pathways for dietary lipids.The third group comprises retinoic acid (vitamin A),1,25-dihydroxyvitamin D3,and thyroid hormone receptors.These also function as RXR heterodimers,but their ligands are synthesized in the body from precursors that are derived from the diet.The fina group includes receptors with no known ligands,which are referred to as orphan nuclear receptors[150].
These orphan receptors[151]have unknown endogenous ligands.Some,such as farnesoid X receptor(FXR),liver X receptor(LXR),and peroxisome proliferator-activated receptors(PPARs,3 types of PPARs have been identified α,γ,and δ(β)) bind a number of metabolic intermediates such as FAs,bile acids,and/or sterols with relatively low affinit .These receptors may thus function as metabolic sensors.Other nuclear receptors,such as constitutive androstane receptor (CAR) and pregnane X receptor(PXR),appear to function as xenobiotic sensors by upregulating the expression of cytochrome P450 enzymes that metabolize these xenobiotics[152].The major functions of the enteric nuclear receptors,namely the vitamin D receptor(VDR),the bile acid receptor(FXR),and the xenobiotic receptors(PXR and CAR),are summarized below.
(1) Absorption of beneficia nutrients and maintenance of homeostasis.Although it is well known that VDR regulates mineral homeostasis,probably the most important site of VDR action is in the intestine.Here,the transcriptional regulation of ion channels and transport proteins by VDR is essential for the uptake of calcium.Insuffi cient calcium absorption is the primary cause of decreased bone mineralization resulting from a loss of VDR activity [153].In the intestine,VDR directly regulates calcium transport and binding proteins.It has become clear that the calcium-sensing receptor(CaSR)has a number of important functions because of its ability to activate many different signaling pathways.This means that the receptor has a number of different but crucial roles in human health.Recently,Ward et al.[154]emphasized that the CaSR may have multiple roles not only in disorders associated with calcium homeostasis,but also in unrelated diseases,such as colorectal cancer,Alzheimer’s disease,diabetes mellitus,hypertension,and GI disorders,because of its involvement in so many signaling pathways.The traditional role of bile acids is simply to facilitate the absorption and digestion of lipid nutrients,but bile acids also act as endocrine signaling molecules that activate nuclear and membrane receptors to control integrative metabolism and energy balance.Recent evidence has shown that transcriptional cofactors sense metabolic changes and modulate gene transcription by mediating reversible epigenomic post-translational modification (PTMs) of histones and chromatin.Importantly,targeting these epigenomic changes has been a successful approach for treating human diseases.Smith et al.[155]reviewed the roles of transcriptional cofactors in the epigenomic regulation of metabolism,and focused on bile acid metabolism in particular.Targeting PTMs of histones and chromatin remodelers together with the bile acid-activated receptors may provide new therapeutic options for bile acidrelated diseases such as obesity and diabetes.
(2) Elimination of toxic compounds.Although only a subset of the enteric nuclear receptors regulates nutrient absorption,they all play a role in the elimination of toxic substances,drugs,and xenobiotics absorbed by the gut.As xenobiotic sensors,PXR and CAR are the principle modulators of detoxifying pathways involving phase I,II,and III enzymes and transporters.While the liver is the major organ associated with bile acid and xenobiotic metabolism,it is now known that the intestine expresses many of the same enzymes and contributes to the metabolism of orally ingested foodstuffs and drugs/xenobiotics in the lumen.A number of studies have focused on the transcriptional targets and drug metabolizing roles of nuclear receptors such as PXR,CAR,and FXR,particularly with respect to drug interactions and metabolism,and it is likely that these evolved to protect the body from toxins.In the firs phase of drug/xenobiotic metabolism,a substrate is typically modifie through the addition of a hydroxyl group by the cytochrome P450(CYP)superfamily.The reactions carried out by this family of monooxygenases include oxidation,reduction,and hydrolysis.In phase II,the chemicals are further modifie by conjugating enzymes that add a larger,more hydrophilic group such as glutathione,glucuronic acid,taurine,or acetate.Phase III involves the transport of the modifie and water-soluble compounds out of the cells.All components of this system are under transcriptional regulation by the enteric nuclear receptors:VDR,PXR,FXR,and CAR.These four nuclear receptors act as the sensors of the intestinal detoxifying system,and thus have the important role of activating the effectors protecting enterocytes from dietary toxins.Enterocytes express many members of the two major classes of membrane transporters,the ABC transporters and the solute carrier(SLC)transporters.Both are regulated by PXR,whereas multidrug resistanceassociated proteins-2 (MRP2) is also regulated by CAR[156,157].Since the ion pumps are located in the apical membranes of the enterocytes,they return toxic compounds directly to the gut lumen[158].
(3) Protection of the gut against pathogens.The enteric tract is host to a large microbial community.In addition to the mucosal barrier formed by tight junctions between epithelial cells and mucus,gastric acid,pancreatic juices,biliary secretions,secretory IgA,and antimicrobial peptides form the major mucosal defense arsenal.It is well known that bile acids are toxic to microbes.The microbicidal activities of steroids also have intrinsic detergent properties.Research has shown that the antimicrobial activity of bile acids can be mediated,in part,by their activation of FXR in the intestine [159].This discovery was made from a genome-wide survey of FXR targets in the intestine that identifie a group of genes involved in innate mucosal immunity.VDR is also expressed in cells of the adaptive immune system,and vitamin D has been shown to prevent or reduce autoimmune disease [160].Vitamin D inhibits the activation of T cells,and promotes tolerance in DCs[161].VDR has also been implicated in TLR signaling and in the regulation of the innate immune response in macrophages [162].Recently,Agace and Persson [163]published a review of vitamin A metabolism in DCs generated in the gut mucosa.They reported that several mediators directly induce vitamin A metabolism in DCs,including certain TLR ligands,cytokines,PPARγligands,and RA itself.The small intestine and mesenteric lymph nodes (MLNs)contain higher concentrations of retinol than other tissues,including the colon,as a result of dietary intake and probably also bile secretion.This in turn leads to enhanced RA generation and signaling in the small intestine environment,and offers a likely explanation for the efficien imprinting of small intestine DCs (SIDCs) compared with DCs in other tissues.
(4) Control of proliferation,differentiation,and carcinogenesis.Diets high in animal fats are believed to increase the risk of colon cancer by elevating levels of fecal bile acids.A unifying hypothesis to explain the effect of VDR and bile acids on tumor promotion was proposed when it was discovered that VDR is activated by the toxic secondary bile acid lithocholic acid (LCA) [164].In a normal diet,LCA levels are low and circulating vitamin D can prime the system to ensure a basal level of catabolic activity.In a high-fat diet and/or in a vitamin D-deficien state,the rate of LCA metabolism is inadequate,and so LCA builds up to toxic levels,damages the colonic epithelium and causes cancer [165].Like VDR,other enteric nuclear receptor groups could have direct effects on proliferation/differentiation as well as indirect effects such as the detoxificatio of carcinogens or reduction of oxidative stress.
(5) Endocrine regulation of enteric nuclear receptors.Recent discoveries have focused on transcriptional regulation of a subfamily of FGFs.Most FGF signals act in an autocrine or paracrine fashion,However,members of the FGF19 subfamily are released into circulation and function as endocrine hormones [166].FXR controls the synthesis of FGF19,which signals in an intestine-liver and intestinegallbladder axis to regulate bile acid synthesis and secretion.Regulation of secreted FGF19 and FGF23 led to the discovery of a third member of this subfamily—FGF21,a fasting hormone regulated by the FA receptor PPARα[167].These discoveries have drawn attention to the importance of enteric nuclear receptor signaling not only within the intestine,but also in the coordination of complex multi-organ physiological processes.More recently,Jakobsson et al.[168]discussed our current understanding of LXR biology and pharmacology with an emphasis on the molecular aspects of LXR signaling that constitute the potential of LXRs as drug targets.They suggested that the recent discoveries of LXR-regulated pathways in inflam mation and proliferation have ignited interest in LXRs as drug targets for metabolic,chronic inflammator ,and neurodegenerative disorders.This means that successful drug development must focus on limiting the range of LXR signaling.
4.12.The transient receptor potential vanilloid 1
The transient receptor potential (TRP) channels are a family of ion channels that sense stimuli from chemical substances.Most TRP channels include 6 membrane-spanning helices and are expressed in many tissues and organs.TRPs have been subdivided into 6 main subclasses based on their ligands:TRPC,TRPV,TRPM,TRPP,TRPML,and TRPA[169].The transient receptor potential vanilloid(TRPV)1,also known as capsaicin receptor due to its sensitivity to capsaicin,is an ion channel that is predominantly expressed in sensory nerves [170].TRPV1 has been found to be widely distributed in a number of different tissues and organs [171],and its effects on health and disease have been investigated.As an ion channel,it is able to sense a series of stimuli and triggers multiple signaling pathways.It has been shown that endogenous arachidonic acid derivatives and lypoxygenase products exert a highly potent stimulatory effect on TRPV1[172].The FA amide arachidonylethanolamide,also known as anandamide,is known as a cannabinoid receptor 1(CB1) agonist.TRPV1 is highly sensitive to capsaicin,which is an exogenous agonist of TRPV1 [173].Rutaecarpine has extensive pharmacological actions from beneficia effects on GI functions to anti-inflammator and anti-obesity effects [174].Evidence suggests that TRPV1 is involved in many other physiological or pathophysiological processes:(1) it takes part in the regulation of appetite and body weight [175];(2) it plays a role in body temperature maintenance[176];and(3)TRPV1 also has effects on GI and cardiovascular systems due to the activities of its agonists [177].Jun et al.discussed the role of TRPV1 in pain signaling,body temperature maintenance,fat distribution,and respiratory inflammatio in their review[178].A number of TRPV1 blockers have been developed,and some are in clinical trials[179].It is obvious that TRPV1 is a useful target when searching for active constituents of functional foods or medicines for the treatment of various diseases including GI disorders.
There are many other receptors involved in GI systems,including lactoferrin receptors(LfR)[180],somatostatin receptors [181],histamine receptors (the latter two belong to the rhodopsin-like family of GPCRs)[182],seven-transmembrane receptors[183],and chemokines and their receptors[184].We focused on the receptors briefl introduced above because these are,in our opinion,the main receptors of importance for functional foods.
5.Interactions between the gut microbiota and dietary nutrients
Prebiotics,polyunsaturated fatty acids (PUFAs),and phytochemicals are the most well-characterized dietary bioactive compounds.The beneficia effects of probiotics mainly relay on their influenc on the composition of the gut microbiota and their ability to generate fermentation products with numerous bioactive roles.PUFAs include the ω-3 and ω-6 FAs,the balance of which may influenc diverse aspects of immunity and metabolism[185].Moreover,interactions between PUFAs and components of the gut microbiota may also influenc their biological roles.Phytochemicals are bioactive non-nutrient plant compounds that are of interest because of their anti-estrogenic,anti-inflammator ,immunomodulatory,and anticarcinogenic effects.However,the bioavailability and effects of polyphenols is very dependent on their transformation by components of the gut microbiota.Prebiotics are mainly non-digestible food ingredients,mostly oligosaccharides,that beneficiall affect the host by stimulating the activity of specifi intestinal bacteria.Possible beneficia effects of prebiotics include the control of intestinal transit and bowel habits,and a reduction in the risk of obesity,atherosclerosis,type 2 diabetes,and allergies,although their effectiveness in humans remains controversial[186].Galacto-oligosaccharide (GOS) and inulin-derivatives(e.g.,fructo-oligosaccharide)are prebiotics that have been commercialized in Europe.GOS is a non-digestible oligosaccharide derived from lactose that is found naturally in human milk and consists of chains of galactose monomers.Consumption of this probiotic may increase the total amount of bifidobacteri in the gut[187].Inulin and its hydrolytic product(oligofructose)are fructans that are linked by different numbers of fructose monomers.These occur naturally at high concentrations in plant foods such as asparagus and wheat,and have different functions,including the induction of anti-inflammator effects and the regulation of lipid and glucose metabolism[187].
