LC-MS/MS determination and pharmacokinetic study of bergenin, the main bioactive component of Bergenia purpurascens after oral administration in rats
Bo-Hong Li, Jin-Dong Wu, Xing-Lu Li
aSchool of Pharmacy, Guangdong Medical College, Dongguan 523808, PR China
bNature's & US Limited Liability Corporation, St. Louis 63132, USA
1. Introduction
Yanbaicai,the rhizome of Bergenia purpurascens,is a well-known and widely used traditional Chinese medicine (TCM) for relief against cough and asthma in Chinese folk [1,2]. Biological and pharmacological studies had shown that B. purpurascens extract showed antioxidant, anti-inflammatory and immunologic enhancement activities [3-5]. Phytochemical investigation indicated that more than 20 compounds had been identified from the plant B. purpurascens, in which bergenin was the principal compound contributing to the biological activity[6-9].Bergenin,a C-glucoside of 4-O-methyl gallic acid isolated from B. purpurascens has a variety of pharmacological activities, including antiarrhythmic, antihepatotoxic, antiulcerogenic, anti-inflammatory,anti-HIV,antimicrobial,immunomodulatory and neuroprotective properties [10-13]. To date, there is no report about the pharmacokinetics of the B. purpurascens extract in vivo, even though several reports for pharmacokinetics have estimated the concentration of bergenin after oral administration of bergenin tablets by LC-MS/MS [14,15]. Compared with the published papers [14,15], in the present study, we have developed a much more sensitive (LLOQ, 1.00 ng/mL) and rapid (running time,3.5 min) LC-MS/MS method to measure bergenin in rat plasma for the first time after oral administration of aqueous B.purpurascens extract.
2. Materials and methods
2.1. Chemicals and reagents
Bergenin (purity>99.0%, determined by HPLC) was isolated from B. purpurascens in our laboratory. The structure of bergenin (Fig.1A) was fully characterized by1H,13C NMR and MS data (seeing the supporting information, Table S1).Isovitexin (purity>98%, determined by HPLC, Fig.1B) was used as an internal standard (IS), which was isolated from the leaves of Santalum album L. and characterized by NMR technique (seeing the supporting information, Table S1).HPLC-grade methanol and acetonitrile were purchased from Merck (Darmstadt, Germany). Deionized water was prepared by a Milli-Q system (Millipore, MA, USA).
2.2. Instrumentation
An Agilent 1200 LC system consisting of a degasser, a binary pump, an SL autosampler and a thermostatic column compartment was coupled with a 6410 triple-quadrupole mass spectrometer (Agilent Technologies, USA), which was equipped with an electrospray ionization (ESI) source and operated with Agilent MassHunter B.01.03 software (Agilent Technologies, USA).
2.3. Chromatographic and mass spectrometric conditions
Chromatographic separation was performed on a Diamonsil?C18column (150 mm×4.6 mm, 5 μm; Beijing, China) protected by a Phenomenex C18guard column (Torrance, CA, USA).The mobile phase consisted of water(A)and methanol(B)using an isocratic elution with a mixture of 30 volumes of solvent A and 70 volumes of solvent B.The flow rate was 0.6 mL/min,and the injection volume was 20 μL. The time for each analysis run was only 3.5 min,and a divert valve was used to divert the eluent to waste from 0 to 1.9 min,and to MS from 1.9 min to 3.5 min.
The mass spectrometer was operated in negative ESI scan mode for bergenin.Quantification was obtained using multiplereaction monitoring (MRM) transition, which was selected as m/z 327.3→m/z 192.0 for bergenin and m/z 431.1→m/z 311.1 for IS (Fig.2). MS/MS conditions were optimized as follows:gas temperature, 300°C; gas flow, 10 L/min; nebulizer, 30 psi;collision gas (N2), 0.35 Pa. The fragment energy was set at 110 V and 160 V for the analyte and IS, respectively. The collision energy was optimized to be 15 eV and 18 eV for the analyte and IS, respectively.
