Minimally invasive local therapies for liver cancer
David Li, Josephine Kang, Benjamin J. Golas, Vincent W. Yeung, David C. Mado ff
1Department of Radiology, Division of Interventional Radiology, New York-Presbyterian Hospital/Weill Cornell Medical Center, New York, NY 10065, USA;
2Department of Medicine, NYU Langone Medical Center, New York, NY 10016, USA;
3Flushing Radiation Oncology Services, Flushing, New York, NY 11354, USA;
4Department of Surgery, Division of Surgical Oncology, New York-Presbyterian Hospital/Weill Cornell Medical Center New York, New York, NY 10065, USA
Minimally invasive local therapies for liver cancer
David Li1, Josephine Kang2,3, Benjamin J. Golas4, Vincent W. Yeung3, David C. Mado ff1
1Department of Radiology, Division of Interventional Radiology, New York-Presbyterian Hospital/Weill Cornell Medical Center, New York, NY 10065, USA;
2Department of Medicine, NYU Langone Medical Center, New York, NY 10016, USA;
3Flushing Radiation Oncology Services, Flushing, New York, NY 11354, USA;
4Department of Surgery, Division of Surgical Oncology, New York-Presbyterian Hospital/Weill Cornell Medical Center New York, New York, NY 10065, USA
Primary and metastatic liver tumors are an increasing global health problem, with hepatocellular carcinoma (HCC) now being the third leading cause of cancer-related mortality worldwide. Systemic treatment options for HCC remain limited, with Sorafenib as the only prospectively validated agent shown to increase overall survival. Surgical resection and/or transplantation, locally ablative therapies and regional or locoregional therapies have fi lled the gap in liver tumor treatments, providing improved survival outcomes for both primary and metastatic tumors. Minimally invasive local therapies have an increasing role in the treatment of both primary and metastatic liver tumors. For patients with low volume disease, these therapies have now been established into consensus practice guidelines. This review highlights technical aspects and outcomes of commonly utilized, minimally invasive local therapies including laparoscopic liver resection (LLR), radiofrequency ablation (RFA), microwave ablation (MWA), high-intensity focused ultrasound (HIFU), irreversible electroporation (IRE), and stereotactic body radiation therapy (SBRT). In addition, the role of combination treatment strategies utilizing these minimally invasive techniques is reviewed.
Liver; hepatocellular carcinoma (HCC); metastasis
Introduction
Primary liver cancer, particularly hepatocellular carcinoma (HCC), continues to be a growing global health problem and has risen to become the third most common cause of cancer-related deaths worldwide, accounting for over >800,000 deaths/year1,2. HCC is the fastest growing cause of cancer-related mortality both in the United States and worldwide over the past several decades due to both its increased incidence and poor prognosis3,4. In addition, the liver remains a common site of metastases, particularly from colorectal carcinoma5. It is estimated that up to two-thirds of patients with colorectal liver metastases ultimately die from their disease due to liver involvement6,7. Hence, there isan ever increasing need to develop e ff ective treatments for both primary and metastatic liver tumors.
An extensive range of therapeutic options have been developed for the treatment of both primary and metastatic liver tumors.is has been, in part, driven by the limited e ff ectiveness and range of available systemic treatments, particularly for HCC. Limitations of the effectiveness of systemic therapy are thought, in part, to be due to the frequent coexistence of HCC with cirrhosis and its high molecular heterogeneity. A signi fi cant advance was made in systemic therapeutic options with the randomized prospective validation of sorafenib in providing survival benefit in patients with HCC8; however, more recent randomized prospective controlled trials evaluating the use of Everolimus (EVOLVE-1) and Brivanib (BRISK-PS) have failed to demonstrate improvement in overall survival as compared to current standards of care9,10. Hence, the role of systemic therapies remains limited. Surgical resection and/or transplantation, locally ablative therapies and regional or locoregional therapies have fi lled the gap in liver tumor treatments, providing improvedpatient survivals as validated by prospective trials for both primary and metastatic tumors.
Surgical resection has been shown to result in improved survival outcomes and thus is the mainstay of curative therapy whenever amenable for both primary and metastatic liver tumors11,12. For example, in the seing of curative hepatectomy for colorectal metastases (CRM), the overall 5-year survival rate of surgical groups has approached >50%, as compared to approximately 15% for medical groups undergoing systemic chemotherapy alone13,14. However, surgical resection has potential complications, particularly in diseased liver states such as cirrhosis or prolonged chemotherapy15-18. There is reduced functional reserve to compensate for the resected hepatic parenchyma and in these settings, local ablation serves as a potentially curative option with the ability to induce complete cytotoxicity to the targeted tumor.
In addition to the increased incidence of HCC, improved screening, follow-up and imaging algorithms have led to the earlier detection and diagnosis of low volume disease primary and metastatic liver tumors amenable for minimally invasive local techniques. These factors have fueled an ever increasing need for local treatment options for both primary and metastatic liver tumors. The improved imaging algorithms have occurred in conjunction with advances in ablative techniques towards targeting of lesions. For percutaneous ablations, a wide range of guidance techniques have been developed including using ultrasound, computed tomography, magnetic resonance, fusion of imaging modalities, and needle tracking for optimal applicator positioning. Improved guidance and lesion localization has improved upon the technical feasibility of all minimally invasive local treatment options, further solidifying their use in current practice.
A wide range of minimally invasive local treatment techniques such as laparoscopic liver resection (LLR) and ablation have been developed. This article will highlight their role in current treatment paradigms, their principles of action, their relative di ff erences in implementation, and the evidence for their use.
Minimally invasive local therapies in current treatment paradigms
The management of both primary tumors and metastases confined to the liver is best served with a multidisciplinary approach as adopted by multiple consensus guidelines, including the National Comprehensive Cancer Network (NCCN) and the European A ssociatio n for the Study of the Liver (E ASL)11,12,19. For HCC, multiple clinical staging systems have been developed in order to stratify patients towards appropriate treatments dependent on prognosis20. These staging systems typically stratify patients according to three generalized parameters: clinical performance status, extent of tumor, and liver function. One of the most widely adopted prognostic staging systems is the Barcelona Clinic Liver Cancer (BCLC) treatment strategy (Figure 1)21,22.
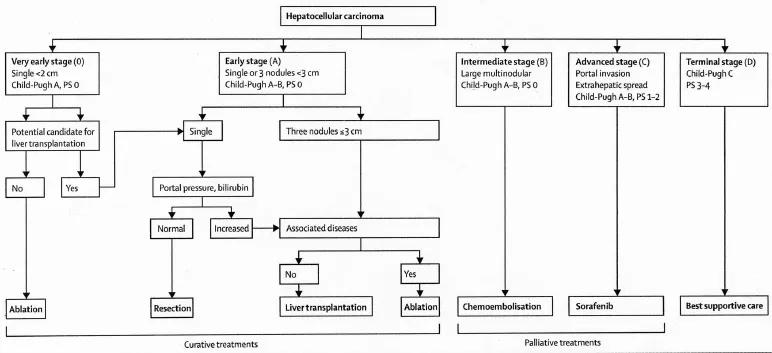
Figure 1 Barcelona clinic liver cancer treatment strategy. (Reprinted with permission from Forner et al.2).
Volume of disease is an important determinant of prognosis and treatment options in patients with disease isolated to the liver.In the BCLC treatment strategy, tumor size and number directly alters disease staging, with a single tumor <2 cm considered very early stage (0), single or up to three tumors <3 cm considered early stage (A), or a large and/or multinodular tumor considered intermediate stage (B) in patients with equivalent Child-Pugh scores (A) and Performance Status (0). Potentially curative options such as surgical resection, liver transplantation (LT), or locally ablative therapies are reserved for patients with low volume disease (i.e., patients who are either BCLC stage 0 or A). For large or multinodular tumors with preserved liver function, locoregional therapies (i.e., transarterial embolotherapy) serve as the preferred treatment option.
Numerous locoregional treatment options have been explored for the treatment of large or multinodular tumors. Transarterially delivered therapies include bland embolization, chemoembolization, drug-eluting beads, and radioembolization, each with their relative advantages and drawbacks which are beyond the scope of this review23-25.
Yau et al. recently analyzed a series of 3,856 patients from a single Asian center and created a new prognostic classification system and treatment strategy through statistical modelling methods termed the Hong Kong Liver Cancer (HKLC) schema (Figure 2)26. In their study, the HKLC was directly compared to the BCLC treatment strategy and was found to have increased ability to provide discriminable prognosis in terms of overall survival (area under receiver operating characteristic curve values, approximately 0.84 vs. 0.80; co ncordance index, 0.74 vs. 0.70). More importantly, HKLC identified subsets of BCLC intermediate and advanced stage patients who demonstrated a significantly increased survival benefit if classified under the HKLC system, where they would be stratified to receive more aggressive therapies such as surgical resection or local ablation.is new classi fi cation system further validates the e ff ectiveness of surgical resection and local ablation in improving the overall survival in patients with primary liver cancer, and expands upon the eligibility criteria for patients to receive these more“aggressive” therapies.
Surgical resection has been shown to result in improved survival outcomes and thus is the mainstay of curative therapy whenever amenable for both primary and metastatic liver tumors11,12. However, surgical resection has potential complications, particularly in diseased liver states such as cirrhosis or prolonged chemotherapy15-18. In these settings, there is reduced functional reserve to compensate for the resected hepatic parenchyma. Even though portal vein embolization has been well-established to increase hepatic reserve in the anticipated future liver remnant, thus increasing the number of potential surgical candidates27; there still exists a large population of patients who are ineligible to safelyundergo hepatic resection. In addition, comparison of surgical series to radiofrequency ablation (RFA) is limited by selection bias towards patient populations with greater comorbidities in the RFA cohorts7. Hence, locally ablative therapies maintain an important role in the treatment of liver tumors in patients with low volume of disease.
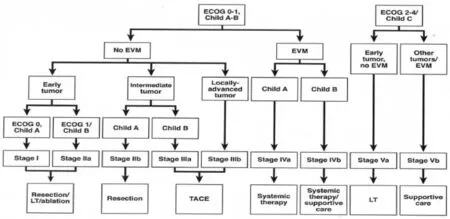
Figure 2 Hong Kong Liver Cancer prognostic classi fi cation and treatment strategy. (Reprinted with permission from Yau et al.26). Early tumor:≤5 cm, ≤3 tumor nodules, and no intrahepatic venous invasion; Intermediate tumor: (1) ≤5 cm, either >3 tumor nodules or with intrahepatic venous invasion, or (2) >5 cm, ≤3 tumor nodules and no intrahepatic venous invasion; and Locally advanced tumor: (1) ≤5 cm, > 3 tumor nodules with intrahepatic venous invasion, or (2) >5 cm, >3 tumor nodules or/and with intrahepatic venous invasion, or (3) di ff use tumor. EVM, extrahepatic vascular invasion/metastasis; LT, liver transplantation.
Traditional, open liver resection (OLR) is also affected by significant morbidity which directly impacts upon patient’s quality of life. As compared to surgical resection, local ablation is of relatively low cost, has minimal morbidity, and can be usually performed with shorter hospital stays7. Huang et al. prospectively evaluated 389 patients eligible for either surgical resection or percutaneous RFA for solitary HCC <3 cm in diameter for quality of life measures28. The authors found no difference in the two groups in disease-free and overall survival, however the RFA group demonstrated a statistically signi fi cant beer health-related quality of life score (HRQL) as compared to the surgical group using the Functional Assessment of Cancer Therapy–Hepatobiliary (FACT-Hep) instrument. In their study, participants underwent open surgical technique with a right subcostal incision, with no comparison to laparoscopic approaches performed. As quality of life measures become increasingly important in decision of making of treatment paradigms, minimally invasive approaches such as laparoscopic resection and local ablation will continue to demonstrate increased clinical utility.
