Pentoxifylline enhances the protective effects of hypertonic saline solution on liver ischemia reperfusion injury through inhibition of oxidative stress
Rafael Soraes Pinheiro, Telesforo Bacchella, Marcel CC Machado and Luiz AC D'Albuquerque
S?o Paulo, Brazil
Pentoxifylline enhances the protective effects of hypertonic saline solution on liver ischemia reperfusion injury through inhibition of oxidative stress
Vinicius Rocha-Santos, Estela RR Figueira, Joel A Rocha-Filho, Ana MM Coelho,
Rafael Soraes Pinheiro, Telesforo Bacchella, Marcel CC Machado and Luiz AC D'Albuquerque
S?o Paulo, Brazil
BACKGROUND: Liver ischemia reperfusion (IR) injury triggers a systemic infammatory response and is the main cause of organ dysfunction and adverse postoperative outcomes after liver surgery. Pentoxifylline (PTX) and hypertonic saline solution (HTS) have been identifed to have benefcial effects against IR injury. This study aimed to investigate if the addition of PTX to HTS is superior to HTS alone for the prevention of liver IR injury.
METHODS: Male Wistar rats were allocated into three groups. Control rats underwent 60 minutes of partial liver ischemia, HTS rats were treated with 0.4 mL/kg of intravenous 7.5% NaCl 15 minutes before reperfusion, and HPTX group were treated with 7.5% NaCl plus 25 mg/kg of PTX 15 minutes before reperfusion. Samples were collected after reperfusion for determination of ALT, AST, TNF-α, IL-6, IL-10, mitochondrial respiration, lipid peroxidation, pulmonary permeability and myeloperoxidase.
RESULTS: HPTX signifcantly decreased TNF-α 30 minutes after reperfusion. HPTX and HTS signifcantly decreased ALT,AST, IL-6, mitochondrial dysfunction and pulmonary myeloperoxidase 4 hours after reperfusion. Compared with HTS only, HPTX signifcantly decreased hepatic oxidative stress 4 hours after reperfusion and pulmonary permeability 4 and 12 hours after reperfusion.
CONCLUSION: This study showed that PTX added the benefcial effects of HTS on liver IR injury through decreases of hepatic oxidative stress and pulmonary permeability.
(Hepatobiliary Pancreat Dis Int 2015;14:194-200)
pentoxifylline;hypertonic saline solution; hepatic oxidative stress; ischemia reperfusion injury; pulmonary permeability
Introduction
Despite improvements in organ storage, liver ischemia reperfusion (IR) injury remains an important issue and is a major risk factor for postoperative liver dysfunction after liver surgery and transplantation.[1,2]The degree of liver injury has a direct association with the infammatory cascade triggered during the ischemic phase. Interruption of oxygen delivery leads to a reduction in oxidative phosphorylation and cellular ATP storage.[3]In the early phase of reperfusion, reactive oxygen species (ROSs) are formed because of oxidation of hypoxanthine by xanthine oxidase. ROSs ultimately result in lipid peroxidation.[4,5]It is well established that lipid peroxidation of cellular membranes plays a major role in hepatic oxidative stress.[6,7]In the later phase of reperfusion, further activation of Kupffer cells and consequent release of infammatory moleculespromote and amplify hepatocyte injury.[5]Furthermore, local infammatory disorders are associated with distant organ damage which worsens patient outcomes.[8-10]Lung injury that causes pulmonary edema, intraalveolar hemorrhage, and neutrophil accumulation is also known to occur after liver IR.[11]
Therefore, several strategies have been proposed to avoid this complex adverse event. Experimental models demonstrated decreased IR injury when treatments including the use of hypertonic saline solution (HTS) or pentoxifylline (PTX) are used before revascularization. Studies[12-14]showed that small-volume resuscitation with HTS infusion improved hemodynamic parameters, microcirculation disturbances, liver edema, and pulmonary permeability. PTX is a methylxanthine phosphodiesterase inhibitor that prevents vasodilation of the microcirculation, including that of the liver.[15]It has been shown that PTX increases tissue oxygenation and intestinal blood fow while decreasing TNF and microvascular leukocyte accumulation, thereby reducing the infammatory response.[16-19]The present study aimed to investigate if the addition of PTX to HTS is superior to the use of HTS alone in of the treatment of liver IR injury.
Methods
Animals
Male Wistar rats were used according to the recommendations of the National Council Guide (1996) for the Care and Use of Laboratory Animals. The rats weighing 230-270 g were housed in the LIM37 from University of S?o Paulo School of Medicine. They were placed at a room temperature of 22 ℃ in a 12-hour light/dark cycle, while receiving food and waterad libitum. The experimental protocol was approved by the Ethics Committee of our institution.