2.4. Preparation of B. purpurascens extract
Ten grams B. purpurascens shattered into powder was extracted three times with 50% ethanol under reflux. After the removal of the major solvent in vacuum,the aqueous residue was then diluted with water to get the B.purpurascens extract with a concentration equivalent to 1.58 g/mL of the raw B. purpurascens material.
To calculate the administration dose, the content of bergenin in aqueous B.purpurascens extract was quantitatively determined using the LC-MS/MS method described above.The content of bergenin in the extract was 16.3 mg/mL.
2.5. Preparation of standard and quality control (QC)samples
Standard stock solution of 100 μg/mL bergenin was accurately prepared by dissolving appropriate amounts of the standard in acetonitrile. A 1000 ng/mL isovitexin solution as IS was also prepared in acetonitrile. All solutions were stored at 4°C.
Calibration standards were prepared by spiking appropriate amount of the standard solutions in 100 μL of blank rat plasma to yield final concentrations of 1.00, 3.00, 10.0, 30.0,100, 300, 1000, and 2000 ng/mL for bergenin. Three concentration levels of QC samples were prepared containing bergenin (3.00, 30.0, 1800 ng/mL) in the same manner.
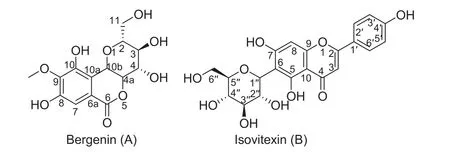
Fig.1 Chemical structure of bergenin and isovitexin (IS).

Fig.2 MS and MS/MS spectra of (A) [M-H]- at m/z 327.3 of bergenin and (B) [M-H]- at m/z 431.1 of isovitexin (IS).
2.6. Sample preparation
100 μL of rat plasma samples (Calibration standards, QC samples, and pharmacokinetic plasma samples) was mixed with 100 μL of acetonitrile and 100 μL of IS working solution.The mixture was vortexed for 1 min and then centrifuged at 10,000 rpm for 5 min. Then about 100 μL of the supernatant was transferred to the autosampler vials ready for injection into the LC-MS/MS system.
2.7. Method validation
The method performance was validated according to the FDA recommendations proposed in 2001 [16,17]. Several validation characteristics were investigated, such as selectivity, linearity,lower limit of quantification (LLOQ), precision, accuracy,matrix effect, extraction recovery and stability.
2.8. Pharmacokinetic study
Six male Wistar rats (200±20 g) were purchased from the Experimental Animal Center of Guangdong Medical College(Dongguan, China). The rats were housed in an airconditioned room at temperature of 22±2°C and a relative humidity of 50±10%with a 12 h dark-light cycle and allowed food and water spontaneously. Rats were fasted for 12 h before the experiment with water freely available.
After an oral administration of aqueous B. purpurascens extract at a dose of 6 mL/kg (equivalent 97.8 mg bergenin/kg body weight), blood samples were harvested into 1.5 mL heparinized eppendorf tubes from each rat via the orbital vein before dosing and at 0.083,0.25,0.50,0.75,1.0,1.5,2.0,3.0,5.0,8.0, 12.0 and 24.0 h. After centrifugation at 3000 rpm/min for 10 min,plasma samples were transferred to neat tubes and stored at -20°C until analysis. All pharmacokinetic parameters were evaluated by noncompartmental analysis using DAS 2.0 software(Chinese Pharmacological Society, China).
3. Results and discussion
3.1. Method development
In the preliminary experiment the ESI and atmospheric pressure chemical ionization (APCI) sources were chosen to obtain good and stable MS response. Bergenin and IS could be ionized under negative ESI or APCI conditions. It was found that ESI can provide higher sensitivity for the analyte and IS than that of APCI.
Different chromatographic conditions,especially the composition of mobile phase, were assessed to achieve good resolution and strong MS intensity, as well as short run time. Various combinations of acetonitrile, methanol and water were tested and compared to identify the appropriate mobile phase. As a result, a mixture consisting of water and methanol (30:70, v/v)was finally adopted as the mobile phase.Flow rate of 0.6 mL/min produced good peak shapes and short run time (3.5 min).
