Catalytic Dehydration of 4-Hydroxy-3-hexanone to 4-Hexen-3-one over HZSM-5 Zeolite
Huang Kai; Zheng Haitao; Tao Keyi
(1. School of Enνironmental and Chemical Engineering, Tianjin Polytechnic Uniνersity, Tianjin 300160; 2. Institute of New Catalytic Materials Science, College of Chemistry, Nankai Uniνersity, Tianjin 300071)
Catalytic Dehydration of 4-Hydroxy-3-hexanone to 4-Hexen-3-one over HZSM-5 Zeolite
Huang Kai1; Zheng Haitao1; Tao Keyi2
(1. School of Enνironmental and Chemical Engineering, Tianjin Polytechnic Uniνersity, Tianjin 300160; 2. Institute of New Catalytic Materials Science, College of Chemistry, Nankai Uniνersity, Tianjin 300071)
A study on catalytic dehydration of 4-hydroxy-3-hexanone (HH) to 4-hexen-3-one (HO) was carried out through conversion of HH over HZSM-5 zeolite catalyst in a fixed-bed reactor (FBR) operating under atmospheric pressure. The test indicated a relatively high activity of the HZSM-5 zeolite capable of achieving a HH conversion of 99.2% and a HO yield of 83.5%. Catalyst deactivation could be prevented by increasing the reaction temperature by 10 ℃ for every 20 h and adding 2.0% of piperidine in the feed. A catalyst stability test (for 100 h) in FBR showed that the catalyst was active even after 100 h of time-on-stream with HH conversion remaining at 99.2% and HO yield still reaching over 83.5%. Regeneration experiment showed that the regenerated catalyst demonstrated a catalytic performance comparable to the fresh one.
HZSM-5; dehydration; 4-hydroxy-3-hexanone; deactivation; regeneration
1 Introduction
According to international standards, 4-hexen-3-one (HO) is a feedstock of important food additives, an edible perfume and a kind of perfume with fruity fragrance, and is widely used as a component of edible flavor and cosmetic fragrance as well as a pharmaceutical intermediate[1-2]. Therefore, it can find an extremely wide application in the field of chemical industry and fine chemicals. To our knowledge, the similar catalytic dehydration of 4-hydroxy-3-hexanone (HH) over the HZSM-5 zeolite was not reported before. So, it is necessary to develop a catalytic system for affording an economical and environmentally friendly technology with high catalytic performance to meet the industrial demand for HO.
In this paper, HH, also named as propionoin, which was used as a starting material, was dehydrated to produce HO in a fixed-bed continuous-flow reactor. Our previous research indicated that the HZSM-5 zeolite was an alternative catalyst for the dehydration of HH among several acidic zeolite catalysts, which exhibited the highest conversion of HH and selectivity of HO. Moreover, the effects of reaction conditions, such as temperature, space velocity, and addition of organic base on catalytic activity of the HZSM-5 catalyst were investigated. The stability of catalyst was also studied, and the test result showed that the catalyst deactivation out of carbon deposition on the HZSM-5 zeolite occurred during the dehydration process. The catalytic activity of deactivated catalyst could be recovered via regeneration process.
2 Experimental
Commercial reagents were all used as received without further purification.
The NaZSM-5 zeolite was prepared by a hydrothermal crystallization method according to the method referred to in the previous literature[3]. The molar ratio of SiO2/Al2O3was 26. By extruding a mixture consisting of 80 % of the nanoscale NaZSM-5 zeolite and 20 % of γ-alumina, a strip form of nanoscale NaZSM-5 catalyst (1 mm×2 mm) was obtained. After the NaZSM-5 catalyst had been dried at 120 ℃ for 12 h, it was then calcined at 540 ℃ for 3 h, followed by ion exchange for three times with 0.3 mol/L of NH4NO3, washing with deionized water, drying at 120 ℃for 6 h, and then being subjected to calcination in a flowing air at 550 ℃ for 5 h. The resulted HZSM-5 powder waspressed under 20 MPa to form pellets which were crushed and sieved to particles of 20—40 mesh for the evaluation of catalytic performance.
Dehydration of HH was carried out in a fixed-bed continuous-flow reactor which has been described elsewhere[4]. A FBR (made of stainless steel tube, 10 mm in inside diameter) was used for reaction tests. 6.5 g of catalyst was sieved to the particle size of 20—40 mesh and put in the reactor. The feed was quantified and preheated before entering the reactor. The exit gas passed through a trap for collecting liquid products. During the mass balance period (usually 14 h), the liquid products were analyzed by an on-line gas chromatograph using a silicone rubber (SE-54) capillary column and one step programmed temperature (65—220 ℃) for every 2 h.