The effects of these prebiotics on immune functioning may be due to their impact on the gut microbiota and the generation of short-chain fatty acids (SCFAs) by binding to SCFA receptors[188].SCFAs may also regulate intestinal fat absorption as butyrate,for instance,impairs lipid transport[189].Inulin and inulin-type fructans are soluble fiber that can modulate the gastric emptying and intestinal transit time,delaying absorption of glucose and improving glucose metabolism[190].Furthermore,dietary fiber including some non-digested polysaccharides such as chitins,pectins,beta-glucans,and lignin can modulate the transit time through the gut.
Recently,Haiser and Turnbaugh [191]reported that the gut microbiota interact with xenobiotics directly by catalyzing various biotransformations.In turn,many xenobiotics inhibit microbial growth.Indirect interactions are also ubiquitous,including microbial effects on the expression and activity of components of host xenobiotic metabolism.Multiple studies have provided a wealth of information by elucidating the rates of absorption,distribution,metabolism,and excretion of xenobiotics including therapeutic drugs,antibiotics,and diet-derived bioactive compounds[192].
A neglected but critical component of xenobiotic metabolism is the influenc of the trillions of microorganisms that inhabit our gastrointestinal tract.Members of the gut microbiota can also influenc xenobiotic metabolism by altering host gene expression and producing compounds that interfere with metabolism outside of the gut [193].A review of the pharmacological literature revealed several cases in which a particular biotransformation was suspected to result directly from a reaction carried out by gut microbes [194].The gut microbiome also affects the metabolism of food-derived bioactive compounds.A metabolomic screen of human plasma revealed that the phosphatidylcholine metabolites choline,trimethylamine N-oxide,and betaine serve as predictive biomarkers for the development of cardiovascular disease [195].These finding highlight the critical role of the gut microbiota in metabolism and also serve as an example of how the composition of commensal microbial communities can directly affect our health.
5.1.Host-microbiota metabolic interactions in the gut
More recently,Nicholson et al.published a review inScience[196]where they emphasized that the influenc of the gut microbiota on human health is continuous from birth to old age.The maternal microbiota may also influenc both the intrauterine environment and the postnatal health of the fetus.At birth,about 100 microbial species populate the colon.Early environmental factors,nutritional factors,and epigenetic factors have been implicated in the composition of the gut microbial symbiont community.Gut microbial composition in early life can influenc the risk for developing diseases in later life.Shifts of microbial diversity occur along with the transition from childhood to adult life.There is a decrease inBacteroidetesand an increase inFirmicutes.The gut microbiota are important for maintaining normal physiology and for energy production throughout life.Body temperature regulation,reproduction,and tissue growth are energy-dependent processes that may partly depend on microbial energy production in the gut.Extrinsic environmental factors and the host genome influenc the diversity and function of the gut microbiota and health.Disruption of the gut microbiota can lead to diseases including inflam matory bowel disease,colon cancer,irritable bowel syndrome,non-alcoholic fatty liver disease,obesity,metabolic syndromes,and hypertension,among others.
The gut microbiota are involved in the regulation of a number of host metabolic pathways,giving rise to interactive host-microbiota metabolic,signaling,and immune axes that physiologically connect the gut,liver,muscles,and brain.Crosstalk between the microbes and the host immune system is transmitted through a series of signaling pathways beyond the immune system.These signaling processes,together with direct metabolic interactions between the microbe and host,act upon the gut,liver,and nervous system.These interactions constitute a complex host-microbe metabolic network.The host and its gut microbiota coproduce a large array of small molecules during the metabolism of food and xenobiotics,many of which play critical roles in shuttling information between host cells.Dietary fibe can be digested and subsequently fermented in the colon by gut microbes into SCFAs such as butyrate,propionate,and acetate,and are sensed by the GPCRs GPR41 and GPR43,which are expressed by gut enteroendocrine cells[197].Butyrate has been shown to regulate energy homeostasis by stimulating leptin production in adipocytes and by inducing glucagonlike peptide-1 secretion by the intestinal enteroendocrine L cells [197].Butyrate regulates neutrophil function,induces expression of vascular cell adhesion molecule-1,increases the expression of tight junction proteins in the colon epithelia,and exhibits anti-inflammator effects by reducing cytokine and chemokine release.SCFAs have been reported to regulate the function of histone deacetylases and to stimulate the sympathetic nervous system[198].SCFAs have also been shown to stimulate gut motility and intestinal transit at physiological concentrations,which have been shown to induce multi-fold increase in serotonin release[199].The colonization of a germ-free mouse with the intestinal microbiota from an obese mouse donor induced a body weight gain that was more substantial than when the microbiota from a lean mouse was transferred [200].This result provided the firs insight into the potential contribution of the microbiota to obesity.Thus,prebiotics can be define are nondigestible food substrates that promote the growth of intestinal bacteria that confer health benefit on the host.
Another metabolic disease associated with obesity and metabolic syndrome is nonalcoholic fatty liver disease,which occurs in 20–30% of the general population,but in 75–100% of obese individuals.The intestinal microbiota may contribute to the development of nonalcoholic fatty liver disease through complex cooperation of two microbe-sensing protein families:nucleotide oligomerization domain receptors(NLRs)and TLRs[201].These regulate metabolic events through inflammasom[202],and stimulate TLR4 and TLR9,respectively,leading to increased secretion of tumor-necrosis factor TNF-α,which in turn drives progression of nonalcoholic steatohepatitis.Prebiotics and other dietary interventions have been shown to have an obvious effect on the expression of TLRs [203].The gut microbiota may constitute a complex metabolic network of communications between dietary nutrients and the host.Perhaps the greatest challenge will be to understand the temporal dynamics of metabolic communication between the host,dietary compounds,and the gut microbiota,and to evaluate the real effects of functional foods and medicinesin vivo.
5.2.Interactions between the microbiota and the immune system
In the lower intestine,the gut microorganisms reach extraordinary densities and have evolved to degrade a variety of plant polysaccharides and other dietary substances [204].This not only enhances host digestive efficien y,but also ensures the nutrient supply for the microbes.These interconnections are particularly clear in the relationships between the microbiota and the immune system.Several immune effectors are used to limit bacterial–epithelial contact.These include the mucus layer,secretory immunoglobulin A(SIgA),and epithelial antibacterial peptides[205].These microbes and complex antigens are sampled by intestinal DCs.Mature DCs migrate to the mesenteric lymph nodes through the intestinal lymphatics.This compartmentalizes live bacteria and the induction of immune responses in the mucosal immune system.Induced B and T cell subsets are recirculated through the lymphatics and the bloodstream and home back to the mucosa,where B cells differentiate into IgAsecreting plasma cells.RegIIIγ is an antibacterial lectin that is expressed in epithelial cells under the control of TLRs[206].RegIIIγ limits bacterial penetration of the small intestinal mucus layer,thus restricting the number of bacteria that contact the epithelial surface[207].These mature DCs interact with B and T cells in the PP,inducing B cells to produce SIgA directed against intestinal bacteria[208].The SIgAs can bind to luminal bacteria,preventing microbial translocation across the epithelial barrier[209].The innate lymphoid cells reside in the lamina propria and control the stimulations of T helper cells to regulate activation of adaptive immunology and development through cytokine shape[210].These lymphoid cells that produce IL-22 are essential for preventing the spread of microbes to systemic sites [211].Nutrients derived from the host diet are critically important in shaping the structure of host-associated microbial communities[212].Epithelial cells also secrete antibacterial substances that can shape the composition of intestinal microbial communities.Some antimicrobial proteins,such as α-defensins,can shape the overall community composition.RegIIIγ has restricted effects on surface-associated bacteria and thus controls the location of microbes relative to host surface tissues.
Sensing of commensal microbiota through the TLR-MyD88 signaling pathway triggers several responses critical for maintaining host-microbial homeostasis.Homeostasis is maintained by a system of checks and balances between pro- and antiinflammator cytokines.Th1 cells produce interferon-λ,and Th17 cells produce IL-17α,IL-17f,and IL-22.These cytokines act as effector features resembling Th2,Th17,and Treg cells.Polysaccharide A (PSA) ofBacteroides fragiliscan induce an IL-10 response in intestinal T cells,which prevents potential damage to the mucosal barrier [213].A recent study revealed that the microbiota have a role in the control of the function of invariant NK T cells(iNKTcells),which bear an invariant T cell receptor specifi for lipid antigens presented by the atypical class I molecule.Germ-free mice were found to have increased susceptibility to iNKT cell-mediated oxazolone-induced colitis and ovalbumin-induced asthma.More interestingly,this effect could be reversed only if mice were exposed to the microbiota during the neonatal period.The regulation of iNKT cell expansion was ascribed to reduced expression of the chemokine CXCL16 in the presence of microbiota.Thus,signals elicited by commensals may repress systemic expression by epithelial cells of a chemokine that interacts with CCR6 and is selectively expressed by iNKT cells[214].This may make us believe that“it’s okay to let your toddler lick the swing set and kiss the dog”.
There are a series of monogenic conditions of the nucleotidebinding oligomerization domain(NOD)receptor family that are considered to be autoinflammator .Studies have shown that TLR ligands can trigger proinflammator IL-1β secretion in the presence of activated NLR family pyrin domain containing 3 (NLRP3) mutations [215].TLR4 and 9 signal transduction increases TNFα expression.In humans,TNFα promotes insulin resistance and the accumulation of fat in the liver.Together,these examples show that innate immune system defects can result in dysbiosis of the intestinal microbiota with downstream metabolic consequences for the host.
There is a primary question as to whether probiotics exert their beneficia fuctions by themselves or through metabolic products.Fukuda et al.[216]concluded that bifidobacteri can protect against enteropathogenic infection by the production of acetate[217].The human gut is colonized with a wide variety of microorganisms,such asBifidobacterium,that have benefi cial effects on human physiology and pathology[218].Studies using this model have shown that Stx(both Stx1 and Stx2)produced byEscherichia coliO157 is a crucial factor in lethal infection [219],and that pretreatment with certain probiotics,including bifidobacteria can protect mice against death [220].When germ-free mice were fed withE.coliO157,they died within 7 days.However,mice survived if they had been colonizedBifidobacterium longumsubsp.Longum JCM 1217T(BL)7 days before inoculation withE.coliO157.By contrast,another strain of bifidobacteriaBifidobacterium adolescentisJCM1275T(BA),failed to preventE.coliO157-induced death under the same conditions.The researchers also found that serum concentrations of Stx2 were markedly lower in BL1O157 mice than in BA1O157 mice,suggesting that BL,but not BA,promotes epithelial defence functions that prevent the translocation of Stx2 into the blood.SCFAs are the major end products of carbohydrate metabolism by bifidobacteria Indeed,the concentration of acetate alone was found to be significantl higher in the feces of mice with the preventive strain compared with those with the non-preventive strain.These results suggest a positive correlation between the amount of fecal acetate and the resistance of mice to infection withE.coliO157.These data also suggest that acetate produced in large amounts by the preventive bifidobacteri exerts its action on the colonic epithelium by inducing anti-inflammator and/or anti-apoptotic effects,blocking the translocation of the lethal dose of Stx2 into the blood.SCFAs generated by commensal bacteria have long been implicated in a variety of beneficia effects on the host,including trophic and anti-inflammator effects on the gutin vivo[214].The latest finding strongly suggest that bacterial acetate actsin vivoto promote the defence functions of host epithelial cells.
