Antioxidant Activities of Nine Selected Culinary Spices from China
Liang Ying ,Ding Ying,Zhang Liu-juanand Liu Xian-jin
1 Key Laboratory of Food Quality and Safety of Jiangsu Province,Nanjing 210014,China
2 Key Laboratory of Control Technology and Standard for Agro-product Safety and Quality,Nanjing 210014,China
Introduction
Deterioration of food quality occurs during processing and storage and is related to oxidative processes.Degradation affects lipids,carbohydrates and proteins (Halliwell,1997).Usually synthetic antioxidants,such as,butylhydroxyanisole (BHA) or butylhydroxytoluene (BHT) are used to decelerate these processes.Yet,these antioxidants suffer from the drawback which they are volatile easily and decompose at high temperature.Additionally,it is still unclear whether chronic consumption can lead to health risks (Martinez-Tome et al.,2001).
Many antioxidant compounds,naturally occurring in plant sources,have been identified as free radical or active oxygen scavengers.Recently,considerable interest has increased in naturally-occurring antioxidants that can be used to protect human beings and food from oxidative stress damage (Scalbert et al.,2005).Spices are natural plant products,which have been used not only as flavoring and coloring agents,but also as food preservatives and folk medicines throughout the world for thousands of years.Many spices have been recognized as having digestive stimulant action,carminative action,antimicrobial,antiinflammatory,anti-mutagenic,and anti-carcinogenic potential,etc (Ceylan and Fung,2004;Srinivasan et al.,2005).Besides,many spices also are an excellent source of phenolic compounds that have been reported to show superior antioxidant activity (Carlsen et al.,2010;Wojdy?o et al.,2007;Mata et al.,2007;Konczak et al.,2010).However,the knowledge about the antioxidant capacity and phenolic compounds of spices commonly consumed in China is limited (Lu et al.,2011).
More than 400 native spices have been used for a long time in "Chinese cuisine",not only to improve or modify the flavour of foods,but also to avoid its deterioration.Some common spices,such as cinnamon,star anise,Sichuan pepper and dried ginger,are widely used in daily Chinese household cooking (Tapsell et al.,2006).The purpose of this study was to evaluate the antioxidant activity and total phenolic contents of selected culinary spices to find out potential sources of natural antioxidants for food application.Nine spices growing in China and commonly used in food preparations were collected,water and/or ethanol extracts were prepared to screen for antioxidant activity and total phenolic content.
Materials and Methods
Plant materials and chemicals
Star anise (Illicium verum),Sichuan pepper (Pericarpium zanthoxyli),cinnamon (Cinnamomum aromaticum),bay leaf (Laurus nobilis),fennel (Foeniculum vulgare),amomum tsaoko fruit (Fructus tsaoko),galangal (Alpinia officinarum Hance),dried ginger (Rhizoma zingiberis) and dried tangerine peel (Pericarpium Citri Reticulatae) were collected from local supermarkets (Nanjing,Jiangsu,China).The collected samples were ground to fine powder by a universal high-speed smashing machine and pass though a 50 mesh screen.The ground samples were stored at -20℃ until analyzed.
1,1-diphenyl-2-picrylhydrazyl (DPPH),2,2'-azinobis (3-ethylbenzthiazoline-6-sulphonic acid) (ABTS),2,4,6-tri (2-pyridyl)-1,3,5-triazine (TPTZ) and 2-deoxy-D-ribose were purchased from Sigma Chemical Co (St.Louis,MO,USA).Ethylenediaminetetraacetic acid (EDTA),thiobarbituric acid (TBA),trichloroacetic acid (TCA),gallic acid,Folin-Ciocalteu reagent,butylated hydroxytoluene (BHT),ascorbic acid,and 6-hydroxy-2,5,7,8-tetramethyl-2-chromanecarboxylic acid (trolox) were from Sinopharm Group Chemical Reagent Co.,Ltd (Shanghai,China).All other chemicals used were of analytical grades.