During the dehydration process of HH, three isomers apart from HO may be produced according to the structure of raw material, which cover ethyl cyclopropyl ketone, 2-methyl-1-amylene-3-ketone and 5-hexene-3-ketone. The reaction equation is described as shown in Scheme 1. Ethyl cyclopropyl ketone and 5-hexene-3-ketone are difficult to be formed because of limitation of thermodynamics. As 2-methyl-1-amylene-3-ketone and ethyl cyclopropyl ketone can be used as the feed for preparation of oak moss, the total target products consist of 2-methyl-1-amylene-3-ketone and HO.
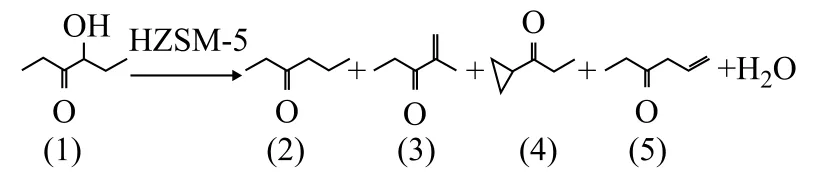
Scheme 1 Reaction for conversion of HH on HZSM-5 catalyst
3 Results and Discussion
In our study, the dehydration of HH (with a purity of>94.6%) to HO was investigated over various solid acid catalysts, such as SAPO-11, HZSM-5 and silica-alumina at temperatures ranging from 320 ℃ to 420 ℃ under atmospheric pressure. HZSM-5 zeolite was the most active for catalytic dehydration based on the test results, which exhibited relatively high activity with a HH conversion of 80.0%—90.0% and a HO selectivity of 50.0%—60.0% at a temperature of 380 ℃, a pressure of 0.1 MPa and a space velocity of 1.0 h-1. Besides, the deactivation rate of the HZSM-5 zeolite was lower compared with other acid catalysts. In our previous work, we found out that the catalyst was continuously deactivated, which led to a loss of activity in the course of reaction. The catalyst deactivation is caused by the coke formation on the catalyst surface, which blocked the micropores of zeolite[5]. It is also suggested that the coke formation could be correlated with the amount of strong Br?nsted acid sites over the catalyst[6]. The stronger the acid sites are, the more serious the carbonization would be. The cause of carbon deposition is mainly ascribed to polymerization of products containing C=C bonds over acid sites. A part of polymerized products covers the active sites of the catalyst which leads to the deactivation of catalyst, and the remainder will be collected as by-product and will form the high-boiling residue (HBR) in the final product. The high content of HBR will increase the difficulty of purification during the subsequent separation of crude products.
3.1 Effects of reaction temperature
Figure 1 shows the dependence of conversion, yield and HBR on reaction temperature over the HZSM-5 zeolite for dehydration of HH at a liquid hourly space velocity (LHSV) of 2.25 h-1. It is well known that the increase of temperature accelerates the reaction rate, and the high temperature contributes to mass transfer. The conversion of HH remains constant (99.9%) when the reaction temperature is over 380 ℃, while the yield of all target products has a slight increase from 76.7% to 80.6%, and the content of HBR decreases from 14.5% to 8.70% with the temperature increasing from 380 ℃ to 420 ℃, as shown in Figure 1.

Figure 1 Dependence of conversion, yield and HBR on reaction temperature over HZSM-5
3.2 Effect of space velocity
Figure 2 shows the effect of space velocity on catalytic activity. It can be seen from Figure 2 that with LHSV increasing from 1.5 h-1to 2.5 h-1at a reaction temperature of 400 ℃, the content of HBR sharply dropped from 30.2% to 6.40%. Meanwhile, the yield of HO increased from 44.2% to 82.9%. Space velocity almost had no influence on conversion, which was close to 99.9% under the present conditions. It means that dehydration reaction of HH over the HZSM-5 zeolite achieved a high reaction rate, and the effect of diffusion had little limitation on the reaction. Meanwhile, the polymerization of olefins was limited due to the low contact time, resulting in a decreasing content of HBR with the increase in the LHSV. Then the yield of target product was also enhanced. We also connected the reactor with a vacuum pump after the products had been condensed in a condenser in order to further decrease the contact time between the raw material and catalyst. The reaction results showed that conversion of HH decreased from 99.9% to 92.2% because of the reduction of contact time. However, no noticeable decrease in the content of HBR was detected. Based on the above experiments, the reasonable LHSV should be 2.5 h-1.