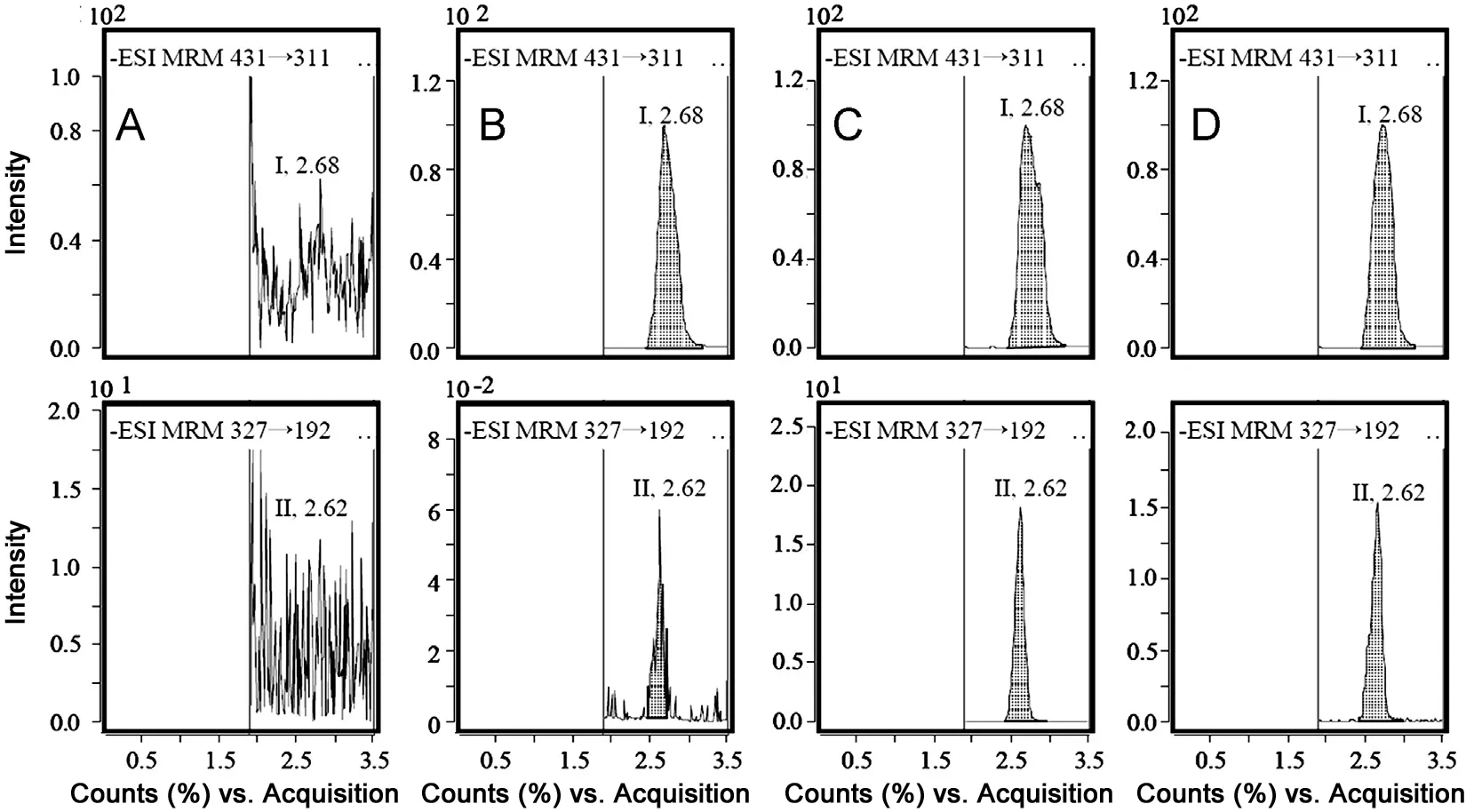
Fig.3 Typical MRM chromatograms of bergenin and isovitexin (IS) in rat plasma: (A) blank plasma; (B) plasma spiked with bergenin at 1.00 ng/mL and I.S. at 1000 ng/mL; (C) plasma spiked with bergenin at 2000 ng/mL and and I.S. at 1000 ng/mL; and (D) plasma at 0.75 h after oral administration of aqueous Bergenia purpurascens extract. Peaks I and II refer to IS and bergenin, respectively.
