Evaluation on nitrogen isotopes analysis in high-C/N-ratio plants using elemental analyzer/isotope ratio mass spectrometry
HU Jing(胡婧)and LIU Wei-Guo(劉衛(wèi)國(guó)),2,
1State Key Laboratory of Loess and Quaternary Geology,
Institute of Earth Environment,Chinese Academy of Sciences,Xi’an 710075,China
2Xi’an Jiaotong University,Xi’an 710049,China
Evaluation on nitrogen isotopes analysis in high-C/N-ratio plants using elemental analyzer/isotope ratio mass spectrometry
HU Jing(胡婧)1and LIU Wei-Guo(劉衛(wèi)國(guó))1,2,?
1State Key Laboratory of Loess and Quaternary Geology,
Institute of Earth Environment,Chinese Academy of Sciences,Xi’an 710075,China
2Xi’an Jiaotong University,Xi’an 710049,China
Elemental analyzer/isotope ratio mass spectrometry(EA/IRMS)has been widely applied to analyze the15N/14N isotope composition(δ15N)of plants and soils,but theδ15N results may be inaccurate due to incomplete combustion of the high-C/N-ratio plant samples by EA.Therefore,it is necessary to develop a method to solve the problem of imperfect combustion.In this study,we used two methods:1)adding copper oxide powder to the samples,and 2)increasing the O2f l ow(from 100mLmin?1to 200mLmin?1)for the auto sampler inlet purge line of the EA.Theδ15N values of the plant samples became more positive and tended to be stable after complete combustion.Also,the required blank samples for each plant sample decreased with increasing amount of the added CuO powder.However,at 200mLmin?1of the oxygen f l ow in the EA,complete combustion could not be achieved without adding copper oxide,but this was done with decreased amount of CuO powder.Therefore, mixing cupric oxide into the high-C/N-ratio samples was an eff i cient,simple and convenient way to solve the problem of imperfect combustion in the EA.
Nitrogen isotopes,Cupric oxide,O2f l ow of EA/IRMS,High-C/N-ratio plants,EA/IRMS
I.INTRODUCTION
Continuous-f l ow elemental analyzer/isotope ratio mass spectrometry(EA/IRMS),which analyzes nitrogen isotopes in plant and soil samples[1–6],has been extensively used to study the global nitrogen cycle[7–11].The EA analysis is based on f l ash combustion.The nitrogen content in a sample is converted to N2and NOxin the oxidation furnace by instantaneous and complete oxidation of the sample[12].However,Wanget al.[13]found that natural plant samples with high C/N ratios could not be combusted completely,resulting in nitrogen isotopic compositions being less than expected. Therefore,methods should be developed to ensure complete combustion of the plant samples.
In order to make sure enough N(≥100μg)is introduced into the mass spectrometer,larger sample amounts are required for the nitrogen isotopic analysis of the high-C/N-ratio plants,but the samples are tightly loaded in Ag(or Sn)foil capsules to avoid air contamination.The plant powder in the packet core may not contact with oxygen,hence the incomplete oxidation and nitrogen isotope fractionation[14]. Vanadium pentoxide(V2O5)has been added to samples to enhance combustion in sulfur stable isotope measurements by EA/IRMS[15–17].This method,however,was ineffective for measuring high C/N ratios samples,because the background intensity of the m/z 28 ion even increased with the V2O5content,and the plant-V2O5mixture could not be reacted completely[13].In this paper,copper oxide powder is used as a combustion-supporting reagent,aimed at f i nding a simple and effective way to obtain accurate isotope results for analyzing plant samples of high-C/N-ratio by EA/IRMS.
II.EXPERIMENTAL
A.Materials
Copper oxide was ground into powder in particle size of<110μg after 650°C sintering for two hours.The high-C/N-ratio plant samples,i.e.Plant 1(P1)and Plant 2(P2), were collected from Xinjiang,China.The plant samples were soaked in deionized water for 24 hours(the deionized water was renewed every 8 hours)to remove salt in the sample surface.They were dried at 40°C in an oven for 48 hours.The dried samples were ground into powder of<74μm in particle size.The carbon and nitrogen contents,and the C/N ratio, were determined using an elemental analyzer.The carbon content was 44.14%for the P1 samples,and 44.77%for the P2 samples,while the nitrogen content was 1.37%for P1 and 0.75%for P2.So,the C/N ratios were 32.22 and 59.69 for P1 and P2,respectively.
