Inhibition of citrus fl vonoids on 12-O-tetradecanoylphorbol 13-acetate-induced skin inflammatio and tumorigenesis in mice
Min-Hsiung Pn Shiming Li Ching-Shu Li Yutk Miyuhi Mihiko Suzw Chi-Tng Ho
a Department of Seafood Science,National Kaohsiung Marine University,Kaohsiung 811,Taiwan,China
b Department of Food Science,Rutgers University,New Brunswick,NJ 08901,USA
c Miyauchi Citrus Research Center,324-10 Shigoka-Machi,Takasaki,Gunma 370-0845,Japan
Abstract The inhibitory effects of a formulated product fromcitrus peel extract,Gold Lotion(GL),on 12-O-tetradecanoylphorbol 13-acetate(TPA)-induced expression of inducible nitric oxide synthase(iNOS)and cyclooxygenase-2(COX-2)in mouse skin was reported in this study.It is found that in the TPA-induced skin inflammatio model,the topical application of GL effectively inhibited the transcriptional activation of iNOS and its mRNA and protein in mouse skin.It is also discovered that GL significantl inhibited TPA-induced mouse skin inflammatio by decreasing inflammator gene parameters.Furthermore,GL dramatically inhibited 7,12-dimethylbenez[a]anthracene(DMBA)/TPA-induced skin tumor formation and reduced tumor incidence,tumor weight and tumor multiplicity of papillomas at 20 weeks.In essence,these in vivo data have revealed that GL is an effective anti-tumor agent that functions by down-regulating the protein levels of COX-2,ornithine decarboxylase(ODC),and vascular endothelial growth factor (VEGF) in mouse skin,suggesting that GL is a novel functional natural product capable of preventing inflammation-associate tumorigenesis.
Keywords: Inhibition;Skin cancer;Citrus fl vonoids;Inflammation Tumorigenesis
1.Introduction
Citrus peel is rich in fl vonoids including methylated derivatives such as polymethoxyfl vones (PMFs).Flavonoids are typically found throughout the whole fruit whereas PMFs are found exclusively in the peels ofCitrusgenus,particularly in the peels of sweet oranges (Citrus sinensis) and mandarin oranges(Citrus reticulate).PMFs have been shown to exhibit a broad spectrum of biological activity including cancer prevention,inhibition of inflammation and anti-tumor properties[1–4].It has been suggested that the consumption of citrus fruits may prevent the development of certain human cancers.It is also commonly recognized that cancer induction can be prevented by certain food phytochemicals,and fl vonoids inCitrusfruits and juices have been recognized as prominent cancer preventing agents[5,6].
Gold lotion (GL),made from the peels of 6citrusfruits available in Japan,namely,navel oranges,citrus hassaku,citrus limon,citrus natsudaidai,citrus miyauchiandsatsuma,was initially developed as a cosmetic product to protect skin from UV irradiation.Consumers later reported anecdotal evidence that GL possessed anti-cancer property based on topical application of GL to treat melanoma,and oral ingestion for prostate,lung and liver cancers.After two years(1984–1986)of safety evaluation,GL was found to be non-toxic by the Huntingdon Research Center in UK(Reports from Miyauchi Citrus Research Center).Subsequent chemical analysis of GL has revealed the abundant existence of fl vonoids and PMFs,which stimulated us to further investigate its anti-inflammatio and anti-cancer activity systematically in animal models.
Previous research has shown that inflammatio is causally linked to carcinogenesis and acts as a driving force in premalignant and malignant transformation of cells[7].In the past two decades,mouse skin models have been developed to elucidate the mechanism of inflammatio as it relates to tumor promotion and progression [8].In skin,the epidermis is the main target for various initiators and promoters.In the mouse skin model of acute inflammation TPA has been used as a potent tumor promoter.In addition to tumor promotion,TPA also causes responses in mouse skin such as arachidonic acid cascade and epidermal ornithine decarboxylase (ODC) induction [9,10].Moreover,TPA also induces epidermal cell proliferation,recruits inflammator cells,increases production of reactive oxygen species (ROS) leading to oxidative DNA damage,and reduces DNA repairing capability.More importantly,topical application of TPA to mice leads to edema and papilloma formation by enhancing the expression of inflammator enzymes such as inducible nitric oxide synthase(iNOS)and cyclooxygenase-2(COX-2)[11].Studies show that COX-2 and iNOS are important enzymes that mediate a majority of known inflammator processes.Additionally,up-regulation of COX-2 and/or iNOS has been associated with the pathophysiology of certain types of human cancers as well as inflammator disorders[12].