Anesthesia and surgical procedures
Intraperitoneal anesthesia was induced in all animals with ketamine 30 mg/kg (Ketalar, Cristália, SP, Brazil) and xylazine 30 mg/kg (Rompum, Bayer, SP, Brazil), followed by orotracheal intubation. Mechanic ventilation was instituted with a tidal volume of 0.08 mL/g body weight, a respiratory rate of 60/min and 21% FiO2(Small Animal Ventilator Model 683, Harvard Apparatus, Holliston, MA, USA). Body temperature ranging from 35-37 ℃was monitored with a rectal digital thermometer (YSI Precision 4000A Thermometer, USA). A midline laparotomy was performed and the pedicles of the left lateral and median hepatic lobes were occluded with a 2.5 mm microvascular clamp. Partial ischemia of 70% of the total liver volume was performed to avoid splanchnic congestion.[20,21]Abdominal wall was closed to prevent dehydration during the ischemic period. Intravenous drugs were given via the dorsal penile vein. After 60 minutes of ischemia, the clamp was removed and liver reperfusion was started. Blood and tissue samples were collected at 30 minutes, 4 and 12 hours after the reperfusion. The blood was collected through cardiac puncture, and the animals were euthanized by exsanguination under anesthesia.
Study groups and experimental protocol
Animals submitted to liver IR were allocated into three groups (Fig. 1). In the control group (n=24), animals were subjected to 60-minute ischemia. In the HTS group (n=24), animals were subjected to 60-minute ischemia and then received 0.4 mL/kg of intravenous 7.5% NaCl 15 minutes before reperfusion. And in the HPTX group (n=24), animals were subjected to 60-minute ischemia and received 0.4 mL/kg of intravenous 7.5% NaCl plus 25 mg/kg of PTX 15 minutes before the reperfusion. Additionally, 14 animals from a sham-operated group underwent the surgical procedure without pedicled clamping or treatment. The total number of animals including those underwent sham operation was 86.
Liver aminotransferases
Serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were used as indicators of liver injury. AST and ALT activities were assayed 4 hours after reperfusion by the optimized ultraviolet method (COBAS MIRA, Roche, Rotkrenz, Switzerland) and optimized accordingly to International Federation of Clinical Chemistry. The results were expressed in units per liter (U/L).
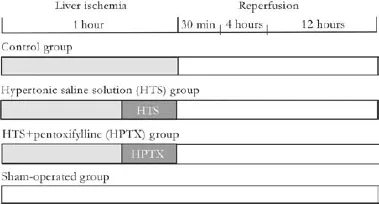
Fig. 1.Study design. Control group: animals subjected to 1 hour of liver ischemia and 4 hours of reperfusion. HTS group: animals were treated with 0.4 mL/kg of intravenous 7.5% NaCl 15 minutes before reperfusion. HPTX group: animals were treated with 7.5% NaCl plus 25 mg/kg of PTX 15 minutes before reperfusion. Shamoperated group: animals subjected to surgical manipulation without liver ischemia.
Serum TNF-α and interleukins
TNF-α was quantifed 30 minutes after reperfusion using specifc ELISA kits (Biosource International Cytoscreen, Camarillo, CA, USA). IL-6 and IL-10 were quantifed 4 hours after reperfusion using specifc ELISA kits (Biosource International Cytoscreen).
Lipid peroxidation
Lipid peroxidation was used as an indirect measurement of oxidative damage induced by ROS. Lipid peroxidation was determined 4 hours after reperfusion in ischemic and non-ischemic liver samples by the thiobarbiturate reaction measuring the formation of malondialdehyde (MDA) spectrophotometrically. Samples of 100 mg liver tissue were homogenized (Polytron PT-2100 homogenizer, Kinematica AG, Luzern, Switzerland) for 60 seconds in 1 mL of 1.15% KCl buffer, and centrifuged (Sorvall RC-5C Plus centrifuge, Thermo Fischer Scientific Inc., Waltham, MA, USA) at 14 000×g for 20 minutes at 4 ℃. Two hundred μL of supernatant was added to 1.5 mL of 0.8% thiobarbituric acid, 200 μL of 8.1% sodium dodecyl sulfate, 1.5 mL of 20% acetic acid (pH 3.5), and 600 μL of distilled water. The mixture was heated for 45 minutes at 90 ℃. After cooling, the focculent precipitate was removed by centrifugation at 10 000×g for 10 minutes. Absorbance was measured at 532 nm (Ultra Microplate Reader ELX 808 from Bio-Tek Instruments, Winooski, VT, USA) using MDA bis (dimethyl acetal) as external standard.[22]
Polarographic study of liver mitochondria
The mitochondrial oxygen consumption was evaluated 4 hours after reperfusion with a closed reaction vessel (Gilson Medical Electronics, Middleton, WI, USA) ftted with the Clark oxygen electrode (Yellow Springs Instruments, Yellow Springs, OH, USA) at 28 ℃ as described previously.[23]The respiratory control ratio calculated as the rate of oxygen consumption in state 3/oxygen consumption in state 4. The adenosine diphosphate/oxygen consumption ratio (ADP/O) was calculated as moles of ATP formed per moles of oxygen consumed by mitochondria.[24]
Analysis of lung permeability
The lung microvascular permeability was quantifed 4 and 12 hours after reperfusion by the Evans blue extravasation technique. Evans blue (Sigma Chemical Co.) dissolved in saline was injected via the dorsal penile vein at 20 mg/kg, 15 minutes before euthanasia. Blood samples were collected, the lungs were perfused via the pulmonary artery with 30 to 50 mL of 0.9% NaCl at 10 mL/min, using a syringe pump (975 Harvard Apparatus, Holliston, MA, USA), and after that the lung was weighed. One small piece of tissue was removed and dried at 60 ℃ for calculation of total dry weight. The perfused lung (4 μL/mg of tissue) was immersed in formamide (Merck, Darmstadt, Germany) for 24 hours at room temperature. The Evans blue concentration extracted in formamide was quantifed spectrophotometrically at 620 nm (ELX808 Ultra Microplate Reader, Thomas Scientifc, USA).[25]
Lung myeloperoxidase assay
The levels of myeloperoxidase activity were determined in lung samples 4 hours after reperfusion. Samples of 300 mg lung tissue were homogenized for 60 seconds with Polytron PT-2100 homogenizer in 1 mL of sodium phosphate buffer at pH 6.2. The samples were sonicated and centrifuged (3000×g, 30 minutes) at 4 ℃. Ten microliters of supernatant was added to 500 μL of sodium phosphate buffer (pH 6.2), 700 μL of 0.25% PBS/BSA, 100 μL ofo-dianisidine, and 100 μL of 0.05% H2O2. Myeloperoxidase activity was analyzed by measuring the change in absorbance at 490 nm caused by the metabolism of H2O2in the presence ofo-dianisidina.[12,26]The results were expressed as optical density (OD) at 490 nm.