Techniques within the treatment armamentarium are not mutually exclusive and there is extensive reported evidence on the role of ablation in conjunction with surgical techniques.
For example, ablative techniques are commonly used to limit disease progression in patients awaiting transplantation (“bridge to transplantation”), with multiple studies demonstrating its utility in this seing29-31. RFA is most commonly used for bridge to transplantation, though there are several small reported series using microwave ablation (MWA) or stereotactic body radiation therapy (SBRT)32,33. Ablative techniques can be also performed in conjunction with major hepatectomy to o ff er curative options for select patients who have bilobar metastatic liver disease13,34. In addition, ablation can be performed in conjunction with locoregional therapies for improved therapeutic e ffi cacy, as will be further elucidated below.
Principles of action for local ablation
Multiple locally ablative techniques for the treatment of tumors have been developed and adopted into standard clinical practice. Each technique is based u pon a di ff erent principle of action to induce cytotoxicity (Table 1).
The locally ablative techniques that rely on tissue heating for cytotoxicity result in a common pathway towards acute coagulative necrosis. Temperatures greater than 60 ℃ result in protein denaturation and near-instantaneous cell death. Temperatures in the range of 42-60 ℃ result in irreversible cell damage due to microvascular thrombosis, ischemia, and hypoxia35. Conversely, cryoablation results in cell death attemperatures less than –40 ℃ through both cell membrane disrup tions by ice crystal formation, and rapid fl uid shis from osmotic gradients with associated cell membrane rupture. Irreversible electroporation (IRE) promotes cytotoxicity through irreversible damage to the cell membrane through the formation of permanent porous channels, stimulating apoptosis35. SBRT delivers high doses of photon beam radiation in a limited number of fractions (typically 3-6) to the target.e primary mechanism of cell death is through ionization of DNA, resulting in direct DNA damage and lethal double strand DNA breaks. Radiation also causes indirect DNA and cell damage through free radical formation36. Tumor cells are less efficient than normal cells in repairing radiation-induced damage, resulting in preferential killing of malignant cells37.

Table 1 Ablative techniques and their mechanisms of action
Technical considerations and outcomes
Laparoscopic liver resection (LLR)
While minimally invasive approaches have been widely adopted in many areas of surgery, there was initial apprehension regarding LLR38. These concerns were based on lack of familiarity with advanced laparoscopic techniques and equipment, challenges replicating open techniques, fears regarding bleeding and gas embolism, the possibility of port site metastases and peritoneal dissemination, unknown long-term outcomes, and lack of data from randomized trials.
Despite this initial trepidation, over 3,000 LLR cases have been performed worldwide38. Most early LLRs were nonanatomic resectio ns fo r p eripherally lo cated, benign lesions. Now, upwards of 50% of LLRs are performed for malignancy, with lobectomies and extended resections being undertaken in specialized centers (Figure 3). To date, no prospective randomized controlled trials have been conducted that compare LLR with OLR.erefore, all existing comparisons are based on retrospective studies as well as meta-analyses. Taken together, published data purport the clinical bene fi ts of LLR, without any oncologic compromise, particularly in the treatment of colorectal cancer (CRC) liver metastases.
Nguyen et al. performed a comparative analysis examining the benefits of LLR versus OLR in 31 case-cohort matched studies, which encompassed 2,473 patients39. For LLR versus OLR, there was significantly less blood loss (15 studies), less transfusions (4 studies; 0% LLR vs. 17.3% OLR; P=0.04), fewer postoperative days of narco tic medicatio n (8 studies; 1 day LLR vs. 5 days OLR; P=0.001), quicker resumption of diet (7 studies; 1-2.4 days LLR vs. 2-4.3 days OLR; P=0.001 to<0.01), and decreased time to ambulation (1 study; 2.8 days LLR vs. 3.8 days OLR; P<0.005). While length of stay (LOS) information for liver surgery is variable secondary to cultural and institutional biases, almost all of the studies reported significantly reduced LOS after LLR (50% shorter) compared with OLR. Shorter LOS translated to reduced costs for LLR. The majority of studies reported comparable morbidity rates, while seven showed signi fi cantly lower complication rates with LLR (6%-13.8% LLR vs. 28.9%-47.8% OLR; P=0.001-0.04).ere were no di ff erences in operative time or mortality. While this review harbors the limitations inherent to any retrospective analysis of selected patients, it supports LLR as safe, feasible, and cost-e ff ective with demonstrable bene fi ts.
Of the nearly 3,000 published reports of LLR, approximately 50% were performed for malignant lesions, 35% of which were CRC metastases38. Multiple retrospective series have reported the safety, feasibility, and oncologic integrity of LLR for CRC38. Castaing et al. reported the only matched prospective comparison in patients undergoing resection of CRC liver metastases via laparoscopic (n=60) and open (n=60) approaches40. LLR was comparable to, and in some cases superior to, OLR in terms ofoncologic outcomes. Speci fi cally, the margin-free resection rate was greater in LLR vs. OLR (87% vs. 72%, P=0.04), while there was no signi fi cant di ff erence in overall and disease-free survival.e 1-, 3-, and 5-year overall survival was 97%, 82%, and 64%, respectively, with LLR, versus 97%, 70%, and 56% with OLR (P=0.32). Furthermore, 1-, 3-, and 5-year disease-free survival was 70%, 47%, and 35%, respectively with LLR, versus 70%, 40%, and 27% in OLR (P=0.32).
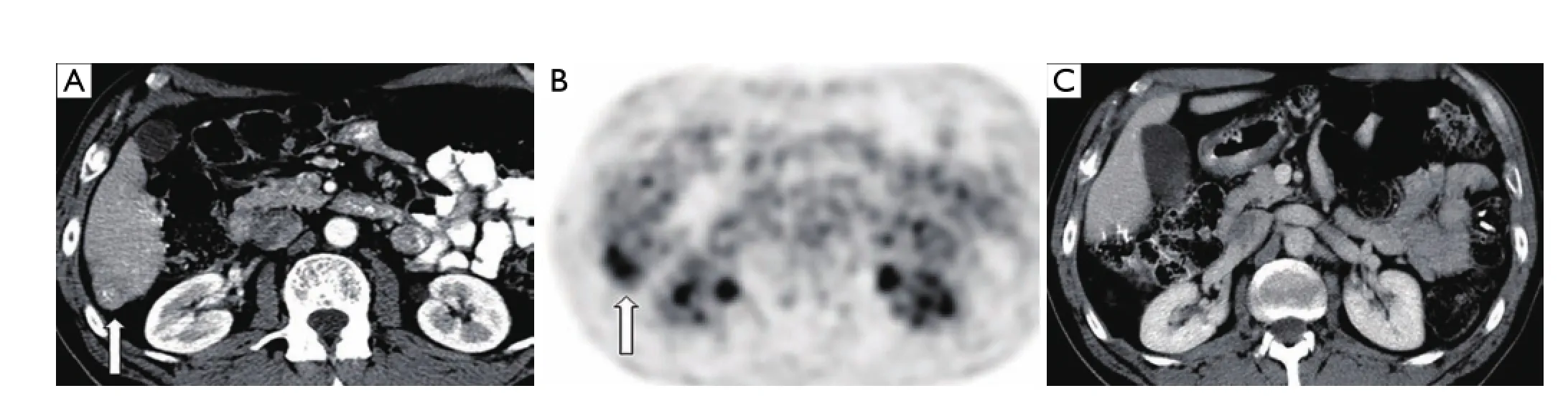
Figure 3 A 64-year-old female s/p right hemicolectomy for adenocarcinoma presenting with solitary segment 6 metastases discovered 3 years post-operatively on routine surveillance imaging. (A,B) Axial contrast enhanced CT image and corresponding PET image demonstrating FDG-avid segment 6 liver metastasis (arrow); (C) post-operative axial CT image obtained 8 years after laparoscopic liver resection demonstrating no residual or recurrent disease.
The nearly 3,000 reported cases of LLR indicate that it is safe, feasible, and cost-e ff ective with demonstrable short-terms benefits and no negative impact on long-term outcomes. LLR is a reasonable fi rst-line approach in the treatment of CRC liver metastasis in select patients. LLR should only be performed in specialized centers by surgeons intimately acquainted with open and minimally invasive techniques. While a randomized prospective trial would be optimal to validate these results, no such study has been reported to date.
Radiofrequency ablation (RFA)
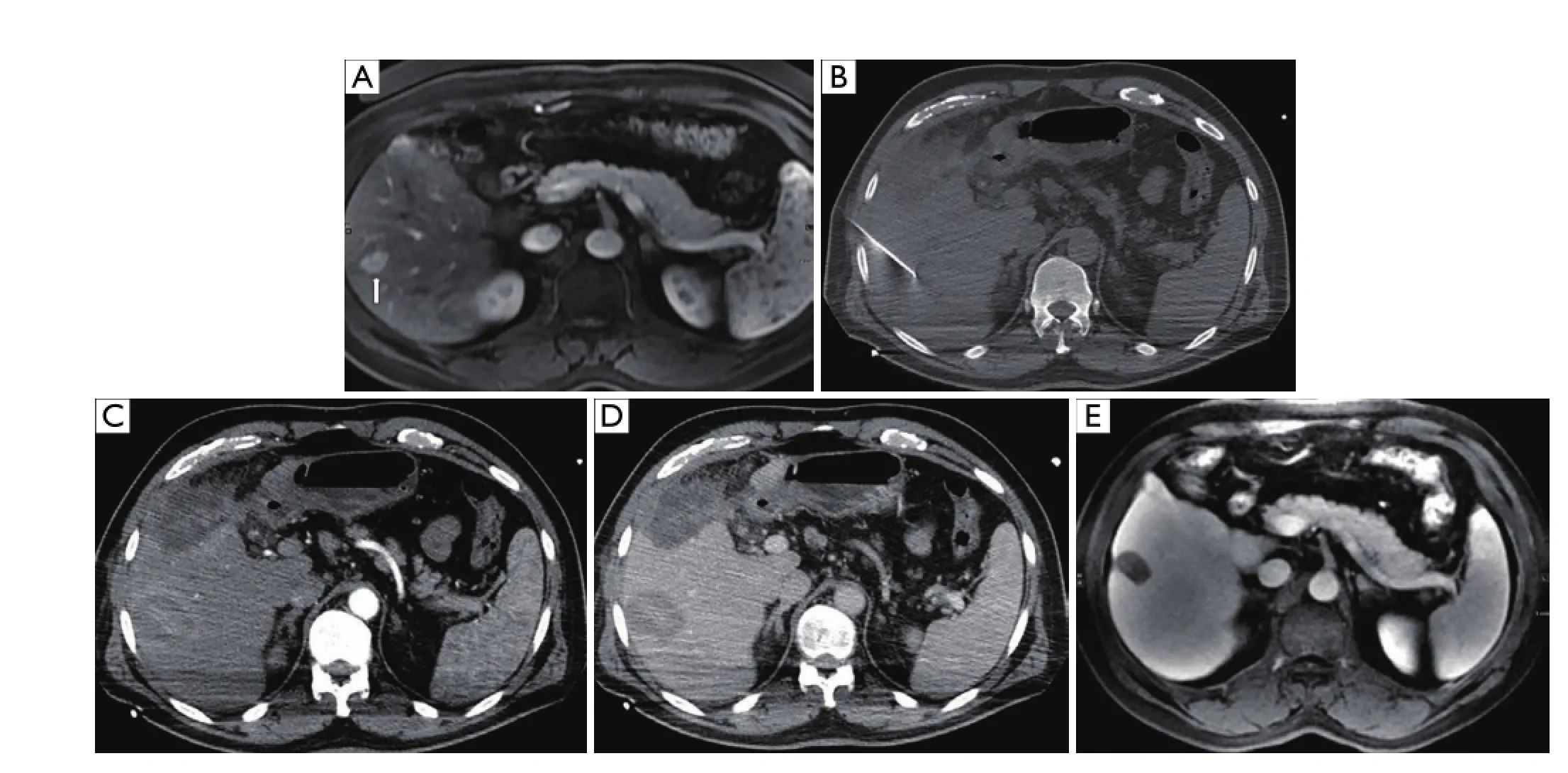
Figure 4 A 56-year-old male with hepatitis C complicated by hepatocellular carcinoma, not a surgical candidate, presenting with an isolated tumor in segment 6 of the liver. (A) T2 weighted axial MRI image demonstrates lesion in segment 6 (arrow). (B) Non-contrast axial CT image during procedure with applicator in the hypoattenuating mass. Immediate contrast-enhanced post-RFA axial CT images in arterial (C) and portal venous (D) phases show complete ablation in the area of the tumor. (E) T2 weighted axial MRI image at two year follow-up demonstrates complete necrosis of the segment 6 tumor. RFA, radiofrequency ablation.