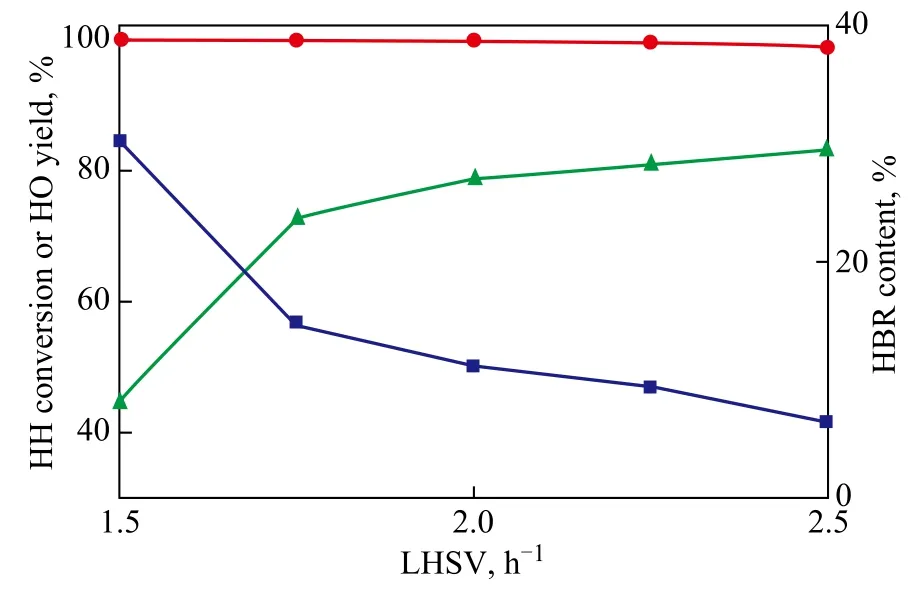
Figure 2 Effect of space velocity on the catalytic activity (at 400℃)
3.3 Effect of addition of piperidine in feed
During the dehydration process of keto-alcohol, the coke deposition and formation of HBR can be ascribed to acid strength of HZSM-5. It is well known that the carbonaceous accumulation deposited on the catalyst is one of the main causes of catalyst deactivation[7-9], and olefin can easily polymerize on the strong Br?nsted acid sites of HZSM-5 zeolite[10]. The nature of acid sites may be defined by the presence of surface protons, leading to the Br?nsted acid sites, or cationic centers arising out of unsaturation in coordination, which is explained as Lewis acidity. Piperidine is a strong base which will be adsorbed on all types of acid centers and can be used as a probe molecule to characterize total acidity of the samples[11]. Therefore, HH mixed with 2.0% of piperidine was used as the feed material, and this small amount of piperidine would be competitively adsorbed with HH over the surface acid sites, which may partly destroy the environment of polymerization and decreased the content of HBR. The experimental result of dehydration reaction is shown in Figure 3. The addition of piperidine in the feed slightly decreased the conversion of HH and yield of HO, meanwhile the content of HBR was also decreased. Especially, the HBR content obviously decreased at the reaction temperatures ranging from 390 ℃ to 410 ℃. At a higher reaction temperature, the decrease of HBR content was not notable. Although piperidine would decrease the yield of target product and increase the cost on separation of products due to the introduction of organic base, the addition of piperidine could extend the life of catalyst.