Liver histology
Samples of liver ischemic lobes were collected 4 hours after reperfusion and fxed in 10% buffered formalin for standard hematoxylin and eosin (HE) staining. A single blinded pathologist performed histologic evaluation. Histologic injury was evaluated according to the scoring system proposed by Quireze et al.[27]
Statistical analysis
Data were expressed as mean±standard deviation (SD). Statistical analysis was performed using theRStatistical Software for Windows, version 2.12. ANOVA was used to evaluate differences in the control, HTS and HPTX groups. When both normality and homogeneity assumptions of variances were fulflled, ANOVA was applied. If only homogeneity was satisfed, the Kruskal-Wallis test was applied. After that, multiple comparisons were performed using the parametric Tukey's test or the nonparametric Tukey's test.[28]In cases that both normality and homogeneity were not satisfed, the non-parametric Tukey's test was used for two-by-two comparisons to identify differences between the groups. Student'sttest or the Mann-Whitney test was used to evaluate differences between the control and Sham-operated groups. Differences were considered statistically signifcant whenPvalue was <0.05.
Results
Hepatocyte injury
Compared with the rats of the sham-operated group, the rats that were subjected to IR showed a signifcant increase in serum levels of liver aminotransferases 4 hours after reperfusion (P<0.05), indicating that liver enzymes were released in response to the extension of liver injury. A signifcant increase in serum levels of AST and ALT was observed in the control group compared with the HTS and HPTX treated groups (P<0.05, Fig. 2).
Infammatory mediators
Serum TNF-α level was signifcantly decreased in the HPTX group compared with the control group (P<0.05, Table 1). IL-6 was signifcantly decreased in the HTS and HPTX groups compared with the control group (P<0.05, Fig. 3A). IL-6 was signifcantly increased in the HPTX group compared with the HTS group (P<0.05). There was no difference in IL-10 among the groups (Table 1 and Fig. 3B).
Hepatic lipid peroxidation
MDA levels from liver samples were signifcantly decreased in ischemic and non-ischemic lobes of the HPTX group compared with the control and HTS groups (P<0.05, Fig. 4).
Mitochondrial respiration
ADP/O ratio from liver samples was signifcantly increased in the ischemic and non-ischemic lobes of the HTS (1.91±0.05 and 1.93±0.05) and HPTX (1.92±0.08 and 1.88±0.08) groups compared with the control group (1.59±0.32 and 1.74±0.08) (8 rats in each group,P<0.05).
Lung permeability and myeloperoxidase activity
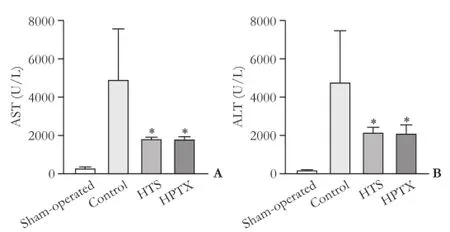
Fig. 2.Serum AST (A) and ALT (B) levels 4 hours after reperfusion (12 rats in each group). *:P<0.05, vs control group.
Lung microvascular permeability quantifed by Evans blue was signifcantly decreased in the HPTX group compared with the control and HTS groups 4 and 12 hours after reperfusion (P<0.05, Fig. 5). Lung myeloperoxidase activity was evaluated as an index of neutrophil recruitment. Myeloperoxidase activity was signifcantly decreased in the HTS and HPTX groups compared with the control group (P<0.05, Fig. 6).