RFA has been used extensively in the setting of both primary and metastatic liver tumors (Figure 4). Weis et al. recently published a Cochrane database analysis on the use ofRFA for the treatment of HCC49. The authors identified and included 11 randomized clinical trials with a total of 1,819 participants with four comparisons: RFA vs. hepatic resection (three trials, 578 participants50-52); RFA vs. PEI (six trials, 1,088 participants53-58); RFA vs. MWA (one trial, 72 participants59), and RFA versus percutaneous laser ablation (PLA) (one trial, 81 participants60) with the primary outcome measure being overall survival. After analysis, the authors concluded that there was moderate quality of evidence that hepatic resection is superior to RFA regarding survival; however, RFA might be associated with fewer complications and shorter hospital stay. They also found moderate quality evidence that RFA is superior to PEI in regards to survival.ere was insu ffi cient evidence to make fi rm conclusions regarding RFA, in comparison to locally ablative techniques such as MWA or PLA. Similar conclusions can be drawn regarding the clinical utility of RFA for treatment of metastatic lesions to the liver. A summary of studies comparing RFA versus surgery for liver metastases published since 2007 is shown in Table 261-72. Surgical resection was found to be superior to RFA in overall survival when feasible.
However, understanding the role of RFA in comparison to surgical resection has been confounded by both inhomogeneity in both treatment techniques and patient populations between the two groups. Two prospective randomized studies for RFA versus surgical resection were determined by Weis et al.49to have the smallest degree of potential bias: 168 patients collated by Feng et al.52and 230 patients by Huang et al.50. In both studies, the authors achieved RFA zones with greater than 0.5 cm margins on the initial hospitalization prior to discharge, and open surgical resection was performed with the assistance of intraoperative ultrasound to ensure complete tumor resection. Though these studies demonstrated improved overall local recurrence rates with resection as compared to RFA, it came at the cost of increased morbidity with increased hospital stays and adverse events. A recent study by Lee et al. demonstrated that the cohort who underwent surgical resection was younger, withbeer liver function reserve and performance status than those who underwent RFA73. When accounting for this discrepancy in populations using propensity score analysis, RFA was found to be superior to surgical resection for patients with small HCC and Child-Pugh Turcoe scores of 5.
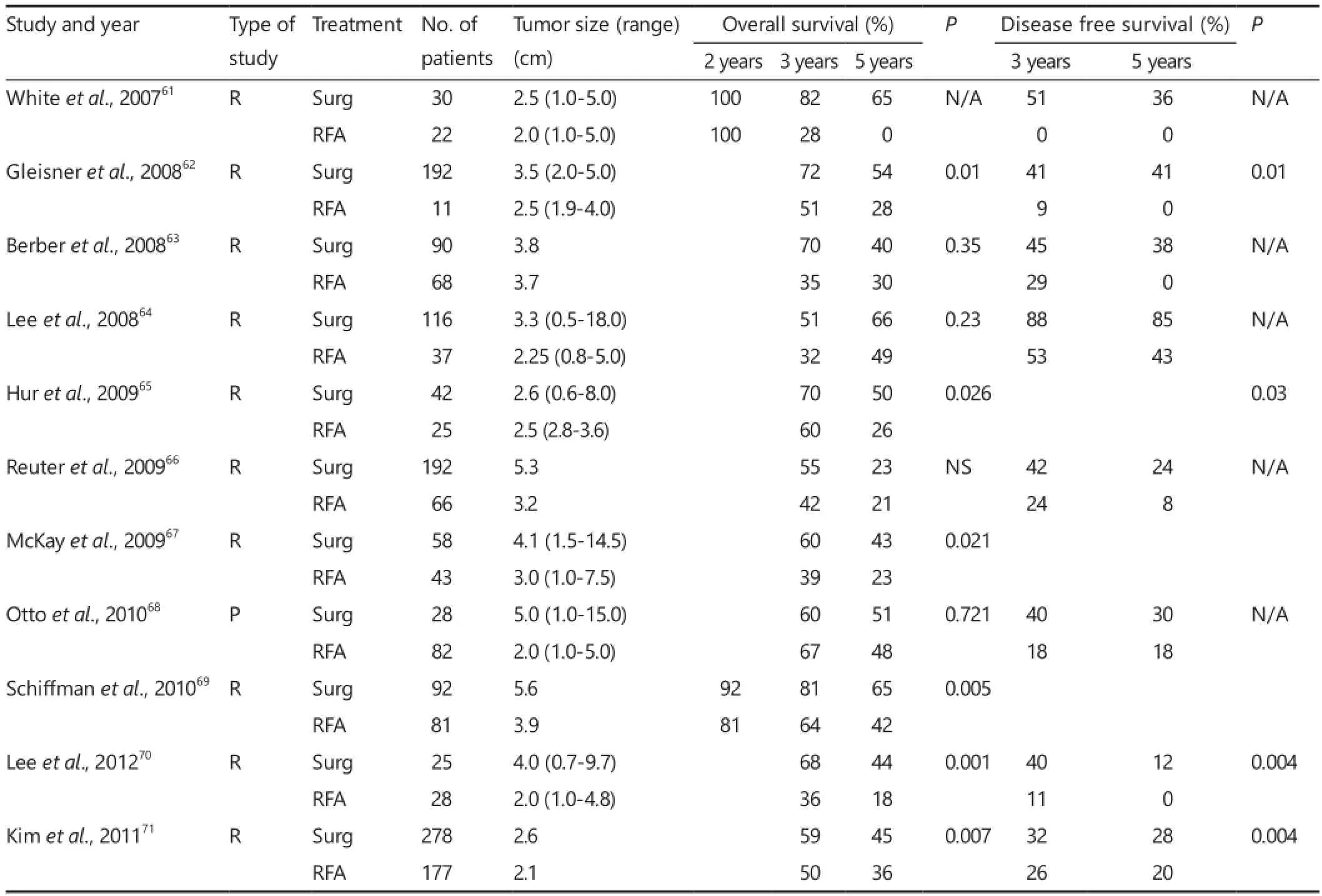
Table 2 Surgery versus RFA
Microwave ablation (MWA)
In MWA, local tissue hyperthermia is created through the direct application of an electromagnetic field which causes dielectric hysteresis35. As such, MWA can readily penetrate through various tissue types including those with high impedance such as lung or char tissue, where RFA is limited35,41. High tissue temperatures can be achieved with MWA, allowing for increased efficacy of ablations as compared to RFA. In addition, preclinical data suggests MWA is not as influenced by “heat-sink”effects next to major vessels as compared to RFA , where heat dissipation occurs as a result of blood fl ow74.ough grounding pads are not necessary for MWA, burns still remain as a potential complication since high temperatures can be achieved which propagate along the microwave applicator shaft that result in entry site injury41.
Given its increased efficacy of ablation and shorter time to achieve ablations, MWA has increasingly been used in the treatment of both primary and metastatic tumors of the liver (Figure 5). Ding et al. recently evaluated a series of 198 patients (85 RFA/113 MWA) with HCC meeting Milan criteria and found similar disease-free survival, cumulative survival, and complication rates between the two groups75,76. In their series, all patients were BCLC Stage A, and tumor size was equivalent between the two groups (mean tumor diameter 2.38±0.81 cm RFA cohort; 2.55±0.89 MWA cohort). Shibata et al. compared the efficacy of MWA versus RFA in a series of 72 patients (36 RFA/36 MWA) in a randomized fashion from a cohort of patients with equivalent background demographics and mean tumor size and concluded that therapeutic effects, complication rates, and rates of residual untreated disease were equivalent between the two modalities59. Zhang et al. evaluated overall survival, complete ablation, local tumor progression and distant recurrence in a series of 155 patients (mean tumor size 2.3±0.4 cm RFA cohort and 2.2±0.4 cm MWA cohort) and also found that RFA and MWA were equivalent77.
External beam radiation therapy including SBRT
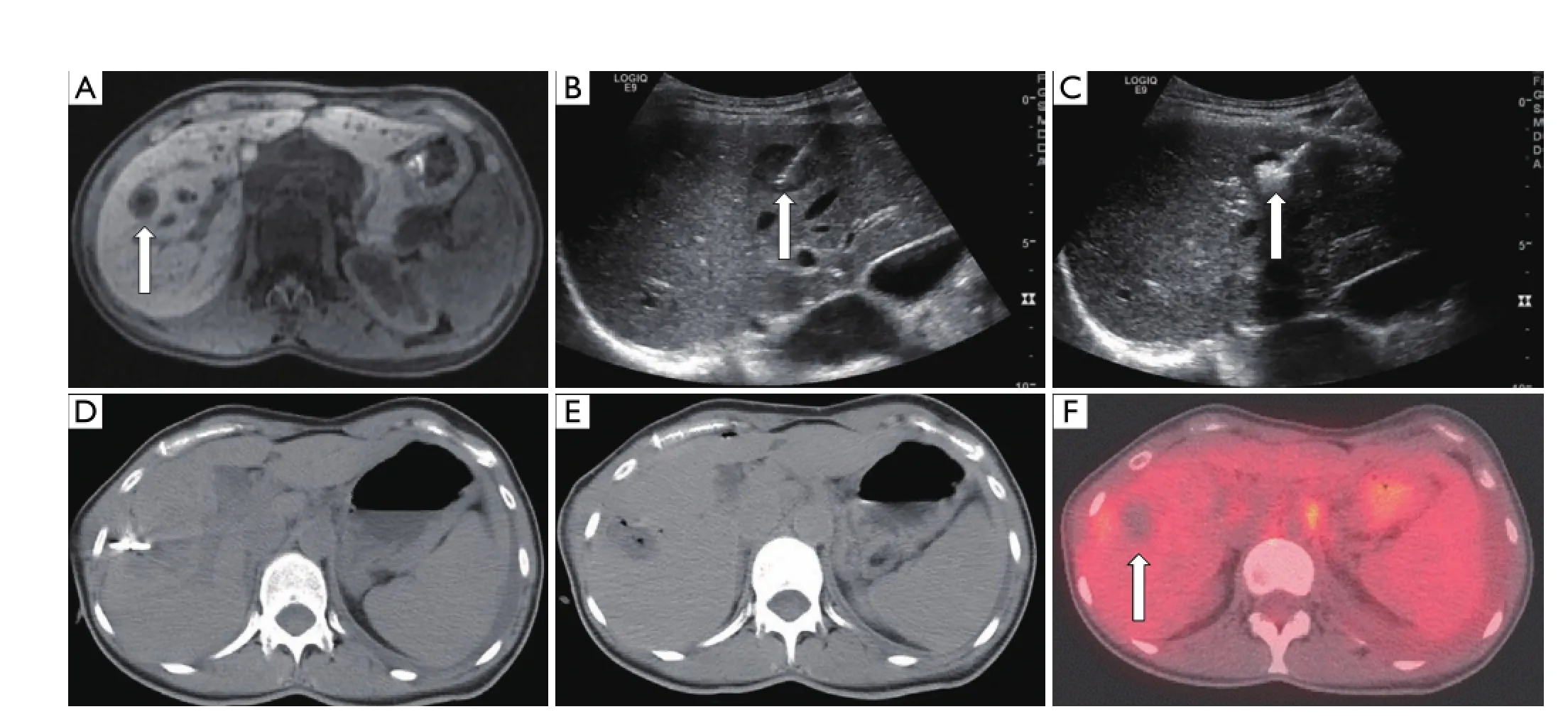
Figure 5 A 40-year-old female with metastatic breast carcinoma who presents with a focal metastatic tumor to segment 7 of the liver. (A) Axial post-contrast MRI image demonstrates 1.7×1.1 cm2hypointense mass in segment 7 (arrow). Intraprocedural ultrasound images (B) During needle placement (arrow), (C) During ablation. Note the hyperechoic area which represents microbubble formation during heating (arrow). (D) Non-contrast axial CT image during procedure demonstrates applicator in place. (E) Non-contrast axial CT image immediately after ablation shows a hypodense region with focal air bubbles indicative of the ablation zone. (F) Fused axial PET-CT image 3 months post-ablation demonstrating ablation cavity with no evidence of residual FDG-avidity (arrow).