The mouse skin model has been used for decades as a reliable and conventional model for studying the mechanisms of carcinogenesis and modulation of sequential steps involved in the process[13].Skin tumors can be induced by the sequential application of a sub-threshold dose of a carcinogen (initiation stage)followed by repetitive treatment with a non-carcinogenic promoter[14].Topical application of TPA,the classical tumor promoter to mouse skin,has been shown to generate a number of biochemical alterations,changes in cellular functions,and histological changes leading to skin tumor promotion[15].The changes that have exhibited the highest correlation with skin tumor-promoting activity of TPA include skin edema,epidermal hyperplasia,inflammation proliferation and oxidative stress[16].The skin changes have been define as biomarkers of skin tumor promotion,and could be used to evaluate the potency of chemo-preventive agents against tumorigenesis[17,18].
In the present study,we have investigated the effectiveness of GL on TPA-induced iNOS,COX-2,and ODC expression in mouse skin;evaluated the anti-inflammator activity of GL in mouse skin following TPA application,and explored the inhibitory effect of GL on mouse skin tumorigenesis using a two-stage skin carcinogenesis model by the evaluation of tumor incidence,multiplicity,and volume.
2.Materials and methods
2.1.Animal treatment
Female ICR mice at 5–6 weeks’ old were supplied by the BioLASCO Experimental Animal Center (Taiwan Co.,Ltd.,BioLASCO,Taipei,Taiwan).All animals were housed in a controlled atmosphere(25±1?C at 50%relative humidity)and with a 12-h light/12-h dark cycle.The dorsal skin of each mouse was shaved with surgical clippers prior to application of each tested compound.GL and TPA were dissolved in 200 μL of acetone and applied topically to the shaved area of each mouse.
2.2.Measurement of epidermal hyperplasia
To measure epidermal thickness,skin samples from different treatment groups were fi ed in 10%formalin and embedded in paraffi for histological examination.Sections (4 μm in thickness)of the skin samples were cut and mounted on polylysinecoated slides.Each section was de-paraffinize in xylene,rehydrated through a series of graded alcohols and stained with hematoxylin and eosin.The thickness of the epidermis was measured using a Nikon light microscope (Japan) equipped with an ocular micrometer at 400× magnificatio and 15 field per section.The number of leukocytes infiltratin the dermis was determined by counting the stained cells at 5 different locations.
2.3.Western blot analysis
The female ICR mice were topically treated on their shaved backs with GL applied 30 min prior to treatment with 10 nmol of TPA.The mice were killed by cervical dislocation at scheduled time.For protein isolation from mouse skin,the mouse dorsal skins derived from different experiments were excised.Following fat removal from the dorsal skin on ice,the skin samples were immediately placed in liquid nitrogen.The epidermal protein was homogenized on ice for 15 s with a polytron tissue homogenizer and lysed in 0.5 mL of ice-cold lysis buffer followed by centrifugation at 10,000×gfor 30 min at 4?C.Proteins in the cytosolic fraction (supernatant) were measured by Bio-Rad protein assay.A total of 50 μg protein was boiled in sodium dodecyl sulfate (SDS) sample loading buffer for 10 min before electrophoresis on 12%SDS–polyacrylamide gel.After electrophoresis for 2 h,proteins in SDS–polyacrylamide gel were transferred to PVDF membrane(Gelman Laboratory,Ann Arbor,MI),and the blots were blocked with 5% non-fat dry milk-PBST buffer [phosphate–buffer saline (PBS) containing 0.1% Tween-20]for 60 min at room temperature.The membranes were incubated for 2 h at room temperature with iNOS,COX-2,COX-1 and β-actin antibodies at a dilution ratio of 1:1000.
2.4.Reverse transcription-polymerase chain reaction(RT-PCR)
Total RNA was isolated from scraped colon mucosa using Trizol Reagent according to the manufacturer’s instruction(Invitrogen,Carlsbad,CA,USA).Changes in the steady-state concentration of mRNA in iNOS,COX-2 and-actin were assessed by RT-PCR.A total of 2g RNA was transcribed into cDNA using SuperScript II Reverse Transcriptase (Invitrogen,Renfrewshire,UK)in a fina volume of 20L.RT reactions were performed at 50?C for 50 min and 70?C for 15 min using a Gene Cycler thermal cycler(Bio-Rad).The thermal cycle conditions were initiated at 95?C for 1 min,followed by 30 cycles of amplificatio (94?C for 30 s,58?C for 25 s,and 72?C for 1 min),and a fina extension at 72?C for 3 min.The complementary DNA was amplifie by PCR with the following primers:iNOS,forward primer 5'-CCCTTCCGAAGTTTCTGGCAGCAGC-3'(2944–2968),reverse primer 5'-GGCTGTCAGAGAGCCTCGTGGCTTTGG-3'(3416–3440);COX-2,forward primer 5'-GGAGAGACTATCAAGATAGTGATC-3' (1094–1117),reverse primer 5'-ATGGTCAGTAGACTTTTACAGCTC-3'(1931–1954);and-actin,forward primer 5'-A AGAG AGGCATCCTCACCCT-3',reverse primer 5'-TACATGGCTGGGGTGTTGAA-3'.PCR products were analyzed by 1% agarose gel and visualized by ethidium bromide staining.