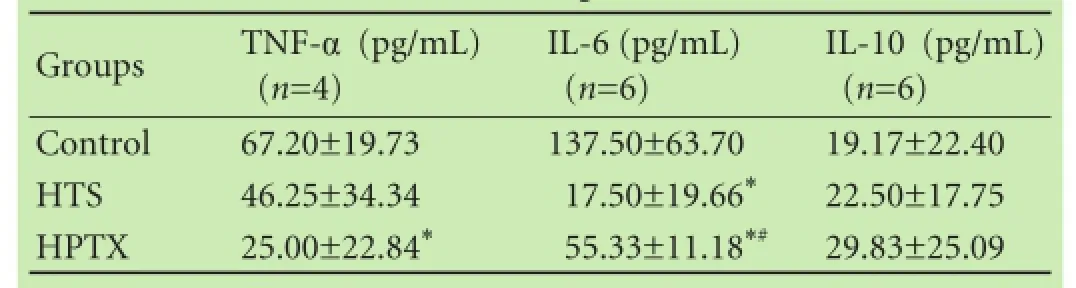
Table 1.Serum levels of TNF-α 30 minutes after reperfusion, and IL-6 and IL-10 4 hours after reperfusion
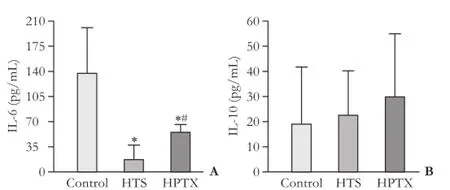
Fig. 3.Serum IL-6 (A) and IL-10 (B) levels 4 hours after reperfusion (6 rats in each group). *:P<0.05, vs control group; #:P<0.05, vs HTS group.
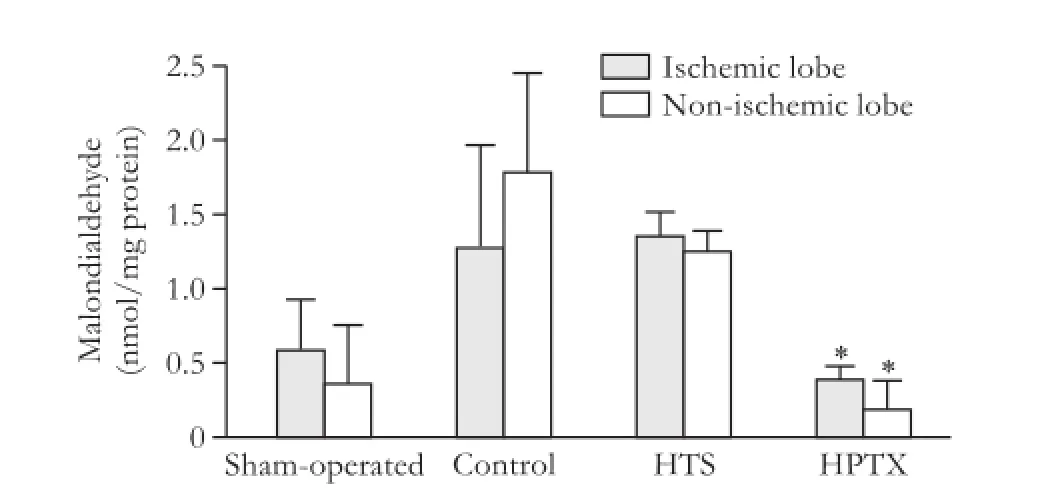
Fig. 4.Malondialdehyde in ischemic and non-ischemic liver samples 4 hours after reperfusion (6 rats in each group). *:P<0.05, vs control and HTS groups.
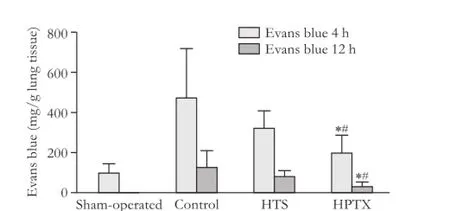
Fig. 5.Evans blue in lung tissue 4 and 12 hours after reperfusion (8 rats in each group). *:P<0.05, vs control group; #:P<0.05, vs HTS group.