Historically, the use of external beam radiation therapy in treatment of liver tumors has been limited due to the overall low tolerance of liver tissue to radiation78. Radiation producestumor cell kill by depositing energy within atoms, causing transformation into free radicals. This results in direct DNA damage, as well as indirect DNA and cellular damage through generation of reactive oxygen species. Ultimately, generation of DNA double strand breaks leads to tumor cell death. Radiation can achieve excellent tumor control when delivered to ablative doses79, but dose is limited due to the radiation tolerance of the surrounding normal liver tissue and adjacent organs. Radiationinduced liver disease is a feared complication of treatment, classically manifesting as a triad of anicteric hepatomegaly, ascites, and elevation of alkaline phosphatase.
With recent advances in technology, radiation can be directed to the tumor while minimizing exposure of surrounding normal liver. Imaging techniques have improved, allowing for precise delineation of hepatic tumors. Breathing motion control and image guidance both before and during treatment delivery permit tumor-directed treatment with accurate localization80,81, reducing treatment uncertainty and decreasing the margin of error. Treatment planning techniques and machines has also improved, allowing highly conformal treatment delivery. With increased conformality comes the potential to deliver higher doses of radiation and thereby increase local control without increasing toxicity. As a result of these advances, radiation is being re-explored as a treatment modality for both primary and metastatic liver tumors.
Over the past decade, multiple studies have been published on the use of conformal radiation treatment for hepatic malignancies, and results have been favorable with high rates of local control79,82-88. Though majority of these studies were small, and many are retrospective, they have provided ample background data to establish current prospective studies and randomized trials. Currently open to accrual is Radiation Therapy Oncology Group (RTOG) 1112, a phase III protocol randomizing patients with unresectable HCC to monotherapy with sorafenib, or sequential tumor-directed radiation followed by sorafenib89.
SBRT is a recently developed technique that allows high conformal radiation treatment by utilizing multiple, noncoplanar beams or arcs to target the tumor with millimeter precision. Compared to standard external beam radiation (3-D or intensity modulated RT), SBRT can create a rapid radiation dose fall off, allowing ablative radiation doses to be delivered to gross tumors while sparing adjacent tissue (Figure 6). As a result, SBRT has emerged as the primary technique of delivering radiation to liver tumors.
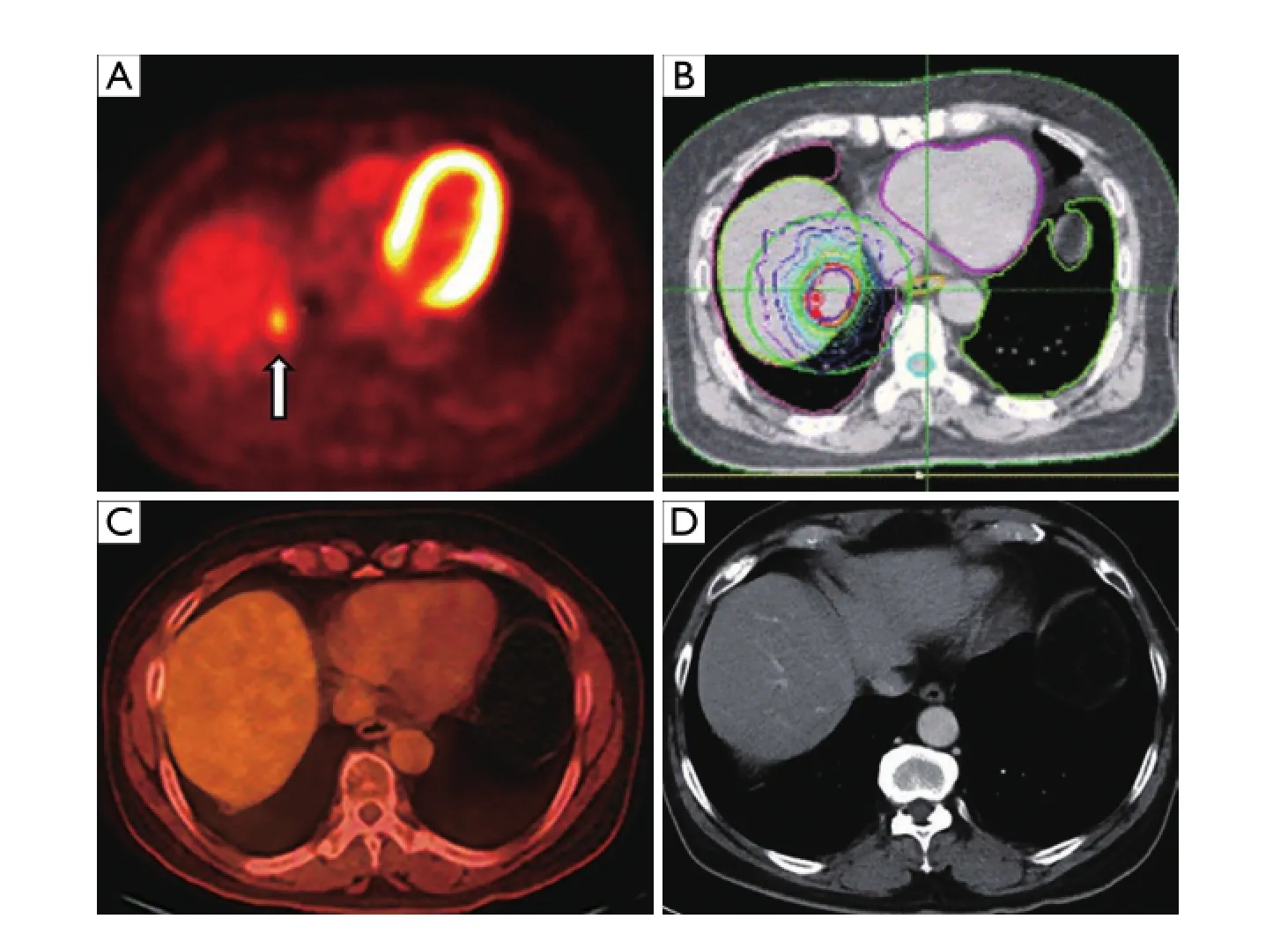
Figure 6 A 61-year-old male with history of localized rectal cancer treated with chemoradiation and surgery; subsequently diagnosed 2 years later with isolated liver lesion growing in size and avidity, biopsied positive for metastatic rectal cancer. Patient initially refused all invasive procedures including surgery, opting for chemotherapy alone, then ultimately agreed to SBRT. (A) Pretreatment axial fused PET-CT image demonstrating metastatic segment 7 liver lesion with SUV 3.8 (arrow). (B) Treatment plan depicting prescription isodose line (in red) with rapid dose fall o ff around the tumor. (C) Axial fused PET-CT obtained 18 months after SBRT demonstrating no evidence of disease. (D) Axial contrast enhanced CT image obtained 4 years after SBRT demonstrating no evidence of disease. SBRT, stereotactic body radiation therapy.
SBRT treatment is delivered as follows. First, to reduceuncertainty from breathing motion, internal fiducial marker placement is utilized in many institutions to allow tumor tracking. Additional ways to account for or restrict liver movement include use of breath hold technique, abdominal compression or respiratory gating. Internal placement of fi ducial markers is often performed to facilitate tumor targeting and tracking. Typically, gold fi ducials are used and placed in vicinity of the tumor approximately 1 week before treatment planning CT, and typically at least two or more fiducials are placed in non-co-planar fashion. Markers are placed percutaneously with image guidance under local anesthesia; it is an outpatient procedure with standard risks from introducing a needle into the liver (bleeding, infection, seeding, pain) and small risk of fiducial migration90. After fiducial placement, a pre-treatment CT is obtained for radiation planning purposes; this is ideally performed with multi-phasic IV contrast in exhale breath-hold po sition. A diagn ostic MRI o r CT is also utilized to de fi ne the tumor volume. The gross tumor volume (GTV) is contoured by the radiation oncologist on each slice of the pre-treatment CT. A clinical target volume (CTV) can be added to account for microscopic extension; in many cases, there will be no CTV expansion necessary. Finally, a planning target volume (PTV) expansion is added to the GTV to account for daily setup error and internal organ motion.e size and number of lesions that can be targeted, and dose of radiation that can be delivered, is dependent primarily on normal liver reserve and estimated risk of liver complications. Patients with poor liver function may require dose reduction to reduce the likelihood of complication85. Childs Pugh class is one measure of estimating normal liver function; for Childs Pugh category B, reduction in radiation dose may be a consideration. Childs Pugh category C is less commonly treated to ablative doses, given poor functional reserve and high risk of toxicity. An alternate method of estimating normal liver function is measurement of Veff, and is currently utilized in RTOG 111289. Ve ff is utilized as an aid in dose prescription, along with standard metrics such as the mean liver dose. For example, for a fi ve fraction treatment, the prescribed total dose ranges from 27.5 to 50 Gy depending on the e ff ective liver volume89. At least 700 cc of normal liver should receive less than 15 Gy in order to maintain a <5% risk of RILD78.
Typically, treatment is delivered in 3-6 fractions, with minimum 1-3 days between each fraction. Depending on location, it is possible to target multiple tumors in a single fraction. The actual radiation treatment is less than 1 hour in duration. Because it is non-invasive and painless, no sedation or anesthesia is required.