Spices extraction
Ethanol extraction: 20 g of ground sample was extracted with 150 mL ethanol under refluxing for 30 min.The mixture was cooled to room temperature and filtered to get the clear supernatant.The solvent was removed by vacuum distillation to obtain the equivalent dry weight.The dried extract was stored at -20℃ until use.
Water extraction: the water extraction was carried out as described in the ethanol extraction procedure,except that ethanol was replaced by water.The solvent was removed by vacuum distillation to obtain the equivalent dry weight.The dried extract was stored at -20℃ until using.The amomum tsaoko fruit,galangal,dried ginger and dried tangerine peel had no water extract,for a kind of jelly was observed during the water extraction process.
Free radical scavenging by using DPPH radical
DPPH radical scavenging activity of spice extracts was determined using the method described by Borneo et al.(2009) with slight modifications.One mL of various concentrations of the extracts was mixed with 3 mL of 0.003% ethanol solution of DPPH.After 30 min of incubation in dark,at room temperature,the absorbance at 517 nm was measured against blank.IC50(half-maximal inhibitory concentration,μg ? mL-1) was calculated as the concentration of the sample necessary to decrease by 50% the initial absorbance of DPPH.BHT,ascorbic acid and trolox were tested as references.The values were presented as the mean of the triplicate analyses.
Free radical scavenging by using ABTS radical
Total antioxidant activity assay was carried out by ABTS method modified by Re et al (1999).ABTS was dissolved in water to a concentration of 7 mmol ? L-1.ABTS radical cation was produced by reacting ABTS stock solution with 2.45 mmol ? L-1potassium persulfate (final concentration) and allowing the mixture to stand in the dark room for 16 h before use.ABTS radical cation solution was diluted with 80% ethanol to an absorbance of 0.700±0.02 at 734 nm.The extract was diluted with 80% ethanol at various concentrations.ABTS radical cation solution (2.0 mL;absorbance of 0.700±0.02) was added to 0.1 mL of the tested samples and mixed thoroughly.The reactive mixture was allowed to stand at room temperature for 6 min,and the absorbance was immediately recorded at 734 nm using a UV spectrophotometer.IC50(half-maximal inhibitory concentration,μg ? mL-1) was calculated as the concentration of sample necessary to decrease by 50% the initial absorbance of ABTS.BHT,ascorbic acid and trolox were tested as references.The values were presented as the mean of triplicate analyses.
Ferric reducing antioxidant power assay (FRAP)
The total antioxidant potential of a sample was determined using the ferric reducing antioxidant power assay by Benzie and Strain (1996).A potential antioxidant will reduce the ferric ion (Fe3+) to the ferrous ion (Fe2+);the latter forms a blue complex (Fe2+/TPTZ),which increases the absorption at 593 nm.FRAP reagent consisted of 10 mmol ? L-1TPTZ solution in 40 mmol ? L-1HCl,20 mmol ? L-1FeCl3solution,and 0.3 mol ? L-1acetate buffer (pH 3.6) in proportions of 1 : 1 : 10 (v/v/v).FRAP reagent was prepared and warmed to 37℃ in a water bath prior to use 5 μL of each appropriate diluted samples were mixed with 200 μL FRAP reagent and the reactive mixture was incubated at 37℃ for 10 min.The absorbance was measured at 593 nm using a UV spectrophotometer.All the solutions were used within the day for preparation.The standard curve was prepared accordingly using a stock iron (Ⅱ) sulphate solution.Results were expressed as μmol of Fe (Ⅱ)/g extracts.BHT,ascorbic acid and trolox were tested as references.The values were presented as the mean of the triplicate analyses.