The plant sample powders were weighed out on a fresh sheet of paper.The CuO powder was mixed with the sample powder at various mass ratios.The prepared samples were loaded into Ag and Sn capsules(5mm×9mm),which were tightly crimped to avoid any trapping of air that would perturb the combustion.
B.Sample analysis using EA/Conf l o III/IRMS
Determination of15N/14N isotope ratios was performed with a FLASH 1112 Elemental Analyzer(CE Instruments, Rodano,Italy)equipped with an AS200 auto sampler interfaced to a DeltaPLUSisotope ratio mass spectrometer(ThermoQuest Finnigan,Bremen,Germany)via a Conf l o III interface(ThermoQuest Finnigan,Germany).The elementalanalyzer was composed of an oxidation furnace f i lled with chromium(III)oxide and silvered cobalt oxide for combustion and a reduction furnace f i lled with copper to reduce the nitric oxide compounds.A CO2adsorption trap, employed to avoid the interference of CO2,and a water trap to protect the GC column from humidity,were connected between the reduction reactor and the GC column. The mixed gas that f l owed from the furnace was separated via the chromatographic column.The following EA conditions were used:temperatures of oxidation reactor,1000°C, temperatures of reduction reactor 650°C,and GC column temperature 40°C[18].High-purity helium was used as a carrier gas at a f l ow rate of 85mLmin?1.All15N/14N isotope ratios were expressed in the conventionalδnotation in per mil versus air,def i ned asδ15N(‰)(air)= [(15N/14Nsample/15N/14Nstandard)?1]×1000.
A tank of high-purity nitrogen with knownδ15N value was used as the working reference gas.Theδ15N standards of IAEA-NO3and a soil standard SN-2 were measured on a daily basis to monitor the analytical accuracy.The standard deviation for duplicate analyses was less than 0.3‰.
C.Improvement of the oxidizing condition
In order to supply enough oxygen for high-C/N-ratio plant samples to be completely converted by the f l ash combustion in the EA,two methods were tried.The f i rst method was to add CuO to the samples.The second method was a direct increase in the oxygen injection of the EA from 100mLmin?1to 200mLmin?1.Because excess O2would shorten lifetime of the reduction reactor[19],we did not continue to increase the oxygen f l ow.
III.RESULTS AND DISCUSSION
A.Checks on the CuO and capsules
In order to determine whether the copper oxide would induce contamination,we monitored the blank of CuO reagent. Different amounts of CuO powder were used to determined N signal intensities of the blank.Also,CuO powder was mixed into the SN-2 standard and theδ15N data were compared with that of the SN-2(Table 1).The results showed that the N intensity of the CuO blank was zero and theδ15N results of SN-2 were not affected by the added CuO.In addition,CuO and the CuO/SN-2 mixture were loaded in the Ag and Sn capsules,indicating that the Ag and Sn capsules contributed no N contamination.
In order to examine whether Ag capsules will prevent the sample from being completely combusted,theδ15N values for samples wrapped in Ag capsules and Sn capsules were compared.As the results shown in Table 2,there was no difference between the plant samples that were loaded into Ag capsules and those in Sn capsules.