6.The gut is not only a site of nutrient transfer,but also an important information exchange system
The gut is the largest endocrine organ in the body.Gut hormones can be classifie into many families based on their structure,and each family originates from a single gene.A hormone gene is often expressed in multiple peptides due to tandem genes,alternative splicing,or differentiated posttranslational processing.By these mechanisms,more than 100 different hormonally active peptides are produced in the GI tract.In addition,gut hormones are widely expressed outside the gut.The different cell types often express different products of the same gene and release the peptides in different ways.Therefore,the same peptide may act as a hormone as well as a local growth factor or neurotransmitter.This suggests that GI hormones should be conceived of as intercellular messengers of major general impact.From the beginning,GI endocrinology has been central to our understanding of multicellular life,health,and disease.In other words,the functions of the body are known to be regulated not only by nerves,but also by hormones.The regulation of pancreatic secretion(exocrine and endocrine)remains a major issue in gut endocrinology[221].
Recently,novel cell surface and soluble signaling molecules produced by cells of the immune system have been discovered that regulate host responses to microorganisms found mainly in the gut.It is now widely appreciated that these molecules interact in a concerted fashion to maintain a balance that governs an appropriate response to infectious organisms.Several classes of these compounds,including proteins,peptides,lipopolysaccharides,glycoproteins,and lipid derivatives,have been characterized as molecules that have potent effects on the host immune system.Peptides such as cytokines and chemokines are well-known examples of such molecules.Whereas polysaccharides have been believed to have beneficia functions,certain phytochemicals have recently been shown to act as potent immunomodulating agents.However,the unavoidable question is how polysaccharides exhibit biological activity under non-digestion conditions.With recent advances in the understanding of how cells communicate with each other to signal effector functions,it has become possible to conceive of strategies to manipulate these signaling pathways and influenc host responses.Compounds that are capable of interacting with the immune system to upregulate or downregulate specifi aspects of the host response can be classifie as immunomodulators or biologic response modifiers These compounds are more likely to be found in the gut than be absorbed into circulation and reach a target site.Recently,certain polysaccharides have been described as potent immunomodulators with specifi activity for both T cells and APCs.
6.1.The information exchange system of the gut/liver axis
Enterohepatic circulation serves to capture bile acids and other steroid metabolites produced in the liver and secreted to the intestine for reabsorption back into circulation and reuptake by the liver.This process is under tight regulation by nuclear receptor signaling.Bile acids produced from cholesterol can alter gene expression in the liver and small intestine by activating the nuclear receptors farnesoid Xreceptor(FXR;NR1H4),pregnane X receptor(PXR;NR1I2),vitamin D receptor(VDR;NR1I1),G protein-coupled receptor TGR5,and other cell signaling pathways(JNK1/2,AKT and ERK1/2).Among these controls,FXR is known to be a major bile acid-responsive ligand-activated transcription factor and a crucial control element for maintaining bile acid homeostasis.FXR has a high affinit for several major endogenous bile acids,notably deoxycholic acid,cholic acid,lithocholic acid,and chenodeoxycholic acid.By responding to excess bile acids,FXR acts as a bridge between the liver and small intestine to control bile acid levels and regulate bile acid synthesis and enterohepatic fl w.FXR is highly expressed in the liver and gut,and contributes to the maintenance of cholesterol/bile acid homeostasis by regulating a variety of metabolic enzymes and transporters.FXR activation also affects lipid and glucose metabolism,and can influenc drug metabolism[222].
Several structurally diverse compounds show high-affinit binding and agonist activity toward FXR,including steroids,aromatics,terpenoids,alkaloids,and FAs.Many compounds unrelated to bile acids can also act as FXR ligands,including androsterone,guggulsterone,stigmasterol,and the exogenous natural plant sterol forskolin[223].In the intestine,FXRcontrols the absorption of bile acids,lipids,vitamins,certain drugs,and other xenobiotics through the regulation of expression of four important transporters,apical sodium-dependent transporter,FA-binding protein subclass 6 (FABP6),which is also known as intestinal bile acid-binding protein (I-BABP),and organic solute transporters that are responsible for the transport of bile acids from the intestine to the portal system.The major bile acid transport (ASBT) system in ileal enterocytes transports bile acids into the ileal enterocyte brush border(apical)membrane[224].In humans,the ASBT gene is activated by retinoic acid,which has implications for the treatment of patients with cholestasis or chronic diseases of the GI system with vitamin A and retinoic acid-based drugs [225].Intestinal FXR activation also affects hepatic events.FGF19 is highly expressed in the small intestine[226].When secreted from the intestine,FGF19 circulates to the liver and suppresses bile acid through the binding and activation of the FGF receptor 4 (FGFR4)/β-Klotho complex located on the surface of hepatocytes and other epithelial cells [227].Activation of the FGFR4/β-Klotho complex stimulates the c-Jun N-terminal kinase (JNK) pathway,eventually suppressing the gene encoding CYP7A1,the cholesterol 7β-hydroxylase,and the rate-limiting bile acid synthetic enzyme[228].
In addition to being a major regulator of bile acid homeostasis,FXR plays an important role in the intestinal defense against inflammation interacting with nuclear factor-kappaB(NF-κB)signaling.Exposure of LPS-activated macrophages to an FXR ligand leads to the reciprocal regulation of NF-κB-dependent genes such as TNFα and IL-1β[229].Intestinal FXR activation,responding to bile acids,controls bacterial growth and maintains mucosal integrity,regulating the expression of a variety of genes involved in defense against inflammatio and mucosal protection.Therefore,FXR might be a critical factor regulating intestinal innate immunity and homeostasis.FXR is also expressed in pancreatic β-cells and regulates insulin signaling.Members of the CYP3A family of cytochrome P450 expressed in the liver and intestine are also involved in bile acid metabolism by catalyzing hydroxylation of bile acids at different positions[230].Human hepatic CYP3A4,the dominant CYP3A in the human liver,metabolizes a number of xenobiotic compounds including many drugs in clinical use[231].This enzyme is also highly expressed in the intestine where it plays an important role in first-pas metabolism of many orally administered drugs[232].
Once taken up,endogenous chemicals and toxic and xenobiotic compounds pass though the small intestine and liver,and diffuse into the whole bodyviathe circulatory system.In these two sites,FXR plays an important role in protecting against potential toxicity.Recent discoveries have suggested that alteration of FXR signal transduction really plays important roles between the liver and gut.We believe that most phytochemicals exert their benefi roles through interactions with these FXRs.
6.2.The information exchange system of the gut/brain axis
Peptide hormones released from the gastrointestinal tract communicate information about the current state of energy balance to the brain.These hormones regulate appetite and energy expenditureviathe vagus nerve or by acting on key brain regions implicated in energy homeostasis such as the brainstem and hypothalamus.Sam et al.[233]published an overview of the main gut hormones implicated in the regulation of food intake.Peripheral signals from the gut and adipose tissue constitute feedback mechanisms that enable maintenance of a steady body weight despite daily variations in energy expenditure and nutrient intake.
The role of peripheral hormones and the gut/brain axis in the regulation of appetite has been of interest in recent years because of the growing crisis of global obesity and metabolic disease.Obesity has become a major public health problem worldwide[234].Gut-brain cross-talk is involved in the regulation of food intake.
(1) Neuroendocrine control of appetite.The hypothalamus and the brainstem are the main central nervous system regions responsible for the regulation of energy homeostasis.These brain areas receive peripheral neural and hormonal signals that relay information about acute nutritional state and adiposity[235].Neural afferents and hormonal signals from the periphery are integrated with higher brain center signals to regulate appetite and energy expenditure [236];(2) there are at least 15 different types of enteroendocrine cells diffusely distributed throughout the GI epithelium.These cells produce and release a variety of hormones and signaling molecules,which together constitute the largest endocrine organ of the body [237];(3)hormone peptides,like neuropeptide Y (NPY) and pancreatic polypeptide (PP),belongs to the ‘PPfold’ family of proteins.These peptides are 36 AAs in length and share a common tertiary structural motif known as the PP-fold.C-terminal amidation of these proteins is necessary for biological activity.PYY exists endogenously in two forms:PYY1-36 and PYY3-36[238].The enzymatic cleavage of secreted PYY1-36 at the amino terminal by the cell surface enzyme dipeptidyl peptidase IV (DPP-IV)gives rise to PYY3-36,the predominant form of circulating PYY immunoreactivity [239];(4) glucagon-like peptide-1 (GLP-1).The 2 bioactive forms of GLP-1,GLP-17-37 and GLP-17-36 amide,are released into circulation from L cells of the GI tract in response to an oral glucose load.Physiologically,GLP-1 is an important incretin,stimulating glucose-dependent insulin release.In addition to its incretin effect,GLP-1 also inhibits the secretion of glucagon,thereby inhibiting endogenous glucose production.The effect is to reduce blood glucose after a meal.GLP-1 also delays gastric emptying,and increases satiety;(5)gut hormones regulating food intake.The GI tract releases more than 20 different regulatory peptide hormones that influ ence a number of physiological processes.Gut hormones act on tissues such as smooth muscle,exocrine glands,and the peripheral nervous system[240].The release of gut hormones such as PYY,GLP-1,and oxyntomodulin(OXM)is stimulated by distension of the stomach and interactions between nutrients and the luminal wall of the intestine;(6) oxyntomodulin (OXM).OXM is a 37 AA peptide that is released post-prandially from L cells in proportion to caloric intake.OXM also delays gastric emptying and reduces gastric acid secretion.In addition,it was shown to reduce food intake in normal-weight human volunteers when administered intravenously.When administered to obese subjects,it reduced both food intake and body weight [241];(7)pancreatic polypeptide(PP).PP is released post-prandially under vagal control by pancreatic islet PP cells [242].PP is secreted in proportion to caloric intake.Circulating levels rise after meals and remain elevated for up to 6 h post-prandially;(8) glucagon.Glucagon is a 29 AA peptide secreted from the α-cells of the pancreatic islets of Langerhans.It is a further product of pre-proglucagon cleavage alongside OXM and GLP-1.Glucagon is released into the portal vein in fasting states and also in response to exercise,and acts on the liver to promote hepatic glycogenolysis and gluconeogenesis to maintain glycaemic balance.Glucagon mediates its effectsviathe glucagon receptor,GPCR.It is expressed in the gut,brain,and many other organs[243];(9)cholecystokinin(CCK).CCK is released postprandially from the small intestine,and has also been shown to co-localise with PYY in L cells.Two types of CCK receptor have been identifie in the CNS and peripheral tissues.CCK1 receptors are present in peripheral tissues such as the pancreas,gallbladder,and on vagal afferent nerve fiber innervating the gut [244].CCK1 receptors within the CNS and dorsomedial hypothalamus have been shown to be involved in the regulation of food intake.The CCK2 receptor is found in the hypothalamus,vagal afferents,and gastric mucosa,and is known to be involved in appetite regulation;(10)Ghrelin.Ghrelin is a 28 AA acylated peptide secreted in the stomach.It was originally identifie as an endogenous ligand and a growth-hormone-releasing peptide[245].