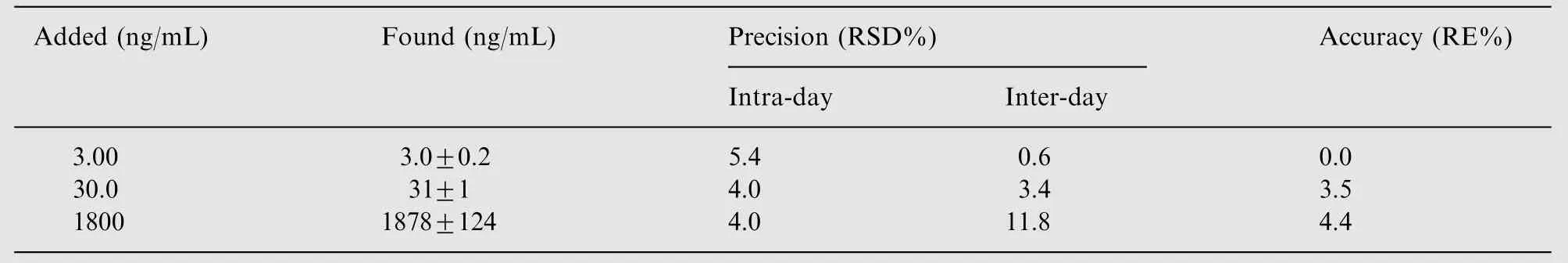
Table 1 Precision and accuracy of the LC-MS/MS assay for bergenin in rat plasma using isovitexin as IS(method validation for 3 days, mean±SD, n=6 for per day).
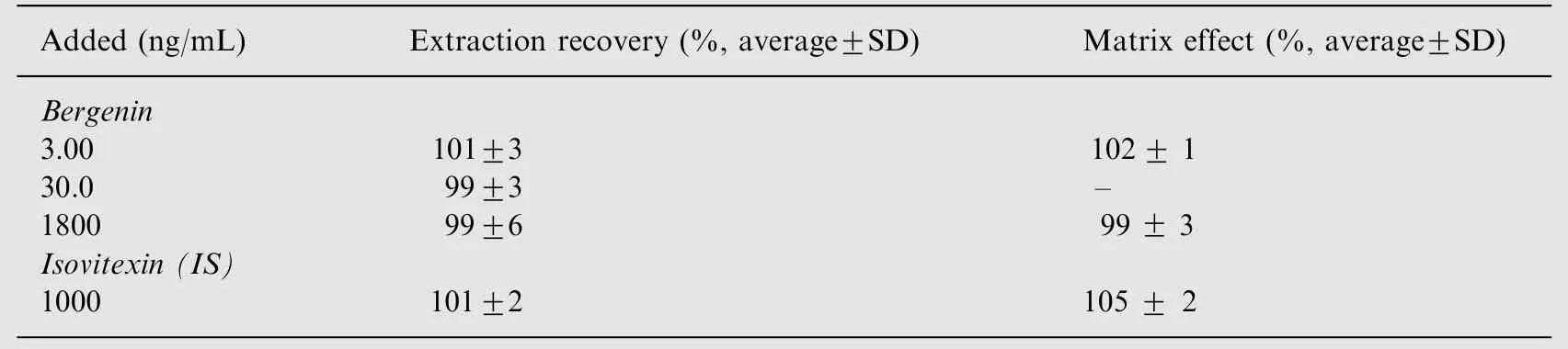
Table 2 Extraction recoveries and matrix effects of bergenin in rat plasma (n=3).