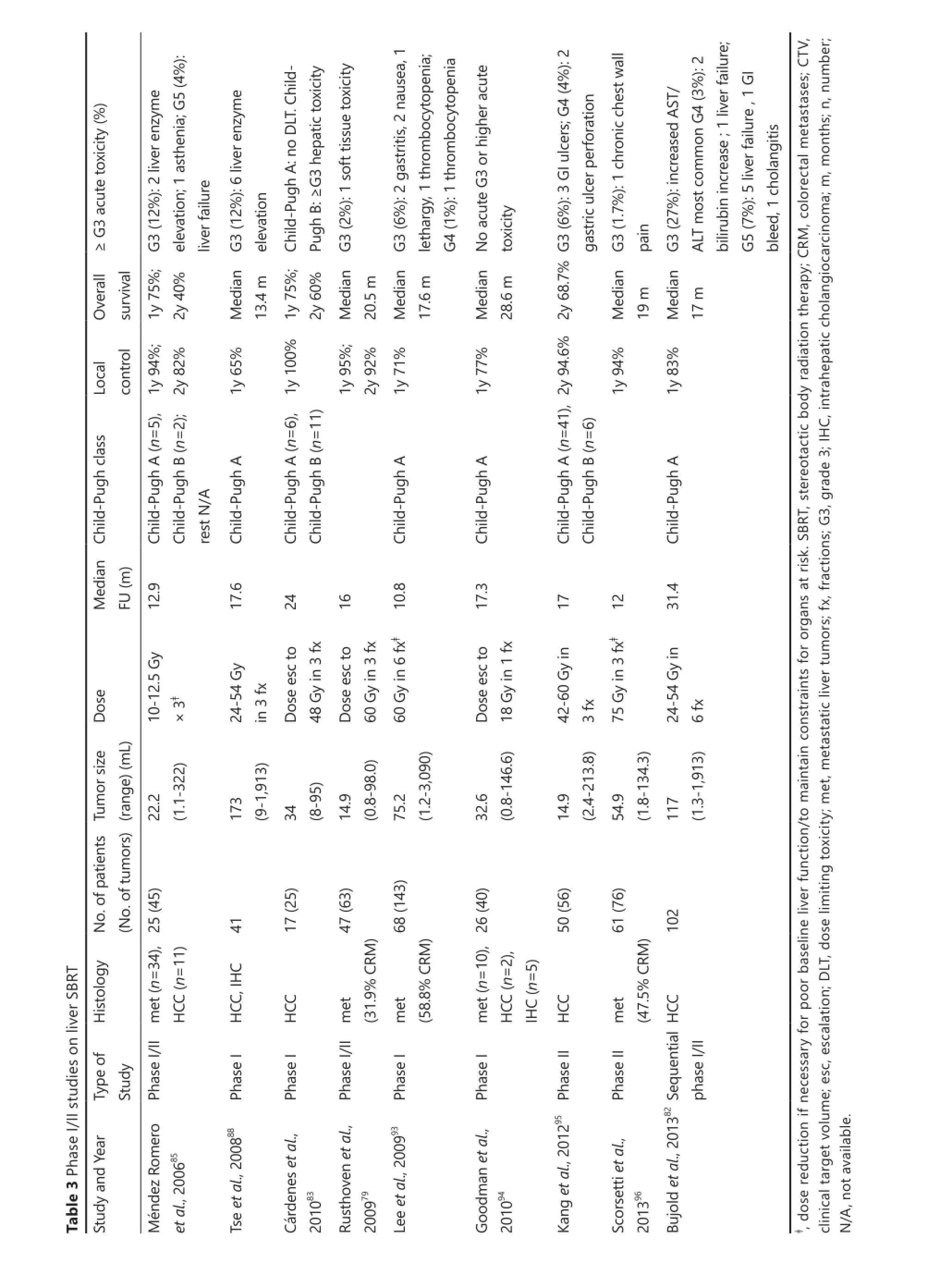
The utility of SBRT as a treatment for unresectable liver tumors was fi rst reported in 1995 by Blomgren and colleagues91. Since then, there have since then been several additional series reporting excellent local control outcomes demonstrating low toxicity, feasibility and e ffi cacy83-85,87,91,92. In general, treatment is delivered in 3-6 fractions, to total doses ranging from 30-60 Gy. With the more generally adopted dosing regimens, overall local control rates for small liver tumors (6 cm or less) ranges from 70%-90% at 2 years. It has also been shown that higher doses are associated with improved local control82. Toxicity rates are associated with poor baseline liver dysfunction, stressing the im p o rtance o f careful patient and d o se selectio n85. Table 3 provides a summary of recent phase I/II studies that have reported treatment outcomes for primary and/or metastatic liver
The single institution phase I/II trial by Mendez Romero et al.85was an initial study with promising results, that provided rationale for subsequent investigation. In this report, 34 metastatic and 11 primary liver tumors with treated with SBRT. Median tumor size was 3.2 (range, 0.5-7.2) cm. Dose prescribed ranged from 10-12.5 Gy × 3 fractions, or 5 Gy × 5. Local control rates were 94% and 84% at 1 and 2 years, respectively. Four patients had grade 3 or higher acute toxicity, including a grade 5 toxicity in a Child-Pugh class B HCC patient, who developed liver failure. A phase I trial by Goodman et al. recently explored the use of single fraction SBRT for primary and metastatic liver tumors, demonstrating com parable e ffi cacy with low toxicity94. However, single fraction treatment has not been widely adopted into clinical practice outside the co n fi nes of a clinical trial.e current ongoing RTOG 1112 trial delivers SBRT to unresectable HCC in 5 fractions of 5.5 to 10 Gy, depending on liver reserve, or e ff ective liver volume irradiated (Ve ff).
The impact of SBRT on overall survival is yet to be determined. The hetereogeneity of baseline liver function, variation in tumor size and comorbidities in the patient population of reported series precludes an accurate assessment of overall survival and local control outcomes across varying treatment modalities. This will need to be addressed in the future through randomized clinical trials. Preliminary studies suggest that liver SBRT is a non-invasive and e ff ective treatment modality for liver tumors in non-surgical candidates who are ineligible, or have failed other treatment modalities.
High dose rate (HDR) CT guided brachytherapy
Radiation has a dose-dependent effect on local control, but deliverable dose is limited by surrounding critical structures. Another technique of radiation delivery that has shown promising outcomes in single-institution series is HDR CT-guided interstitial brachytherapy97-99. Radiation is deliveredusing an iridium-192 source as a single fraction.e bene fi t of this technique is that radiation dose fall-o ff from the iridium-192 source is quite dramatic, allowing greater protection of the surrounding healthy liver compared to external radiation techniques. A prospective phase II trial by Ricke et al.100demonstrated favorable results with this technique for patients with liver tumors near the hilum, or large (>5 cm) tumors. With average dose of 17 Gy, local control at 12 months was 71% and 40%, respectively. Subsequent series have shown promising local control for large tumors. Collettini et al. reported outcomes of 35 patients with HCC, ranging in size from 5-12 cm, treated with HDR brachytherapy. All patients were Childs Pugh A or B. At 12 months, local control was 93% and no major toxicity was reported101. In conclusion, HDR brachytherapy may be another alternative for liver tumors that are not amenable to traditional ablative techniques, and further studies with longer follow up are warranted.
High-intensity focused ultrasound (HIFU)
HIFU incorporates multiple ultrasound beams produced by piezoelectric or piezoceramic transducers directed into a threedimensional focal point typically 1 to 5 mm in diameter and 10 to 50 mm in length102. The ultrasound beams delivered to the focal point are both thermally ablative and causes cavitation to the underlying tissues. Coupling of the ultrasound source and the patient is achieved through a degassed water bath to achieve minimal reflection or absorption of the sound waves prior to reaching the focal point. Motion needs to be minimized during the entire procedure and the focal zone is shifted sequentially to cover an entire area of interest for ablation. Hence, patients are typ ically in a con fi ned space for at least several hours for the procedure, and general anesthesia is recommended for patient comfort.
Several series have evaluated the safety and e ffi cacy of HIFU for the treatment of HCC103-109. Ng et al. reported on the safety and e ffi cacy of HIFU on the treatment of HCC (median tumor size 2.2 cm; range, 0.9 to 8 cm) for a series of 49 patients who were not surgical candidates and concluded that HIFU was an effective modality in this setting, with 1- and 3-year overall survival rates reported as 87.7% and 62.4%, respectively106. Wu et al. reported on the safety and efficacy of large HCCs (mean tumor diameter 8.1 cm; range, 4 to 14 cm) treated with HIFU and found favorable overall survival rates of 86.1%, 61.5%, and 35.3% at 6, 12, and 18 months, respectively107. Recently, Cheung et al. reported on the outcomes of HIFU performed for the treatment of HCC as a bridge to transplantation in 10 patients as compared to 29 patients who received transarterial chemoembolization and found excellent efficacy with HIFU (90% complete response/10% partial response) with none of the patients on the liver transplant list (n=5) dropping out103. Studies investigating the use of HIFU for the treatment of metastatic liver tumors are limited. Most studies do not address outcomes related to this defined subset of patients. Wu et al. reported on the largest series of patients who have undergone HIFU (n=1,038); however histologies and tumor locations were variable108. In their study, primary and metastatic liver tumors were reported on as a single cohort without analysis of the subgroups. Overall, these studies indicate that HIFU may serve as an excellent locally ablative technique for the treatment of HCC; however, technical implementation of the procedure serves as a signi fi cant barrier to widespread use.
Irreversible electroporation (IRE)
IRE is an ostensibly non-thermal technique in which the direct placement of electrodes is used to create a pulsed direct current inducing cytotoxicity in tumor cells by altering transmembrane potentials which irreversibly disrupt cell membrane integrity110. As compared to other percutaneous approach techniques, IRE requires the placement of at least two applicators in parallel to create ablation zones in the range of 1.5-2.0 cm per electrode pair111. The zone of ablation created by IRE is dependent on multiple factors including electrode spacing, relative positions, active tip length, pulse number, pulse duration, and applied voltage110,112. Hence, precise placement of at least two probes is necessary to create appropriate zone of ablations, making IRE more technically challenging than other locally ablative techniques. The current generated by IRE is known to cause whole-body muscle contractions and general anesthesia with the use of neuromuscular blockage is obligatory for its clinical use. In addition, IRE has been shown to induce cardiac arrhythmias, though this potential complication can be averted with the use of cardiac synchronization of the administered pulses to the complete refractory period of the cardiac cycle113.
IRE has a theoretical safety advantage over other locally ablative techniques in the treatment of tumors close to structures susceptible to thermal injury, such as major bile ducts in the liver. In addition, IRE may be more e ff ective in cytotoxicity for tumors next to major vessels, due to reduction in the heat-sink effect. Several small series have reported on the use of IRE for the treatment of liver tumors in locations thought to be unfavorable for other ablative therapies112,114-117. In a series of 18 HCC lesions in 11 patients treated with IRE by Cheung et al., 13/18 (72%) of lesions were completely ablated [13/14 (93%) of lesions<3 cm] with a local recurrence-free period of 18±4 months115.
Cannon et al. reported on 44 patients with both primary (n=14) and metastatic (n=20 colorectal; n=10 other) liver tumors treated with IRE; with local recurrence free survival of 97.4%, 94.6%, and 59.7% at 3, 6, and 12 months, respectively114. In their series, a trend towards higher recurrence rates for tumors>4 cm was identified (HR, 3.236; 95% CI, 0.585-17.891; P=0.178). Kingham et al. reported on the use of IRE in a series of 28 patients with 65 tumors (median 1 cm) and found a local recurrence rate of 5.7% at median follow-up of 6 months116. These small series suggest that IRE has improved efficacy in smaller lesions; and though local tumor control is excellent at 3-6 months, recurrence rates are higher aer 12-18 months.
Percutaneous ethanol injection (PEI)
PEI involves the direct instillation of ethanol into tumors ultimately resulting in coagulation necrosis. As such, the technique is relatively simple and inexpensive. In practice, PEI is limited by poor and uneven distribution of the ethanol within the tumor and di ff usion into the adjacent normal tissues. Ultrasound guidance allows for real-time monitoring of the ethanol dispersion and is generally used to help compensate for these limitations. Even so, multiple intra-procedural needle repositions are sometimes needed, given the uneven distribution of the ethanol118.e longest clinical experience and follow-up for patients is available for PEI in the treatment of HCC, with some studies documenting observation periods greater than 15 years119,120. However, as discussed in the RFA section, multiple studies have demonstrated superiority of RFA to PEI in patient overall survival. Hence, other locally ablative therapies have been favored in clinical practice as compared to PEI.
Cryoablation
Cryoablation involves the direct application of a cryoprobe into a tumor. The thermal contact with the tumor results in ice crystal formation and osmotic shock. One distinct advantage of cryoablation as compared to other ablative techniques is that the zone of ablation is readily visible (i.e., “iceball”) using noncontrast CT, ultrasound, or MRI monitoring, allowing for precise targeting of a zone of ablation to the tumor121. In addition, multiple probes can be used in tandem to create larger ablation zones and shorten procedural times. Despite the technical advantages of cryoablation, its complication pro fi le has limited its general use in the treatment of liver tumors.ough uncommon, cryo-shock is a potentially life-threatening complication distinct to cryoablation, characterized by thrombocytopenia, acute renal failure, adult respiratory distress syndrome and disseminated intravascular
coagulopathy121. A meta-analysis performed by Huang et al. investigated the role of cryoablation in comparison to RFA in the treatment of unresectable HCC122. Outcomes analyzed included mortality, complication rate and local recurrence; and RFA was found to be superior to cryoablation, particularly in regards to complication rates (OR 2.80; 95% CI, 1.54-5.09) and local tumor recurrence (OR 1.96; 95% CI, 1.12-3.42).
