A simple method for the isolation and purification of resveratrol from Polygonum cuspidatum
Dong-Geng Wng, Wen-Ying Liu, Gung-Tong Chen,,*
aMedical School, Nan Tong University, Nan Tong 226001, China
bDepartment of Pharmaceutical Analysis, China Pharmaceutical University, Nanjing 210009, China
1. Introduction
Polygonum cuspidatum Sieb.et Zucc.,a well-known traditional Chinese medicine and officially listed in the Chinese Pharmacopoeia [1], has been traditionally used for treatment of various inflammatory diseases, hepatitis, tumors, and diarrhea in East Asian countries such as China, Korea and Japan.Its major constituents are stilbenes and anthraquinones such as polydatin, resveratrol (Fig.1), anthraglycoside B,emodin, physcion and chrysophanol [2-4].
As one of its main active components, resveratrol is also found in many other families of plants such as peanuts [5],grapes [6] and wine [7], but the content of resveratrol in P. cuspidatum is much higher than that in grape and other plants. Now resveratrol has widely been used in medicine,health products and cosmetic industries on account of their various pharmaceutical properties such as anti-inflammatory,anticancer [8] and cardioprotective activities [9].
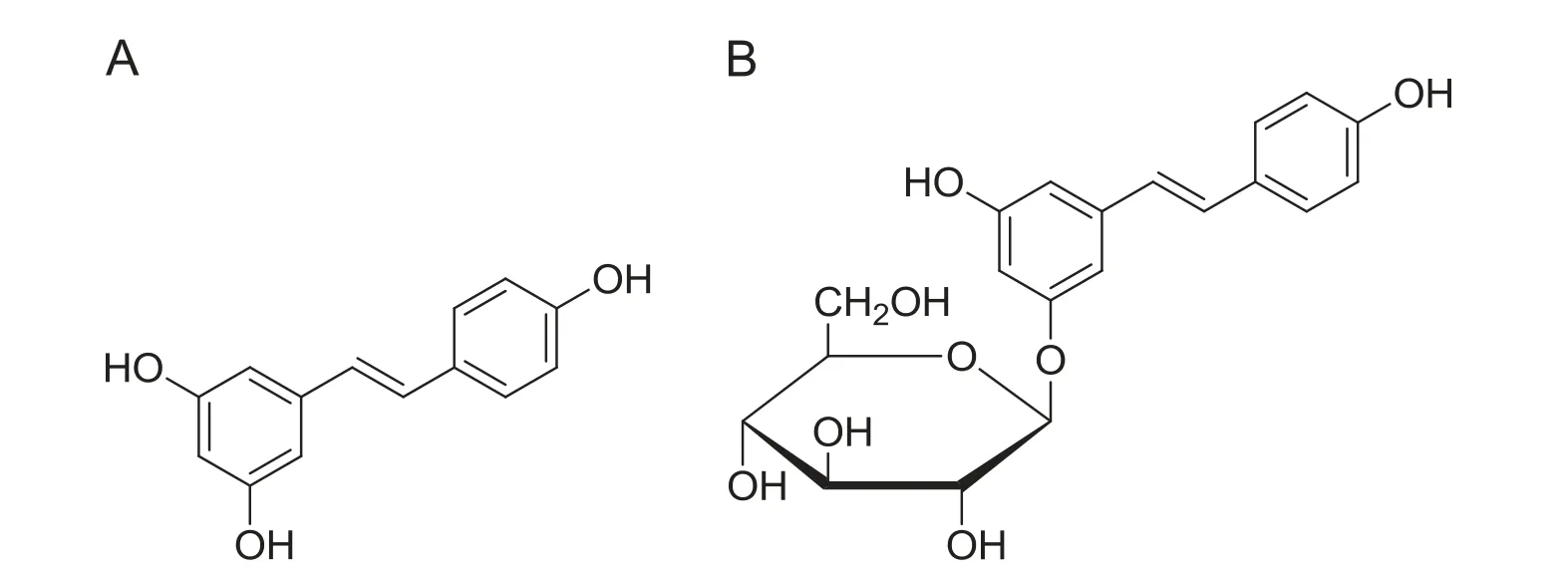
Fig.1 Chemical structures of trans-resveratrol (A) and trans-polydatin (B).