2.5.Two-stage skin carcinogenesis model
The inhibition of tumor promoting activity of GL was examined by a standard initiation promotion with DMBA and TPA,as reported previously [19].A group of 12 female ICR mice were given commercial rodent pellets and fresh tap waterad libitum,both of which were changed twice a week.The dorsal region of each mouse was shaved with an electric clipper 2 days before initiation.Mice at the age of 6 weeks were administrated with 200 nmol DMBA in 200L of acetone,while control mice received only 200L of acetone.One week after initiation,the mice were topically treated with 200L of acetone or promoted with TPA (5 nmol in 200L acetone) twice a week for 20 weeks.For the other 2 groups,the mice were treated with GL(100L)30 min before each TPA treatment.Tumors of at least 1 mm in diameter(measured using an electronic digital caliper)were counted and recorded twice weekly and the diameters of skin tumors were measured at the same time.The results were expressed as the average number of tumors per mouse,percentage of tumor-bearing mice and size distribution of tumors in each mouse.
2.6.Molecular mechanism analysis(inflammatory and angiogenic events)
The skin/papilloma tissue was removed using a scalpel,and samples were placed on ice prior to removal of fat by scraping.The samples were then homogenized in the lysis buffer [50 mM Tris–HCl,pH 7.4,1 mM NaF,150 mM NaCl,1 mM ethylene glycol-bis(aminoethylether)-tetraacetic acid,1 mM phenylmethanesulfonyl fluoride 1% NP-40 and 10g/mL leupeptin].The homogenate was clarifie by centrifugation at 12,000×gfor 20 min at 4?C.COX-2,iNOS,VEGF and ODC were detected with western blot analysis using specifi antibodies.
2.7.HPLC(high performance liquid chromatography)system and conditions
2.7.1.Separation and quantification of polymethoxyflavones
The HPLC was equipped with a reversed phase amide C16column (Ascentis RP-Amide,3m,150 mm×4.6 mm ID),from Supelco(Bellefonte,PA,USA).Gradient elution was used with a mobile phase composed of water(solvent A)and acetonitrile(ACN,solvent B).The optimized condition is as follows:a 20 min gradient was started with 40% of B,linearly increased to 55% of B in 10 min,then linearly increased to 70%in 15 min,and finall ramped to 80%in 20 min.Flow rate was 1.0 mL/min and the column temperature was maintained at 35?C.Detection wavelength was 326 nm and the injection volume was 10L.
2.7.2.Measurement of other flavonoids
The HPLC was equipped with a reversed phase C18column(Luna C18(2),3m,150 mm×4.6 mm ID),from Phenomenex,Inc.(Torrance,CA,USA).Gradient elution was used with a mobile phase composed of water containing 0.2% acetic acid(solvent A)and methanol containing 0.2%acetic acid(solvent B).The optimized conditions were as follows:a 100 min run was started with 0% of B and kept as isocratic for 2 min,linearly increased to 6% of B in 6 min and kept as isocratic for 6 min,then to 20% in 5 min and kept as isocratic for 18 min,then to 40% of B in 50 min.The fl w rate was 0.8 mL/min and the column temperature was maintained at ambient temperature.The detection wavelength was 283 nm and the injection volume was 10L.
3.Results
3.1.Inhibitory effects of GL on TPA-induced iNOS and COX-2 expression in mouse skin
Using TPA-induced acute inflammator response model in mouse skin,we investigated the anti-inflammator effects of GL.Having induced both iNOS and COX-2 expression by TPA treatment in mouse skin,we measured the protein expression of iNOS and COX-2 by western blot analysis.Female ICR mice were topically treated on their shaved backs with GL(100 and 200L,respectively) 30 min prior to treatment with 10 nmol TPA.The mice were killed by cervical dislocation at 1 h,2 h,and 4 h.For protein and RNA isolation from mouse skin,the dorsal skin of mice derived from different experiments was excised.The epidermal protein and RNA were extracted and subjected to western blot and RT-PCR analysis.
As shown in Fig.1A,when TPA was applied topically on the shaved area(backs)of female ICR mice,the levels of iNOS and COX-2 proteins were markedly increased compared to the negative control group.However,topical application of 100 μL and 200L GL 30 min prior to TPA treatment resulted in a dramatic reduction in the level of iNOS protein.In the RT-PCR analysis (Fig.1B),the topical application of GL also significantl reduced TPA-induced iNOS gene expression in mouse skin.Hence,we have found that GL inhibited both iNOS gene and protein expression in a dose-dependent manner in TPA-treated mouse skin,but not COX-2(Fig.1A and B).