Hydroxyl radical-mediated 2-deoxy-D-ribose degradation
The ability of the extracts to inhibit nonsite-specific hydroxyl radical-mediated peroxidation was carried out according to Hinneburg et al.(2006) with some adaptions.The reaction mixture contained 100 μL of extract dissolved in water,300 μL of 5.6 mmol ? L-12-deoxy-D-ribose in KH2PO4-NaOH buffer (50 mmol ? L-1,pH 7.4),150 μL of premixed 100 μmol ? L-1FeCl3and 104 mmol ? L-1EDTA (1:1 v/v) solution,80 μL of 1.0 mmol ? L-1H2O2and 80 μL of 1.0 mmol ? L-1water ascorbic acid.Tubes were vortexed and incubated at 50℃ for 30 min.Thereafter,0.5 mL of 2.8% TCA and 0.5 mL of 1.0% TBA were added to each tube.The samples were vortexed and heated in a water bath at 50℃ for 30 min.The extent of oxidation was estimated from the absorbance of the solution at 532 nm.The percentage inhibition values were calculated from the absorbance of the control (Ac) and of the sample (As) using Eq.(1),where the controls contained all the reaction reagents except the extract or positive control substance.The antioxidant activities of the extracts were expressed as trolox equivalents (μmol trolox/g dried extract).BHT,ascorbic acid were tested as references.The values were presented as the means of the triplicate analyses.
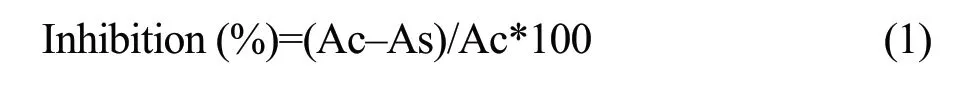
Determination of total phenolic content
Total phenolic content was determined using the Folin-Ciocalteu colorimetric method described previously by Cai et al (2004).Briefly,5 mL water dilutions of the extracts (the ethanol extracts were diluted with 200 μL ethanol and 4.8 mL water) were mixed with 1 mL Folin–Ciocalteu reagent for 3 min and the reaction solution was neutralized with 3 mL saturated sodium carbonate (75 g ? L-1).The absorbance of the resulting blue color was measured at 760 nm with a UV spectrophotometer after interval shaking for 2 h,at room temperature.Quantification was conducted based on the standard curve of the gallic acid (5-80 mg ? L-1).Results were expressed as milligram of the gallic acid equivalent (GAE) per one gram of the dried extracts.
Results and Discussion
DPPH radical scavenging
DPPH? is a stable nitrogen-centered free radical,its colour changes from blue/purple to yellow upon reduction by either the process of hydrogen-or electron-donation.Substances that are able to perform this reaction can be considered as antioxidants and therefore radical scavengers (Brand-Williams et al.,1995).Values obtained for this DPPH assay are shown in Table 1.BHT,ascorbic acid and trolox were tested as references.
Both ethanol and water extracts exhibit good radical scavenging activities.Ethanol extracts of Sichuan pepper,cinnamon and bay leaf were more active than the water ones.Water extracts of the star anise and fennel were more active than those of the corresponding ethanol extracts.The best results were obtained with ethanol extract of Sichuan pepper,which followed by cinnamon,amomum tsaoko fruit,bay leaf and water extract of cinnamon (IC50=82.02 μg ? mL-1,89.98 μg ? mL-1,110.97 μg ? mL-1,203.15 μg ? mL-1,and 237.67 μg ? mL-1,respectively).The spice extract with the lowest DPPH radical scavenging effect was the ethanol extract of star anise (IC50=5 064.28 μg ? mL-1).Between those high and low values,wide ranges of DPPH radical scavenging abilities were obtained among the species.DPPH radical scavenging effects of the references were higher than those of the nine spices.