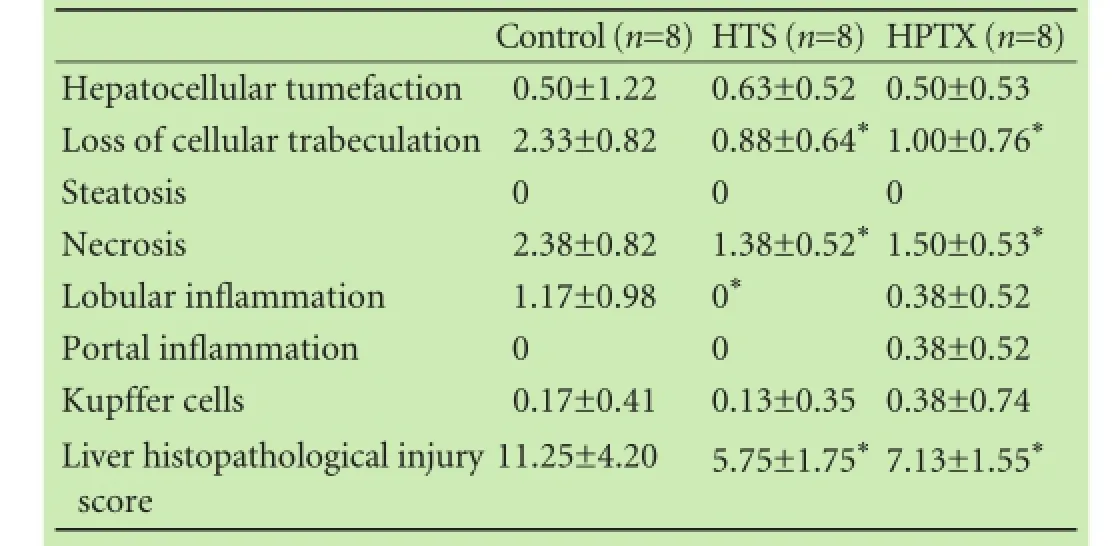
Table 2.Liver histological analysis 4 hours after reperfusion
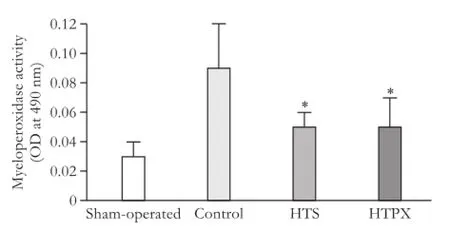
Fig. 6.Myeloperoxidase activity in lung tissue 4 hours after reperfusion (8 rats in each group). *:P<0.05, vs control group.
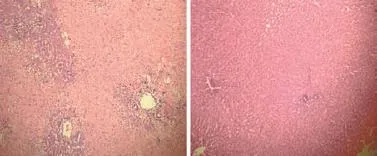
Fig. 7.Histologic evaluation of liver of ischemic lobes 4 hours after reperfusion (HE staining, original magnifcation ×40).A: showing areas of extensive necrosis in the control group;B: showing preserved liver tissue with decreased liver histologic score in the HPTX group.
Liver histology
Liver necrosis and the loss of hepatocellular trabeculation in the ischemic lobes were signifcantly decreased in the HTS and HPTX groups compared with the control group 4 hours after reperfusion (P<0.05). The liver histopathological injury score in the ischemic lobes was signifcantly decreased in the HTS and HPTX groups compared with the control group (P<0.05, Table 2, Fig. 7).
Discussion
This study revealed that HPTX has hepatoprotective effects on liver IR injury as demonstrated by lower levels of liver aminotransfereases and reduced local and distant injury. The precise mechanisms of liver IR injury remain unclear. Liver IR results in free radical formation and lipid peroxidation generating intermediate products such as MDA that is observed when oxidative stress is elevated.[29]Activated Kupffer cells produce massive amounts of ROS, and lipid peroxidation impairs normal functions of mitochondrial respiratory chains.[4]The liberation of infammatory molecules into the systemic circulation leads to further remote organ injury and liver dysfunction.[30,31]
In our previous study,[12]we found a signifcant decrease in aminotransfereases levels in animals treated with HTS. Filho et al[13]found benefcial effects of HTS for liver transplantation in patients with fulminant hepatitis. Oreopoulos et al[32]further demonstrated that HTS reduces hepatic ICAM-1 expression and thus leads to protection against liver IR injury. Based on the results and conclusions of previous studies, it is possible that HTS induces endothelial cell shrinkage and leads to reduced microvascular disturbances by reducing infammation of the liver.
El-Ghoneimi et al[33]reported signifcantly lower levels of aminotransfereases, decreased levels of serum TNF-α, and a lower necrotic area in liver tissue in the PTX treated group compared to the lactate Ringer's group. Moreover, Arnault et al[34]suggested that PTX improves steatotic liver function and reduces hepatic damage after long preservation times. PTX is a compound that has been shown to restore cardiac output, downregulate the synthesis of proinfammatory mediators like IL-6, improve microvascular hepatic and intestinal blood fow, and increase survival after hemorrhagic shock.[35-38]Tian et al[39]concluded that the change of IL-6 levels is one of the reasons for superior outcomes in the PTX treated group. Our data confrmed this fnding. Thus, HPTX in the treatment of liver IR injury reduced the levels of IL-6 compared to the control group. IL-10 has a crucial role in preventing infammatory pathologies. In our study, we did not fnd any increase in IL-10 levels. But HPTX was given before reperfusion and not before ischemia. In addition, the decrease in oxidative stress evidenced by lower levels of IL-6 and MDA could downregulate the IL-10 production.
Other strategies, such as the simultaneous administration of drugs during fuid resuscitation, aimed at modulating organ dysfunction triggered by trauma and hemorrhage have been evaluated previously.[40]Yada-Langui et al[11]reported that HTS or PTX signifcantly attenuated bacterial translocation and lung injury after hemorrhagic shock.