3.2. Assay validation
3.2.1. Selectivity
Under the chromatographic conditions described above, bergenin and IS were eluted at 2.62 and 2.68 min (Fig.3),respectively. No obvious interfering endogenous substances were observed at the retention times of bergenin and IS in blank plasma sample.
3.2.2. Linearity and LLOQ
The linear regressions of the peak area ratios versus concentrations were fitted over the concentration range 1.00-2000 ng/mL for bergenin in rat plasma.A typical equation of the calibration curve was y=3.98 ×10-5x+ 6.14×10-5, r2= 0.9936,where y represents the ratio of bergenin peak area to that of IS and x represents the plasma concentration.The LLOQ of bergenin in plasma was 1.00 ng/mL. The present method exhibited a good linearity and sensitivity.
3.2.3. Precision and accuracy
The intra- and inter-day precision and accuracy of bergenin are summarized in Table 1. In the present assay, the intra- andinter-day precision (RSD)were within 5.4% and 11.8%, respectively;the accuracy did not exceed 4.4%.The obtained data were within acceptable criteria and conformed to the guidelines for bioanalytical method validation [16], which indicated that the present method was reproducible and reliable for the determination of bergenin in rat plasma samples.

Table 3 Stability of bergenin in rat plasma in various conditions (n=3).
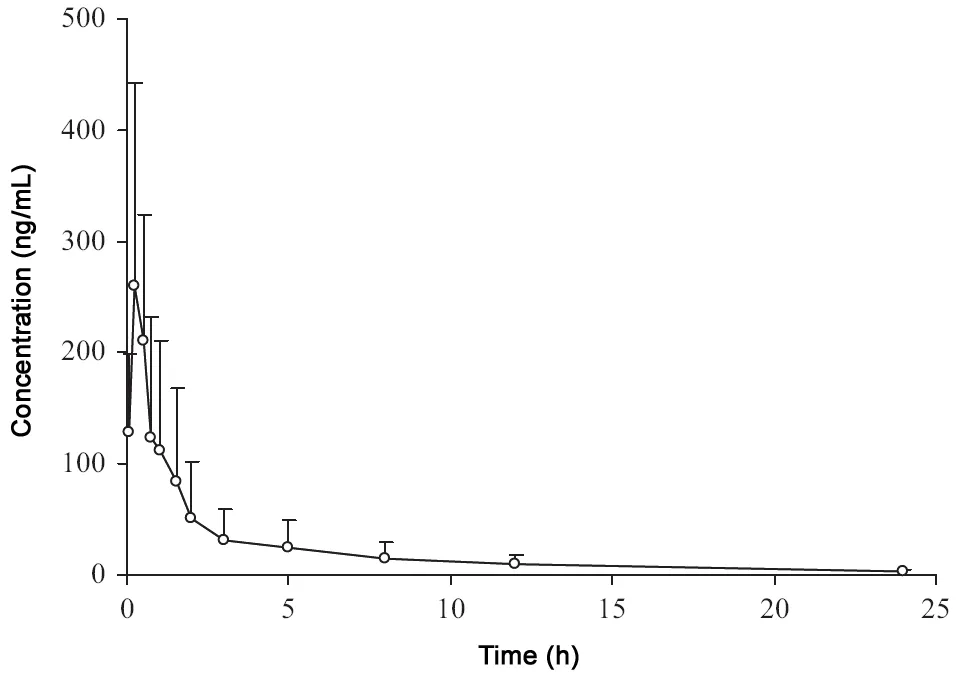
Fig.4 Mean plasma concentration-time profile of bergenin after oral administration of aqueous Bergenia purpurascens extract.
3.2.4. Matrix effect (ME)
The MEs for bergenin at concentrations of 3.00 and 1800 ng/mL were measured to be 102±1% and 99±3% (n = 3),respectively. The ME for IS was 105±2% (n=3). As a result, the ME from plasma was negligible in this method(Table 2).