More recently,Duraffourd et al.[246]showed that μ-opioid receptors (MORs) present in nerves in the portal vein walls respond to peptides to regulate a gut-brain neural circuit that controls intestinal gluconeogenesis and satiety.Peptides and protein digests behaved as MOR antagonists in competition experimentsin vitro.These stimulate MOR-dependent induction of intestinal gluconeogenesis by activation of brain areas receiving inputs from GI ascending nervesin vivo.MOR-knockout mice do not carry out intestinal gluconeogenesis in response to peptides and are insensitive to the satiety effect induced by protein-enriched diets.Thus,the regulation of portal MORs by peptides triggering signals to and from the brain to induce intestinal gluconeogenesis are links in the satiety associated with dietary protein assimilation.MORs expressed in the mesenteric-portal area control a gut-brain neural circuit involved in the regulation of intestinal gluconeogenesis[247].The regulatory role of MORs in the control of food intake has been largely documented for the central nervous system,and is related to their roles in the so-called “reward” system [248].Duraffourd et al.[246]also demonstrated that MORs play a role in mediating the satiety effects of diet proteins,acting within a neural gut-brain circuit.Oral intake of various μ-opioid antagonists decrease hunger in humans despite the fact that they do not reach the brain due to extensive first-pas hepatic metabolism[249].
Harrold et al.[250]also showed that key peripheral episodic and tonic signals from orexigenic or anorexigenic agents can also control the central nervous system.Research suggests that fluctuation in the availability or utilization of energy-yielding substrates (mainly glucose and FAs) control eating behavior.Reduced ATP levels due to a decrease in FA oxidation or glucose utilization increases the AMP/ATP ratio and activates the ubiquitous cellular energy sensor AMP kinase(AMPK),which exists in the periphery and in the brain.AMPKactivation or deactivation in the hypothalamus increases or decreases food intake[251].Pathways in the CNS that are sensitive to this metabolic signaling have begun to be elucidated.However,direct entry of metabolites and their action on receptors in the CNS may also contribute to their effects on satiety.Chemicals released by the GI tract during digestion also act as satiety signals in the control of appetite.
6.3.Gut cannabinoid signaling regulates inflammation and energy balance
The control of energy balance is one of the most highly integrated and complex functions of the body.Disturbances in the regulation of energy balance frequently lead to the development of obesity.The major control systems of energy balance lie in the brain.These centers integrate information from the body and initiate appropriate behavioral,humoral,and neural outputs.The energy balance centers of the brain receive important inputs from the GI tract,liver,pancreas,adipose tissue,and skeletal muscle,and are mediated by a series of different signaling molecules[252].The role of the gut has been particularly highlighted in the control of energy balance.Obesity is now characterized as a systemic low-grade inflammator condition with cells of the immune system directly involved in the metabolic and homeostatic abnormalities that lead to many of its co-morbidities,including diabetes and liver disease [253].A question that arises is whether there is a connection between the microbiota of the GI tract,the development of an inflamma tory state,and the control of energy balance.Recent evidence suggests that the endocannabinoid system may be a major mediator.Endogenously produced lipid-mediators that act on the cannabinoid(CB)1 and CB2 receptors have been implicated in the control of food intake and energy balance,the regulation of inflammation and in gut-adipose tissue signaling.Cluny et al.[252]reviewed and discussed how the endocannabinoid system,intestinal microbiota,and the brain-gut axis are involved in the regulation of energy balance and the development of obesityassociated systemic inflammation The endocannabinoid system plays a major role in the regulation of energy homeostasis and is generally upregulated in obese states.It is also implicated in the development of low-grade inflammatio in obesity by controlling intestinal permeability through actions mediated by the immune system.The fact that the microbiota influenc signaling in the endocannabinoid system to regulate inflammatio and adipose tissue metabolism suggests that dietary interventions to alter gut bacteria may be a novel avenue to explore alternative means of activating the endocannabinoid system while avoiding direct stimulation of global CB1 receptors.
6.4.CCR6 as a mediator of immunity in the lungs and gut
Chemokines constitute a family of structurally related chemotactic cytokines that direct the migration of leukocytes throughout the body under both physiological and inflammator conditions[254].While most chemokine receptors bind to multiple chemokines,the chemokine receptor CCR6 has only one chemokine ligand,CCL20(macrophage inflammator protein-3α,MIP-3α)[255].CCL20 is expressed by a variety of epithelial cell types including pulmonary epithelial cells and IECs[256].CCL20 is typically expressed at a low basal level,but can be strongly induced by pro-inflammator signals including primary cytokines (e.g.,TNF-α) and TLR agonists.The production of CCL20 by human bronchial epithelial cells is regulated by the pro-inflammator cytokines TNF-α and IL-1β,and also by proallergic cytokines IL-4 and IL-13,which are known to influenc CCL20 expression by activation of both the ERK1/2 and p38 MAPK pathways [257].CCR6 is also expressed on immature DCs,most B cells,subsets of CD4+ and CD8+ T cells,and NKT cells[258].Studies have also shown that CCR6 is a specifi marker for Th17 cells and Treg cells and distinguishes them from other T helper cells[259].DCs are the most potent class of APCs in the immune system that induce primary immune responses against invading pathogens,suggesting that DCs are a key leukocyte population involved in driving the innate immune response.Immature DCs express various chemokine receptors such as CCR1,CCR2,CCR3,CCR5,CCR6,and CXCR4[260].Once immature DCs take up antigens in mucosal tissues,these DCs gain a mature status by down-regulating CCR6 expression [261].They then home to regional lymph nodes through afferent lymphatic vesselsviathe interaction of CCR7 with its ligands.Upon arrival in the regional lymph nodes,the mature DCs become effective APCs.
The CCR6/CCL20 axis plays an important role in intestinal immunity.During normal development and immune homeostasis,CCR6-mediated signals help to organize lymphoid tissues such as Peyer’s patches,mesenteric lymph nodes(MLNs),and GALT by recruiting lymphoid and myeloid cells,including DCs and macrophages.In addition,CCR6-mediated signals are central to innate immune responses to normal intestinal flora The relative CCR6-dependent chemotactic response of DCs and macrophages,and the subsequent activation and effector function of these cell populations,may also play an important role in intestinal immune responses[262].In the gut,areas of PPs,isolated lymphoid follicles(ILFs),MLNs,and GALTs show constitutive expression of CCL20,which is important for the chemotaxis of immature DCs [263].CCR6/CCL20-mediated signals can induce chemotaxis of CCR6-expressing DCs and macrophages to sites of infection to help in the immune response.These finding suggest that CCR6-mediated signals in macrophages and DCs may be important for cell activation during exposure to microbes and microbial products in gut.
Conventional and plasmocytoid DC subsets are found in many lung compartments including the airway epithelium,lung parenchyma,visceral pleura,and the bronchoalveolar space,which attests their importance in the maintenance of respiratory health[264].Immature DCs are highly abundant in human lung parenchyma where they express low levels of the costimulatory molecules CD80 and CD86,actively display antigen uptake properties,and constitutively express the chemokine receptors CCR1 and CCR5.DCs are constantly recruited into the lungs,where they recognize inhaled antigens that transform them into APCs and migrate to the draining pulmonary lymph nodes where they activate antigen-specifi CD4+ and CD8+ T cells.Many cells in the lung produce a wide array of chemokines that orchestrate the recruitment of DCs into the lungs based on the original stimulus.Various cytokines have been shown to regulate CCR6 expression in lung DCs.Studies have shown that epithelial airway cells exposed to the inflammator cytokines IL-1β,TNF-α,and IFN-γ produce IL-15,which cause monocytes to differentiate into partially mature DCs that have characteristics of plasmocytoid DCs[265].These data suggest that a pathogenic Th2 response is decided by CCR6/CCL20-dependent recruitment of conventional DCs to the lungs[266].
6.5.Ghrelin’s actions on the reproductive axis
Ghrelin is a peptide hormone secreted from the stomach into circulation,but it can also be synthesized in several other tissues and exert both endocrine and paracrine effects.To date,its expression and activity have been documented in various reproductive tissues,indicating that ghrelin regulates several aspects of reproductive physiology and pathology[267].The evidence indicates that ghrelin participates in the regulation of reproductive physiology by 2 distinct and probably overlapping actions:(i) through systemic release of the stomach-derived peptides,which act at different levels of the reproductive system,and(ii)through biological effects on reproductive organs induced by locally expressed ghrelin[268].Besides of its acute effects on the reproductive axis,ghrelin may have a more chronic or long-term action on various aspects of reproduction,such as puberty,which is highly sensitive to energy stores.Ghrelin’s central effects on the reproductive system also involve modulation of prolactin secretion.
It has been suggested that rat gonadal ghrelin expression is a function of the estrous cycle as its present at increased concentrations in the cytoplasm of luteal-phase steroidogenic luteal cells[269].A number of growth factors and cytokines released from the reproductive tract and the preimplantation embryo exert a paracrine and autocrine influenc on the rate of embryo development,the proportion of embryos developing to the blastocyst stage,the cell number in the blastocyst,and the rate of apoptosis [270].Ghrelin may also be involved in these processes as a chemical messenger mediating intercellular communication.Research has revealed strong ghrelin expression in the human placenta during the firs trimester,especially in extravillous trophoblasts (EVT) on the tips of the chorionic villi [271].Since maternal ghrelin crosses the feto-placental barrier[272],it could be critical for fetal development.A pattern of testicular expression of ghrelin and its cognate receptor has been demonstrated in humans [273].Ghrelin’s actions may have implications not only for the control of spermatogenesis,but also for Leydig cell proliferation,with an inhibitory role in reproductive functioning during states of malnutrition.Since the reproductive axis is highly dependent on body energy status,ghrelin could be a signal linking nutritional status to the hypothalamus–pituitary–gonad axis(HPGA).
Proper maturation and function of the reproductive axis are essential for perpetuation of the species,and so they are subjected to the fin regulation of different central and peripheral signals affecting the so-called HPGA [274].Reproductive function is highly energy demanding,but not crucial for the survival of the individual.During evolution,sophisticated mechanisms have been selected to allow the shutting down of the HPGA in conditions of energy insuffi ciency.Several neuroendocrine integrators jointly participate in the physiological–pathophysiological control of energy balance and reproductive development [275].Mucciolia et al.[274]summarized ghrelin’s actions on the reproductive axis as follows:(1) effects on gonadotropin secretion.A complex mode of action of circulating ghrelin on the HPGA has been documented that includes inhibition of hypothalamic gonadotropin-releasing hormone (GnRH) release;(2) effects on puberty.Ghrelin delays pubertal onset in both males and females,but males appear to be more sensitive than females;(3)effects on prolactin.Ghrelin’s central effects on the reproductive system also involve modulation of the secretion of prolactin,a pituitary hormone that inhibits gonadotropin secretion;and(4) intratesticular ghrelin administration has also been shown to inhibit the proliferative rate of immature Leydig cells during puberty and after selective ablation of mature Leydig cells by ethylene dimethane sulfonate treatmentin vivo.