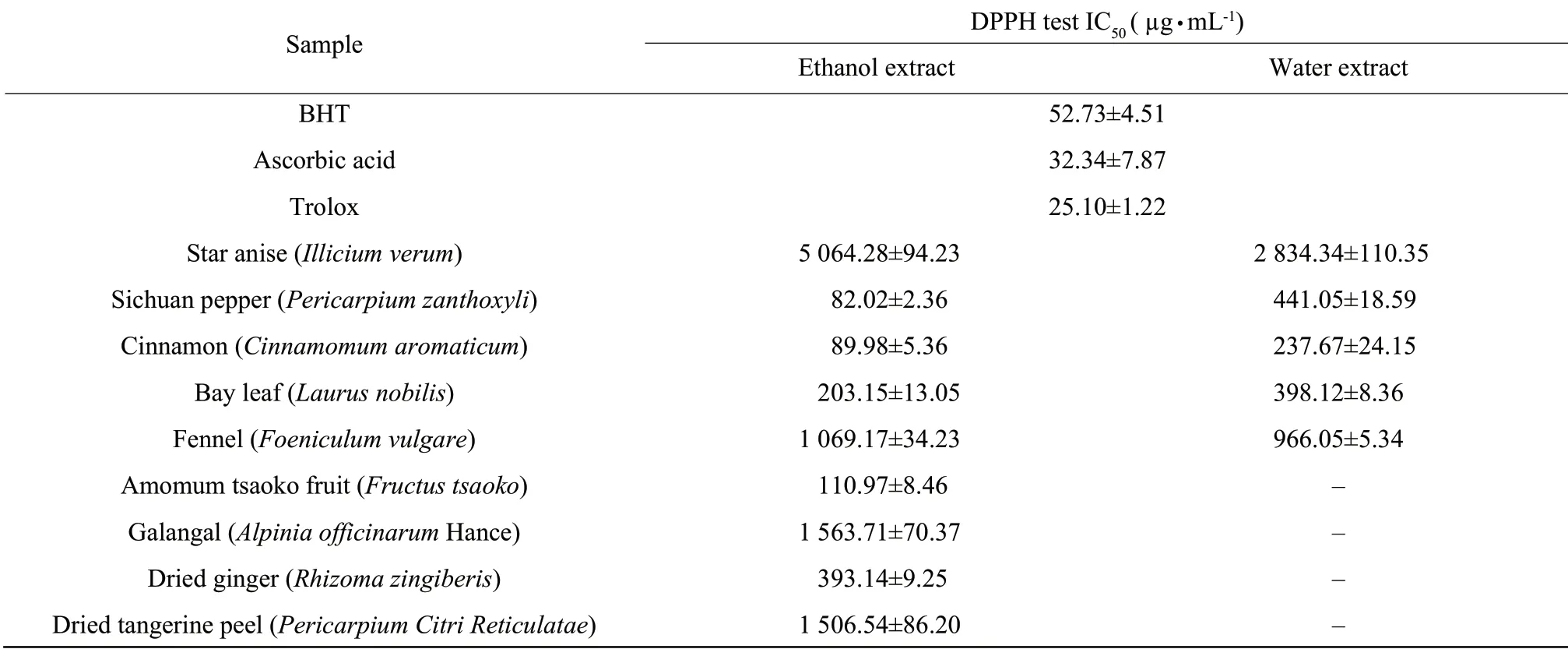
Table1 DPPH radical scavenging activity of water and/or ethanol extracts of spices
ABTS radical scavenging
This is to monitor the decay of the radical-cation ABTS?+produced by the oxidation of ABTS caused by the addition of a phenolic-containing sample.ABTS?+has a strong absorption in 734 nm and can be easily determined spectrophotometrically (Roginsky and Lissi,2005).
Table2 illustrated the effects of spices on the uppression of the absorbance of ABTS?+.Ethanol extract of the cinnamon possessed the highest ABTS radical scavenging ability,followed by ethanol extract of dried ginger,amomum tsaoko fruit,water extract of cinnamon and Sichuan pepper (IC50=171.39 μg ? mL-1,400.98 μg ? mL-1,411.07 μg ? mL-1,420.43 μg ? mL-1,and 549.25 μg ? mL-1,respectively).Ethanol extract of the star anise demonstrated the lowest ABTS radical scavenging ability (IC50=3 192.54 μg ? mL-1).ABTS radical scavenging ability of ethanol extract of cinnamon was just a little lower than that of BHT and higher than that of ascorbic acid and trolox.It will probably be further investigated in the future to determine which chemical groups are responsible for such high antioxidant capacity value.