3.2.5. Extraction recovery
The extraction recoveries of bergenin at three QC levels (3.00,30.0, 1800 ng/mL) were determined by comparing the peak area of the analyte to which the analyte was added postprotein precipitation at equivalent concentrations. The recovery of the IS (1000 ng/mL) was determined in a similar way.At three concentration levels of the analyte, the recoveries were between 99±6% and 101±3% (Table 2).
3.2.6. Stability
The freeze-thaw stability was determined after three freezethaw cycles at -20°C with a minimal interval of 24 h.The long-term stability was evaluated by analyzing samples kept at -20°C for 30 days. The short-term stability was assessed after keeping the samples at ambient condition for 2 h. Post-preparative stability was evaluated by analyzingpost-protein precipitation samples kept in the autosampler at ambient condition for 12 h. The results of the stability study are presented in Table 3, which confirm the high stability of bergenin throughout the determination.
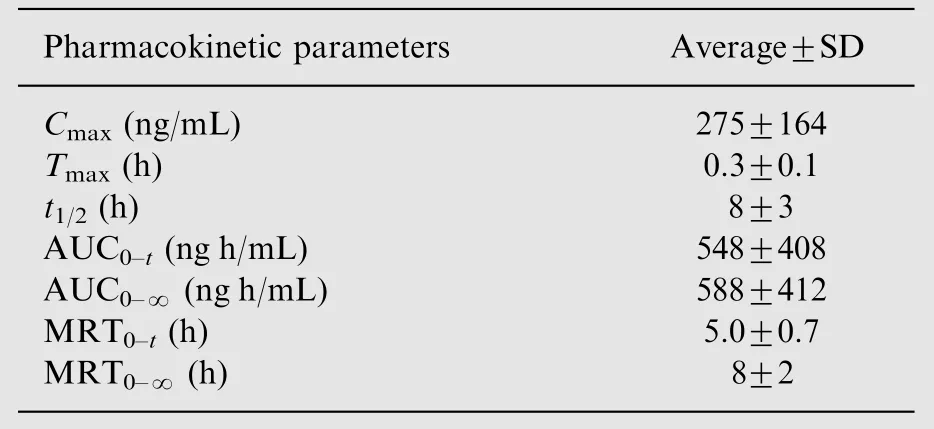
Table 4 Pharmacokinetic parameters of bergenin in rats after oral administration of Bergenia purpurascens extract(n=6).
3.3. Pharmacokinetics application
After oral administration of B. purpurascens extract at a dose of 6 mL/kg (equivalent 97.8 mg bergenin/kg body weight) to six Wistar rats, their plasma concentration of bergenin was determined by the described LC-MS/MS method. The mean plasma concentration-time profile is represented in Fig.4 and the main pharmacokinetic parameters are listed in Table 4.Plasma concentration of bergenin was detectable as early as 10 min after administration, with a mean peak concentration in plasma (Cmax) of 275±164 ng/mL and the time to peak(Tmax) of 0.3±0.1 h. The elimination half life (t1/2), mean residence time (MRT) and area under the concentration-time curve from time 0 to24 h (AUC0-24) were 8±3 h,5.0 ±0.7 h and 548±408 ng h/mL, respectively.
4. Conclusion
In general, a rapid and sensitive LC-MS/MS method was developed and validated for the determination of bergenin in rat plasma after oral administration of B.purpurascens extract.The pharmacokinetics obtained from this study and the method developed herein might provide useful information for clinical application of this TCM.
The work was financially supported by the Natural Science Foundation of Guangdong Province(Nos.9151008018000003).
Appendix A. Supporting information
Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.jpha.2013.01.005.
[1] D.K. Patel1, K. Patel, R. Kumar1, et al., Pharmacological and analytical aspects of bergenin: a concise report, Asian Pac. J.Trop. Dis. 2 (2012) 163-167.
[2] Zhonghua Bencao Committee of State Traditional Chinese Medicine Administration, Zhonghua Bencao, vol. 10, Shanghai Scientific and Techincal Publishers, Shanghai, 1999, pp. 48-49.
[3] I.Mazhar,F.Azhar,K.Mazhar,et al.,Evaluation of antibacterial activity of Bergenia ciliata, Pak. J. Pharm. Sci. 15 (2002) 21-27.