Given the above findings it appears there are a series of signaling pathway networks between the gut/brain,gut/endocrine system,gut/liver,gut/lungs,gut/cardiovascular system,and even the gut/reproductive system.This raises the question of how for the GI tract communicates with these organs and systems.We strangly suggest that it is simply a cyclic system.
7.Some functional foods cannot be absorbed,but have great effect on cytokines and chemokines
The intestinal mucosa is constantly exposed to the luminal contents,which include microorganisms and dietary components.Probiotic non-digestible oligosaccharides may be supplemented to modulation immune responses in the intestine.Intestinal epithelial cells lining the mucosa are known to express carbohydrate (glycan)-binding receptors that may be involved in the modulation of mucosal immune responses.The GI immune system is the largest and most complex immunological tissue in the human body,and it can constantly discriminate between harmless and dangerous compounds.IECs express several receptors that recognize antigens and semiantigens present in the intestinal lumen as well as the receptors described above.These receptors could be the most important information delivery systems and could play critical roles in the bioactive effects of functional foods or oral vaccines and/or medicines.
7.1.Polysaccharides
Numerous dietary polysaccharides,particularly glucans,appear to elicit diverse immunomodulatory effects in numerous animal tissues,including the blood and GI tract[276].Glycanbinding receptors,which are also called lectins,include the family of the C-type lectin receptors,galectins,and siglecs[277],which recognize different glycan structures.APCs and IECs are important sensors and regulators of potential danger signals within the intestine and are crucial in the cross-talk between luminal antigens and immune cells.Dietary supplementation with non-digestible oligosaccharides has been shown to reduce the risk of developing allergic diseases and to suppress acute allergy symptoms such as acute allergic skin responses in animal and clinical studies[278].Dietary supplementation with a specifi probiotic mixture of short-chain galacto-oligosaccharides (scGOS) and long-chain fructo-oligosaccharides(lcFOS)in a 9:1 ratio was shown to suppress the development of acute allergy symptoms,possibly involving the induction of Treg cells[279].
7.1.1.Glycan recognition
Different subsets of intestinal DCs have been described that can induce Th1,Th2,Th1,or Treg cell effector responses upon activation[280].In order to induce an immune response or oral tolerance,antigens must be recognized,and DCs as well as IECs express receptors that recognize glycan structures.These sensors include:(1)C-type lectins.Expression of membrane-bound Ctype lectins is mainly restricted to APCs,including DCs and macrophages [281].C-type lectins are classifie into groups of proteins that contain one or more carbohydrate recognition domain (CRD) and proteins that contain a C-type lectin-like domain without a CRD.DCs express a wide variety of receptors,including TLRs.C-type lectins recognize specifi carbohydrate antigens and are mainly associated with antigen uptake and processing [282].Several studies have shown that glycan recognition may be essential as activation of a specifi C-type lectin,DC-specifi intercellular adhesion molecule-3-grabbing non-integrin (DC-SIGN),on DCs integrates TLR signaling,resulting in an IL-10-mediated immune response and induction of Treg cells [283];(2) siglecs:sialic acid-binding immunoglobulin-like lectins are type I trans-membrane proteins.Like the membrane-bound C-type lectins,siglecs are expressed on DCs and macrophages as well as almost all immune cell types.Siglecs recognize sialylated glycans of which Nacetylneuraminic acid is the most common;and (3) galectins:although APCs can respond to carbohydrate structures directly,antigens firs encounter a monolayer of epithelial cells.Like APCs,IECs also express various TLRs and it has been shown that the expression of TLRs at the apical surface is increased under inflammator conditions [284].IECS also express various galectins.After secretion,galectins bind to glycoproteins or receptors at cell surfaces and so regulate cell functions.Galectins are secreted by IECs as well as various immune cells,including DCs,macrophages,and granulocytes[285].Glycans can thus be recognized by different families of receptors that are expressed on APCs and IECs.Non-digestible oligosaccharides are known to be involved in maturation of the immune response of young infants,cytokine secretion,and oral tolerance induction,and they can reduce the risk of developing allergic diseases [286].These oligosaccharides are known to function as probiotics,stimulating the growth of lactic acid bacteria andBifidobacteriain the colon of a host[287].Although the beneficia effects of probiotic oligosaccharides are known,the exact mechanisms by which these occur are unclear.Human milk oligosaccharides have been shown to bind glycan-binding receptors including C-type lectin receptors and galectins[288].The recognition of glycans by C-type lectins or galectins may regulate immune responses[289].Although TLRs and glycan-binding receptors are widely expressed by different cell types in the intestine,the communication and interactions between these cells are just beginning to be understood.The known glycan-binding receptors,ligands,signal transductions,and biological effects are summarized in Table 1.
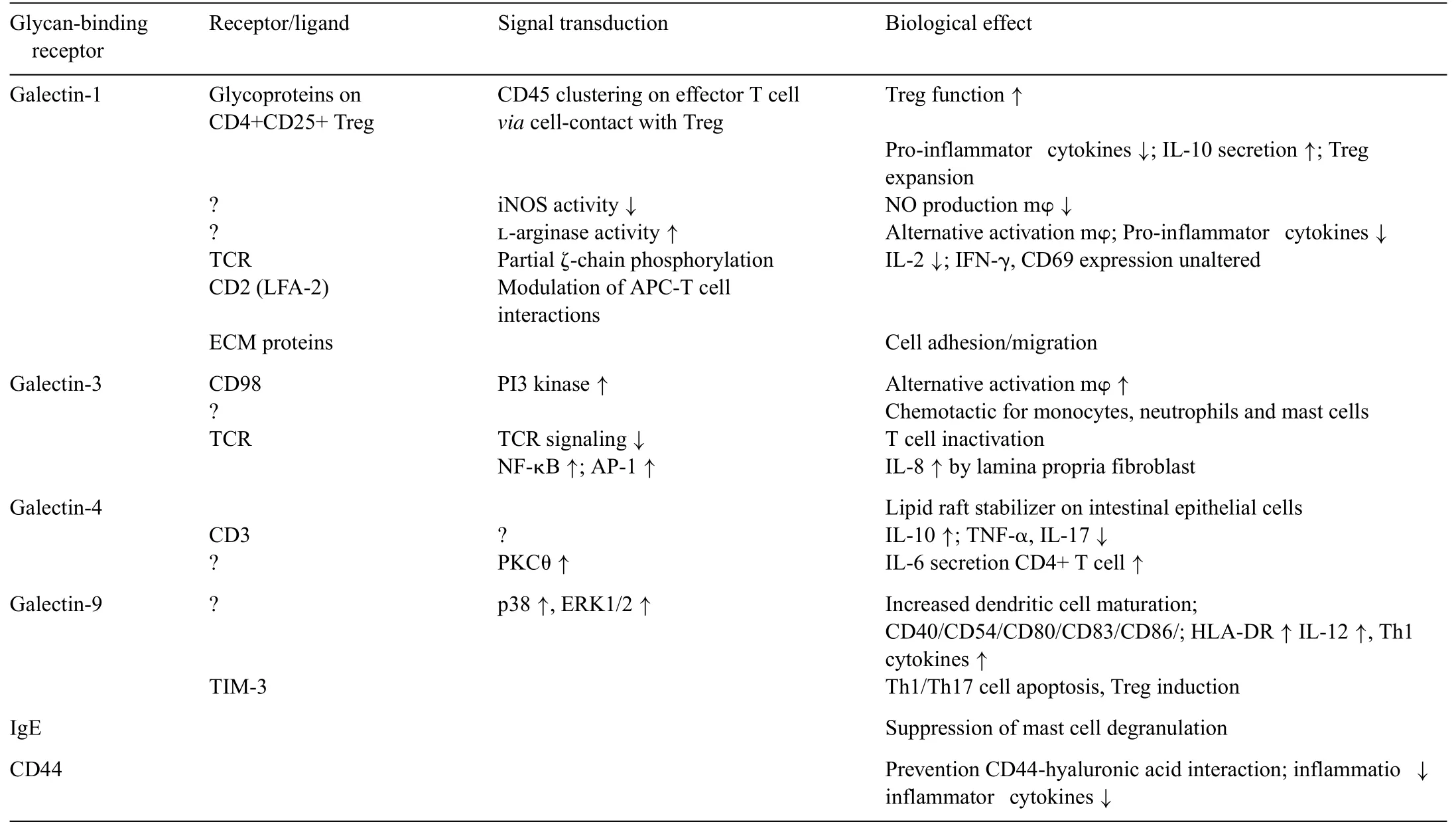
Table 1 Immune modulation by galectins.
7.1.2.Cytokine secretions induced by polysaccharides
In recent years,many studies have focused on the immunoregulation of polysaccharides,which have mainly been isolated from plants or probiotics.A number of different effects on immunoregulation and cytokine secretion have been elucidated in multiple studies.Table 2 displays data published since 2011.
Polysaccharides,especially the non- and poorly digestible polysaccharides,have been shown to beneficiall affect one or more targeted cellular functionsin vitro,but a fewin vivostudies have also been published.Scientists are interested in the immunologic effects of dietary intake,but it is unclear whether polysaccharides that elicit effectsin vitroor by injection are ineffective or have different effects when taken orally [315].One can only speculate on the mechanisms involved,particularly when one considers the exceedingly complex environment of the GI tract.It is possible that fragments of polysaccharides partially hydrolyzed by gut bacteria may either bind to the gut epithelia and exert localized and/or systemic immune effects or be absorbed into the bloodstream (but even here these may be rapidly catabolized!)where they can potentially exert systemic effects.Further studies are needed to determine the optimal timing and duration of polysaccharide ingestion.That is,should polysaccharides be consumed continuously,before,at the time of,or after exposure to a pathogen or environmental insult?Only a few studies have actually investigated the impact of the timing of polysaccharide intake on the achievement of optimal benefits Daily feeding of certain polysaccharides appears to result in tolerance(and diminished benefits in some mushroomβ-glucans[316].
Most studies have suggested that certain polysaccharides affect immune system functions.However many arein vitrostudies or studies of the injection of polysaccharides and,as mentioned earlier,their immunologic effects following oral administration are less clear.Functional foods are ingested and not injected.So it is really incredible for the results by adding specimens to the cultured cell lines,the reason just because of the complex cell communicationsin vivo,nether in the cultured cell lines! Ramberg et al.[317]published a review evaluating the available data regarding the specifi immunologic effects of dietary polysaccharides.They found 62 publications reporting statistically significan effects of orally ingested glucans,pectins,heteroglycans,glucomannans,fucoidans,galactomannans,arabinogalactans,and mixed polysaccharide products in rodents.Only 15 controlled human studies reported that oral glucans,arabinogalactans,heteroglycans,and fucoidans exerted significan effects.Although some studies investigated anti-inflammator effects,most investigated the ability of oral polysaccharides to stimulate the immune system.Taken as a whole,the literature on oral polysaccharides is highly heterogeneous and is insufficien for broad structure/function generalizations.Numerous dietary polysaccharides,particularly glucans,appear to elicit diverse immunomodulatory effects in numerous animal tissues,including the blood,GI tract,and spleen,but the mechanisms of these effects remain unclear.