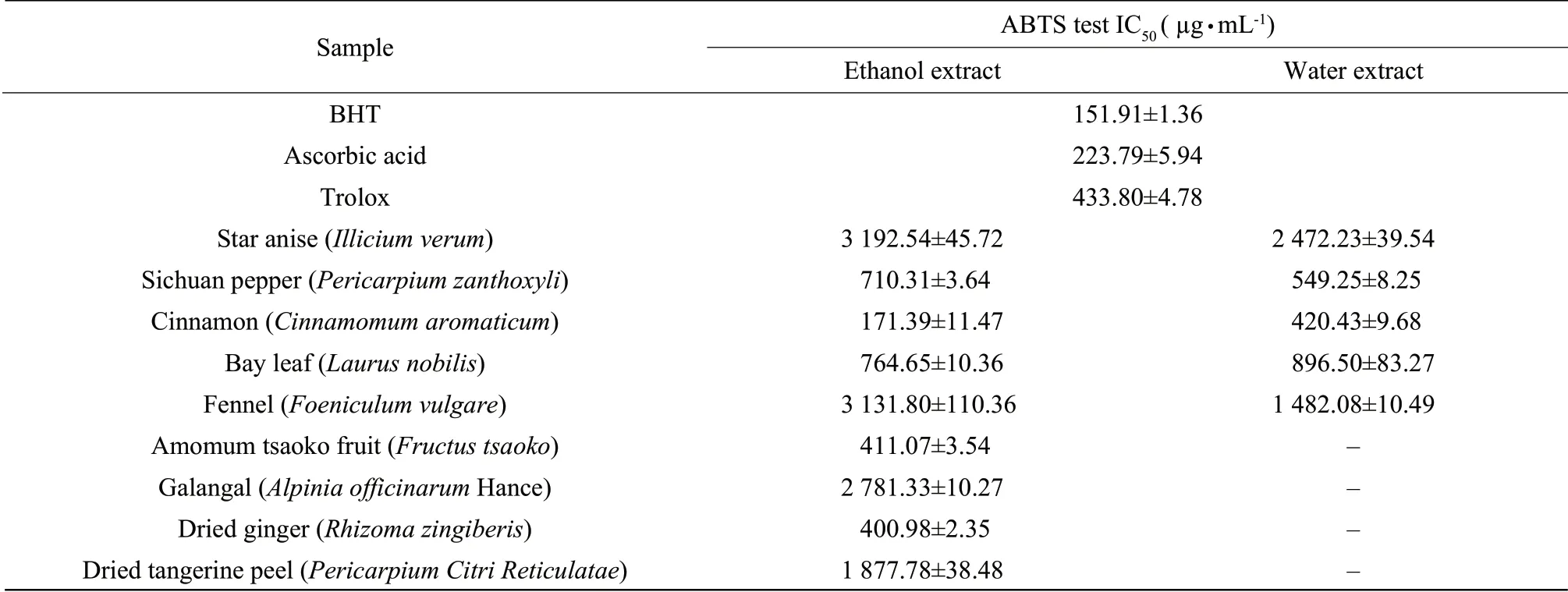
Table2 ABTS radical scavenging activity of water and/or ethanol extracts of spices
Ferric reducing antioxidant power
In this method,the antioxidant activity was determined based on the ability to reduce ferric (III) iron to ferrous (II) iron (Benzie et al.,1996).Results were expressed as μmol Fe (II) equivalent per g of dried extract.As shown in Table 3,there was a wide range of ferric reducing/antioxidant power within the extracts analyzed.FRAP antioxidant activity ranged from the lowest value of 127.16 μmol Fe (II)/g (ethanol extract of star anise) to the highest value of 4 541.87 μmol Fe (II)/g (ethanol extract of cinnamon).The extracts with high FRAP antioxidant capacity were as follows: ethanol and water extracts of cinnamon (4 541.87 and 1 134.52 μmol of Fe (II)/g,respectively),ethanol extract of dried ginger (1 180.10 μmol of Fe (II)/g),amomum tsaoko fruit (830.47 μmol of Fe (II)/g) and water extracts of bay leaf (609.33 μmol of Fe (II)/g).This trend was similar to ABTS assay,which was in agreement with Lu et al (2011).This might due to the basis on the similar redox reaction for ABTS and FRAP assays,and the comparable redox potential of Fe (Ⅲ) salt (0.70 V) to that of ABTS radical (0.68 V)(Huang et al.,2005).BHT,ascorbic acid and trolox were also quantified FRAP values.As it can be seen,all the extracts analyzed for FRAP had much lower FRAP values when compared to BHT,ascorbic acid and trolox.However,we should keep in mind that those high FRAP values obtained for BHT,ascorbic acid and trolox were because the assay performed on highly purified reference standards and not in complex matrices,such as those of the extracts analyzed.