Although there is considerable evidence that transresveratrol possesses strong biological activity, P. cuspidatum has a lower level of trans-resveratrol in comparison to its glucoside transpolydatin (piceid, trans-3,5,40-trihydroxylstilbene-3-mono-D-glycoside).In fact,the level of trans-polydatin in P. cuspidatum is 5-8 times greater than the level of transresveratrol. Therefore, if we can hydrolyze polydatin into resveratrol, the output of resveratrol will be greatly increased.
Because these polyphenols present a wide range of possible health benefits, a huge effort has been made towards the development of isolation, purification and quantification methodologies. Conventional methods entail extraction by heating under reflux with ethanol, followed by filtration, concentration and purification.This procedure is time-consuming and requires a large amount of solvent [10]. Other authors have experimented with molecular imprinted polymers for the selective extraction and purification of resveratrol and piceid from the same plant [11].Supercritical fluid extraction (SFE), which primarily uses CO2as its extraction medium, has been widely used for the extraction of non polar substances such as oils from natural plants and recent literature has shown that it can also be used for the extraction of resveratrol from P.cuspidatum when either ethanol or acetonitrile is added as a modifier[12,13].Enzymic hydrolysis technology has been recoursed to increase in the recovery of resveratrol [14,15].Other methods such as high-speed counter-current chromatography(HSCCC)[2,16,17]and eco-friendly extraction technology[18]were also used in the preparation of resveratrol. These new technologies mentioned above are simple and intuitive, but expensive and time-consuming.
After refluxing extraction with 95% ethanol, the dried extract from P. cuspidatum contains resveratrol, polydatin,emodin and many other components. According to their different chemical properties such as solubility, acidity and hydrolysis, we designed a new method for the preparation of resveratrol from P. cuspidatum. This method not only greatly improved the yield of resveratrol, but also reduced organic solvent consumption and had a lower cost.
2. Experimental
2.1. Reagents and materials
The AR grade solvents used for the extraction were 95%ethanol, ethyl acetate, and petroleum ether (Guoyao Group of Chemical Reagents Ltd., China). Acetonitrile (Tedia Company INC., USA) and acetic acid were of HPLC grade.All other reagents used were also of AR grade,and water was distilled three times.
The reference compounds of trans-resveratrol, polydatin and emodin were all of purity >99% (National Institute for the Control of Pharmaceutical and Biological Products).
P. cuspidatum was purchased from a local drug store and identified by Dr. Li-Na Chen (School of Pharmacy, Nanjing Medical University, Nanjing, China).
2.2. HPLC
The analysis was carried out on an HPLC system (Shimadzu,Japan) equipped with an LC-10ATvp pump, an SPD-10Avp detector, and a CTO-10ASvp column oven. A Nucleosil 100 C18 reverse-phase column (150 mm×4.6 mm; particle size,5 μm; Knauer, Berlin, Germany) protected by a pre-column was used.
For determination of samples obtained in this experiment,we used acetic acid:acetonitrile:water (2:20:80, v/v) as solvent A, and 100% acetonitrile as solvent B, at a flow rate of 1.0 mL/min with the following gradient: 0-30% B linear(0-14 min), 30-100% B linear (14-18 min), 100% B(18-23 min).This was followed by a 15 min equilibrium period with initial conditions prior to injection of the next sample.Samples were filtered (0.45 μm, Millipore) and 20 μL was directly injected. Chromatograms were monitored at 290 nm using the UV detector.
Chromatographic peaks were identified through comparison with retention times of resveratrol,polydatin and emodin standards. Quantitative determination of resveratrol was performed using an external standard based on the area of peak under the optimal HPLC analytical conditions.
2.3. HPLC-MS/MS and other equipments
In order to confirm the main component of the final product to be resveratrol, it was purified on a preparative HPLC system and its chemical structure was further identified by HPLC-MS/MS and1H-NMR.
The preparative HPLC system consisted of two LC-8A pump (Shimadzu, Japan), an SPD-20A detector (Shimadzu,Japan), a Sapphire C18 column (10.0 mm×250 mm, 5 μm;Sepax Technologies, Inc.) protected by a pre-column, and N2000 workstation (Saier Tai Technology Co. Hangzhou,China). For the preparation of resveratrol, we used methanol:water (45:55, v/v) as the mobile phase, and the detective wavelength was set at 290 nm.