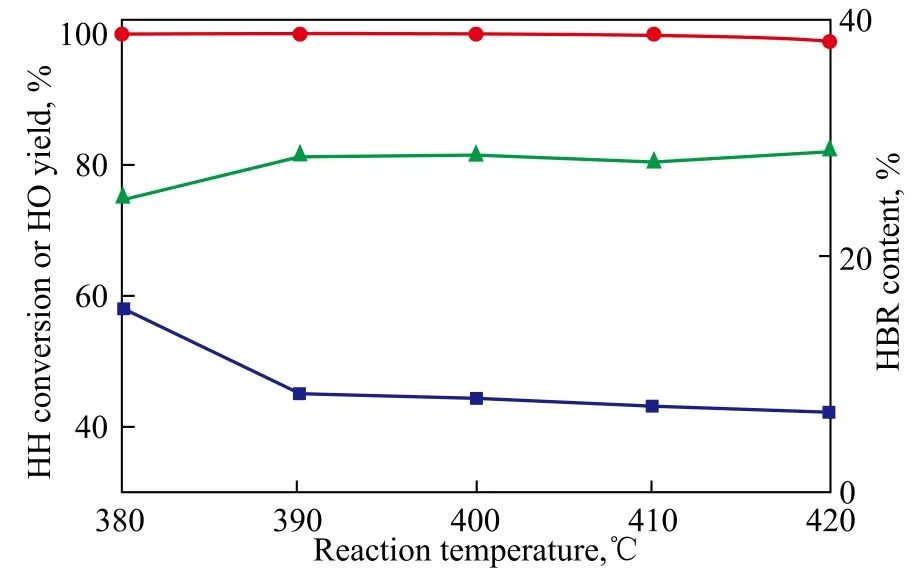
Figure 3 Effects of addition piperidine in feed (at a space velocity of 2.50 h-1
3.4 Catalyst stability test in a fixed bed reactor
Long-duration evaluation test was carried out to investigate the stability of catalyst and its performance. Figure 4 presents the reaction activity and HBR content with time-on-stream over the HZSM-5 catalyst. It can be seen form Figure 4 that conversion of HH reached 99.9% at an initial temperature of 368 ℃, verifying a high initial activity of the catalyst. However, the catalyst lost a part of its activity with the conversion of HH dropping from 99.7% to 88.4% after running for 25 h, indicating to thedeactivation of catalyst. However, the catalyst activity became stable when the reaction temperature increased by 10 ℃. The conversion maintained between 85.0% and 95.0% for another 80 h test. In order to keep the catalytic stability, the reaction temperature should be increased by 10 ℃ for every 20 h continuously, so that the catalyst activity could be recovered after a 10 ℃increase in reaction temperature, and there was no indication of further catalyst deactivation at the end of the test.
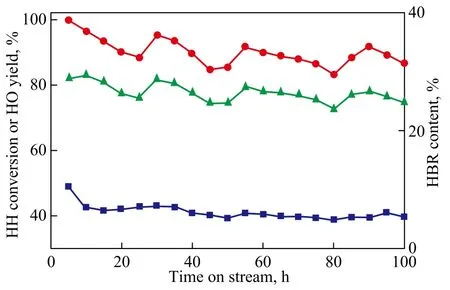
Figure 4 Consecutive test with time-on-stream (at a space velocity of 2.50 h-1, a temperature in the range of 368—450℃, and a pressure of 0.1 MPa)
3.5 Comparison of catalytic performance between fresh catalyst and regenerated catalyst
After the stability testing of catalyst for 100 h, the used catalyst was regenerated by calcining at 550 ℃ for 5 h. A comparison of catalytic performance between fresh catalyst and regenerated catalyst was investigated under the same reaction conditions as shown Figure 5. It can be seen from the results that little difference in the catalyst activity was detected before and after catalyst regeneration, denoting that the coke formation was the major reason leading to catalyst deactivation. So ex-situ regeneration of deactivated catalyst can recover the activity of catalyst, which will further decrease the cost of production. The product which was obtained through catalytic dehydration of HH should be fractionated immediately in order to avoid its polymerization after a long-time retention. A few Na2CO3was added into the product to adjust the solution pH value to be close to 7 (neutral), and then the product was distilled after having been mixed with a definite amount of triethanolamine (TEA) and butylated hydroxytoluene (BHT). The distilled product was collected and analyzed by the gas chromatograph, and the result showed that a total of 98.2% of target product was obtained.