The ODC protein is a rate-limiting enzyme involved in the synthesis of polyamines that play a pivotal role in cell growth and proliferation[20].Induced ODCexpression is also recognized as a biochemical hallmark of tumor promotion[21].As illustrated in Fig.1A,topical application of 100 μL and 200 μL of GL 30 min prior to TPA treatment resulted in a dramatic reduction in the levels of ODC proteins.
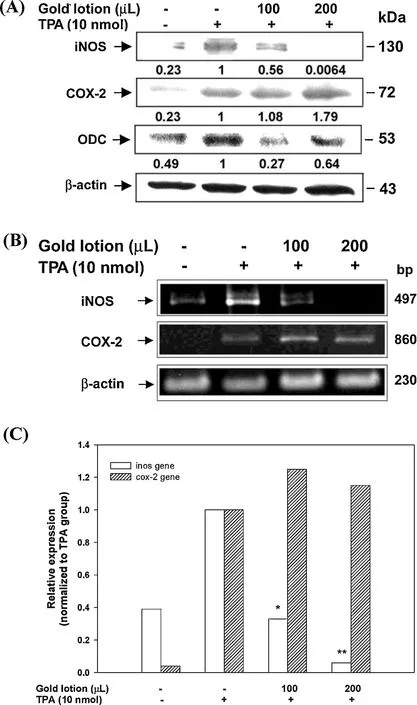
Fig.1.Inhibitory effects of GL on the expression of TPA-induced iNOS and COX-2 genes and proteins.(A)Mice were treated topically with 100 or 200 μL of GL 30 min prior to the treatment of 10 nmol TPA,and the mice were killed at 2 and 4 h,respectively,after the TPA treatment.The epidermal proteins were analyzed for iNOS,COX-2 and ODC by western blotting analysis.The western blot is a representative of at least three independent experiments.(B)Mice were treated as described in the text,and animals were killed at 1 and 2 h,respectively,after TPA treatment.A total of 2 g of complementary DNA were subjected to RT-PCR analysis for iNOS and COX-2 gene expression.(C)Quantificatio of iNOS and COX-2 gene expression was performed by densitometric analysis of the agarose gel.*P<0.01,**P<0.001 compared to the TPA group.
3.2.Inhibitory effect of GL on TPA-induced epidermal hyperplasia in mouse skin
An inhibition study of GL against TPA-induced inflammatio was also performed.As shown in Fig.2,TPA application at a dose of 10 nmol led to marked edema,and an increase in epidermal thickness of 13.2±0.7 μm was observed in the TPA treated group,versus5.4±1.9 μm in the acetone group.Pre-treatment with 100 μL and 200 μL of GL prior to TPA application dramatically suppressed epidermal thickness in a dose-dependent manner(10.1±1.3 μm in 100 μL GL group and 8.9±1.4 μm in 200 μL GL group;P<0.05,respectively).In addition,histological examination analysis(Fig.2A,arrow)revealed a higher number of leukocytes with infiltrate dermis due to the TPA application as compared with the acetone group.Hence,pretreatment with 100 μL and 200 μL of GL reduced leukocyte infiltration
3.3.Inhibition of tumor promotion by GL(two-stage skin carcinogenesis model)
The mouse skin model of multi-stage carcinogenesis,which is divided into 3 distinct stages,initiation,promotion,and progression [8],serves as a majorin vivomodel for studying the sequential and stepwise evolution of the cancer process by chemical and physical carcinogens.In this well-established and standardized model,a single sub-carcinogenic dose of skin carcinogen such as DMBA,which causes irreversible DNAdamage,is firs applied topically.Then,the repeated application of skin promoters,most commonly used phorbol esters such as TPA,to induce cell proliferation and inflammatio was followed[22].This process promotes the selective clonal expansion of initiated epidermal cells and leads to the formation of multiple squamous papillomas.The mouse skin carcinogenesis model not only establishes a link between genetic pathways and histological stages of tumor development but also serves as a model for function evaluation of phytochemicals and natural products[23].
3.4.Tumor inhibitory activity of GL on mouse skin
Up regulation of iNOS,COX-2 and ODC occurs in many pathological conditions,such as in tumorigenesis.Because the application of GL(100 and 200 μL)to mouse skin significantl inhibited various molecular targets that play significan roles in the progression of skin tumors(Fig.1),we employed the twostage skin carcinogenesis model to assess the inhibition of tumor promoting potential of GL.As shown in Table 1,no differences were observed in the body weights of mice in each group.Similarly,no any unhealthy symptoms were observed throughout the study.Furthermore,no significan difference was observed in the mean weights of liver,spleen and kidney,and no pathologic alternations were found among the groups (Table 1).These results suggested no noticeable side effects and no toxicity caused by the administration of GL during a period of 20 weeks in the two-stage skin carcinogenesis model.
