Simulation of Low-Temperature Coal Tar Hydrocracking in Supercritical Gasoline
Zhang Lei; Liu Zongkuan; Gu Zhaolin
(1. Department of Chemical Engineering and Technology, Xi’an Jiaotong Uniνersity, Xi’an 710049; 2. School of Human Settlements and Ciνil Engineering, Xi’an Jiaotong Uniνersity)
Simulation of Low-Temperature Coal Tar Hydrocracking in Supercritical Gasoline
Zhang Lei1; Liu Zongkuan1; Gu Zhaolin2
(1. Department of Chemical Engineering and Technology, Xi’an Jiaotong Uniνersity, Xi’an 710049; 2. School of Human Settlements and Ciνil Engineering, Xi’an Jiaotong Uniνersity)
The aim of this paper was preliminary design of the process for low-temperature coal tar hydrocracking in supercritical gasoline based on Aspen Plus with the concept of energy self-sustainability. In order to ensure the correctness and accuracy of the simulation, we did the following tasks: selecting reasonable model compounds for low-temperature coal tar; describing the nature of products gasoline and diesel accurately; and confirming the proper property study method for each block by means of experience and trial. The purpose of energy self-sustainability could be possibly achieved, on one hand, by using hot stream to preheat cold stream and achieving temperature control of streams, and on the other hand, by utilizing gas (byproduct of the coal tar hydrocracking) combustion reaction to provide energy. Results showed that the whole process could provide a positive net power of about 609 kW·h for processing the lowtemperature coal tar with a flowrate of 2 268 kg/h. The total heat recovery amounted to 2 229 kW·h, among which 845 kW·h was obtained from the gas combustion reaction, and 1 116 kW·h was provided by the reactor’s outlet stream, with the rest furnished by hot streams of the products gasoline, diesel and residue. In addition, the process flow sheet could achieve products separation well, and specifically the purity of product gasoline and diesel reached 97.2% and 100%, respectively.
coal tar; supercritical solvent; hydrocracking; process design; energy self-sustainability; Aspen Plus
1 Introduction
It is a pressing task to look for new oil substitute amid an increasingly deepening petroleum crisis globally. Using coal tar to prepare fuel would alleviate the energy crisis because of its considerable output, e.g., the output only in China in 2009 was about 15 million tons[1]. Among all kinds of coal tars, the low-temperature coal tar is the most suitable one for preparing fuel due to its high alkane content (35%) and lower pitch content[1-2].
Hydrocracking reaction taking place in supercritical solvent can convert the conventional heterogeneous reaction into homogeneous reaction, which means that the reactants and even catalyst and gas can be dissolved in supercritical solvent. As a result, diffusion limitation among reactants, as well as between reactants and catalyst, can be weakened dramatically. The final effects of hydrocracking in supercritical solvent mainly manifest in two aspects: one is to inhibit or delay the generation of coke and gas[3-5]through improving product selectivity and yield of middle distillate[6-8]; the other is to prolong the service life of catalyst. Research of Chang, et al.[7]showed that the activity of Ni-Mo-Pd/Y zeolite catalyst was still very high after 8 hours of operation for coal tar hydrocracking in supercritical solvent, indicating to its great significance for extending the operating cycle.
At present, supercritical water is widely used in hydrocracking research because of its zero emission and cheapness, along with its pollution and toxicity-free nature. However, its critical temperature of 374 ℃ and critical pressure of 22.78 MPa are harsh, which would impose high demandingness for the equipment and accelerate the decomposition of organic substances within minutes[9-11]. Recently, many researchers have been paying attention to organic supercritical solvents which require relatively mild critical conditions[9-10], and the study results showthat when their molecular weight, boiling point and critical temperature are higher, the yield of light oil would be also higher[3-4,11], although no close relationship with critical pressure and density is mentioned.
Gasoline was selected as the supercritical solvent in the study thanks to its mild critical conditions (requiring a critical temperature of 316 ℃, and a critical pressure of 3.47 MPa)[8]. Specifically, it didn’t require separation as solvent after the completion of reaction. Since there was no literature concerning the simulation of coal tar hydrocracking in supercritical gasoline based on Aspen Plus, a flow sheet was designed based on preliminary experiments which had confirmed the optimal technological parameters for the maximum yield of light oil[8]. The flow sheet should, on one hand, well achieve coal tar hydrocracking in supercritical gasoline and realize separation of the products; and on the other hand, explore the possibility of energy self-sustainability, which could be a concept for future commercial development.