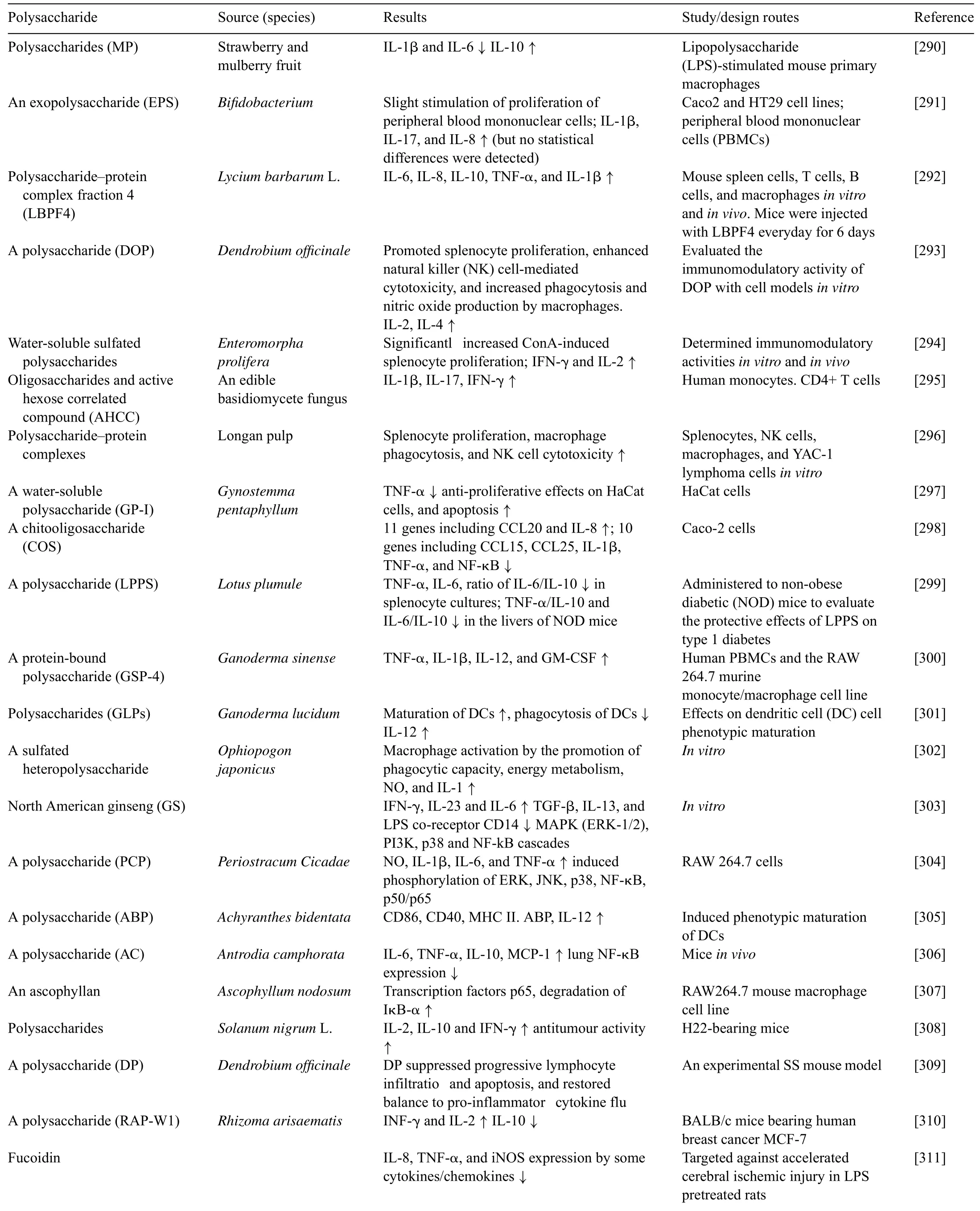
Table 2 The cytokine secretions and immunoregulations induced by polysaccharides(since 2011–present).
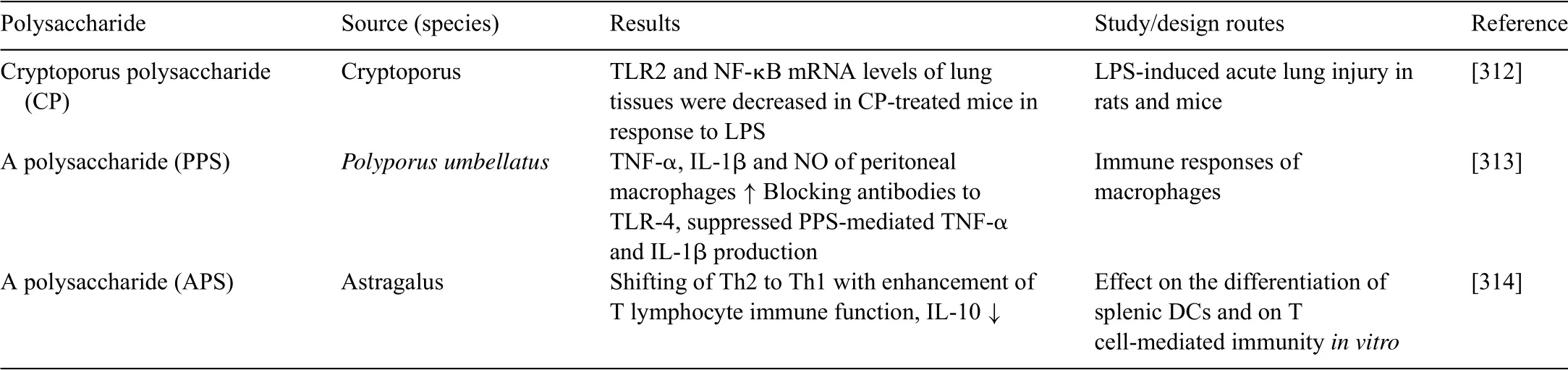
Table 2(Continued)
7.2.Peptides and their receptors and functions
Bioactive peptides derived from food and other sources have been a focus of functional food research.Their activityin vitroandin vivohas mostly revealed antioxidant and antihyper/hypotensive effects.In order to exert such bioactivity,food peptides must be either ingested and then reach the intestine in their intact form,or be liberatedin situ,that is,in the intestine,fromtheir parent proteins to act locally,that is in the gut.Ingested food peptides rarely make it into the gut and across the mucosal barrier.The proteins are degraded to very short peptides or are completely hydrolyzed to AAs and few proteins are transported intact across the gut mucosal barrier[318].Even those that do are rapidly hydrolyzed by enzymes or eliminated by innate immunological cells such as macrophages.As a result,they rarely reach their targets.A promising delivery route for specifi bioactivity through food proteins isin situpeptide release (by host or gut microbial enzymes)with local activity in the gut;the effects of such proteins appear to be a result of interactions with numerous receptors.Bioactive peptides are usually either released through processes such as fermentation or enzymatic hydrolysis to enrich a fraction or extract for specifi bioactive peptides or their precursors[319]or proteins are left intact in the fina product and the peptide bioactives are liberatedin situby the host digestive system or gut microbial enzymes[320].
Identificatio and characterization of bioactive peptides has been of increasing interest over the past decade.More than 150 scientifi papers including“bioactive peptide(s)”in their title or abstract are published a year whereas roughly 50 were published only ten years ago[321].Bioactive peptides are of most interest to researchers who are studying cancer,cardiovascular diseases,diabetes,apoptosis,and angiogenesis.Although many peptides derived from food proteins have been detected in the stomach or small intestine,and even the cardiovascular system[322],the presence of these peptides alone is not sufficien to establish their bioavailability.The biggest threat to any bioactive peptide is:(i) the lumen of the small intestine,which contains large quantities of proteinase and peptidases,and(ii)the brush border of the epithelial cells,which contains a series of peptidases[323].Bioactive peptides can only survive when their sequence resists degradation.An alternative strategy is to reach the site of action quickly after digestive releasein situand thus target receptors located in the gut.
However,bioactive peptides generated from food proteins and even intact proteins can be absorbed in the intestine and induce biological functions at the gut or tissue level.For example,many reports have described a role for food-derived peptides in increasing gut secretory and absorptive capacity or gut tissue growth.In relation to milk,this has particular importance in delivering bioactive proteins and peptides to infants with an immature GI tract mucosal barrier.Human milk contains not only antibodies and immune cells,but also many other substances that can interfere with bacterial colonization and prevent antigen penetration[324].The gut has a complex receptor system that can transduct multiple signals and actually constitutes a signaling pathway.Functional foods,medicines,and even nutrients can be censored and stringently controlled by the gut through these receptors and ensure both homeostasis and health.
Many bioactive peptides are produced through preprandial fermentation by bacteria,and the extent to which these are producedin vivoby luminal bacteria has yet to be directly studied[325].Their effects on the host intestine include mucin production and reduced blood pressure.Many of the effects of bioactive peptides are receptor mediated and some such receptors have been identified However,many remain unknown.Interestingly,some attempts to isolate the bioactive fermentation products ofLactobacillus helviticushave lead to confusing results,as all 3 peptide fractions collected had the same bioactive effects in the host[326].There are several examples of bioactive peptides produced by fermentation.β-Casomorphin 7,a product of milk fermentation,caused a dramatic increase in mucin production in rat intestinal explants[327].This bioactive peptide acts directly on the intestinal epithelium after absorption through μ-opioid receptors present on the basolateral side of goblet cells [328].Observations have been madein vivo,with goblet cell hypertrophy and hyperplasia of Paneth and goblet cells being induced with the administration of yoghurt fermented withL.casei[329].Fermentation products ofL.rhamnosusalso suppress the release of prostaglandins through an opioid receptor-mediated response in IECs [330].L.johnsonniiappears to produce a metabolite that lowers blood pressureviaa histaminergic receptor present in the intestinal epithelium[331].
Bioactive peptide production is not limited to milk products.Fermentation of fis proteins also leads to potentially bioactive peptides [332].Consumption of bioactive peptides produced from the fermentation of milk and fis leads to an increased number of IgA (+) B cells in the lamina propria [333].Fermented fis protein also leads to increased production of proand anti-inflammator cytokines IL-4,IL-6,IL-10,IFNγ,and TNFα.
Soybeans,an excellent source of dietary peptides,have beneficia effects on health.Dilshat-Yimit et al.[334]investigated the effect of soybean peptide on immunoregulation,brain function,and neurochemistry in healthy volunteers.They found that cell numbers were upregulated in the group who had fewer leukocytes but downregulated in the group with more leukocytes.For the lymphocyte-rich type,lymphocyte counts tended to decrease,accompanied by an increase in granulocyte numbers.For the granulocyte-rich type,granulocyte counts tended to increase,but lymphocyte counts also increased.
7.3.Phytochemicals
Phytochemicals have been shown to exert multiple bioactive functions including antioxidant scavenging of ROS and modulation of protein function.They dynamically regulate the metabolic functions of proteins,enzymes,transporters,receptors,and signaling transduction proteins related to various lifestyle-related diseases [340–342].These compounds form a very varied group:around 8000 species of phenolics including fl vonoids,anthraquinones,and phenylpropanoids;about 25,000 terpenoids including terpenes,carotenoids,xanthophylls,and iridoids;12,000 alkaloids;and several sulfatecontaining chemicals such as isothiocyanates.To date,numerous studies of phytochemicals have been published.Furthermore,new compounds beneficia to human health are constantly being discovered[335].It is rather interesting that most phytochemicals are similar to Chinese herbal medicines[336].This indicates that the mechanism of action of phytochemicals is similar to that of traditional Chinese herbal medicines and healthy plant foods[337–339].