At the end of the study,the skin tumors of each group were collected.In the analysis of tumor incidence,the animals in Ac/TPA group exhibited 16±3 papillomas per mouse and 100%incidence of skin tumors at 20 weeks(Fig.3A),whereas mice treated with acetone exhibited no tumor development.When GL (100 μL) was applied to the shaven backs of mice 30 min prior to each TPA application,the number of papillomas was 12±4 (reduced by 25% compared to the Ac/TPA group;P<0.05).In addition,we also found that the tumor incidence was 100%in the Ac/TPA group,whereas the GL-treated group showed significan reduction by 18%(Fig.3B).Comparing the weight of the tumors in all groups,tumor weight in the Ac/TPA group was 0.31±0.12 g,whereas it was significantl decreased to 0.11±0.07 g in the GL-treated group(Fig.3C).
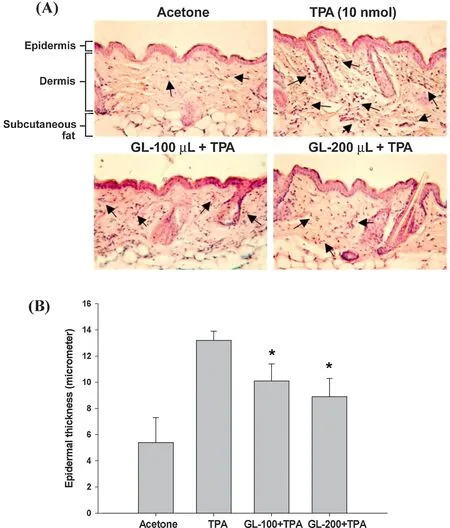
Fig.2.Inhibitory effects of GL on TPA-induced skin thickness in mouse skin.GL (100 or 200 μL) was topically applied to the dorsal skin 30 min prior to the treatment of 10 nmol TPA.After 24 h,all mice were killed for histological examination and analysis of epidermal thickness(μm)as described in Section 2.
3.5.Inhibition of GL on tumor size and the expression of angiogenic molecules in mouse skin tumors
Tumor promotion data were analyzed in terms of size distribution of papillomas and compared with the Ac/TPA group.The number of papillomas(≥5 mm in diameter)per mouse was significantl reduced in the GL treated group(9±2 in Ac/TPA groupvs.6±1 in GL treated group,Fig.4A).Additionally,in mouse skin tumors initiated by DMBA and promoted by TPA for 20 weeks,the protein expression of ODC,COX-2 and VEGF were markedly increased compared to skin tissue from the Ac/Ac group (Fig.4B).However,treatment with GL (100 μL) resulted in the strong reduction of ODC,COX-2 and VEGF protein levels in skin tumors.Because ODC is involved in cancer proliferation and both COX-2 and VEGF contribute to angiogenesis,these results suggested thatGL reduced tumor size by inhibiting both tumor growth and angiogenesis.
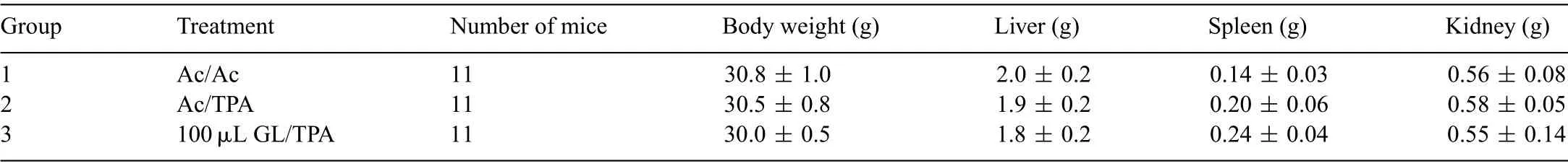
Table 1 Effects of GL on body weight and organ weight in two-stage skin carcinogenesis model.