2 Methods
This model was established on the basis of preliminary experiments, and the experimental results showed that the optimal temperature, MRSC (mass ratio of solvent gasoline to coal tar) and MRHC (mass ratio of hydrogen to coal tar) were 380 ℃, 1.0, and 0.06, respectively, for obtaining a maximum yield of light oil[7-8]. The product yield of gas, gasoline and diesel reached 9.21%, 19.17%, and 48.54%, respectively[7-8].
2.1 Simulated compounds and products
It is pretty important for a successful simulation to select some suitable model compounds. The low-temperature coal tar is made up of tens of thousands of compounds, in which only about five hundred compounds can be identified. However, it is amazing that the content of most compounds is very low. Specifically, the number of compounds whose contents are higher than 1% is only between 10—30[1-2]. So, it is possible to select the model compounds, and meanwhile the accuracy can be guaranteed. According to the existing literature[1,12], components included in coal tar can be divided into acidic substance, alkaline substance and neutral substance (<420 ℃ fraction), as shown in Figure 1.
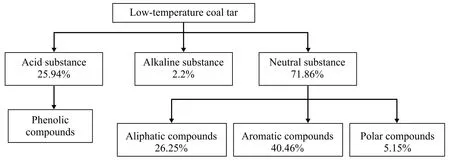
Figure 1 Composition of low temperature coal tar
The polar compounds contained in neutral substance and alkaline substance should not be considered in the simulation study due to their extremely low content, and thus the simulation mainly focused on aliphatic and aromatic compounds included in the neutral substance, as well as the main components included in the acidic substance (mainly phenolic compounds). So, model compounds and their corresponding content can be determined by two steps, viz.: selecting the high content compounds as the model compounds of coal tar, and then computing the content of model compounds according to their relative content in coal tar. Table 1 shows the model compounds and their corresponding content.
The products of gasoline and diesel were inputted as pseudo-components. Table 2 presents their nature, which is in good agreement with the literature[13]. Product gas was inputted as real components, i.e., CH4, C2H6, C3H8, C4H10, H2S, and NH3.
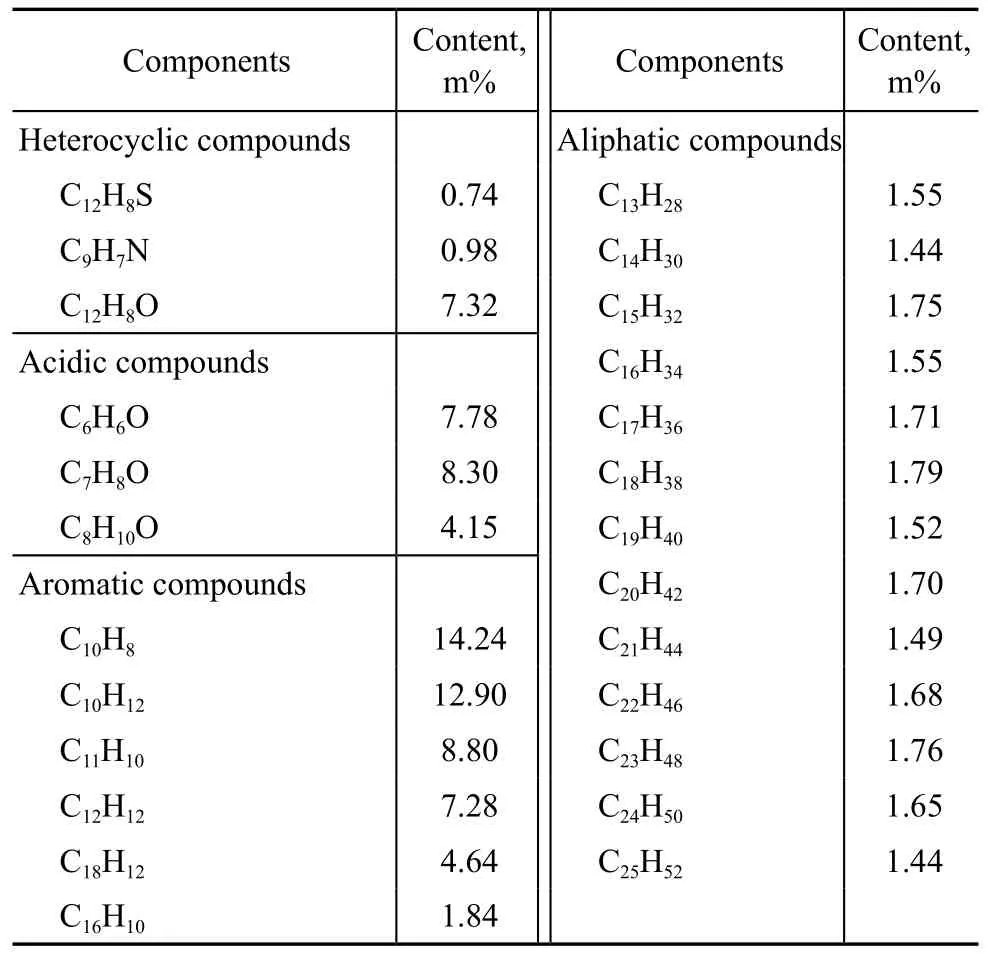
Table 1 Model compounds of low-temperature coal
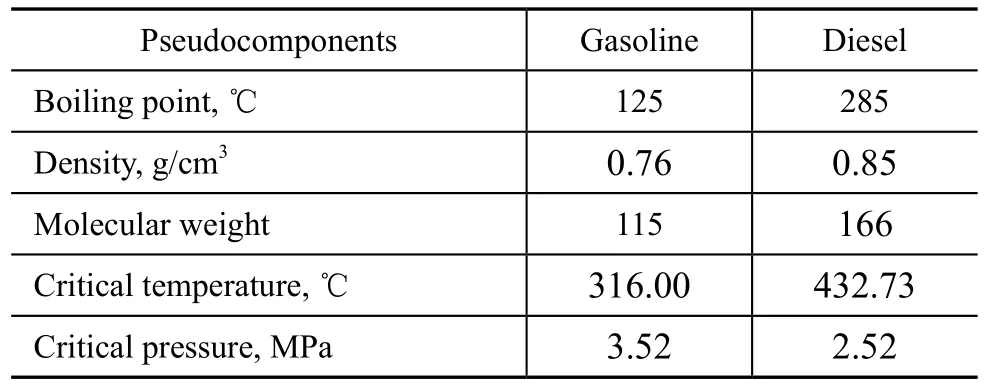
Table 2 The nature of product gasoline and diesel
2.2Model and simulation
2.2.1 Flow sheet