In the present study, the protective effects of HPTX were also demonstrated in liver histology showing decreased histopathological injury score and necrosis in theHTS and HPTX groups. Additionally, HPTX was shown to be superior to HTS alone when we analyzed lipid peroxidation in ischemic and non-ischemic lobes and the early and late lung permeability after reperfusion. Compared with other groups, the HPTX group had superior results. It was reported that there was the occurrence of remote lung injury secondary to intestinal and liver IR, verifying an increased transvascular permeability leading to interstitial edema.[35]In experimental hemorrhagic shock models, resuscitation with lactate Ringer's solution was associated with increased lung damage when compared with HTS and PTX treated animals. This may be due to the proinfammatory properties of lactate Ringer's solution.[11,17,41]This fnding suggests that HPTX may modulate systemic infammation by preventing the remote organ injury.
It was reported that lipid peroxidation initiated by ROS is strongly associated with hepatocellular damage.[5-7,42]Okuda et al[43]found that ROS reached peak levels between 17 to 20 minutes during organ reperfusion following ischemia. Measurement of MDA production revealed a two-fold increase in lipid peroxidation in liver tissue and mitochondria in revascularized tissue after 90 minutes of ischemia.[44]On the other hand, ROS generation after IR is responsible for NF-κB activation and transcription of κB-dependent protective genes in the hepatocyte.[45]In this study, we observed decreased MDA levels in ischemic and non-ischemic lobes in the HPTX treated group after 4 hours of reperfusion when compared to other groups.
Our data showed that HPTX administration had a protective effect in different phases of liver IR injury, and that HPTX was better than HTS alone. Lipid peroxidation was signifcantly decreased in the liver 4 hours after reperfusion. We believe that HTS and PTX have strong protective effects against liver injury in the early phase of ischemia, especially in oxidative burst which is the main period of ROS liberation.
In conclusion, our data suggest that HPTX administration is an optimal regimen to decrease deleterious effects of liver IR injury in a rat model because of the decrease of oxidative stress and pulmonary permeability. These fndings may have implications in future liver surgery and transplantation.
Acknowledgements:We thank Sandra Nassa Sampietre, Cinthia Lanchotte (Laboratory of Medical Investigations LIM37 FMUSP) for assistance in data processing, Dr. Katia Ramos Moreira Leite (Department of Surgery HCFMUSP) for histological evaluation, Marcio Augusto Diniz (Institute of Mathematics and Statistics USP) for statistical analysis and Professor Eleazar Chaib (Director of the Laboratory of Medical Investigations LIM37 FMUSP) for research consultation.
Contributors:R-SV, FERR and R-FJA conceived and designed the study, contributed to the acquisition of data and supervised the experimental work, analysed the data and wrote the manuscript. PRS and BT helped to design the study and participated in animal preparation and performance of experimental work. CAMM, MMCC and DLAC participated in experimental design and helped to draft the manuscript. All authors read and approved the fnal manuscript. R-FJA is the guarantor.
Funding:This study was supported by a grant from S?o Paulo Foundation Research FAPESP 2011/05214-3.
Ethical approval:The experimental protocol was approved by the Ethics Committee of Hospital das Clinicas, University of S?o Paulo School of Medicine, Brazil (No. 0339/09).
Competing interest:No benefts in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Belghiti J, Noun R, Malafosse R, Jagot P, Sauvanet A, Pierangeli F, et al. Continuous versus intermittent portal triad clamping for liver resection: a controlled study. Ann Surg 1999;229:369-375.
2 Nativ NI, Maguire TJ, Yarmush G, Brasaemle DL, Henry SD, Guarrera JV, et al. Liver defatting: an alternative approach to enable steatotic liver transplantation. Am J Transplant 2012;12: 3176-3183.
3 Carini R, Albano E. Recent insights on the mechanisms of liver preconditioning. Gastroenterology 2003;125:1480-1491.
4 Lee WY, Koh EJ, Lee SM. A combination of ischemic preconditioning and allopurinol protects against ischemic injury through a nitric oxide-dependent mechanism. Nitric Oxide 2012;26:1-8.
5 Lentsch AB, Kato A, Yoshidome H, McMasters KM, Edwards MJ. Infammatory mechanisms and therapeutic strategies for warm hepatic ischemia/reperfusion injury. Hepatology 2000;32:169-173.
6 Yokota R, Fukai M, Shimamura T, Suzuki T, Watanabe Y, Nagashima K, et al. A novel hydroxyl radical scavenger, nicaraven, protects the liver from warm ischemia and reperfusion injury. Surgery 2000;127:661-669.
7 Zar HA, Tanigawa K, Kim YM, Lancaster JR Jr. Rat liver postischemic lipid peroxidation and vasoconstriction depend on ischemia time. Free Radic Biol Med 1998;25:255-264.
8 Colletti LM, Kunkel SL, Walz A, Burdick MD, Kunkel RG, Wilke CA, et al. The role of cytokine networks in the local liver injury following hepatic ischemia/reperfusion in the rat. Hepatology 1996;23:506-514.
9 Glantzounis GK, Salacinski HJ, Yang W, Davidson BR, Seifalian AM. The contemporary role of antioxidant therapy in attenuating liver ischemia-reperfusion injury: a review. Liver Transpl 2005;11:1031-1047.
10 Jaeschke H. Molecular mechanisms of hepatic ischemia-reperfusion injury and preconditioning. Am J Physiol Gastrointest Liver Physiol 2003;284:G15-26.