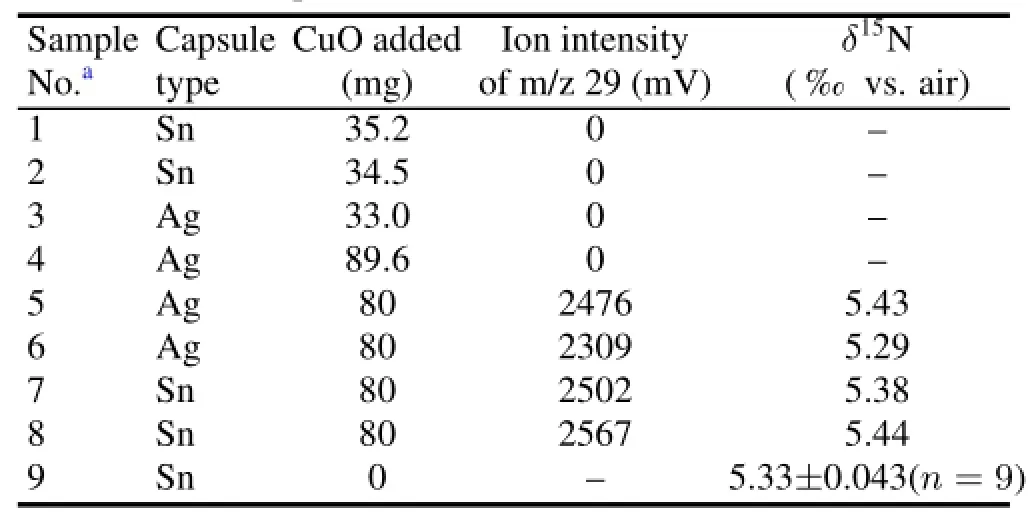
TABLE 1.Analysis of the N blank of CuO powder in Sn and Ag capsules,and nitrogen isotope results of the SN-2 standard and its mixture with CuO powder(mean values±standard deviation)
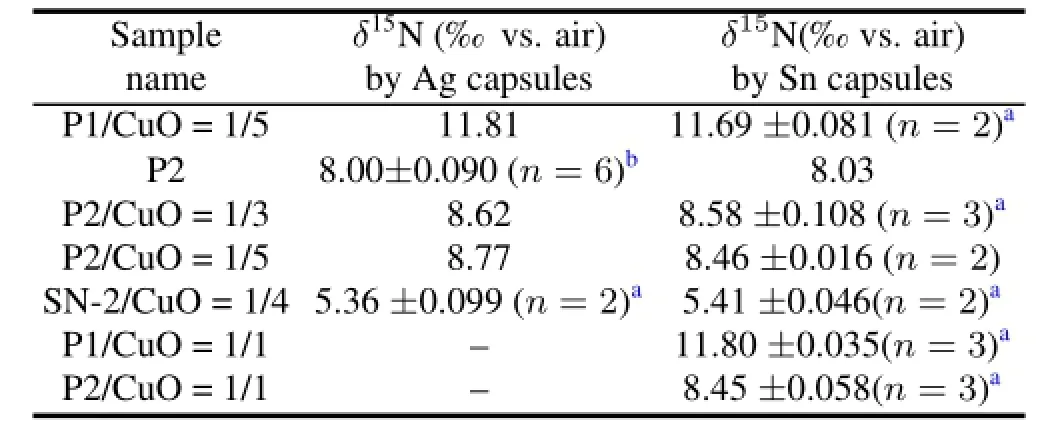
TABLE 2.Comparison ofδ15N data between the samples in Ag and Sn capsules and the intra-assay precision assessed by replicate analysis in a single day and several days(mean values±standard deviation)
B.The precision of EA-IRMSδ15N analysis
The precision of the EA/IRMS system must be guaranteed to studyδ15N values,so replicate analyses,in which the plant samples mixed with different amounts of CuO were analyzed, were performed in a single day or in several days.And the results are of high precision(Table 2),with the standard deviation being less than 0.2‰.
C.δ15N linearity range of the IRMS system
The ion intensity of N2of the plant samples increases with the degree of combustion completeness of the plant samples. It is essential that the range in which the ion intensity changed was within theδ15N linearity range of IRMS system.Theδ15N linearity range of IRMS system was tested by investigating the relationship between the ion intensity and theδ15N values[20].First,the pressure controller of reference gas N2of the Finnigan Conf l o III interface was adjusted.Eleven pulses of various amounts of N2were measured to achieve a diverse range of ion intensity without any change in the EA conditions.The measuredδ15N values did not change in the ion intensity range of 500–6600mV(Fig.1(a)).Next,differ-ent amounts of the SN-2 standard were loaded in Sn or Ag cups to examine the linearity of the EA/IRMS system.In the N2ion intensity range of 750–4300mV,the measuredδ15N value showed a linear correlation with the ion intensity.In the range of 1500–4000mV,theδ15N value did not vary signif i cant(Fig.1(b)).These results demonstrated that the increase in theδ15N values as a function of increasing size of plant samples was not caused by any non-linearity offset of the mass spectrometer.