Figure 2 shows the basic plant scheme that was developed by means of the software Aspen Plus. This model can be divided into three parts in terms of functionality: the first part covers coal tar hydrocracking in supercritical gasoline; the second part covers the separation of the products; and the third part covers the heat transfer scheme.
The input of the plant was made up of four flows, i.e., coal tar, solvent gasoline, hydrogen and air. All fluxes were at standard conditions, i.e. 25 ℃ and 0.1 MPa. Coal tar was pre-heated orderly by products gasoline and diesel, and then mixed with solvent gasoline which was preheated by stream RESIDUE1 and mixed with the recycled residue. Then the mixed flow was pressurized to 3.9 MPa by P1 and then was heated by the stream BURN-OUT. After the completion of the reaction, the generated water and gas were separated, respectively. Gas was partly fed into the burner, and the liquid product was heated in turn by stream 1, REA-OUT and HEATER.
When these heating steps finished, the temperature of flow H-OUT should be high enough to transform the liquid phase into vapor only, or otherwise some complications would occur[9]. Then the liquid was fed into the rectification tower by COMP. Four product streams can be obtained after separation, i.e., gas, gasoline, diesel and residue. Gas was discharged from the system, gasoline and diesel were used to pre-heat the flow of coal tar as the feedstock, while a part of residue was directly used to heat the solvent gasoline, with the rest used as the recycled flow after being cooled down by COOLER.
2.2.2 Property method
Property method is a decisive factor for a successful simulation study. Because the system included the reaction section and the separation section, and real components and pseudocomponents, as well as air system, it was crucial for each block to choose appropriate property method. Table 3 shows the property methods for some blocks. Property method for the rest adopted the one which was devised by Redlich-Kwong-Soave. The fact was that the simulation on the whole was a process involving hydrogen and hydrocarbons. The Soave-Redich-Kwong method, which was suitable for air system, was selected to study Blocks HEATEX1, HEATETX3 and BURNER; the Chao-Seader method, which was suitable for pseudocomponents, was selected to study HEATEX5; the Braun K-10 method, which was suited to both the separation of petroleum products and pseudocomponents, was selected as the property method to study blocks DIST, FLASH, HEATEX2 (cold side), HEATEX3 (cold side), HEATEX4 (hot side).