11 Yada-Langui MM, Coimbra R, Lancellotti C, Mimica I, Garcia C, Correia N Jr, et al. Hypertonic saline and pentoxifylline prevent lung injury and bacterial translocation after hemorrhagic shock. Shock 2000;14:594-598.
12 Figueira ER, Bacchella T, Coelho AM, Sampietre SN, Molan NA, Leit?o RM, et al. Timing-dependent protection of hypertonic saline solution administration in experimental liver ischemia/reperfusion injury. Surgery 2010;147:415-423.
13 Filho JA, Machado MA, Nani RS, Rocha JP, Figueira ER, Bacchella T, et al. Hypertonic saline solution increases cerebral perfusion pressure during clinical orthotopic liver transplantation for fulminant hepatic failure: preliminary results. Clinics (Sao Paulo) 2006;61:231-238.
14 Garrido Adel P, Cruz RJ Jr, Poli de Figueiredo LF, Rocha e Silva M. Small volume of hypertonic saline as the initial fuid replacement in experimental hypodynamic sepsis. Crit Care 2006;10:R62.
15 Kozaki K, Egawa H, Bermudez L, Keefe EB, So SK, Esquivel CO. Effects of pentoxifylline pretreatment on Kupffer cells in rat liver transplantation. Hepatology 1995;21:1079-1082.
16 Barroso-Aranda J, Schmid-Sch?nbein GW. Pentoxifylline pretreatment decreases the pool of circulating activated neutrophils, in-vivo adhesion to endothelium, and improves survival from hemorrhagic shock. Biorheology 1990;27:401-418.
17 Cruz RJ Jr, Yada-Langui MM, de Figueiredo LF, Sinosaki S, Rocha e Silva M. The synergistic effects of pentoxifylline on systemic and regional perfusion after hemorrhage and hypertonic resuscitation. Anesth Analg 2006;102:1518-1524.
18 Müller JM, Vollmar B, Menger MD. Pentoxifylline reduces venular leukocyte adherence (“refow paradox“) but not microvascular “no refow“ in hepatic ischemia/reperfusion. J Surg Res 1997;71:1-6.
19 Nishizawa H, Egawa H, Inomata Y, Uemoto S, Asonuma K, Kiuchi T, et al. Effciency of pentoxifylline in donor pretreatment in rat liver transplantation. J Surg Res 1997;72:170-176.
20 Madrahimov N, Dirsch O, Broelsch C, Dahmen U. Marginal hepatectomy in the rat: from anatomy to surgery. Ann Surg 2006;244:89-98.
21 Yoshizumi T, Yanaga K, Soejima Y, Maeda T, Uchiyama H, Sugimachi K. Amelioration of liver injury by ischaemic preconditioning. Br J Surg 1998;85:1636-1640.
22 Soriano FG, Liaudet L, Szabó E, Virág L, Mabley JG, Pacher P, et al. Resistance to acute septic peritonitis in poly(ADP-ribose) polymerase-1-defcient mice. Shock 2002;17:286-292.
23 Chance B, Williams GR. A simple and rapid assay of oxidative phosphorylation. Nature 1955;175:1120-1121.
24 Correia SC, Santos RX, Perry G, Zhu X, Moreira PI, Smith MA. Mitochondria: the missing link between preconditioning and neuroprotection. J Alzheimers Dis 2010;20:S475-485.
25 Jing-Jing Y, Wei-ze X, Qiang S, Jian-hua L, Xi-Wang L, Guoping J. Real time three-dimensional transthoracic echocardiography evaluation in a patient with congenital pulmonary vein stenosis. Echocardiography 2010;27:E109-111.
26 Warren JS, Yabroff KR, Mandel DM, Johnson KJ, Ward PA. Role of O2- in neutrophil recruitment into sites of dermal and pulmonary vasculitis. Free Radic Biol Med 1990;8:163-172.
27 Quireze C, Montero EF, Leit?o RM, Juliano Y, Fagundes DJ, Polide-Figueiredo LF. Ischemic preconditioning prevents apoptotic cell death and necrosis in early and intermediate phases of liver ischemia-reperfusion injury in rats. J Invest Surg 2006;19:229-236.
28 Brunner E, Puri ML. Nonparametric methods in factorial designs. Statistical Papers 2001;42:1-52.
29 Fukai M, Hayashi T, Yokota R, Shimamura T, Suzuki T, Taniguchi M, et al. Lipid peroxidation during ischemia depends on ischemia time in warm ischemia and reperfusion of rat liver. Free Radic Biol Med 2005;38:1372-1381.
30 Kimura N, Muraoka R, Horiuchi T, Tabo T, Uchinami M, Yokomachi J, et al. Intermittent hepatic pedicle clamping reduces liver and lung injury. J Surg Res 1998;78:11-17.
31 Ohashi I, Kaku R, Fuji H, Nakatsuka H, Matsumi M, Morita K. Severe acute pulmonary edema during living related liver transplantation surgery. Masui 2004;53:925-928.
32 Oreopoulos GD, Wu H, Szaszi K, Fan J, Marshall JC, Khadaroo RG, et al. Hypertonic preconditioning prevents hepatocellular injury following ischemia/reperfusion in mice: a role for interleukin 10. Hepatology 2004;40:211-220.