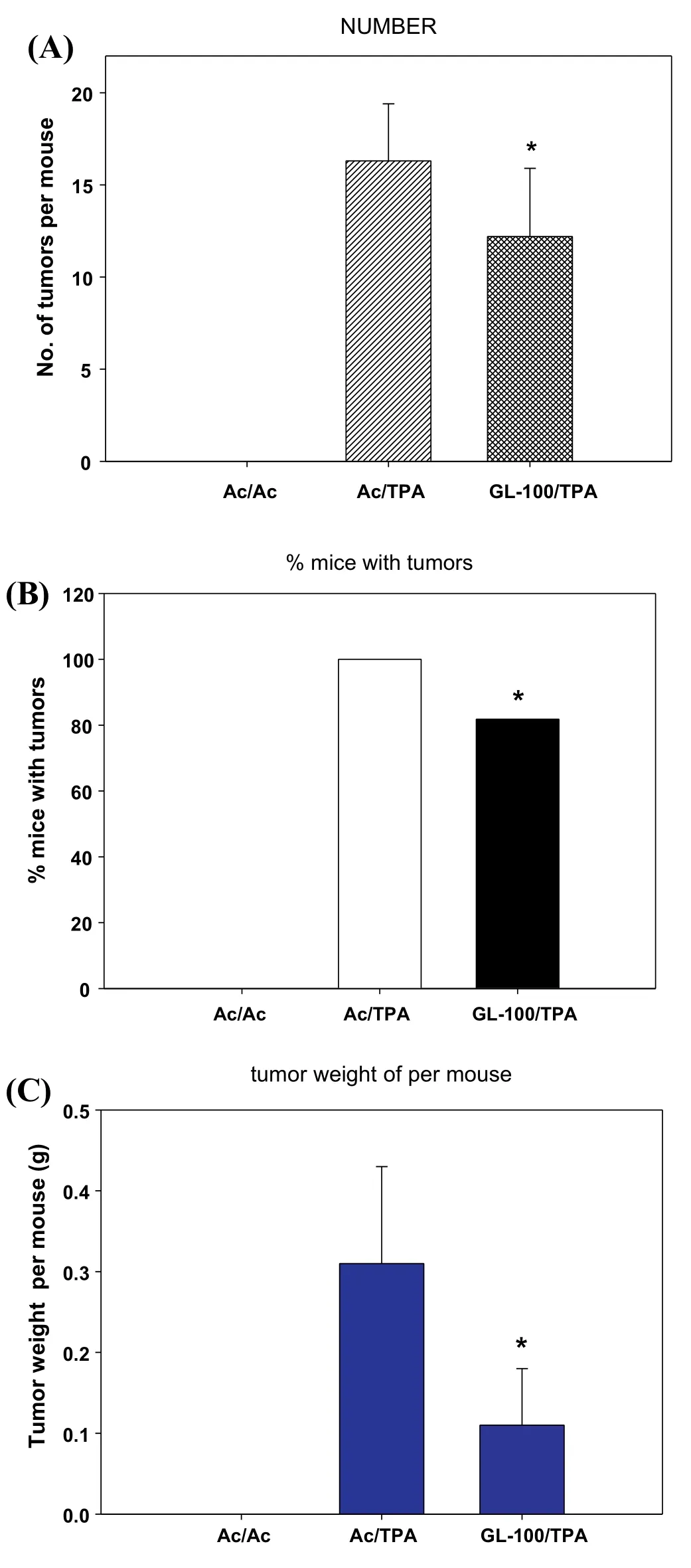
Fig.3.Effect of GL on TPA-promoted skin carcinogenesis.Tumor formation in all mice was initiated with DMBA(200 nmol)and promoted with TPA(5 nmol)twice weekly after 1 week initiation.GL(100 μL)topically applied 30 min prior to each TPA treatment.Tumors with 1 mm or larger in diameter were counted and recorded weekly,as described in Section 2.(A)Average number of papillomas/mouse.(B) Percentage of mice with papillomas (P<0.05).(C) Tumor weight per mouse (g).*P<0.05 indicates statistically significan differences from the Ac/TPA treated group.
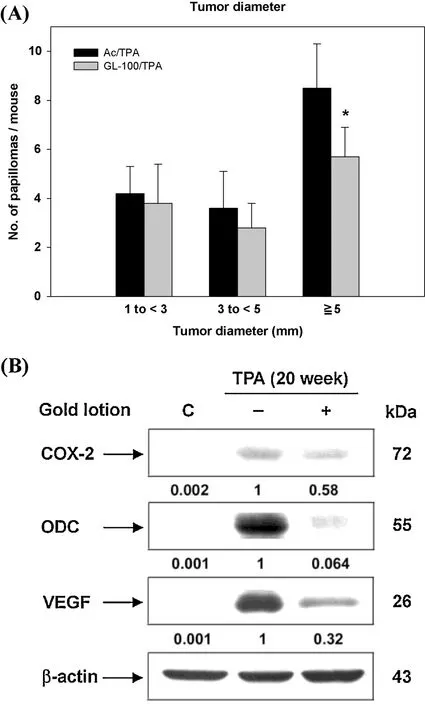
Fig.4.Effect of GL on tumor diameter in DMBA/TPA-induced skin tumorigenesis.During the period of tumor promotion,the diameters(in millimeter)of skin tumors were measured by an electronic digital caliper twice a week.The tumor dimension was recorded as the average of length×width(square millimeter)per mouse.Statistical analysis was completed by Student’s t-test.*P<0.05 indicates statistically significan differences from the Ac/TPA treated group.(B)Effect of GL on angiogenesis in skin tumors.COX-2,ODC,and VEGF were evaluated with western blotting analysis using specifi antibodies.