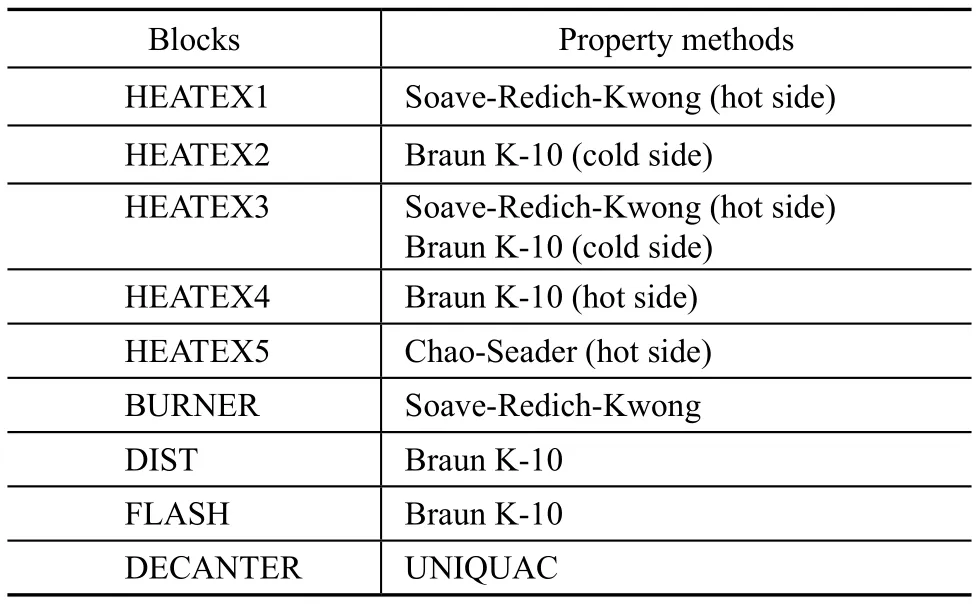
Table 3 Property methods for the relevant blocks
3 Results and Discussion
3.1 Stream analysis
Table 4 shows the simulated results. Coal tar was preheated in turn by gasoline and diesel streams, with its temperature increasing primarily from 25 ℃ to 57 ℃, and then to 188.9 ℃, showing that the diesel stream could through heat exchange increase the temperature of coal tar significantly. The temperature of solvent gasoline increased from 25 ℃ to 77.6 ℃ when it was heated by the residue. The stream’s temperature reached 161.3 ℃ when the two flows were mixed with the recycled residue, and then the stream was pressurized to 3.9 MPa.
After the stream REA-OUT finished the preliminary separation from the generated water and gas, the temperature and pressure of the stream LIQUID 1 dropped to 45 ℃ under a pressure of 0.2 MPa. The stream LIQUID 1 was heated by the stream 1 (the effluent stream from HEATEX1) and REA-OUT in succession before it was fed into the rectification tower, the temperature of which was primarily increased to 95 ℃, and then to 318 ℃. Because the stream H2-OUT contained two phases (mainly gas phase), its temperature should be increased before it was pressurized by block COMP. In fact, the stream H2-OUT should be heated to 390 ℃ which could transform the liquid phase into vapor only.
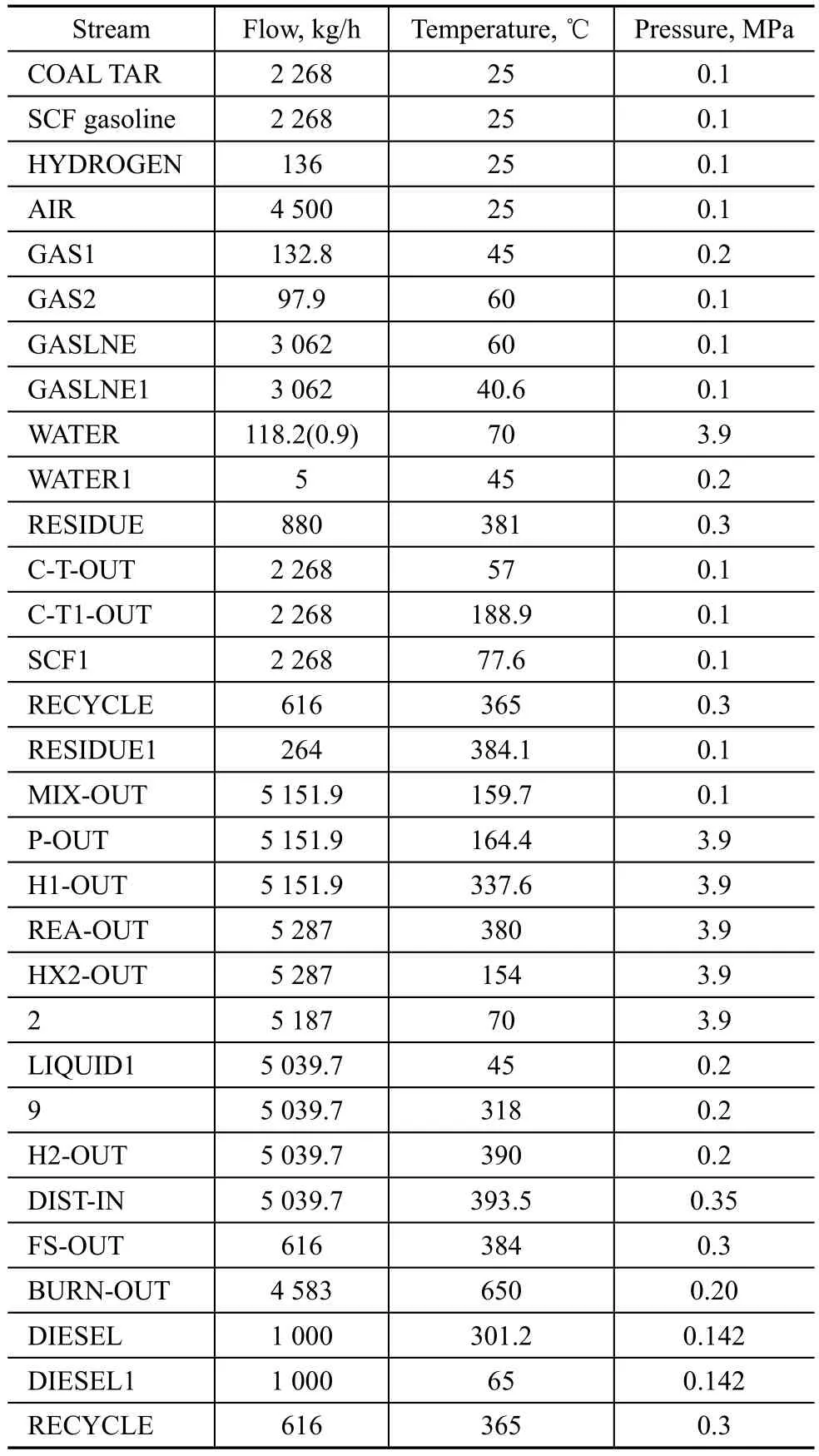
Table 4 Flowrate, temperature and pressure of streams referred to in Figure 2
3.2 Mass balance for each component and rectification column