[4] L. Kokoska, Z. Polesny, V. Rada, et al., Screening of some Siberian medicinal plants for antimicrobial activity, J. Ethnopharmacol. 82 (2002) 51-53.
[5] M. Roselli, G. Lentini, S. Habtemariam, Phytochemical, antioxidant and anti-a-glucosidase activity evaluations of Bergenia cordifolia, Phytother. Res. 26 (2012) 908-914.
[6] X.G. Sun, W.H. Huang, M. Ma, et al., Comparative studies on content of arbutin, bergenin and catechin in different part of Bergenia purpurascens and B. crassifolia, Zhongguo Zhong Yao Za Zhi 35 (2010) 2079-2082.
[7] J.L. Yuan, J.L. Suo, Research progress in medicinal plant genus Bergenia Moench, J. Baoji Univ. Arts Sci. 31 (2011) 46-50.
[8] B.X.Wang,H.Zhao,Y.X.Li,The research progress of the genus Bergenia, Chin. J. Spectrosc. Lab. 29 (2012) 367-370.
[9] P.P. Li, S.C. Yang, Y.H. Zeng, Advances in studies on resources for medicinal source plants with bergenin, Chin. Trad. Herbal Drugs 40 (2009) 1500-1505.
[10] U. Singh, A. Barik, K.I. Priyadarsini, Reactions of hydroxyl radical with bergenin, a natural poly phenol studied by pulse radiolysis, Bioorg. Med. Chem. 17 (2009) 6008-6014.
[11] N.Nazir,S.Koul,M.A.Qurishi,et al.,Evaluation of antioxidant and antimicrobial activities of bergenin and its derivatives obtained by chemoenzymatic synthesis, Eur. J. Med. Chem. 46(2011) 2415-2420.
[12] R. Kumar, D.K. Patel, S.K. Prasad, et al., Type 2 antidiabetic activity of bergenin from the roots of Caesalpinia digyna Rottler,Fitoterapia 83 (2012) 395-401.
[13] M.R.Shah,M.Arfan,H.Amin,et al.,Synthesis of new bergenin derivatives as potent inhibitors of inflammatory mediators NO and TNF-a, Bioorg. Med. Chem. Lett. 22 (2012) 2744-2747.
[14] W.S. Yu, Y.W. Wang, Y.H. Zhang, et al., Quantitation of bergenin in human plasma by liquid chromatography/tandem mass spectrometry, J. Chromatogr. B 877 (2009) 33-36.
[15] J.Wang,B.J.Wang,C.M.Wei,et al.,Determination of bergenin in human plasma after oral administration by HPLC-MS/MS method and its pharmacokinetic study, Biomed. Chromatogr. 23(2009) 199-203.
[16] USDHHS, FDA, CDER, Guidance for Industry: Bioanalytical Method Validation, U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Veterinary Medicine (CV), 2001. Available from: http://www/fda.gov/cder/ guidance/index.htm.
[17] V.S. Ponnuru, B.R. Challa, R. Nadendla, Quantification of desloratadine in human plasma by LC-ESI-MS/MS and application to a pharmacokinetic study, J. Pharm. Anal. 2 (2012)180-187.
 Journal of Pharmaceutical Analysis2013年4期
Journal of Pharmaceutical Analysis2013年4期
- Journal of Pharmaceutical Analysis的其它文章
- Characterization of phloroglucinol derivatives and diterpenes in Euphorbia ebracteolata Hayata by utilizing ultra-performance liquid chromatography/quadrupole time-of-flight mass spectrometry
- Application of analytical instruments in pharmaceutical analysis
- Investigation of the interaction between indigotin and two serum albumins by spectroscopic approaches
- In vitro antibacterial and free radical scavenging activity of green hull of Juglans regia
- Ultra-high-performance liquid chromatography for the determination of exenatide in monkey plasma by tandem quadrupole mass spectrometry
- Fingerprint analysis of Cirsium japonicum DC. using high performance liquid chromatography