HPLC/MS/MS analyses were performed using a system consisting of a Finnigan autosampler (Thermo Electron Corporation, USA), a Finnigan LC pump, a Finnigan TSQ Quantum Ultra equipped with an electrospray ion source and operated by XCalibur software.
1H-NMR spectra were measured on a Bruker DRX-400 spectrometer.
KQ5200 ultrasonic washer (Kunshan Ultrasonic Instrument Co. Ltd., China) and RE 52-86A rotary evaporator(Shanghai Yarong Instrument Co. Ltd., China) were used for extraction.
2.4. Spectral data of trans-resveratrol by mass spectrometry and 1H-NMR
HPLC-ESI-MS/MS was adopted to analyze trans-resveratrol.The data were shown here:parent ion [M-H]-m/z 227,main product ions m/z 185, 143, 117 and 119. The molecular mass of trans-resveratrol is 228 D.
In dimethyl sulfoxide (DMSO), the1H-NMR data were as follows: 6.11 (m, 1H,H-4), 6.37(d, 2H, J=2.0 Hz, H-2, H-6),6.76 (d, 2H, J=8.5 Hz, H-3′, H-5′), 6.90 (d, 1H, J=16.3 Hz,H-a), 6.92 (d, 1H, J=16.3 Hz, H-β), 7.39 (d, 2H, J=8.5 Hz,H-2′, H-6′), 9.17 (s, 2H, 3-OH, 5-OH), 9.52 (s,1H, 4′-OH).From the above data, it was easy to confirm resveratrol.
2.5. Procedures for the preparation of resveratrol from P. cuspidatum
Method for the preparation of resveratrol from P. cuspidatum was based on the chemical properties of compounds in the 95% ethanol extract. The whole process included reflux extraction, filtering, hydrolyzing, liquid-liquid extraction and eluting. The flow chart is shown in Fig.2.
2.5.1. Preparation of the 95% ethanol extract from P. cuspidatum by reflux extraction
Extraction of resveratrol from P. cuspidatum was performed with 95% ethanol by reflux extraction according to the method reported in literature [19]. In this process, the dried roots of P. cuspidatum were ground to powder (about 40 mesh)by using FZ102 plant disintegrator.One hundred grams of powder were placed in distillation flask. After adding 95%ethanol in a proportion of 1:6 (powder: 95% ethanol, g/mL),the mixture was left to rest approximately 12 h at room temperature. Then the soaked powder of P. cuspidatum was extracted by refluxing extraction at about 80°C. This extraction procedure was repeated three times and 1 h for each time.The extract solutions obtained were combined and evaporated to dryness by a rotary evaporator under vacuum at 65°C.The yield was calculated dividing the mass of recovered dry extract (mr) by the initial mass of P. cuspidatum powder (mi)(see Eq. (1)) and the content of resveratrol was detected by HPLC.
2.5.2. Filtering and hydrolyzing
The 95%ethanol extract mentioned above was pulverized into powder form(less than 0.9 mm).A milled sample and water in a proportion of 1:30 (w/v,g/mL) were sealed in a vessel and placed into the ultrasonic cleaning bath for 20 min at 50°C.The mixture finally becomes a uniform suspension solution,and was immediately filtered at lower pressure. The aqueous solution and the residue acquired were used for HPLC detection.
In order to hydrolyze polydatin to resveratrol, the aqueous solution was adjusted with hydrochloric acid to pH=1, and hydrolyzed by refluxing in water bath for 8 h at 75°C.
2.5.3. Liquid-liquid extraction
Liquid-liquid extraction was carried out in a separating funnel.The aqueous solution mentioned above was mixed with the same volume of extraction solvent, and the separation between the aqueous and organic phases was carried out by gravitational sedimentation. To achieve a high recovery extraction was repeated three times.The volumes of aqueous and organic phases were measured and concentrations of resveratrol in each phase were analyzed with HPLC (see Eq. (2)).