Table 5 presents the mass balance for each component, which was computed by means of the following formula: Input=Output-Generation+ Relative error
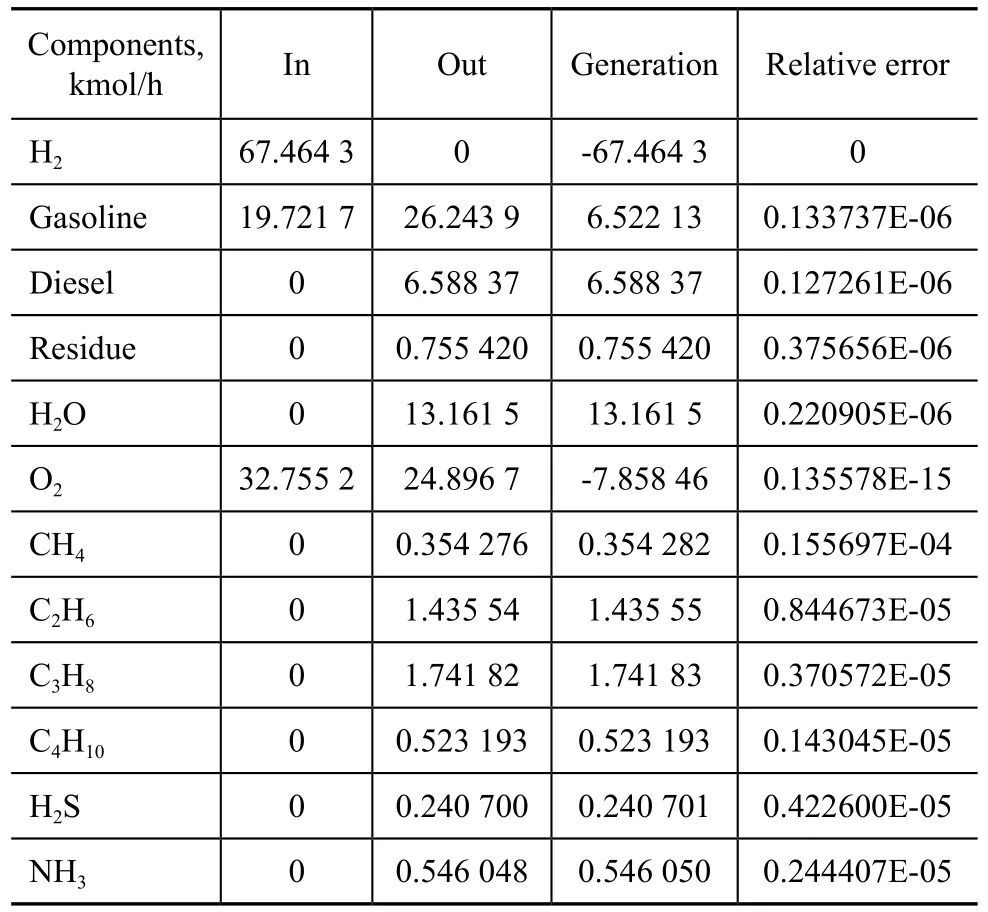
Table 5 Mass balance for each component
3.3 Temperature control
3.3.1 Discharge temperature of block COOLER
It was worth mentioning that the discharge temperature of the block COOLER should be optimized. This is because the energy of stream FS-OUT could not be utilized adequately if the temperature was too low, and conversely, some complications would appear, i.e., the vapor fraction of recycled residue would be higher than 0.01 (e.g., when its temperature was 380 ℃). So, we adopted the temperature to be 365 ℃ in this simulation study. The degree of energy recovery could be re flected by the temperature of stream MIX-OUT. The temperature change of the stream MIX-OUT along with the change in discharge temperature of the block COOLER is shown in Figure 3, implying that the temperature of the stream MIX-OUT would increase by about 2 ℃ when the discharge temperature of the block COOLER increased by 15 ℃.
3.3.2 Temperature control of reactor in-feed stream
The temperature of reactor inlet stream H1-OUT should be controlled strictly. This is because, on one hand, the temperature should be higher than the critical temperature of gasoline (316 ℃), on the other hand, the reactor net duty would increase dramatically coupled with the temperature increase of stream H1-OUT. The change of reactor