4.Discussion
In this study,we evaluated thein vivoanti-inflammator ,anti-tumor efficay of the citrus peel product GL,and a possible mechanism involved in using TPA-induced inflammatio in mouse skin model and DMBA/TPA two-stage skin carcinogenesis model was proposed.Based on the results of the TPA-induced skin inflammatio model,pre-treatment with 100 μL and 200 μL GL prior to TPA application significantl inhibited the inflammator enzyme iNOS gene and protein expression,but not COX-2.These differential effects may be due to the degree of dependency of iNOS and COX-2 promoters on various transcription factors.It has been reported that the promoter regions of iNOS and COX-2 contain different binding sites for transcription factors.Therefore,the transcriptional regulation of iNOS and COX-2 can be differentiated.The iNOS promoter contains severalcis-acting elements such as nuclear factor-κB(NF-κB),activator protein-1(AP-1),CCAAT/enhancer binding protein(C/EBP)β,and signal transducers and activators of transcription protein (Stat),while the COX-2 promoter has NF-κB,C/EBPβ,and cAMP response element(CRE)cis-acting elements[24,25].Thus,the GL generated repression of TPA-induced iNOS expression but not COX-2 may be completely through the regulation of different upstream transcription.It is possible that GL may interfere with the enzyme activity of COX-2,but not at the transcriptional level.Further validation for this proposed mechanism is warranted.In our previous study,it is found similar results with dihydrolipoic acid(DHLA):DHLA significantl suppressed iNOS expression,while only showed weak suppression toward the expression of COX-2 protein induced by TPA.However,it has demonstrated direct inhibition of COX-2 activity[26].
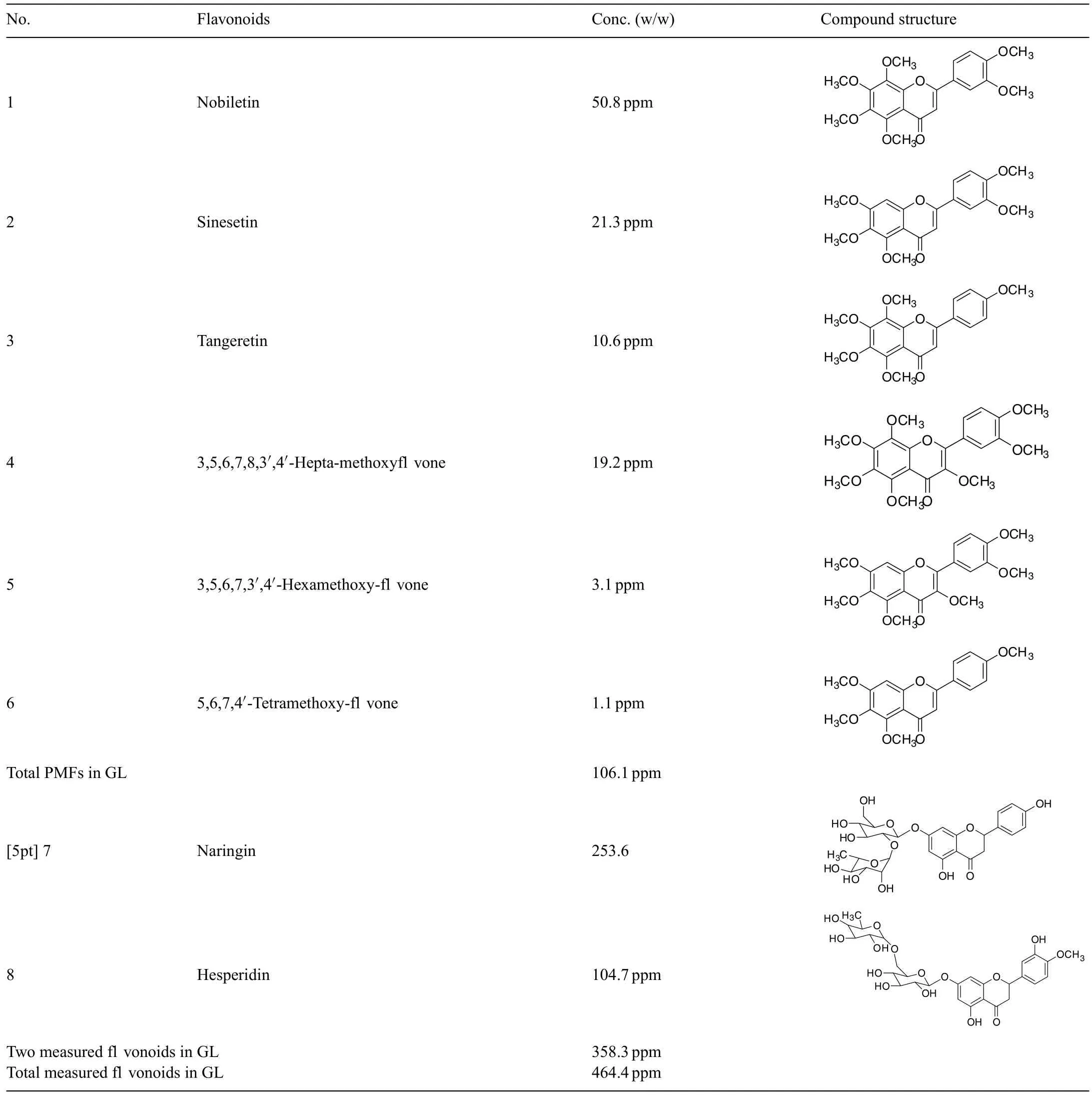
Table 2 Flavonoid contents in citrus peel extract(GL).