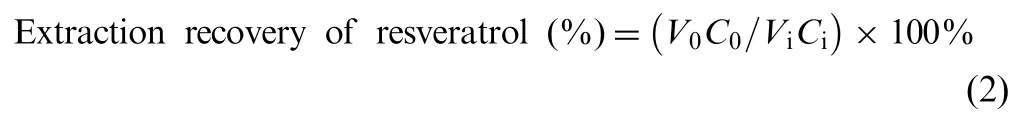
where V0and C0are the volume and concentration of resveratrol in organic phase after extraction respectively, Viand Ciare the initial volume and initial concentration of resveratrol in aqueous solution before solvent extraction.
2.5.4. Eluting
To remove the impurities including strong acidic and watersoluble compounds, the alkaline aqueous solution with pH 8-9(for example,5%sodium bicarbonate solution)was added to the organic phase,and washed(about 2 times,v/v=1)until the color of aqueous phase changed from red to almost colorless. The mixture was directly left to complete phase separation in a funnel. The volumes of aqueous and organic phases were measured and the concentration of resveratrol in each phase was measured by HPLC.
3. Results and discussion
3.1. Extraction of resveratrol from P. cuspidatum
The yield of 95%ethanol extract acquired from P.cuspidatum by refluxing extraction was 13.3%, and the content of resveratrol in the extract was 3.3%.
Fig.3(A) shows the HPLC chromatograms of the 95%ethanol extract. It is clear that there are five major peaks in this chromatogram. Peaks 3 and 4 were identified as resveratrol and emodin with retention time of 14.8, 21.3 min by comparison with the external standards, respectively. Peaks 1,2 and 5 were of unknown components. It was evident that peaks with retention time over 21 min were of non polar or less polar compounds.
3.2. Filtration
3.2.1. Roles of filtration
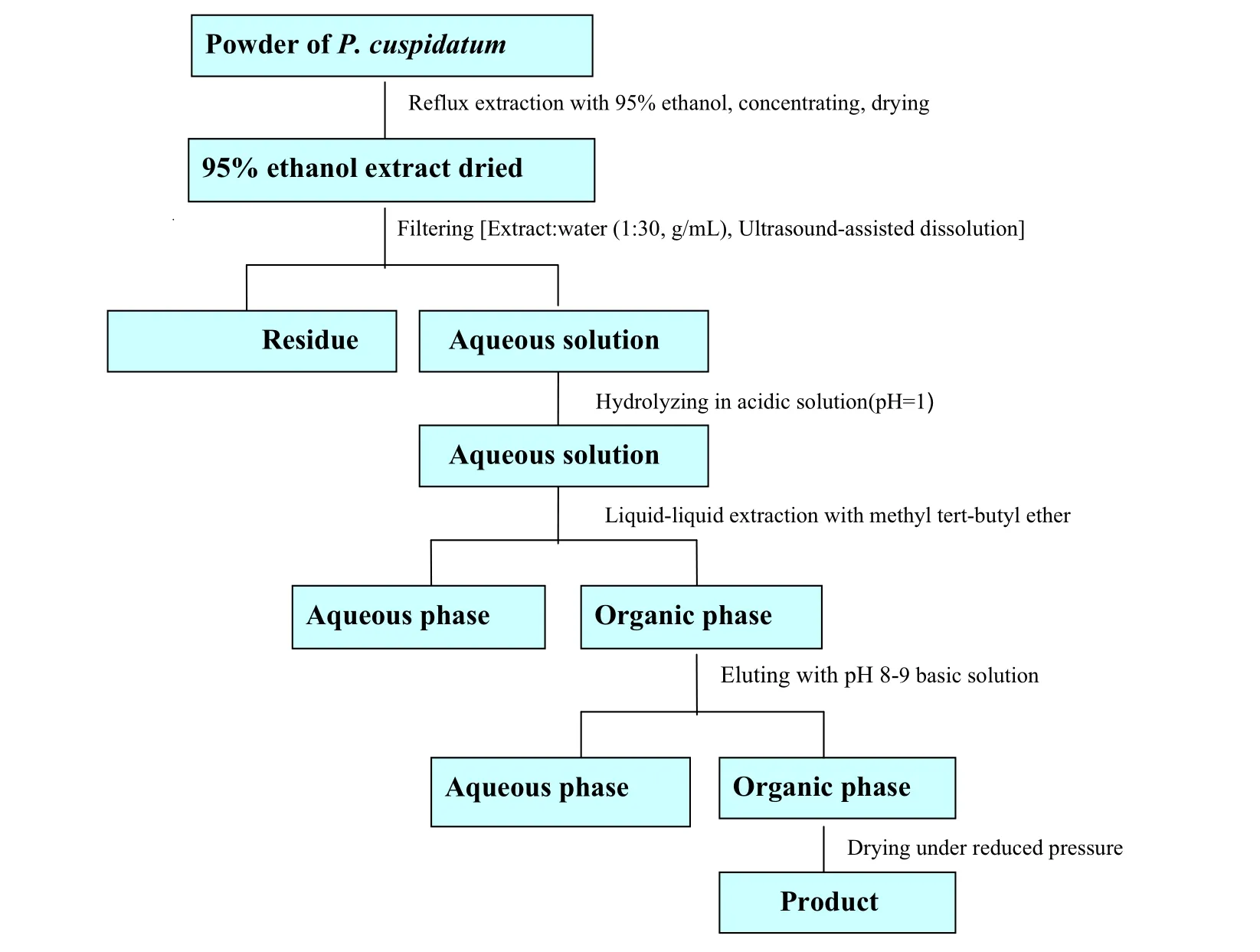
Fig.2 Flow chart representative of the extraction and purification processes of resveratrol.