Hydroxyl radical-mediated deoxyribose degradation
Deoxyribose degradation occurs by hydroxyl radicals generated by a Fenton reaction.Hydroxyl radicals are the main free radicals present in vivo in water environment and easily cross cell membranes,at specific sites.Thus,removing hydroxyl radical is very important for protection of food systems.The activities of the extracts were expressed as μmol trolox equivalent per g of dried extract.Results of hydroxyl radical-mediated deoxyribose degradation are presented in Table 4 and compared with that of BHT and ascorbic acid.In the assay,the trolox equivalents ranked from 3 869.36 μmol trolox/g ethanol extract for cinnamon to 80.07 μmol trolox/g ethanol extract for galangal,which was not significantly different from the values found for water extract of Sichuan pepper.The ethanol extracts had much higher trolox equivalents than the water extracts,respectively.The values higher than 1 000 μmol trolox/g were the ethanol extracts including cinnamon (3 869.36 μmol of trolox/g),bay leaf (2 114.65 μmol of trolox/g),Sichuan pepper (2 074.15 μmol of trolox/g),star anise(1 602.83 μmol of trolox/g) and fennel (1 556.64 μmol of trolox/g).Those values were also lower than those of BHT and ascorbic acid.
Table3 Ferric reducing/antioxidant power of water and/or ethanol extracts of spices
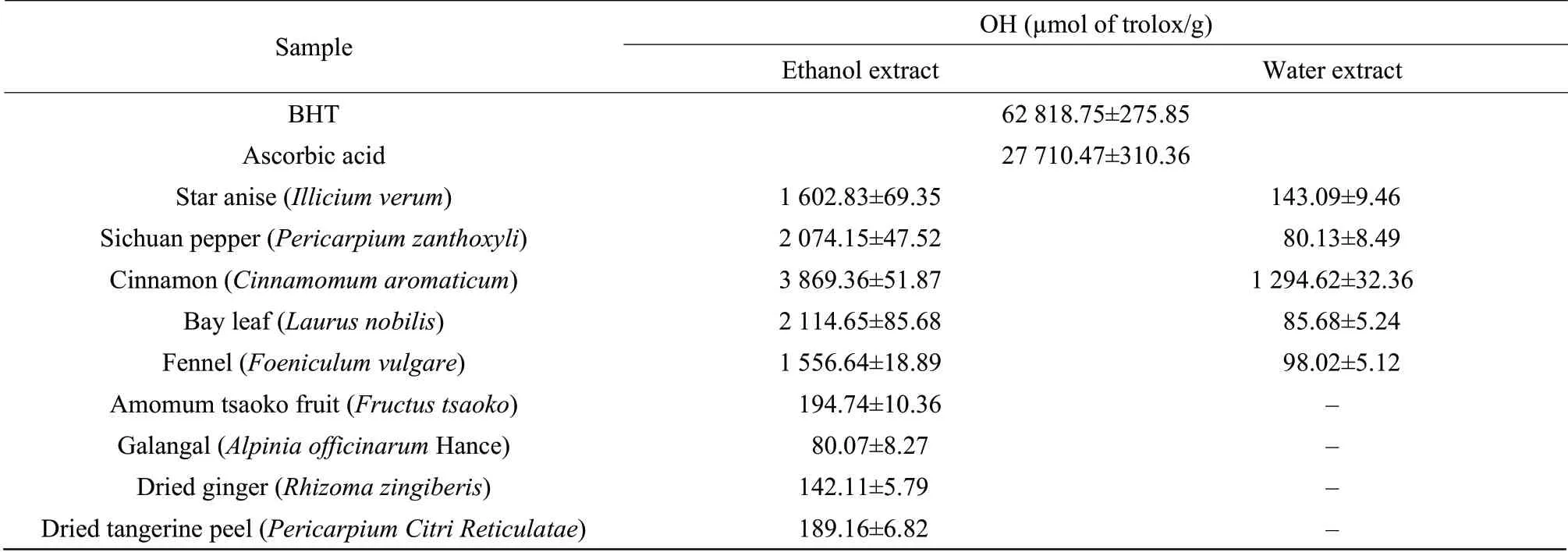
Table4 Hydroxyl radical-mediated deoxyribose degradation of water and/or ethanol extracts of spices
Total phenolic content