Percutaneous laser ablation (PLA)
PLA involves the direct deposition of laser light via fiberoptic applicators to induce tissue hyperthermia in tumors. Proponents of PL A advo cate that the thin fl exible fi beroptic delivery fi bers allow for safer and technically easier approaches to tumors as compared to other ablative techniques123. In addition, ablative zones can be controlled with feedback and dose-planning systems. Hence, low complication rates are associated with PLA. Vogl et al. reported on the use of PLA in 899 patients with 2,520 liver tumors and demonstrated a major complication rate of 2%; the majority being either pleural e ff usion (n=16; 0.8%) requiring thoracentesis or hepatic abscess requiring drainage (n=15; 0.7%)124. Evidence regarding the use of PLA for the treatment of liver tumors is limited. Pacella et al. retrospectively analyzed the use of PLA for the treatment of HCC in a cohort of 432 cirrhotic patients (344 with a single nodule ≤4 cm; 88 with two or three nodule <3 cm)125. An initial complete response was reported in 344 patients (78%) with median overall survival of 47 months (95% CI, 41 to 53 months). Multivariate analysis con fi rmed the achievement of complete ablation (P=0.001; RR =0.517) as an independent predictor of survival. Ferrari et al. evaluated the use of PLA as compared to RFA in a prospective randomized study of 81 cirrhotic patients with HCC60.ere was a homogeneous distribution in age, sex, Child-Pugh class and HCC nodule dimensions between the two cohorts with RFA found to have beer initial tumor ablation as compared to PLA (94% vs. 78%). In addition, it was suggestive that the RFA cohort had improved overall survival as compared to PLA at 1, 3 and 5 years (92.2% vs. 88.6%, 75.0% vs. 70.4%, 40.9% vs. 22.9%; respectively), however the di ff erence did not reach statistical signi fi cance (P=0.3299).ough the evidence is still limited, there is suggestion that PLA is limited in achieving complete tumor ablation as compared to other locally ablative therapies.
Combination treatment approaches
There are multiple theoretical advantages in combining locally ablative therapies with each other or to other treatment options such as transarterial embolotherapy. Several of thedescribed modalities have different principles of action in creating cytotoxicity and combining them may potentiate their effectiveness, improving tumor control. In combination, dose and energy profiles for each locally ablative modality may be potentially reduced without comprising cytotoxicity to tumor cells; increasing the safety margin to adjacent normal tissues. Combination approaches also allow for limitations of each modality on its own to be overcome, thus expanding the number of patients eligible for therapeutic options.
The greatest cumulated evidence exists for the use of RFA in combination with transarterial embolotherapy. In theory, the decreased blood fl ow induced by transarterial embolization reduces heat loss, improving the RFA margins. In addition TACE enhances nearby control of satellite lesions126. Conceptually, the practice is similar to chemotherapy in addition to surgical resection for oncologic treatments. Ablation provides localized curative treatment of the tumor similar to surgery while TAE provides control of micrometastases similar to chemotherapy. In 2013, several meta-analyses cumulated the randomized controlled trials available for RFA plus TACE as compared to RFA alone127-129. All of the meta-analyses concluded that there was high quality evidence suggesting that TACE in combination with RFA improved survival outcomes as compared to RFA alone for patients with HCC, particularly for tumors larger than 3 cm in size. Peng et al.130studied a series of 189 patients with HCC less than 7cm, randomly assigned to TACE plus RFA or RFA alone and found that the TACE plus RFA cohort had improved overall and recurrence free survival (HR, 0.525; 95% CI, 0.335-0.822; P=0.002; HR, 0.575; 95% CI, 0.374-0.897; P=0.009, respectively).
The use of localized radiation in combination with thermal ablatio n is of particular interest fo r future investigation. Hyperthermia has been proven to potentiate the cytotoxic e ff ect of radiation and has been clinically adopted131,132. In addition, recent animal studies have suggested that the use of radiation in combination with RFA resulted in improved tumor growth control as compared to RFA alone133,134. In a review by Ahmed et al., the combination of therapies were found to have increased tumor necrosis, decreased tumor growth and improved overall animal survival in a rat tumor model41. The mechanism for this potentiation is still unknown. One potential etiology is from increased blood flow after ablation resulting in improved oxygenation which potentiates free radical formation with subsequent radiation. Another is increased free-radical formation after radiation resulting in inhibition of tumor cell recovery which potentiates the e ff ects of thermal ablation41.
The combination of thermal ablation in tandem with SBRT is complimentary from a technical standpoint and is readily adaptable into clinical practice.ermal ablative techniques o ff er the added clinical bene fi t of tissue diagnosis that can be obtained immediately prior to the procedure that is usually necessary for radiation oncologists to appropriately initiate treatments. In addition, fi ducial seed placement sometimes necessary for SBRT localization can be performed at the same time. Conversely, SBRT can provide improved tumor control in areas where thermal ablation is known to have high failure rates, such as next to major vessels in the setting of RFA. In addition, doses for radiation and ablation zones for thermal ablation can be reduced to preserve normal tissue parenchyma without compromising efficacy in tumor control. The combination of these therapies serves as an exciting avenue of research with clinical studies necessary to validate proper dosing and timing regimens.
Conclusion
Minimally invasive therapies for the treatment of both primary and metastatic liver tumors continue to highlight the fact that we are privileged to practice medicine during a time of such dynamic change and innovation. As such, both laparoscopic resection and locally ablative therapies have solidified a role in the treatment paradigms of both primary and metastatic liver tumors, including the most recently validated prognostic treatment strategy, the HKLC schema.
Based upon its principle of action and technical aspects of implementation, each local therapy has its benefits and drawbacks in clinical practice.e greatest cumulative evidence exists for surgical resection and RFA. As both cost and quality of life become further incorporated into treatment paradigms, minimally invasive local therapeutic options will continue their trends towards increased utilization.
As summarized in the principles of action section, locally ablative therapies harness the newest technological developments into various devices that are able to induce localized cytotoxicity to tumor cells while minimizing damage to nearby native tissues. MWA, HIFU, and SBRT are all relatively nascent technologies, though preliminary data regarding their use is promising, with overall survivals similar to RFA. These technologies expand upon the patients eligible for local ablation by o ff ering di ff erent technical advantages as compared to RFA.
Tandem approaches with combination of local ablation such as RFA in addition to locoregional therapies such as TACE have already been explored with promising results demonstrating improved overall survival as compared to RFA alone. In addition, new technologies such as IRE are currently being clinically investigated to further expand the patients eligible to safely undergo local ablation.
Con fl ict of interest statement
No potential con fl icts of interest are disclosed.
1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011;61:69-90.
2. Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet 2012;379:1245-1255.
3. Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin 2005;55:74-108.
5. Simard EP, Ward EM, Siegel R, Jemal A. Cancers with increasing incidence trends in the United States: 1999 through 2008. CA Cancer J Clin 2012;62:118-128.
6. Geoghegan JG, Scheele J. Treatment of colorectal liver metastases. Br J Surg 1999;86:158-169.
7. Mahnken AH, Pereira PL, de Baère T. Interventional oncologic approaches to liver metastases. Radiology 2013;266:407-430.
8. Villanueva A, Hernandez-Gea V, Llovet JM. Medical therapies for hepatocellular carcinoma: a critical view of the evidence. Nat Rev Gastroenterol Hepatol 2013;10:34-42.
9. Llovet JM, Decaens T, Raoul JL, Boucher E, Kudo M, Chang C, et al. Brivanib in patients with advanced hepatocellular carcinoma who were intolerant to sorafenib or for whom sorafenib failed: results from the randomized phase III BRISK-PS study. J Clin Oncol 2013;31:3509-3516.
10. Zhu AX, Kudo M, Assenat E, Caan S, Kang YK, Lim HY, et al. E ff ect of everolimus on survival in advanced hepatocellular carcinoma aer failure of sorafenib: the EVOLVE-1 randomized clinical trial. JAMA 2014;312:57-67.
11. Benson AB 3rd, Abrams TA, Ben-Josef E, Bloomston PM, Botha JF, Clary BM, et al. NCCN clinical practice guidelines in oncology: hepatobiliary cancers. J Natl Compr Canc Netw 2009;7:350-391.
12. Benson AB 3rd, Bekaii-Saab T, Chan E, Chen YJ, Choti MA, Cooper HS, et al. Metastatic colon cancer, version 3.2013: featured updates to the NCCN Guidelines. J Natl Compr Canc Netw 2013;11:141-152; quiz 152.
13. Brouquet A, Abdalla EK, Kopetz S, GarreCR, Overman MJ, Eng C, et al. High survival rate aer two-stage resection of advanced colorectal liver metastases: response-based selection and complete resection de fi ne outcome. J Clin Oncol 2011;29:1083-1090.
14. Johnston FM, Mavros MN, Herman JM, Pawlik TM. Local therapies for hepatic metastases. J Natl Compr Canc Netw 2013;11:153-160.
15. Pawlik TM, Olino K, Gleisner AL, Torbenson M, Schulick R, Choti MA. Preoperative chemotherapy for colorectal liver metastases: impact on hepatic histology and postoperative outcome. J Gastrointest Surg 2007;11:860-868.
16. Nathan H, Segev DL, Mayo SC, Choti MA, Cameron AM, Wolfgang CL, et al. National trends in surgical procedures for hepatocellular carcinoma: 1998-2008. Cancer 2012;118:1838-1844.
17. Tanabe G, Sakamoto M, Akazawa K, Kurita K, Hamanoue M, Ueno S, et al. Intraoperative risk factors associated with hepatic resection. Br J Surg 1995;82:1262-1265.
19. European Association of the Study of the Liver. 2011 European Association of the Study of the Liver. 2011 European Association of the Study of the Liver hepatitis C virus clinical practice guidelines. Liver Int 2012;32 Suppl 1:2-8.
20. Chapiro J, Geschwind JF. Hepatocellular carcinoma: have we fi nally found the ultimate staging system for HCC? Nat Rev Gastroenterol Hepatol 2014;11:334-336.
21. Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis 1999;19:329-338.
22. Hsu CY, Hsia CY, Huang YH, Su CW, Lin HC, Lee PC, et al. Selecting an optimal staging system for hepatocellular carcinoma: comparison of 5 currently used prognostic models. Cancer 2010;116:3006-3014.
23. Salem R, Mazzaferro V, Sangro B. Yrium 90 radioembolization for the treatment of hepatocellular carcinoma: biological lessons, current challenges, and clinical perspectives. Hepatology 2013;58:2188-2197.
24. Salem R, Lewandowski RJ. Chemoembolization and radioembolization for hepatocellular carcinoma. Clin Gastroenterol Hepatol 2013;11:604-611; quiz e43-44.
25. Memon K, Lewandowski RJ, Riaz A, Salem R. Chemoembolization and radioembolization for metastatic disease to the liver: available data and future studies. Curr Treat Options Oncol 2012;13:403-415.
26. Yau T, Tang VY, Yao TJ, Fan ST, Lo CM, Poon RT. Development of Hong Kong Liver Cancer staging system with treatment strati fi cation for patients with hepatocellular carcinoma. Gastroenterology 2014;146:1691-1700.
27. Mado ff DC, Hicks ME, Vauthey JN, Charnsangavej C, Morello FA Jr, Ahrar K, et al. Transhepatic portal vein embolization: anatomy, indications, and technical considerations. Radiographics 2002;22:1063-1076.