net-duty coupled with the change in temperature of stream H1-OUT is presented in Table 6, indicating that the reactor net duty would increase about -95 kW·h when the temperature of stream H1-OUT increased 20 ℃. The temperature of the stream H1-OUT could be controlled by gas and air flowrate, which was 81 kg/h and 4 500 kg/h, respectively, in this simulation study. In fact, the temperature of stream H1-OUT would increase 15 ℃when air flowrate increased by every 500 kg/h, implying that the reactor net duty would increase dramatically with an increasing air flowrate. However, the net duty of the block HEATER would decrease 20 kW·h, when the AIR flowrate increased by every 500 kg/h, as discussed in part 3.5.1 of this paper.
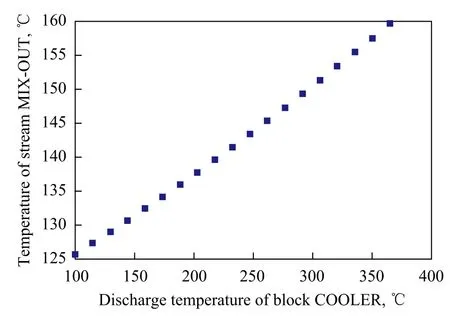
Figure 3 The temperature change of the stream MIX-OUT along with the change in discharge temperature of the block COOLER
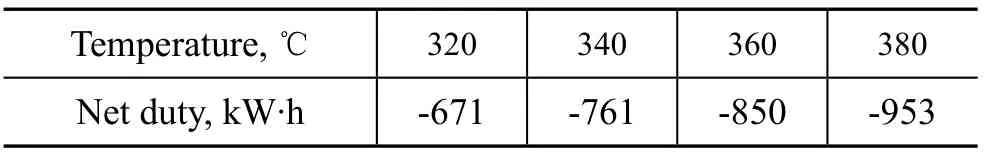
Table 6 The influence of temperature of reactor inlet stream H1-OUT on reactor net duty
3.4 Rectification column
Table 7 shows the operating parameters of rectification column and the corresponding calculated results, implying that, on one hand, the separation effect was very good; and on the other hand, the net duty of bottom reboiler and stripper reboiler was lower.
3.5 Energy analysis
3.5.1 Net heat
The endothermal blocks included the rectification column bottom reboiler (Reb), the stripper reboiler (Str-reb) and HEATER; and the work blocks covered P1, P2 and COMP.