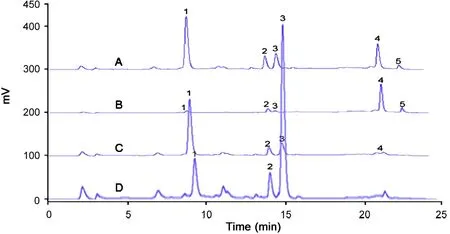
Fig.3 HPLC chromatograms of samples in filtration and hydrolyzing. (A) Chromatogram of 95% ethanol extract, (B)and (C) chromatograms of residue and aqueous solution after filtration, (D) chromatograms of aqueous solution after hydrolyzing for 6 h. Peak1 polydatin, 3 resveratrol, 4 emodin, 2 and 5 unknown components.
Fig.3(A) shows the chromatogram of 95% ethanol extract,Fig.3(B and C) are the chromatograms of residue and aqueous solution after filtering, respectively. It is very clear that resveratrol(peak3)and other compounds with TR15 min were mostly remained in aqueous phase, while the non polar or less polar compounds with TR21 min were mostly or completely left in residue.
The 95% ethanol extract contains some non polar or less polar compounds such as physcion, chrysophanol, emodin and so on. To remove these compounds and debris fragments from the extract, the efficiency of two different filtering approaches was compared. The first one used direct filtration method. The extracting solution acquired from P. cuspidatum by refluxing extraction was concentrated under low pressure until ethanol was mostly or completely evaporated. After that it was diluted with water and then filtrated. It was found that those non polar or less polar compounds,which might exist in single molecular form,were still found in the filtrate and could not be easily removed. However, compared with the first one,the second method was very effective in eliminating the non polar or less polar compounds. The cause might be that these compounds existed in molecular aggregation form and thus could be easily removed by filtering (figures were not given here).
3.2.2. Effect of extract-water ratio on the extraction of resveratrol
The extract-water ratio (w/v, g/mL) has a marked effect on the resveratrol extraction.A plot of extract-water ratio versus the extraction recovery of resveratrol is shown in Fig.4. The extraction recovery of resveratrol started to increase at ratio 1:10 and reached the maximum (86.3%) at about 1:30(Analysis of variance: F=11.847, P=0.008). But when the ratio was over 1:30, it was not significant for the change of extraction recovery value (Analysis of variance: F=0.056,P=0.946).
Obviously, this was concerned with the degree of extracts dissolved in water. When the extract-water ratio was below 1:30, the extraction recovery of resveratrol was comparatively lower.This might be caused by the losses of resveratrol for the formation of coarse particles in the mixture.
3.3. Hydrolyzing
In order to improve the yield of resveratrol, we used a glycoside hydrolysis pathway. First we used sulfuric acid(pH=1),both resveratrol and polydatin can be easily oxidized in this condition. Therefore, we finally used the refluxhydrolysis approach under hydrochloric acid condition(pH=1). TLC results showed that after reflux-hydrolyzing for 8 h, polydatin was hydrolyzed completely.