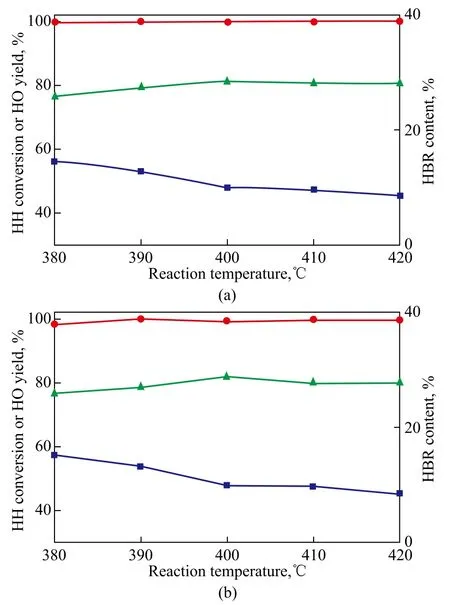
Figure 5 Comparison of catalyst performances between fresh catalyst (a) and regenerated catalyst (b) (at a space velocity of 2.50 h-1, a temperature in the range of 380—420℃, and a pressure of 0.1 MPa)
4 Conclusions
In summary, catalytic dehydration of HH to HO over HZSM-5 zeolite was a feasible method to produce HO in high yield, the conversion of HH could reach as high as 99.9%, the HBR content was as low as 10.0% and the target product yield was above 80.0% with its final purity reaching 98.2% after refining when the reaction was conducted at a temperature of 380—430 ℃, a space velocity of 2.50 h-1, and a reaction pressure of 0.1 MPa. Stability test (for 100 h) showed that the catalyst was active even after 100 h of time-on-stream with the HH conversion equating to 99.2% and the HO yield exceeding 83.5%. Deactivation of catalyst could be prevented by increasing the reaction temperature by 10 ℃ for every 20 h and adding 2.0% of piperidine in the feed. Regeneration experiment showed that regenerated catalyst had a similar catalytic perfor-mance compared with the fresh one. It showed that the exsitu regeneration of deactivated catalyst could recover the activity of catalyst. It was a potential method for dehydration of HH to produce HO with high yield of target product obtained under relatively mild reaction conditions.
Acknowledgement:This work was supported by the Science and Technology Planning Program of Tianjin (Project No. 12ZXCXGX21900).
[1] Susua C, Smolinske. CRC Handbook of Food, Drug, and Cosmetic Excipients[M]. Detroit: CRC Press, 1992, 952.
[2] Branen A L, Davidson P M, Salminen S, et al. Food Additives[M]. New York: Taylor & Francis, 1990, 736.
[3] Zhao X B, Guo X W, Wang X S. Characterization of modified nanoscale ZSM-5 catalyst and its application in FCC gasoline upgrading process[J]. Energy and Fuels, 2006, 20(4): 1388-1391
[4] Jiang S, Hwang J S, Jin T, et al. Dehydration of methanol to dimethyl ether over ZSM-5 zeolite[J]. Bulletin of the Korean Chemical Society, 2004, 25(2): 185-189
[5] Zhou L W, Wang F, Luo M, et al. Deactivation and regeneration of ZSM-5 zeolite catalysts for ethanol dehydration[J]. Petrochemical Technology, 2008, 37(4): 333-337 (in Chinese)
[6] Mclellan G D, Howe R F, Parker L M, et al. Effects of coke formation on the acidity of ZSM-5[J]. Journal of Catalysis, 1986, 99(2): 486-491
[7] Aguayo A T, Gayubo A G, Atutxa A, et al. Catalyst deactivation by coke in the transformation of aqueous ethanol into hydrocarbons. Kinetic modeling and acidity deterioration of the catalyst[J]. Industrial and Engineering Chemistry Research, 2002, 41(17): 4216-4224
[8] Tabak S A, Yurchak S. Conversion of methanol over ZSM-5 to fuels and chemicals[J]. Catalysis Today, 1990, 6(3): 307-327
[9] Phillips C B, Datta R. Production of ethylene from hydrous ethanol on H-ZSM-5 under mild conditions[J]. Industrial and Engineering Chemistry Research, 1997, 36(11): 4466-4475
[10] Guisnet M, Magnoux P. Deactivation by coking of zeolite catalysts. Prevention of deactivation. Optimal conditions for regeneration[J]. Catalyst Today, 1997, 36(4): 477-483
[11] Das D, Mishra H K, Parida K M, et al. Preparation, physico-chemical characterization and catalytic activity of sulphated ZrO2–TiO2mixed oxides[J]. Journal of Molecular Catalysis A: Chemical, 2002, 189(2): 271-282
Recieved date: 2013-07-24; Accepted date: 2013-08-19.
Professor Zheng Haitao, Telephone: +86-22-83955662; E-mail: zhenght999@hotmail.com.
- 中國煉油與石油化工的其它文章
- Study on Preparation of Waterproofing Agent for Mineral Wool Board from Modified C9Petroleum Resin
- Diffusion and Reaction Model of Catalyst Pellets for Fischer-Tropsch Synthesis
- Simulation of Low-Temperature Coal Tar Hydrocracking in Supercritical Gasoline
- Catalytic Cracking and PSO-RBF Neural Network Model of FCC Cycle Oil
- Effect of Impregnation Sequence on Propane Dehydrogenation Performance of PtSnNa/ZSM-5 Catalyst
- Influence of Synthesis Parameters with Low Seed Addition on the Crystallinity of ZSM-5