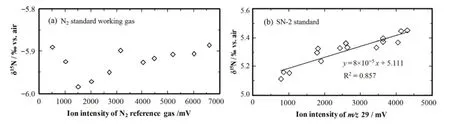
Fig.1.Linearity of the mass spectrometer for nitrogen isotope analysis.(a)δ15N of N2standard working gas,at the ion intensity of N2being 500–6600mV;(b)δ15N of SN-2 at the ion intensity of N2being 750–4300mV.
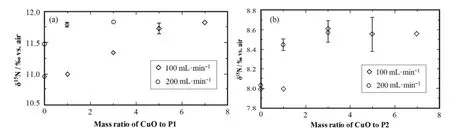
Fig.2.Theδ15N values of samples P1(a)and P2(b)at different mass ratios of CuO/plant-sample
D.Theδ15N values by add CuO and O increase
It is well known that isotope fractionation will occur if the sample is not fully oxidized using EA/IRMS[14,17].The14N isotope can participate in the reaction more easily than15N,hence more negative results ofδ15N when the samples were only partially converted.
In this study,the nitrogen isotopes of the two high-C/N-ratio plant samples had a similar trend in the ion intensity at m/z 29.When the oxygen loop in the EA was set to 100mLmin?1or 200mLmin?1,the nitrogen isotopes of the two samples were more negative without CuO powder,indicating non-complete combustion of the samples.By adding CuO powder into the samples as shown in Fig.2,the isotopic compositions at 100mLmin?1increased until the ratio of CuO/plant of 5/1 for for P1 and 3/1 for P2.However, at 200mLmin?1of the O2fl ow,the isotope results tented to be stable when the mass ratio of CuO/plant was over 3/1 for P1 and 1/1 for P2.And the nitrogen isotopes for P1 and P2 showed the same trend of variation under different experimental conditions.As mentioned above,the stable behavior means complete combustion of the plant samples,and this can be realized by adding CuO powder or increasing in the O2fl ow in the EA.
E.Blank samples after each sample analysis
To verify complete combustion of the plant samples,blank samples were measured after analysis of each plant sample,until no appearance of the nitrogen peak.As shown in Fig.3(a),the ion intensity of the f i rst blank sample was 35–60mV at the O2f l ow of both 100 and 200mLmin?1. It was obvious that the nitrogen in the two plant samples was not converted completely.However,at the O2f l ow of 100mLmin?1,four or f i ve blank samples were required to eliminate the m/z 29 peak without adding CuO(Fig.3(a)), while this was achieved by using just two blank samples at 200mLmin?1.Then,to some extent,increasing the O2f l ow to 200mLmin?1promotes the sample combustion.But complete conversion of the plant samples cannot be achieved by just increasing the O2f l ow.
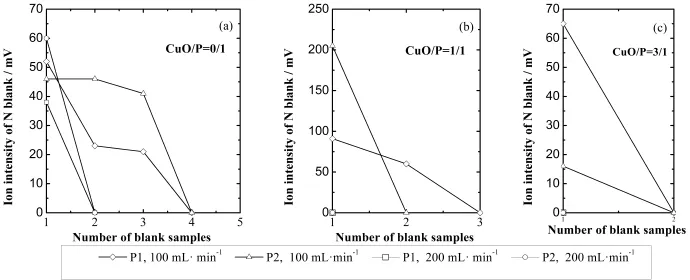
Fig.3.Nitrogen signal intensity from blank samples analyzed after the plant sample analysis at different mass ratios of CuO/plant-sample.
As shown in Figs.3(b)and 3(c),the number of blank samples decreased with increasing amount of added CuO.In the 100-mLmin?1scenario,the number of blank samples required was reduced from three to two when the mass ratio of copper oxide/plant samples increased from 1/1 to 3/1. With a 200-mLmin?1f l ow,the high-C/N-ratio plant samples were combusted completely when the mass ratio of copper oxide/plant samples was greater than 1/1.This indicates that copper oxide was an eff i cient reagent to promote combustion for N isotope analysis of the high-C/N-ratio samples using EA.