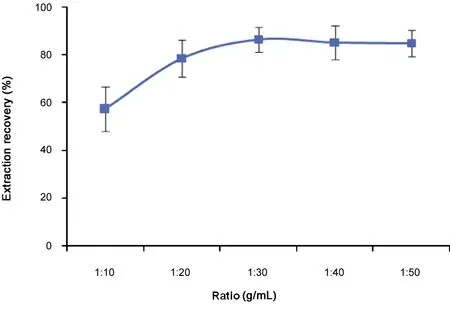
Fig.4 Effect of extract-water ratio (g/mL) on the extraction recovery of resveratrol (n=3).
Fig.3(D) shows the chromatogram of aqueous solution after hydrolyzed in acidic solution (pH=1) for 6 h. It was clear that most polydatin was transformed to resveratrol.
3.4. Liquid-liquid extraction
3.4.1. Selection of extraction solvent
Any components will be distributed between two phases according to the distribution constant,temperature,and the relative volumes of two phases.Therefore,the selection of extractants is one of the most important factors in solvent extraction. Four solvents [ethyl acetate, ethyl acetate:petroleum ether (1:1), petroleum ether and methyl tert-butyl ether] were tested in their extraction efficiency,and the extraction recoveries acquried were 82± 7%,92 ±7%, 11±4%, 93±9%(mean± SD, n=3),respectively. For methyl tert-butyl ether, the extraction recovery of resveratrol was the significantly highest among the four extractants(Analysis of variance: F=93.55, P=0.0001), thus it was finally selected as extraction solvent. Similar results were also obtained when ethyl acetate-petroleum ether (1/1, v/v) was used as extractant.
3.4.2. Chromatograms after liquid-liquid extraction
Fig.5 shows HPLC chromatograms of samples after liquidliquid extraction using methyl tert-butyl ether as extractant. It was evident that resveratrol (peak3) was selectively extracted into the organic phase (Fig.5(A)), and not found in the aqueous phase. Impurities of water soluble compounds(Tr<14.0 min) were mostly remained in the aqueous phase(Fig.5(B)).
3.5. Eluting
3.5.1. Effects of pH on the extraction of resveratrol during the eluting process
Fig.6 shows that pH had a great effect on the extraction of resveratrol. When basic aqueous solution with pH=8-9 was used as elution solvent, the extraction recovery of resveratrol was over 90%.However,the extraction recovery of resveratrol decreased quickly to 0 when the elution solvent with pH>10 was used. The content of resveratrol in the dried product reached to 73.8%.
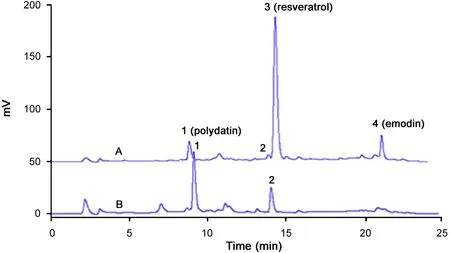
Fig.5 HPLC chromatograms of samples after liquid-liquid extraction. (A) chromatogram of organic phase, and (B) chromatogram of aqueous phase.
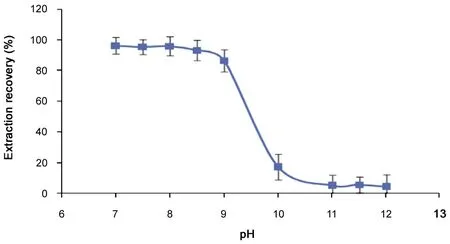
Fig.6 Effect of pH on resveratrol extraction during the eluting process.