Phenolic substances have been shown to be responsible for the antioxidant activity of plant materials (Rice-Evans et al.,1996).Table 5 summarizes the amount of the total phenols in the extracts investigated by the Folin-Ciocalteu method.The content of total phenols was expressed as gallic acid equivalents (mg GAE/g extract).The results demonstrated the presence of the natural antioxidant phenolic compounds in all these extracts and the contents varied widely in the spice extracts,ranging from 11.92 to 227.69 mg GAE/g extract.Significantly the highest results were found in the order of ethanol extracts of dried ginger (227.69 mg GAE/g)>amomum tsaoko fruit (213.54 mg GAE/g)>cinnamon (115.27 mg GAE/g)>Sichuan pepper (106.26 mg GAE/g)>bay leaf (94.07 mg GAE/g).The content of the total phenols in ethanol extracts was higher than that of the water extracts.The values obtained for the water extracts of spices were similar to that reported,while the values found for the ethanol extract of the spices were highly upon that reported in the literature,which the spices were extracted with 60% ethanol (Lu et al.,2011).
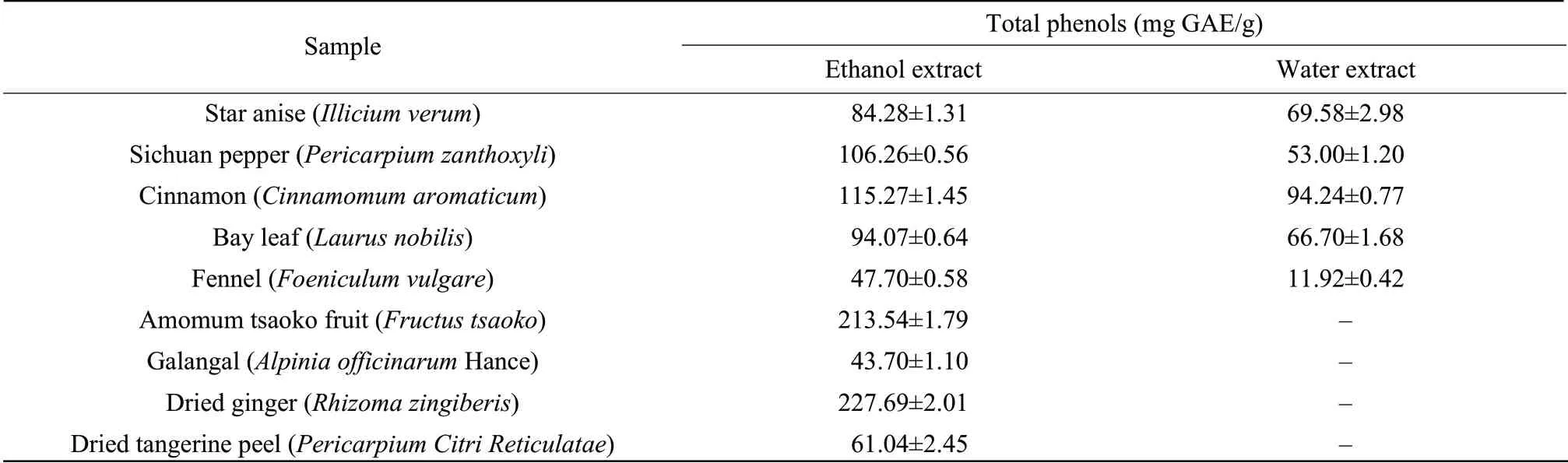
Table5 Total phenolic content of water and/or ethanol extracts of spices
No linear correlation could be established between total phenolic content and antioxidant activities,corroborating results were also found in the literature (Mata et al.,2007;Ou et al.,2003).One can assume that other compounds,without a phenolic structure,may be responsible for the antioxidant activities.Still,the extracts with high content of the phenols,such as dried ginger,amomum tsaoko fruit and cinnamon,also had the high ability of DPPH and ABTS radical scavenging and ferric reducing antioxidant power.