The block HEATER needed a maximum net heat duty of 317 kW·h. The net heat duty of HEATER could be adjusted by the incoming air flowrate of the burner, because when the air flowrate increased, the energy of the burner’s effluent stream would increase also, and thus the temperature of stream 9 increased too. The detail is shown in Figure 4, implying that the net duty would decrease by 20 kW·h when AIR flowrate increased by 500 kg/h. However, the increase of AIR flowrate would result in the increase in temperature of stream H1-OUT, and thus the net duty of reactor would increase. So, we set an intermediate flowrate of 4 500 kg/h in this simulation study.
The net heat duty of Reb and Str-reb was 100 kW·h and 160 kW·h, respectively. The total positive net heat duty was 609 kW·h as shown in Table 8, indicating that the plant would need a positive net heat duty of 609 kW·h for processing low temperature coal tar with a flowrate of 2 268 kg/h.
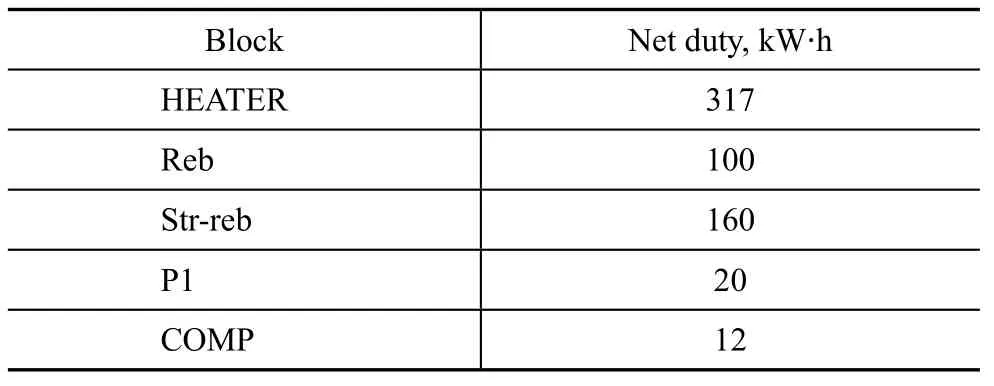
Table 8 Net duty of endothermal block and work block
3.5.2 Energy recovery
There were six heat exchangers in the flow sheet with a total energy recovery of 2 229 kW·h. Figure 5 shows the detail. The energy recovery in HEATEX 1 and HEATEX 2 was higher, which was 680 kW·h and 1116 kW·h, respectively. The total energy recovery by HEATEX 1 and HEATEX 3 was 845 kW·h. The total energy recovery by HEATEX 4, HEATEX 5 and HEATEX 6 was 272kW·h. The energy recovery from hot stream of REA-OUT was 1116 kW·h.
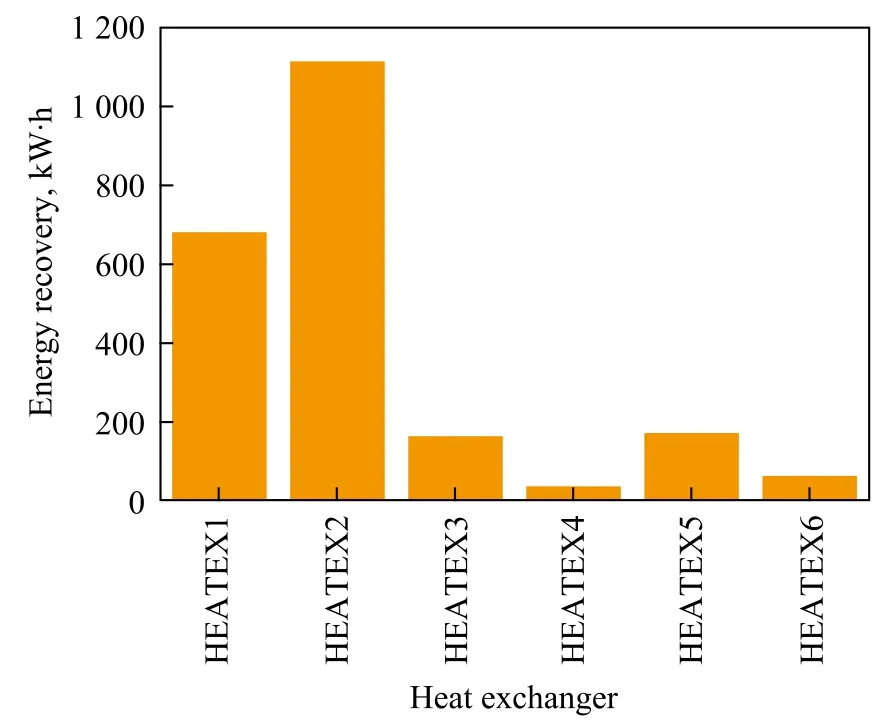
Figure 5 Energy recovery in each heat exchange block
4 Conclusions
The process, on one hand, well realized coal tar hydrocracking in supercritical gasoline and products separation, and on the other hand, probed into the possibility of en-ergy self-sustainability.