Only a very small amount of phytochemicals can be incorporated into the blood circulation,and levels of these chemicals in blood are usually<1 μM[343].Phytochemical levels in blood are independent of ingested amounts since they are regulated by conjugation enzymes such as UDP-glucuronosyl transferases and phenol sulfotransferases below their Km values,which are the concentrations of chemicals that the enzymes express affin ity for on the substrates.Therefore,the phytochemicals in blood are mostly found in an inactive form.Most can interact with IECs and transfer immune signals without uptake.Indeed,Moon et al.[344]found that about two-thirds of quercetin was conjugated in IECs and showed antioxidant potency and prevented several cancers,atherosclerosis,cardiovascular diseases,and osteoporosis [345].Thus,phytochemicals can have beneficia effects on health,but most of these compounds undergo conjugation during the intestinal absorption process and are excreted into feces without being absorbed.Various compounds have been found to be chemopreventive of degenerative diseases including fl vonoids,prenyl chemicals,terpenoids,alkaloids,sulfate-containing chemicals,and epigallocatechin gallate,all of which are effective at very low concentrations.Isofl vone can be converted to active equol by intestinal microorganisms[346].Most phytochemicals exert their effects by interacting with GI systems.Furthermore,phytochemicals play roles similar to other functional compounds,such as polysaccharides and bioactive peptides,which indicates that all of these functionviaa similar mechanism,namely by interacting with GI systems,especially the receptors throughout the GI,rather than by being absorbed into circulation[348,349].
17β-Estradiol (E2),the most active estrogen,has profound effects on the growth,differentiation,and functioning of many reproductive and non-reproductive tissues including the liver,cardiovascular system,and brain.Most of E2 actions are exertedviatwo estrogen receptor subtypes(ERα and ERβ),which are members of the nuclear receptor superfamily and regulate both unique and overlapping physiological effects of E2.Most phytochemicals are xenoestrogens.While these compounds mainly exert their rolesvianuclear receptors,Marino et al.reviewed the rapid responses of estrogen receptors to xenoestrogens [350].Flavonoids are potentially able to protect against the development of E2-dependent pathologies (e.g.,endocrine tumors)by binding to ERα and ERβ[351].Flavonoids show a higher affinit toward ERβ than ERα,but they are able to activate both receptors[352].Xenoestrogens activate gene transcription of both ERα- and ERβ-dependent estrogen response elements(ERE),although different compounds show different degrees of specificit toward ERβ or ERα in terms of promoter activation.In addition to direct interaction with EREs,ERs can regulate gene transcription without directly binding to DNA.In this indirect genomic mechanism,ERs associate with specifi transcription factors,such as Sp1,AP-1,and NFκB,which in turn mediates the binding of the complex to DNA.This mechanism can also be affected by xenoestrogens that impair the ERα interaction with the transcriptional factors Sp1 and AP-1 [353].In addition to transcriptional actions,ERα and ERβ mediate E2-induced rapid effects,which occur within seconds of E2 binding to the receptors.At this level,ligand-activated ERs can interact with several other proteins involved in many different signal transduction cascades,and thus form multi-molecular complexes that mediate the rapid signal transduction events[354].However,to date,the ERα-mediated extracellular regulated kinase/mitogen activated protein kinase(ERK/MAPK)and phosphatidyl-inositol-3-kinase/AKT (PI3K/AKT) pathways as well as the ERβ-mediated p38/MAPK signaling appear to be the unique molecular circuitries activated by E2 in different cell contexts[355].The bulk of the data that demonstrate the involvement of the ER-based extra-nuclear effects in the regulation of different physiological processes have been obtained mainly,if not exclusively,fromin vitromodel systems.Thus,the lack of anin vivomodel raises questions about the physiological relevance of these E2-triggered effects.Nevertheless,recent data have demonstrated that rapid extra-nuclear ER signaling occursin vivoand also affects the regulation of specifi physiological processes in animal models [356].Plant-derived polyphenols have diverse estrogenic biological activities due to their ability to act as either estrogen agonists or antagonists depending on the ER subtype present.These abilities have attracted a lot of attention as these compounds are potentially safe,effective dietary estrogen replacements.
Awell known phytochemical is resveratrol.Resveratrol[357]is a polyphenol found in the skin and seed of grapes,and it can extend the life span of budding yeast[358].Resveratrol has also been linked to many physiological benefits including protection against cardiovascular disease,age-related deterioration,and the pathological consequences of fat-rich diets[359].Resveratrol is reported to exert its effects by directly activating the yeast Sir2 protein and its mammalian homolog Sirt1.These members of the Sirtuin family catalyze NAD+-dependent deacetylation reactions.The Sirtuins have been independently linked to lifespan regulation in budding yeast.However,the observed activation of Sirt1 by resveratrolin vitronow appears to be an artifact of the assay used,casting doubt on the direct resveratrol-Sirt1 connection[360].A series of studies showed that resveratrol activates Sirt1 indirectly through AMPK,another energy-sensing enzyme that is required for many of the adaptations triggered by calorie restriction [361].AMPK promotes the activation of peroxisome proliferator-activated receptor-coactivator 1α(PGC-1α)by Sirt1 through several mechanisms,including deacetylation by Sirt1 and an increase in NAD+concentration,which is rate limiting for Sirt1 activity [362].AMPK activation is the most upstream signal triggered by resveratrol,but Sirt1 and AMPK do not appear to be a direct resveratrol target.More recently,Park et al.[363]found that resveratrol inhibits phosphodiesterases(PDEs),leading to increased cAMP levels,Epac1 activation,elevated intracellular calcium,and AMPK activation.Calorie restriction also activates AMPK.The increase of NAD+levels leads to Sirt1 activation,which promotes beneficia metabolic changes primarily through deacetylation and activation of PGC-1α.In a parallel pathway,increased cAMP levels can also activate PKA,which directly phosphorylates and activates Sirt1.Sirt1 activation by either pathway,as well as the potential activation of other NAD+-dependent enzymes,can lead to numerous physiologic outputs.The metabolic effects of resveratrol result from competitive inhibition of cAMP-degrading phosphodiesterases,leading to elevated cAMP levels.As a consequence,resveratrol increases NAD+and Sirt1 activity.The metabolic benefit of resveratrol mainly include prevention of diet-induced obesity and an increase in mitochondrial function,physical stamina,and glucose tolerance.
8.Which is the agent responsible for the biological function,the molecule or the cell?
The cell is the fundamental unit of structure,function,and organization of living systems—something we have known for more than a hundred years.It is common to all organisms that develop from a single fertilized egg.Unfortunately,along with the rapid progress of molecular biology,many scientists have come to consider molecular bioactive effects as the functions of molecules and not cells.However,we would like to emphasize that all molecules exerts their biological function through target cells.The Nobel Prize-winning Sydney Brenner proposed that the correct level of abstraction is the cell and provided an outline of CELLMAP,a design for a system to organize biological information [364].This holistic approach is based on the idea that complex wholes cannot be understood by a study of their isolated parts.When many components are put together,especially with interactions that are nonlinear,new emergent properties can only be comprehended in the context of the whole system.In essence,molecules tell us nothing about cells and their behavior,and neurons tell us nothing about brains and how they work.The key is that we have a map of the molecules within the cells and a map of the cells in the whole body.Even for microbes,the cells also constitute a complex cell communication network through their interactions,and their cross-talk in signaling pathways and metabolic networks.When choosing the level of the cell we can adopt a uniform conceptual architecture for all levels,viewing the organism as a network of interacting cells in the same way as we view the cell as a network of interacting molecules.Transcription factors and,in particular,assemblages of these,are special devices that not only interact with each other,but also with special DNA sequences in gene promoters.
Based on the approach outlined above,we determined that almost all foods include macronutrients,vitamins,phytochemicals,and even toxins that affect immunomodulation,activation or suppression of signal transduction,and metabolic regulation.Moreover,different experiments performed with the same samples result in remarkable different,and even contradictory,findings So what can these data can tell us?Which agents are beneficia for our health? In order to resolve these problems,we have to be clear as to which component is the agent of the biological function and how it functionsin vivo.We strongly suggest that the cell alone exerts,only cell is the executor of biological functions,and cell exerts its functions through interactions each other cells,and that these interactions constitutes a cellular communications network that occursviaby signaling molecules in circulations.
9.Do we really have a mobile cell communication network in our body?
The immune system is formed by a complex network of different cell types including lymphocytes,DCs,macrophages,and neutrophils.Each has a specifi function,but all share the ability to move continuously throughout the body,constantly surveying peripheral tissues and lymphoid organs for the presence of potential pathogens.These cells are able to change their homeostatic recirculation program when they are informed of an infected site.Recruitment of immune cells to the site of infection is critical for mounting an immune response to figh off and clear away the invaders.Obviously,this is an information communication process,especially for mobile cells,such as leukocytes,and certainly confirm mobile cell communication network.
9.1.The evidence for the objective existence of a mobile cell communication network
The chemokine/chemokine receptor family orchestrates diverse patterns of immune cell migration.Chemokines are small cytokines characterized by their ability to induce directional migration of cells by binding to chemokine receptors.Currently,more than 50 chemokines and 20 chemokine receptors have been described.All chemokines bind to receptors that belong to the seven-transmembrane GPCR family.The signaling pathways initiated by ligand binding to the GPCRs lead to activation of kinases responsible for signal transduction,changes in intracellular calcium levels,and phosphorylation and activation of transcription factors.This results in molecular and functional changes in effector cells that allow them to migrate toward a gradient of chemokines.Genes encoding proteins involved in cell adhesion (integrins,laminins,and cadherins) are of particular significanc to chemokine-induced alterations in gene expression as they mediate the attachment of immune/tumor cells to various target tissues[365].
The classical leukocyte adhesion cascade involves the following key steps:(i)leukocyte capture and rolling,(ii)activation and arrest,and(iii)transendothelial migration.To mediate an effective response,leukocytes must fin their way to sites of infection or inflammation Leukocyte invasion of tissues can be induced by several substances—including IL-1,TNF-α,and LPS—that cause leukocyte emigration when injectedin vivo.All such compounds induce the production of chemoattractants,which in turn cause leukocyte migration.Therefore,chemotactic activity includes receptor-mediated gradient perception and must be measured by the ability of a chemoattractant to induce directed leukocyte migration.The magnitude of the cellular response elicited by chemokines is dictated by the level of receptor expression at the plasma membrane,which is a balance of finel tuned endocytic and recycling pathways.Recent data have revealed that receptor traffickin properties can drive chemokine receptors to lysosomal degradation or recycling pathways,producing opposite effects on the strength of the intracellular signaling cascade[366].
The route for leukocyte emigration to a specifi target site is guided and regulated by adhesive cascades,which are mediated by three sequential and partially overlapping steps:initiation by selectin-mediated,capturing and rolling,and chemokinetriggered activation and integrin-dependent arrest on endothelial immunoglobulin superfamily(IgSF)ligands.Different selectin and integrin family members,together with diverse endothelialdisplayed chemokines,provide large combinatorial specificit to this process.