33 El-Ghoneimi A, Cursio R, Schmid-Alliana A, Tovey M, Lasfar A, Michiels JF, et al. Pentoxifylline inhibits liver expression of tumor necrosis factor alpha mRNA following normothermic ischemia-reperfusion. HPB (Oxford) 2007;9:112-119.
34 Arnault I, Bao YM, Sebagh M, Anjo A, Dimicoli JL, Lemoine A, et al. Benefcial effect of pentoxifylline on microvesicular steatotic livers submitted to a prolonged cold ischemia. Transplantation 2003;76:77-83.
35 Flynn WJ, Cryer HG, Garrison RN. Pentoxifylline restores intestinal microvascular blood fow during resuscitated hemorrhagic shock. Surgery 1991;110:350-356.
36 Hernández E, Bucio L, Souza V, Escobar MC, Gómez-Quiroz LE, Farfán B, et al. Pentoxifylline downregulates alpha (I) collagen expression by the inhibition of Ikappabalpha degradation in liver stellate cells. Cell Biol Toxicol 2008;24:303-314.
37 Marzi I, Maier M, Herzog C, Bauer M. Infuence of pentoxifylline and albifylline on liver microcirculation and leukocyte adhesion after hemorrhagic shock in the rat. J Trauma 1996;40:90-96.
38 Wang P, Ba ZF, Morrison MH, Ayala A, Chaudry IH. Mechanism of the benefcial effects of pentoxifylline on hepatocellular function after trauma hemorrhage and resuscitation. Surgery 1992;112:451-458.
39 Tian Y, Jochum W, Georgiev P, Moritz W, Graf R, Clavien PA. Kupffer cell-dependent TNF-alpha signaling mediates injury in the arterialized small-for-size liver transplantation in the mouse. Proc Natl Acad Sci U S A 2006;103:4598-4603.
40 Corso CO, Okamoto S, Leiderer R, Messmer K. Resuscitation with hypertonic saline dextran reduces endothelial cell swelling and improves hepatic microvascular perfusion and function after hemorrhagic shock. J Surg Res 1998;80:210-220.
41 Coimbra R, Porcides R, Loomis W, Melbostad H, Lall R, Deree J, et al. HSPTX protects against hemorrhagic shock resuscitation-induced tissue injury: an attractive alternative to Ringer's lactate. J Trauma 2006;60:41-51.
42 Seif B, Kadkhodaee M, Delavari F, Mikaeili S, Shams S, Ostad SN. Pretreatment with pentoxifylline and N-acetylcysteine in liver ischemia reperfusion-induced renal injury. Ren Fail 2012;34:610-615.
43 Okuda M, Lee HC, Chance B, Kumar C. Glutathione and ischemia-reperfusion injury in the perfused rat liver. Free Radic Biol Med 1992;12:271-279.
44 Marubayashi S, Dohi K, Ochi K, Kawasaki T. Role of free radicals in ischemic rat liver cell injury: prevention of damage by alpha-tocopherol administration. Surgery 1986;99:184-192.
45 Lluis JM, Buricchi F, Chiarugi P, Morales A, Fernandez-Checa JC. Dual role of mitochondrial reactive oxygen species in hypoxia signaling: activation of nuclear factor-{kappa}B via c-SRC and oxidant-dependent cell death. Cancer Res 2007;67:7368-7377.
Received January 17, 2014
Accepted after revision September 29, 2014
AuthorAffliations:Department of Gastroenterology, Laboratory of Medical Investigations LIM37 Discipline of Liver and Gastrointestinal Transplantation (Rocha-Santos V, Figueira ERR, Coelho AMM, Pinheiro RS, Bacchella T, Machado MCC and D'Albuquerque LAC) and Discipline of Anesthesiology (Rocha-Filho JA), Hospital das Clinicas, University of S?o Paulo School of Medicine, S?o Paulo, SP, Brazil
Joel A Rocha-Filho, MD, Discipline of Anesthesiology, University of S?o Paulo School of Medicine, Rua Manoel da Nóbrega, 489 apt 21, 04001-083, S?o Paulo, SP, Brazil (Tel: +55-11-2661-3323; Email: joelrocha@me.com)
? 2015, Hepatobiliary Pancreat Dis Int. All rights reserved.
10.1016/S1499-3872(15)60348-4
Published online March 10, 2015.
 Hepatobiliary & Pancreatic Diseases International2015年2期
Hepatobiliary & Pancreatic Diseases International2015年2期
- Hepatobiliary & Pancreatic Diseases International的其它文章
- Meetings and Courses
- Current strategies for preventing the recurrence of hepatocellular carcinoma after livertransplantation
- Glypican-3 as a specifc biomarker for hepatocellular carcinoma
- Inhibition of pancreatic stellate cell activity by adipose-derived stem cells
- Mutations in thep16gene in DMBA-induced pancreatic intraepithelial neoplasia and pancreatic cancer in rats
- Contrast-enhanced ultrasound in diagnosis of gallbladder adenoma