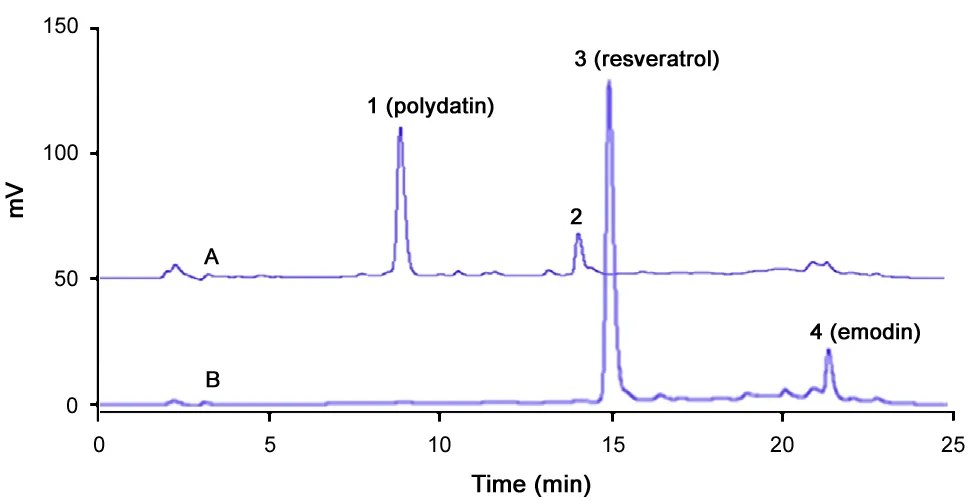
Fig.7 HPLC chromatograms of samples after eluting. (A)chromatogram of aqueous phase, and (B) chromatogram of organic phase.
Resveratrol is a weak acid, so under a certain pH range it existed as un-ionized molecular form. When being eluted with solution (pH<9), resveratrol still remained in the organic phase. However, when an eluent with pH>10 was used,resveratrol (ionized form) was transferred to aqueous phase.An increase of pH (9<pH<10) brought about a considerable decrease of extraction recovery of resveratrol.
3.5.2. Investigation of the eluting efficiency
Fig.7(A and B) are the HPLC chromatograms of aqueous and organic phases after being eluted. Fig.7(A) shows that impurities with retention time less than 14.5 min werecompletely eluted into the aqueous phase, while resveratrol(peak3) and emodin (peak4) with retention time 14.8 and 21.3 min were still remained in the organic phase as displayed in Fig.7(B).

Table 1 Comparision of methods for the isolation and purification of resveratrol from Polygonum cuspidatum.
3.6. Comparison of different technologies for the extraction and purification of resveratrol
According to literatures reported, there are several different methods for the preparation of resveratrol from P. cuspidatum.Among them four major methods are shown in Table 1. The conventional methods such as macroporous resin or silica gel column chromatography are simple,but they are time-consuming and require a large amount of organic solvents. High-speed counter-current chromatography and supercritical fluid extraction are of advanced technologies,but they need expensive equipment.In this study, we used acid hydrolysis combined with alkaline solution washing approach. Because of no participation of precious instrument and less consumption of organic solvents in this method,its cost is naturally lower.By comparing resveratrol yields of extracts before and after acid hydrolysis, we found the yield of resveratrol of the latter increased about four-fold (data were not given here).
4. Conclusion
In this work we developed a simple and effective method for the preparation of resveratrol from P.cuspidatum.By comparing with some traditional and modern methods in separation and purification science, this method brings us several advantages. First, it does not need column packing such as macroporous resin or silica gel. Secondly, it requires only a small amount of organic solvents.And finally,it does not need expensive equipment.
According to this method, the yield of resveratrol increased about 4-fold by acid hydrolysis of glycoside, and the content of resveratrol in the final product was over 73.8%.
This work was supported by Natural Science Foundation of Jiangsu College & University (10KJB350003) and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
[1] China Pharmacopoeia Committee, Pharmacopoeia of the People's Republic of China, the First Division of 2005 Edition, China Chemical Industry Press, Beijing, 2005, pp. 167.
[2] X. Chu, A. Sun, R. Liu, Preparative isolation and purification of five compounds from the Chinese medicinal herb Polygonum cuspidatum Sieb.et Zucc by high-speed counter-current chromatography, J. Chromatogr. A 1097 (2005) 33-39.
[3] D.L. Zhang, X. Li, D.X. Hao, et al., Systematic purification of polydatin, resveratrol and anthraglycoside B from Polygonum cuspidatum Sieb.et Zucc,Sep. Purif.Technol.66(2009)329-339.
[4] B.C.Vastano,Y.Chen,N.Q.Zhu,et al.,Isolation and identification of stibenes of Polygonum cuspidatum, J. Agric. Food Chem.48 (2000) 253-256.
[5] J.M. Ingham, 3,5,4′-Trihydroxystilbene as a phytoalexin from groundnuts (Arachis hypogaea), Phytochemistry 15 (1976)1791-1793.