Induction of ODC occurs in response to TPA treatment in mouse skin,and ODC is constitutively elevated in the resulting skin tumors[27].Applications of TPA to mouse skin led to epidermal hyperplasia and excessive leukocyte recruitment and infiltratio [28,29].In this study,TPA-induced hyperplasia and leukocyte infiltratio were also reduced by pretreatment with GL.Moreover,a decreased protein level of ODC by GL pretreatment was observed as a result of GL pretreatment.These results suggest that GL inhibited TPA-induced skin inflam mationviadown regulation of inflammator iNOS and ODC expression in mouse skin.
It is confirme the inhibitory activity of GL on tumor using two-stage skin carcinogenesis model.At the end of the 20-week study,body weight and gross organ analyses revealed that long-term treatment with GL did not produce noticeable toxicity effects.Pre-treatment with GL suppressed DMBA/TPA-induced skin tumor number and incidence as well as tumor weight.Results of tumor size distribution demonstrated that GL signifi cantly reduced the number of larger tumors(≥5 mm),suggesting that GLmay hinder tumor growth,especially in larger tumors.To investigate the anti-tumor promotion activity of GL,we collected the skin tumors and performed western blot analysis.The results showed that the protein levels of ODC were strongly decreased in skin tumors in GL-treated mice.Angiogenesis is the process of forming new blood vessels from pre-existing vessels and is an essential process for solid tumor growth,invasion and metastasis.Up regulation of VEGF is a major angiogenic stimulus in mouse epidermal carcinogenesis[30].In addition to its role of inflammation COX-2 is also an important mediator of angiogenesis and tumor growth[31].In this study,it is found that both COX-2 and VEGF protein levels were dramatically decreased in the GL-treated group.Thus,these results demonstrated that GL treatment effectively reduced tumorigenesis induced by topical application of DMBA/TPA through inhibiting cell proliferation and tumor angiogenesis.Based on the afore mentioned results,we suggest that GL possesses strong chemo-preventive effect against DMBA/TPA-induced skin carcinogenesisviathe mechanisms of down-regulation of inflammation proliferation and angiogenesis.
Our chemical analysis revealed that the formulated GL product,composed of 6 citrus peels,was rich in fl vonoids with a total measured content of at least 450 ppm or 0.45 mg/mL,in which its polymethoxyfavone content was as high as 106 ppm or 0.1 mg/mL(Table 2).The net amount of fl vonoids present,i.e.dosage level,does not adequately explain the effica y or effectiveness of anti-inflammator and anti-carcinogenic properties of GL in the prevention and treatment of human diseases such as melanoma or prostate cancer.Nonetheless,the overall content and formulation of this specifi citrus peel product presents a unique chemical prof il leading to strong anti-inflammatio and anti-cancer activity in our tested mouse skin model.Therefore,based on the results of this study,we expect that the potential synergistic effects among the rich fl vonoids in this formulated citrus extract,particularly polymethoxyfl vones and traditionally recognized fl vonoids,may account for its effica y and effectiveness against inflammatio and cancer expressed in the well-established mouse skin model.
5.Conclusion
The growing demand and the vast potential for natural products to serve as safe and efficaciou therapeutic agents continues to garner increasing attention.However,plant extracts with complex chemical prof ile must be rigorously evaluated and chemically characterized to insure adequate performance consistency with respect to biological activity.The objectives in this study are to disentangle the complexity of the natural products derived from citrus peels;to comprehend the chemical prof il and effica y of GL;to establish a potential SAR(structure activity relationship);to explore purported synergistic effects;and more importantly,to gain maximum benefit from this safe and efficaciou citrus peel products.Based on our findings it is suggested that GL promotes a strong protective effect against TPA-mediated epithelial carcinogenesisviainhibition of COX-2,ODC,and VEGFexpression.This is the firs investigation with solid evidence indicating the potential of GL as a novel chemopreventive agent in the treatment of inflammation-associate tumorigenesis.
 食品科學(xué)與人類(lèi)健康(英文)2012年1期
食品科學(xué)與人類(lèi)健康(英文)2012年1期
- 食品科學(xué)與人類(lèi)健康(英文)的其它文章
- About the Beijing Academy of Food Sciences
- Hypothesis review:The direct interaction of food nanoparticles with the lymphatic system
- How functional foods play critical roles in human health
- Minireview:Therapeutic potential of myricetin in diabetes mellitus
- GUIDE FOR AUTHORS
- Pharmacological potential of ampelopsin in Rattan tea