28. Huang G, Chen X, Lau WY, Shen F, Wang RY, Yuan SX, et al. Quality of life aer surgical resection compared withradiofrequency ablation for small hepatocellular carcinomas. Br J Surg 2014;101:1006-1015.
29. Cunningham SC, Tsai S, Marques HP, Mira P, Cameron A, Barroso E, et al. Management of early hepatocellular carcinoma in patients with well-compensated cirrhosis. Ann Surg Oncol 2009;16:1820-1831.
30. DuBay DA, Sandroussi C, Kachura JR, Ho CS, BeecroJR, Vollmer CM, et al. Radiofrequency ablation of hepatocellular carcinoma as a bridge to liver transplantation. HPB (Oxford) 2011;13:24-32.
31. Heckman JT, Devera MB, Marsh JW, Fontes P, Amesur NB, Holloway SE, et al. Bridging locoregional therapy for hepatocellular carcinoma prior to liver transplantation. Ann Surg Oncol 2008;15:3169-3177.
32. Katz AW, Chawla S, Qu Z, Kashyap R, Milano MT, Hezel AF. Stereotactic hypofractionated radiation therapy as a bridge to transplantation for hepatocellular carcinoma: clinical outcome and pathologic correlation. Int J Radiat Oncol Biol Phys 2012;83:895-900.
34. Jaeck D, Oussoultzoglou E, Rosso E, Greget M, Weber JC, Bachellier P. A two-stage hepatectomy procedure combined with portal vein embolization to achieve curative resection for initially unresectable multiple and bilobar colorectal liver metastases. Ann Surg 2004;240:1037-1049; discussion 1049-1051.
35. Knavel EM, Brace CL. Tumor ablation: common modalities and general practices. Tech Vasc Interv Radiol 2013;16:192-200.
38. Reddy SK, Tsung A, Geller DA. Laparoscopic liver resection. World J Surg 2011;35:1478-1486.
39. Nguyen KT, Marsh JW, Tsung A, Steel JJ, Gamblin TC, Geller DA. Comparative bene fi ts of laparoscopic vs open hepatic resection: a critical appraisal. Arch Surg 2011;146:348-356.
40. Castaing D, Vibert E, Ricca L, Azoulay D, Adam R, Gayet B. Oncologic results of laparoscopic versus open hepatectomy for colorectal liver metastases in two specialized centers. Ann Surg 2009;250:849-855.
41. Ahmed M, Brace CL, Lee FT Jr, Goldberg SN. Principles of and advances in percutaneous ablation. Radiology 2011;258:351-369.
42. Haemmerich D, Pilcher TA. Convective cooling affects cardiac catheter cryoablation and radiofrequency ablation in opposite directions. Conf Proc IEEE Eng Med Biol Soc 2007;2007:1499-1502.
43. Dupuy DE, Goldberg SN. Image-guided radiofrequency tumor ablation: challenges and opportunities--part II. J Vasc Interv Radiol 2001;12:1135-1148.
44. Goldberg SN. Radiofrequency tumor ablation: principles and techniques. Eur J Ultrasound 2001;13:129-147.
45. Rhim H, Dodd GD 3rd, Chintapalli KN, Wood BJ, Dupuy DE, Hvizda JL, et al. Radiofrequency thermal ablation of abdominal tumors: lessons learned from complications. Radiographics 2004;24:41-52.
46. Hu ff man SD, Hu ff man NP, Lewandowski RJ, Brown DB. Radiofrequency ablation complicated by skin burn. Semin Intervent Radiol 2011;28:179-182.
48. Lu DS, Raman SS, Limanond P, Aziz D, Economou J, Busuil R, et al. In fl uence of large peritumoral vessels on outcome of radiofrequency ablation of liver tumors. J Vasc Interv Radiol 2003;14:1267-1274.
49. Weis S, Franke A, M?ssner J, Jakobsen JC, Schoppmeyer K. Radiofrequency (thermal) ablation versus no intervention or other interventions for hepatocellular carcinoma. Cochrane Database Syst Rev 2013;12:CD003046.
50. Huang J, Yan L, Cheng Z, Wu H, Du L, Wang J, et al. A randomized trial comparing radiofrequency ablation and surgical resection for HCC conforming to the Milan criteria. Ann Surg 2010;252:903-912.
51. Chen MS, Li JQ, Zheng Y, Guo RP, Liang HH, Zhang YQ, et al. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg 2006;243:321-328.
52. Feng K, Yan J, Li X, Xia F, Ma K, Wang S, et al. A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma. J Hepatol 2012;57:794-802.
53. Brunello F, Veltri A, Carucci P, Pagano E, Ciccone G, Moreo P, et al. Radiofrequency ablation versus ethanol injection for early hepatocellular carcinoma: A randomized controlled trial. Scand J Gastroenterol 2008;43:727-735.
54. Giorgio A, Di Sarno A, De Stefano G, Scognamiglio U, Farella N, Mariniello A, et al. Percutaneous radiofrequency ablation of hepatocellular carcinoma compared to percutaneous ethanol injection in treatment of cirrhotic patients: an Italian randomized controlled trial. Anticancer Res 2011;31:2291-2295.
55. Lencioni RA, Allgaier HP, Cioni D, Olschewski M, Deibert P, Crocetti L, et al. Small hepatocellular carcinoma in cirrhosis: randomized comparison of radio-frequency thermal ablation versus percutaneous ethanol injection. Radiology2003;228:235-240.
56. Lin SM, Lin CJ, Lin CC, Hsu CW, Chen YC. Radiofrequency ablation improves prognosis compared with ethanol injection for hepatocellular carcinoma < or =4 cm. Gastroenterology 2004;127:1714-1723.
57. Lin SM, Lin CJ, Lin CC, Hsu CW, Chen YC. Randomised controlled trial comparing percutaneous radiofrequency thermal ablation, percutaneous ethanol injection, and percutaneous acetic acid injection to treat hepatocellular carcinoma of 3 cm or less. Gut 2005;54:1151-1156.
58. Shiina S, Teratani T, Obi S, Sato S, Tateishi R, Fujishima T, et al. A randomized controlled trial of radiofrequency ablation with ethanol injection for small hepatocellular carcinoma. Gastroenterology 2005;129:122-130.
59. Shibata T, Iimuro Y, Yamamoto Y, Maetani Y, Ametani F, Itoh K, et al. Small hepatocellular carcinoma: comparison of radio-frequency ablation and percutaneous microwave coagulation therapy. Radiology 2002;223:331-337.
60. Ferrari FS, Megliola A, Scorzelli A, Stella A, Vigni F, Drudi FM, et al. Treatment of small HCC through radiofrequency ablation and laser ablation. Comparison of techniques and long-term results. Radiol Med 2007;112:377-393.
61. White RR, Avital I, Sofocleous CT, Brown KT, Brody LA, Covey A, et al. Rates and paerns of recurrence for percutaneous radiofrequency ablation and open wedge resection for solitary colorectal liver metastasis. J Gastrointest Surg 2007;11:256-263.
62. Gleisner AL, Choti MA, Assumpcao L, Nathan H, Schulick RD, Pawlik TM. Colorectal liver metastases: recurrence and survival following hepatic resection, radiofrequency ablation, and combined resection-radiofrequency ablation. Arch Surg 2008;143:1204-1212.
63. Berber E, Tsinberg M, Tellioglu G, Simpfendorfer CH, Siperstein AE. Resection versus laparoscopic radiofrequency thermal ablation of solitary colorectal liver metastasis. J Gastrointest Surg 2008;12:1967-1972.
64. Lee WS, Yun SH, Chun HK, Lee WY, Kim SJ, Choi SH, et al. Clinical outcomes of hepatic resection and radiofrequency ablation in patients with solitary colorectal liver metastasis. J Clin Gastroenterol 2008;42:945-949.
65. Hur H, Ko YT, Min BS, Kim KS, Choi JS, Sohn SK, et al. Comparative study of resection and radiofrequency ablation in the treatment of solitary colorectal liver metastases. Am J Surg 2009;197:728-736.
66. Reuter NP, Woodall CE, Scoggins CR, McMasters KM, Martin RC. Radiofrequency ablation vs. resection for hepatic colorectal metastasis: therapeutically equivalent? J Gastrointest Surg 2009;13:486-491.
69. Schi ff man SC, Bower M, Brown RE, Martin RC, McMasters KM, Scoggins CR. Hepatectomy is superior to thermal ablation for patients with a solitary colorectal liver metastasis. J Gastrointest Surg 2010;14:1881-1886; discussion 1886-1887.
70. Lee H, Jin GY, Han YM, Chung GH, Lee YC, Kwon KS, et al. Comparison of survival rate in primary non-small-cell lung cancer among elderly patients treated with radiofrequency ablation, surgery, or chemotherapy. Cardiovasc Intervent Radiol 2012;35:343-350.
71. Kim KH, Yoon YS, Yu CS, Kim TW, Kim HJ, Kim PN, et al. Comparative analysis of radiofrequency ablation and surgical resection for colorectal liver metastases. J Korean Surg Soc 2011;81:25-34.
72. Leblanc F, Fonck M, Brunet R, Becouarn Y, Mathoulin-Pélissier S, Evrard S. Comparison of hepatic recurrences aer resection or intraoperative radiofrequency ablation indicated by size and topographical characteristics of the metastases. Eur J Surg Oncol 2008;34:185-190.
73. Lee YH, Hsu CY, Chu CW, Liu PH, Hsia CY, Huang YH, et al. Radiofrequency Ablation is Beeran Surgical Resection in Patients With Hepatocellular Carcinoma Within the Milan Criteria and Preserved Liver Function: A Retrospective Study Using Propensity Score Analyses. J Clin Gastroenterol 2014. [Epub ahead of print].
74. Schramm W, Yang D, Wood BJ, Raay F, Haemmerich D. Contribution of direct heating, thermal conduction and perfusion during radiofrequency and microwave ablation. Open Biomed Eng J 2007;1:47-52.
75. Ding J, Jing X, Liu J, Wang Y, Wang F, Wang Y, et al. Comparison of two di ff erent thermal techniques for the treatment of hepatocellular carcinoma. Eur J Radiol 2013;82:1379-1384.
76. Ding J, Jing X, Liu J, Wang Y, Wang F, Wang Y, et al. Complications of thermal ablation of hepatic tumours: comparison of radiofrequency and microwave ablative techniques. Clin Radiol 2013;68:608-615.
77. Zhang L, Wang N, Shen Q, Cheng W, Qian GJ.erapeutic e ffi cacy of percutaneous radiofrequency ablation versus microwave ablation for hepatocellular carcinoma. PLoS One 2013;8:e76119.
78. Marks LB, Yorke ED, Jackson A, Ten Haken RK, Constine LS, Eisbruch A, et al. Use of normal tissue complication probability models in the clinic. Int J Radiat Oncol Biol Phys 2010;76:S10-S19.
79. Rusthoven KE, Kavanagh BD, Cardenes H, Stieber VW, Burri SH, Feigenberg SJ, et al. Multi-institutional phase I/II trial of stereotactic body radiation therapy for liver metastases. J Clin Oncol 2009;27:1572-1578.
80. Wagman R, Yorke E, Ford E, Giraud P, Mageras G, Minsky B, et al. Respiratory gating for liver tumors: use in dose escalation. Int J Radiat Oncol Biol Phys 2003;55:659-668.
81. Wunderink W, Méndez Romero A, de Kruijf W, de Boer H, Levendag P, Heijmen B. Reduction of respiratory liver tumor motion by abdominal compression in stereotactic body frame, analyzed by tracking fi ducial markers implanted in liver. Int J Radiat Oncol Biol Phys 2008;71:907-915.
82. Bujold A, Massey CA, Kim JJ, Brierley J, Cho C, Wong RK, et al. Sequential phase I and II trials of stereotactic body radiotherapy for locally advanced hepatocellular carcinoma. J Clin Oncol 2013;31:1631-1639.