Heat consumption case was also analyzed in this paper, indicating that the main heat consumption blocks included the rectification column and HEATER, the net duty of which was 260 kW·h and 316 kW·h, respectively. The total positive net duty of the whole system was 609 kW·h, namely, 609 kW·h of power was needed for processing the low temperature coal tar with a flowrate of 2 268 kg/ h. The total energy recovery of the heat exchange system was 2 229 kW·h, in which 845 kW·h was obtained via the gas combustion, and 1 116 kW·h was originated from the reactor’s outlet stream REA-OUT.
Acknowledgement:We gratefully acknowledge the financial support from the National Natural Science Foundation of China (No. 2117619) and the Shaanxi Province Major Project of Innovation of Science and Technology (No. 2008zkc03205, No. 2011KTZB03-03-01 ).
[1] Ma B Q, Ren P J, Yang Z B, et al. Preparation of Fuel Oil from Coal Tar[M]. Beijing: Chemical Industry Press, 2010 (in Chinese)
[2] Wang S D, Guo S C. Study of structure and components of coal tar from Shenfu coal[J]. Journal of Fuel Chemistry and Technology, 1995, 28(1): 188-204 (in Chinese)
[3] Viet T T, Lee J H, Ryu J W, et al. Hydrocracking of vacuum residue with activated carbon in supercritical hydrocarbon solvents[J]. Fuel, 2012, 94: 556-562
[4] Viet T T, Lee J H, Ma F, et al. Hydrocracking of petroleum vacuum residue with activated carbon and metal additives in a supercritical m-xylene solvent[J]. Fuel, 2013, 103: 553-561
[5] Li W, Pan C Y, Sheng L, et al. Upgrading of high-boiling fraction of bio-oil in supercritical methanol[J]. Bioresource Technology, 2011, 102(19): 9223-9228
[6] Han L N, Zhang R, Bi J C. Reaction property of coal tar and its fractions in supercritical water[J]. Journal of Fuel Chemistry and Technology. 2008, 36(1): 1-5
[7] Chang N, Gu Z L, Wang Z S, et al. Study of Y zeolite catalysts for coal tar hydro-cracking in supercritical gasoline[J]. Journal of Porous Materials, 2011, 18(5): 589-596
[8] Gu Z L, Chang N, Hou X P, et al. Experimental study on the coal tar hydrocracking process in supercritical solvents[J]. Fuel, 2012, 91: 33-39
[9] Luca F, Michele V, Daniele C. Supercritical water gasification of biomass for H2production: Process design[J]. Bioresource Technology, 2012, 121: 139-147
[10] Kruse A, Vogel H. Heterogeneous catalysis in supercritical media: 2. Near-critical and supercritical water[J]. Chemical Engineering & Technology, 2008, 31(9): 1241-1245
[11] Kruse A, Vogel H. Heterogeneous catalysis in supercritical media: 3. Other media[J]. Chemical Engineering & Technology, 2008, 31(10): 1391-1395
[12] Xu C, Hamilton S, Mallik A, et al. Upgrading of Athabasca vacuum tower bottoms (VTB) in supercritical hydrocarbon solvents with activated carbon-supported metallic catalysts[J]. Energy & Fuels, 2007, 21(6): 3490-3498
[13] Pang L. Mathematical simulation of the reaction process of F-T wax hydrocracking[D]. Shanghai: East China University of Science and Technology, 2011 (in Chinese)
Recieved date: 2013-06-09; Accepted date: 2013-09-25.
Liu Zongkuan, E-mail: zkliu@mail. xjtu.edu.cn.
- 中國煉油與石油化工的其它文章
- Study on Preparation of Waterproofing Agent for Mineral Wool Board from Modified C9Petroleum Resin
- Catalytic Dehydration of 4-Hydroxy-3-hexanone to 4-Hexen-3-one over HZSM-5 Zeolite
- Diffusion and Reaction Model of Catalyst Pellets for Fischer-Tropsch Synthesis
- Catalytic Cracking and PSO-RBF Neural Network Model of FCC Cycle Oil
- Effect of Impregnation Sequence on Propane Dehydrogenation Performance of PtSnNa/ZSM-5 Catalyst
- Influence of Synthesis Parameters with Low Seed Addition on the Crystallinity of ZSM-5