[6] P. Landcake, R.J. Price, The production of resveratrol by Vitis vinifera and other members of the Vitaceae as a response to infection or injury, Physiol. Plant Pathol. 9 (1976) 77-86.
[7] E.H. Siemann, L.L. Creasy, Concentration of the phytoalexin resveratrol in wine, Am. J. Enol. Vitic. 43 (1992) 49-52.
[8] A.A. Bertelli, F. Ferrara, G. Diana, et al., Resveratrol, a natural stilbene in grapes and wine,enhances intraphagocytosis in human promonocytes: a co-factor in antiinflammatory and anticancer chemopreventive activity, Int. J. Tissue React. 21 (1999) 93-104.
[9] M.H.Li,J.K.Chen,S.S.Huang,et al.,Cardioprotective effect of resveratrol, a natural antioxidant derived from grapes, Cardiovasc. Res. 47 (2000) 549-555.
[10] H.Y. Xiang, C.S. Zhou, Q.F. Lei, et al., Studies on separation and purification of piceid from Poligonum cuspidatum by macroporus adsorption resin, Chin. Pharm. J. 40 (2005) 96-98 (in Chinese).
[11] X.Zhuang,X.Dong,S.Ma,et al.,Selective on-line extraction of transresveratrol and emodin from Polygonum cuspidatum using molecularly imprinted polymer, J. Chromatogr. Sci. 46 (2008)739-742.
[12] , et al., Supercritical fluid extraction of piceid, resveratrol and emodin from Japanese knotweed, J. Supercrit. Fluids 51 (2010) 325-330.
[13] W. Yu, B. Shu, Y. Zhao, Supercritical CO2extraction of resveratrol and its glycoside piceid from Chinese traditional medicinal herb Polygonum cuspidatum, J. Sci. Food Agric. 85(2005) 489-492.
[14] Z.F. Huang,J.H. Yi, Q.L. Liu, et al., Research of extracting and purifying process of resveratrol from Polygonum cuspidatum extract by enzymatic hydrolysis, Nat. Prod. Res. Dev. 21 (2009)1061-1064 in Chinese.
[15] T.L. Tian, J. Shen, M.M. Xu, et al., Biotransformation of polydatin from Polygonum cuspidatum by Rhizopus sp T-34,Sichuan Daxue Xuebao 2 (2008) 437-440 (in Chinese).
[16] F.Q. Yang, T.Y. Zhang, Y. Ito, Large-scale separation of resveratrol, anthraglycoside A and anthraglycoside B from Polygonum cuspidatum Sieb. et Zucc by high speed counter-current chromatography, J. Chromatogr. A 919 (2001) 443-448.
[17] M. Yang, X.J. Xu, C.Y. Xie, et al., Preparative isolation and purification of 12,13-dihydroxyeuparin from Radix Eupatorii Chinensis by high-speed counter-current chromatography,J. Pharm. Anal. 2 (2012) 258-263.
[18] M. Stefano, B. Arianna, B. Luisa, et al., A one-pot ultrasoundassisted water extraction/cyclodextrin encapsulation of resveratrol from Polygonum cuspidatum, Food Chem. 130 (2012) 746-750.
[19] D.G. Wang, W.Y. Liu, Application of Doehlert design for the optimization of resveratrol extraction from Polygonum cuspidatum, Chin. Pharm. J. 40 (2005) 1138-1142 (in Chinese).
 Journal of Pharmaceutical Analysis2013年4期
Journal of Pharmaceutical Analysis2013年4期
- Journal of Pharmaceutical Analysis的其它文章
- Quantitative analysis of cefixime via complexation with palladium(II) in pharmaceutical formulations by spectrophotometry
- Simultaneous pharmacokinetic assessment of cefadroxil and clavulanic acid in human plasma by LC-MS and its application to bioequivalence studies
- Application of UPLC-MS/MS for separation and quantification of 3α-Hydroxy Tibolone and comparative bioavailability of two Tibolone formulations in healthy volunteers
- Fingerprint analysis of Cirsium japonicum DC. using high performance liquid chromatography
- Ultra-high-performance liquid chromatography for the determination of exenatide in monkey plasma by tandem quadrupole mass spectrometry
- In vitro antibacterial and free radical scavenging activity of green hull of Juglans regia