83. Cárdenes HR, Price TR, Perkins SM, Maluccio M, Kwo P, Breen TE, et al. Phase I feasibility trial of stereotactic body radiation therapy for primary hepatocellular carcinoma. Clin Transl Oncol 2010;12:218-225.
84. Louis C, Dewas S, Mirabel X, Lacornerie T, Adenis A, Bonodeau F, et al. Stereotactic radiotherapy of hepatocellular carcinoma: preliminary results. Technol Cancer Res Treat 2010;9:479-487.
85. Méndez Romero A, Wunderink W, Hussain SM, De Pooter JA, Heijmen BJ, Nowak PC, et al. Stereotactic body radiation therapy for primary and metastatic liver tumors: A single institution phase i-ii study. Acta Oncol 2006;45:831-837.
86. Rule W, Timmerman R, Tong L, Abdulrahman R, Meyer J, Boike T, et al. Phase I dose-escalation study of stereotactic body radiotherapy in patients with hepatic metastases. Ann Surg Oncol 2011;18:1081-1087.
87. Seo YS, Kim MS, Yoo SY, Cho CK, Choi CW, Kim JH, et al. Preliminary result of stereotactic body radiotherapy as a local salvage treatment for inoperable hepatocellular carcinoma. J Surg Oncol 2010;102:209-214.
88. Tse RV, Hawkins M, Lockwood G, Kim JJ, Cummings B, Knox J, et al. Phase I study of individualized stereotactic body radiotherapy for hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J Clin Oncol 2008;26:657-664.
89. Dawson LA. RTOG 1112: Randomized Phase III Study of Sorafenib versus Stereotactic Body Radiationerapy followed by Sorafenib in Hepatocellular Carcinoma.
90. Valentine K, Cabrera T, Roberge D. Implanting metal fi ducials to guide stereotactic liver radiation: McGill experience and review of current devices, techniques and complications. Technol Cancer Res Treat 2014;13:253-258.
91. Blomgren H, Lax I, N?slund I, Svanstr?m R. Stereotactic high dose fraction radiation therapy of extracranial tumors using an accelerator. Clinical experience of the fi rst thirty-one patients. Acta Oncol 1995;34:861-870.
92. Kwon JH, Bae SH, Kim JY, Choi BO, Jang HS, Jang JW, et al. Longterm e ff ect of stereotactic body radiation therapy for primary hepatocellular carcinoma ineligible for local ablation therapy or surgical resection. Stereotactic radiotherapy for liver cancer. BMC Cancer 2010;10:475.
93. Lee MT, Kim JJ, Dinniwell R, Brierley J, Lockwood G, Wong R, et al. Phase I study of individualized stereotactic body radiotherapy of liver metastases. J Clin Oncol 2009;27:1585-1591.
95. Kang JK, Kim MS, Cho CK, Yang KM, Yoo HJ, Kim JH, et al. Stereotactic body radiation therapy for inoperable hepatocellular carcinoma as a local salvage treatment aer incomplete transarterial chemoembolization. Cancer 2012;118:5424-5431.
97. Denecke T, Lopez H?nninen E. Brachytherapy of liver metastases. Recent Results Cancer Res 2008;177:95-104.
98. Ricke J, Mohnike K, Pech M, Seidensticker M, Rühl R, Wieners G, et al. Local response and impact on survival aer local ablation of liver metastases from colorectal carcinoma by computed tomography-guided high-dose-rate brachytherapy. Int J Radiat Oncol Biol Phys 2010;78:479-485.
100. Ricke J, Wust P, Wieners G, Beck A, Cho CH, Seidensticker M, et al. Liver malignancies: CT-guided interstitial brachytherapy in patients with unfavorable lesions for thermal ablation. J Vasc Interv Radiol 2004;15:1279-1286.
102. Mearini L. High intensity focused ultrasound, liver disease and bridging therapy. World J Gastroenterol 2013;19:7494-7499.
104. Shen HP, Gong JP, Zuo GQ. Role of high-intensity focusedultrasound in treatment of hepatocellular carcinoma. Am Surg 2011;77:1496-1501.
105. Xu G, Luo G, He L, Li J, Shan H, Zhang R, et al. Follow-up of high-intensity focused ultrasound treatment for patients with hepatocellular carcinoma. Ultrasound Med Biol 2011;37:1993-1999. 106. Ng KK, Poon RT, Chan SC, Chok KS, Cheung, Tung H, et al. High-intensity focused ultrasound for hepatocellular carcinoma: a single-center experience. Ann Surg 2011;253:981-987.
107. Wu F, Wang ZB, Chen WZ, Zhu H, Bai J, Zou JZ, et al. Extracorporeal high intensity focused ultrasound ablation in the treatment of patients with large hepatocellular carcinoma. Ann Surg Oncol 2004;11:1061-1069.
108. Wu F, Wang ZB, Chen WZ, Wang W, Gui Y, Zhang M, et al. Extracorporeal high intensity focused ultrasound ablation in the treatment of 1038 patients with solid carcinomas in China: an overview. Ultrason Sonochem 2004;11:149-154.
109. Wu F, Wang ZB, Chen WZ, Zou JZ, Bai J, Zhu H, et al. Advanced hepatocellular carcinoma: treatment with high-intensity focused ultrasound ablation combined with transcatheter arterial embolization. Radiology 2005;235:659-667.
110. Lu DS, Kee ST, Lee EW. Irreversible electroporation: ready for prime time? Tech Vasc Interv Radiol 2013;16:277-286.
111. Ben-David E, Appelbaum L, Sosna J, Nissenbaum I, Goldberg SN. Characterization of irreversible electroporation ablation in in vivo porcine liver. AJR Am J Roentgenol 2012;198:W62-W68.
112. Silk MT, Wimmer T, Lee KS, Srimathveeravalli G, Brown KT, Kingham PT, et al. Percutaneous ablation of peribiliary tumors with irreversible electroporation. J Vasc Interv Radiol 2014;25:112-118.
113. Deodhar A, Dickfeld T, Single GW, Hamilton WC Jr,ornton RH, Sofocleous CT, et al. Irreversible electroporation near the heart: ventricular arrhythmias can be prevented with ECG synchronization. AJR Am J Roentgenol 2011;196:W330-W335.
114. Cannon R, Ellis S, Hayes D, Narayanan G, Martin RC 2nd. Safety and early e ffi cacy of irreversible electroporation for hepatic tumors in proximity to vital structures. J Surg Oncol 2013;107:544-549.
115. Cheung W, Kavnoudias H, Roberts S, Szkandera B, Kemp W,omson KR. Irreversible electroporation for unresectable hepatocellular carcinoma: initial experience and review of safety and outcomes. Technol Cancer Res Treat 2013;12:233-241.
116. Kingham TP, Karkar A M, D’Angelica MI, Allen PJ, Demaeo RP, Getrajdman GI, et al. Ablation of perivascular hepatic malignant tumors with irreversible electroporation. J Am Coll Surg 2012;215:379-387.
119. Ebara M, Okabe S, Kita K, Sugiura N, Fukuda H, Yoshikawa M, et al. Percutaneous ethanol injection for small hepatocellular carcinoma: therapeutic e ffi cacy based on 20-year observation. J Hepatol 2005;43:458-464.
120. Taniguchi M, Kim SR, Imoto S, Ikawa H, Ando K, Mita K, et al. Long-term outcome of percutaneous ethanol injection therapy for minimum-sized hepatocellular carcinoma. World J Gastroenterol 2008;14:1997-2002.
122. Huang YZ, Zhou SC, Zhou H, Tong M. Radiofrequency ablation versus cryosurgery ablation for hepatocellular carcinoma: a metaanalysis. Hepatogastroenterology 2013;60:1131-1135.
123. Pacella CM, Francica G, Di Costanzo GG. Laser ablation for small hepatocellular carcinoma. Radiol Res Pract 2011;2011:595627.
124. Vogl TJ, Straub R, Eichler K, Woitaschek D, Mack MG. Malignant liver tumors treated with MR imaging-guided laser-induced thermotherapy: experience with complications in 899 patients (2,520 lesions). Radiology 2002;225:367-377.
125. Pacella CM, Francica G, Di Lascio FM, Arienti V, Antico E, Caspani B, et al. Long-term outcome of cirrhotic patients with early hepatocellular carcinoma treated with ultrasound-guided percutaneous laser ablation: a retrospective analysis. J Clin Oncol 2009;27:2615-2621.
126. Lencioni R. Loco-regional treatment of hepatocellular carcinoma. Hepatology 2010;52:762-773.
127. Lu Z, Wen F, Guo Q, Liang H, Mao X, Sun H. Radiofrequency ablation plus chemoembolization versus radiofrequency ablation alone for hepatocellular carcinoma: a meta-analysis of randomized-controlled trials. Eur J Gastroenterol Hepatol 2013;25:187-194.
128. Ni JY, Liu SS, Xu LF, Sun HL, Chen YT. Meta-analysis of radiofrequency ablation in combination with transarterial chemoembolization for hepatocellular carcinoma. World J Gastroenterol 2013;19:3872-3882.
129. Jiang G, Xu X, Ren S, Wang L. Combining transarterial chemoembolization with radiofrequency ablation for hepatocellular carcinoma. Tumour Biol 2014;35:3405-3408.
130. Peng ZW, Zhang YJ, Chen MS, Xu L, Liang HH, Lin XJ, et al. Radiofrequency ablation with or without transcatheter arterial chemoembolization in the treatment of hepatocellular carcinoma: a prospective randomized trial. J Clin Oncol 2013;31:426-432.
131. Hurwitz MD, Hansen JL, Prokopios-Davos S, Manola J, Wang Q, Bornstein BA, et al. Hyperthermia combined with radiation for the treatment of locally advanced prostate cancer: long-term results from Dana-Farber Cancer Institute study 94-153. Cancer2011;117:510-516.
132. Zagar TM, Oleson JR, Vujaskovic Z, Dewhirst MW, Craciunescu OI, Blackwell KL, et al. Hyperthermia combined with radiation therapy for superficial breast cancer and chest wall recurrence: a review of the randomised data. Int J Hyperthermia 2010;26:612-617.
133. Goldberg SN. Science to practice: Can we expand focal interventional oncologic ablation treatments into an e ff ective systemic therapy? Radiology 2013;267:321-323.
134. Soundararajan A, Dodd GD 3rd, Bao A, Phillips WT, McManus LM, Prihoda TJ, et al. Chemoradionuclide therapy with 186Relabeled liposomal doxorubicin in combination with radiofrequency ablation for e ff ective treatment of head and neck cancer in a nude rat tumor xenogramodel. Radiology 2011;261:813-823.
Cite this article as:Li D, Kang J, Golas BJ, Yeung VW, Madoff DC. Minimally invasive local therapies for liver cancer. Cancer Biol Med 2014;11:217-236. doi: 10.7497/j.issn.2095-3941.2014.04.001
David C. Mado ff
E-mail: dcm9006@med.cornell.edu
Received October 24, 2014; accepted October 28, 2014. Available at www.cancerbiomed.org
Copyright ? 2014 by Cancer Biology & Medicine
 Cancer Biology & Medicine2014年4期
Cancer Biology & Medicine2014年4期
- Cancer Biology & Medicine的其它文章
- Sequential maximum androgen blockade (MAB) in minimally symptomatic prostate cancer progressing after initial MAB: two case reports
- E ff ect of EGFR-TKI retreatment following chemotherapy for advanced non-small cell lung cancer patients who underwent EGFR-TKI
- Argyrophilic nucleolar organizer region in MIB-1 positive cells in non-small cell lung cancer: clinicopathological signi fi cance and survival
- Emerging function of mTORC2 as a core regulator in glioblastoma: metabolic reprogramming and drug resistance
- Recent advances in lymphatic targeted drug delivery system for tumor metastasis
- Combining targeted therapy and immune checkpoint inhibitors in the treatment of metastatic melanoma