Chemokine signals activate leukocyte integrins and actin remodeling machineries critical for leukocyte adhesion and motility across vascular barriers.The arrest of leukocytes at target blood vessel sites depends on rapid conformational activation of their α4 and β2 integrins by the binding of endothelial-displayed chemokines to leukocyte GPCRs.A universal regulator of this event is the integrin-actin adaptor talin-1.Chemokine-stimulated GPCRs can transmit within fractions of second signalsviamultiple Rho GTPases,which locally raise plasma membrane levels of the talin activating phosphatidyl inositolPtdInsP2(PIP2).Additional pools of GPCR-stimulated Rac-1 and Rap-1 GTPases together with GPCR-stimulated PLC and PI3K family members regulate the turnover of focal contacts of leukocyte integrins,induce the collapse of leukocyte microvilli,and promote polarized leukocyte crawling in search of exit cues.Concomitantly,other leukocyte GTPases triggers invasive protrusions into and between endothelial cells in search of basolateral chemokine exit cues[367].
Leukocyte traffickin from the bloodstream to inflame target tissues across the endothelial barrier is an essential response.Leukocyte adhesion,locomotion,and diapedesis induce signaling in endothelial cells,and this is accompanied by a profound reorganization of the endothelial cell surfaces which are only beginning to be unveiled.The coordination between these different endothelial membrane-remodeling events probably provides the road map for transmigrating leukocytes to fin exit points in the vessel wall[368].
To fulfil their duties,leukocytes must be able to counteract the pushing force generated by the fl w,arresting on the surface of endothelial cells and transmigrating into tissues.Everything must be done within a few seconds or less to cope with the timing imposed by fl w dynamics[369].Leukocyte rolling on endothelial cells and other P-selectin substrates is mediated by P-selectin binding to P-selectin glycoprotein ligand-1 expressed on the tips of leukocyte microvilli.Leukocyte rolling is a result of rapid,yet balanced formation and dissociation of selectin–ligand bonds in the presence of hydrodynamic shear forces [370].Rolling initiates with the firs step of capture,this can result in rolling along the vessel endothelium followed by slow rolling,activation,and fir arrest.The free fl wing marginated neutrophils tether and roll along the vessel endothelium through reversible and rapid interactions between the selectin family of adhesion molecules expressed on endothelial cells(P,E-selectin)or neutrophils (L-selectin) with their carbohydrate ligands expressed on the opposing cell to mediate the primary steps of capturing and rolling[371].
9.2.The GI tract is the regulatory center of the mobile cell communication network
Studies exploring the relationships between leucocytes and chemokines/chemokine receptors in the GI are currently underway.Considering the large area of the GI tract(a surface area of almost 400 m2in man),it is not surprising that it has developed both immunologic and non-immunologic means of protection.Furthermore,almost all receptors are expressed throughout the GI tract.As mentioned above,various signals can be secreted and released into bloodstream,including cytokines,chemokines,and hormones,after the ingestion of oral medicines,drugs,and functional foods.
When the GI tract is faced with an antigen,it can be induced to express pro-inflammator cytokines such as IL-1β and TNF-α,which cause leukocyte emigration and cell communication.Therefore,the GI tract should be considered the center of the immunodefence system as well as the center of mobile cell communication.
9.3.How can we build a cell communication network?
Frankenstein et al.[372]analyzed the cytokine network with the aim of uncovering its characteristic features:connection density,motifs and anti-motifs,distinct cell roles,and network super-family associations.In their model,cytokine connections between and among immune and body cells were obtained manually from an internet database,theCytokines Online Pathfinder Encyclopedia.They automatically transformed the raw data into a network format designating cells as nodes and cytokine connections as edges,and used an information-based methodology to uncover the large-scale architecture of the cytokine networks that connect immune and non-immune cells.The density of each of 113 different published networks was also computed,and interestingly,the immune cytokine sub-network was found to be the densest of networks with a density score of 0.61;it was followed by the non-immune (body cell) sub-network which had a density score of 0.4,and the neuronal network of the monkey brain,which had a score of 0.15.These finding indicate that the density of the mobile cell communication network is considerably greater than that of the nerve-mediated wired cell communication network.However,this is only based on data and an ideal system and so cannot be considered representative of the physical facts.
We discovered a method for developing a cell communication network that can be applied with human volunteers and oral administration of medicines,drugs,and functional foods.The hypothesis of a mobile cell wireless communication network was suggested based on immunological data and the complex interactions between dietary constituents and GI mucous receptors as well as the molecular biology of cell signaling and its effects [373].The key points of this hypothesis include:(1) the so-called “cytokine networks” are the natural signal transduction system of cell communications;(2)mobile cells,especially leukocytes,communicate with each other across the area network through paracrine or autocrine signaling,and across the whole communication network(which includes not only mobile cells but also immobilized cells and local and remote communication) by telecrine or endocrine signaling or neurosecretion;(3)three networks of intercellular communication can be associated with cytokine and chemokine secretion;one is limited to the cells of the immune system(immune cells),one to parenchymal cells of organs and tissues(body cells),and one involves interactions between immune and body cells;(4) these different cell communications interact to form a complex network,including wired and wireless connections;(5)bioactive molecules-cytokines/cytokine receptors and chemokines/chemokine receptors play key roles in cell communications;(6)the blood concentration of these signals (cytokines and chemokines) is very low (pg),but they can cascadeviadifferent signaling pathways in target cells;(7) the cytokine and chemokine signals are transportedviathe bloodstream to any part of the body,and they exert different functions in different cells.However,when they are transportedviathe blood,they do not interfere with each other;(8) mobile cells roll through the vessel epidermis and are guided by adhesion proteins;(9) because these cytokines or chemokines are transported by the circulatory system,a 2–3 mL blood sample from a volunteer will be data rich.
We [374]investigated the physiological functions ofPholiota namekopolysaccharides (PNPS-1),and mapped out the cell communication network diagram.This showed that PNPS-1 possessed significan anti-inflammator activity in humansin vivo.
10.Conclusions and future perspectives
10.1.Conclusions
The above data indicate that at least some functional foods exert their effects by interacting with numerous complex receptors located throughout the GI tract.The gut is not only a nutrient recognition and control system,but also a signal transducer,neuroendocrine sensor,and immunological recognition and presentation system.These complex information exchange systems constitute a symphonic signal transduction network between the different cells of the GI tract and immobilized cells in organs as well as mobile cells in the blood.Functional foods may exert their effectsviasome of these networksin vivo.
Nutrients are taken up by complex transport systems that are compactly and stoichiometrically coupled with ion channels,Na+–K+pumps,sensors,and signaling pathways.Nutrients and their homeostasis must therefore be coordinated with integral physiological,metabolic,immunological,and neuroendocrine systems.
In the gut,there is a delicate balance between the need to recognize pathogens and dietary ingredients to prevent unwanted immune responses to food antigens or the normal intestinal flora and the need to simultaneously allow for adequate nutrient uptake.As the GI tract covers a large surface area,it has developed both immunologic and non-immunologic means of protection.
Intestinal cells can express adhesion molecules constitutively,and can be upregulated by cytokines,chemokines,and other pro-inflammator molecules,including dietary ingredients and microbial metabolites.In addition to mediating adhesion,some of these molecules are also costimulatory during intercellular signaling.To date,almost all membrane receptors have been observed on the mucosa,which indicates that these receptors can interact with their specifi ligands in the GI tract and thereby sense the environmental conditions,especially the nutritional status of the body,viathe circulatory system.
Probiotics are the most well characterized dietary bioactive compounds.The beneficia effects of probiotics mainly rely on their influenc on the composition of the gut microbiota and their ability to generate fermentation products with diverse bioactive roles.The gut microbiota may constitute a complex metabolic network for communication relating to dietary nutrition and the host.The challenge may be to determine the temporal dynamics of metabolic communication between the host,diet,and gut microbiota,and to evaluate their real effectsin vivo.
Some functional foods cannot be absorbed,but do impact cytokines and chemokines.Non-digestible oligosaccharides may be supplemented to modulation immune responses in the intestine.IECs lining the mucosa are known to express carbohydrate (glycan)-binding receptors that may be involved in modulation of the mucosal immune response.The GI immune system is the largest and most complex immunological tissue in the human body and it can discriminate between potentially dangerous antigens and beneficia probiotics and microbes.IECs express several receptors that recognize antigens present in the intestinal lumen.These receptors could be the most important information delivering systems and have critical bioactive effects in association with functional foods or oral medicines.
A series of communications occur between the gut/brain,gut/endocrine system,gut/liver,gut/lungs,gut/cardiovascular system,and even the gut/reproductive system.The evidence strongly suggests that the gut functions as an information center in this cyclic system.The cell is the fundamental unit of structure,function,and organization of living systems.So the cell,and only the cell,is the agent of biological function,and it exerts its effects through interactions with other cells,which constitutes a cell communication network operated by signaling molecules in the circulatory system.
The density of the immune cytokine sub-network is the densest of networks,which suggests that the density of the mobile cell communication network is considerably greater than that of the nerve-mediated wired cell communication network.However,this findin was based on an ideal system,and so is far from the physical truth.We discovered a method for developing a cell communication network that can be conveniently applied in human volunteers to experiment with oral administration of different medicines,drugs,and functional foods.Because cytokines and chemokines are transported by circulation,a 2–3 ml blood sample from volunteer can be data rich.
10.2.Future perspectives
Although food intake is required to ensure adequate nutrition,it also has to be stringently regulated according to the physiology of the metabolic systems and homeostasis of each individual.Once excessive nutrients are ingested,or too little,or if nutritional intake is not balanced,then illness occurs.This process is intensively regulated by the GI tract.Furthermore,the GI tract also acts as an information exchange system through which organisms distinguish,recognize,identify,and sense the complex microecological environment of the lumen as well as its molecular structure and that of the world around us.This means that almost everything will affect our healthviathe GI information exchange system,including nutrients,microbes,functional foods,medicines,pathogens,vaccines,and even toxins.Based on these ideas,we believe that future research will focus on the following:(1) the biological roles of functional food components are being investigated since their metabolites and effects may depend on the gut microbiota,which may differ between individuals;(2) advances in our understanding of the interactions that occur between bioactive food compounds and specifi intestinal bacteria can contribute to better elucidation of both positive and negative interactionsin vivo;(3)increased understanding of the intestinal targets of nutritional regulation may extend beyond incretin release to mechanisms entirely confine to ECs.Characterization of these EC-based mechanisms regulating nutrient absorption and metabolite traffickin will help us to distinguish the passive effects of nutrient transport on energetic status from active signaling events;(4) based on the high throughput microarray and molecular interaction approach,screening for more sensors or receptors throughout the GI will be possible.Existing functional food data will need to be complemented by experimentsin vivoin which the expression of the potential nutrient sensor or receptor can be manipulated with temporal resolution and specificall in the intestine;(5)several tasks should be addressed in the near future in order to ensure the safe and successful development and delivery of functional foods.These include the identificatio of the suitable oral delivery systems,improved understanding of conditions affecting their biological activity,and of the development of a gold standard method for the functional evaluation of functional foods or medicines in humansin vivo.The cell communication network described above appears to be the best candidate method currently available.
Acknowledgements
National Natural Science Foundation of China (nos.30871951 and 31000749) and Tianjin Scientifi Research Program (no.10ZCKFNC01800) financiall supported this research.
- 食品科學(xué)與人類健康(英文)的其它文章
- About the Beijing Academy of Food Sciences
- Inhibition of citrus fl vonoids on 12-O-tetradecanoylphorbol 13-acetate-induced skin inflammatio and tumorigenesis in mice
- Hypothesis review:The direct interaction of food nanoparticles with the lymphatic system
- Minireview:Therapeutic potential of myricetin in diabetes mellitus
- GUIDE FOR AUTHORS
- Pharmacological potential of ampelopsin in Rattan tea