IV.CONCLUSION
Adding copper oxide powder to the high-C/N-ratio plant samples ensures complete oxidation of the nitrogen contents in the samples by f l ash combustion in the EA/IRMS,so that the nitrogen isotope values of the plant samples can be accurately determined.While increasing the oxygen f l ow in the EA improves the nitrogen oxidation of the high-C/N-ratio plant samples,complete combustion cannot be achieved by just increasing the oxygen f l ow.In addition,it is necessary to use blank samples after each partially combusted sample to avoid interference of the memory effect from residue nitrogen in the last sample.Therefore,adding CuO powder and increasing the O2f l ow of the EA can greatly reduce the time spent on blank samples if the high-C/N-ratio plant samples can be completely combusted by f l ash combustion.So,in analysis of nitrogen isotope with EA/IRMS,mixing copper oxide in the samples is an easy and effective way to solve the problem of incomplete combustion of high C/N ratio plant samples.The true nitrogen compositions can be obtained and the analysis eff i ciency can be promoted by adding copper oxide and increasing O2f l ow.
[1]Werner R A,Bruch B A,Brand W A.Rapid Commun Mass SP, 1999,13:1237–1241.
[2]Dijkstra P,Williamson C,Menyailo O,et al.Isot Environ Healt S,2003,39:29–39.
[3]Hansen T and Sommer U.Rapid Commun Mass SP,2007,21: 314–318.
[4]Wang X,Feng L J,Zhang F S,et al.Rapid Commun Mass SP. 2008,22:1196–1202.
[5]Liu W G and Wang Z.Chinese Sci Bull,2009,54:272–279.
[6]Liu W G,Wang Z F,Wang Z,et al.China Chin J Geochem, 2011,30:295–303.
[7]Schulze E D,Williams R J,Farquhar G D,et al.Aust J Plant Physiol,1998,25:413–425.
[8]Hietz P,Wanek W,Wania R,et al.Oecologia,2002,131:350–355.
[9]Reich A,Ewel J J,Nadkarni N M,et al.Oecologia,2003,137: 587–590.
[10]Liu W G and Xing M.Chem Geol,2012,296–297:66–72.
[11]Xing M and Liu W G.Appl Geochem,2012,27:831–840.
[12]Elemental Analyzer Operating Manual.Thermo Finnigan Corporation,2003,51–52.
[13]Wang Z F,Wang Z,Hu J,et al.J Chin Mass Spectrom Soc, 2008,29:290–294.(in Chinese)
[14]Grassineau N V.Appl Geochem,2006,21:756–765.
[15]Fry B.Rapid Commum Mass SP.2007,21:750–756.
[16]Delacour A,Fr¨uh–Green G L,Bernasconi S M,et al.Geochim Cosmochim Ac,2008,72:5090–5110.
[17]Hansen T,Burmeister A,Sommer U.Rapid Commun Mass SP, 2009,23:3387–3393.
[18]Wang Z,Liu W G,Wen Q B.J Chin Mass Spectrom Soc,2005,26:71–75.(in Chinese)
[19]Kracht O.Thermo Fisher scientif i c training for f l ash EA 1112 2007,5.
[20]Liu W G,Wang Z,Cui L L,et al.Rapid Commun Mass SP, 2012,26:1746–1752.
10.13538/j.1001-8042/nst.25.020303
(Received May 16,2013;accepted in revised form November 8,2013;published online April 20,2014)
?Corresponding author,liuwg@loess.llqg.ac.cn
Nuclear Science and Techniques2014年2期
- Nuclear Science and Techniques的其它文章
- Ordered water monolayer on ionic model substrates studied by molecular dynamics simulations?
- Upgrade to the front-end electronics of the BESIII muon identif i cation system
- Demonstration of Pm-147 GaN betavoltaic cells
- A method for determination of the s orbital component of12Be ground state?
- Measurement of keffwith an improved neutron source multiplication method based on numerical analysis?
- A hybrid voxel sampling method for constructing Rad-HUMAN phantom?