Our study showed that of 18 tested spice extracts,the cinnamon,amomum tsaoko fruit,Sichuan pepper and dried ginger were the most effective.Ethanol extract of the cinnamon exhibited the highest antioxidant capacity in both ABTS and FRAP assays,the second highest in DPPH assay and the third highest in Fenton reaction,associated with high level of the total phenols.The result was in agreement with Dudonne et al (2009).However,it did not accord with that by Lu et al.(2011),who found that galangal exhibited the highest antioxidant capacity.It might be caused by different extraction methods,including extraction solvent,extraction time and temperature.Cinnamon is widely used as flavoring in China,also processes certain biological activities,including antimicrobial,antiviral,antioxidant,antitumor,antihypertension,antidiabetes,gastroprotective and immunomodulatory (Hlebowicz et al.,2007).Meanwhile,the ethanol extracts of the amomum tsaoko fruit,Sichuan pepper and dried ginger demonstrated high antioxidant capacity and total phenolic contents.It should be noted that further concentration and/or purification is needed to achieve better antioxidant capacities,which is desirable to extent these studies,and to further characterize the most active fractions.
Conclusions
The spices,commonly consumed in cooking,may act as important supplements of the antioxidants in the diet,especially in the cooking for some dishes of the poor nutritional value.The study showed many selected culinary spices from China had high levels of antioxidant capacity;the extracts with high antioxidant activities also had the high content of the phenols.Both ethanol and water extracts exhibited good radical scavenging activities,including DPPH radical scavenging,ABTS radical scavenging and ferric reducing activities.Further researches are needed to investigate what chemical compounds are presented in the spices and their potentials to be used as possible natural substitutes for artificial antioxidants currently used in food processing.
Benzie I F F,Strain J J.1996.The ferric reducing ability of plasma (FRAP) as a measure of "antioxidant power": the FRAP assay.Anal Biochem,239(1): 70-76.
Borneo R,León A E,Aguirre A,et al.2009.Antioxidant capacity of medicinal plants from the Province of Córdoba (Argentina) and their in vitro testing in a model food system.Food Chem,112: 664-670.
Brand-Williams W,Cuvelier M E,Berset C.1995.Use of a free radical method to evaluate antioxidant activity.LWT-Food Sci Technol,28(1): 25-30.
Cai Y Z,Luo Q,Sun M,et al.2004.Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer.Life Sci,74(17): 2157-2184.
Carlsen M H,Halvorsen B L,Holte K,et al.2010.The total antioxidant content of more than 3100 foods,beverages,spices,herbs and supplements used worldwide.Nutr J,9: 3-7.
Ceylan E,Fung D Y C.2004.Antimicrobial activity of spices.J Rapid Methods Autom Microbiol,12: 1-55.
Dudonne S,Vitrac X,Coutiere P,et al.2009.Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH,ABTS,FRAP,SOD and ORAC assays.J Agric Food Chem,57(5): 1768-1774.
Halliwell B.1997.Antioxidants and human disease: a general introduction.Nutr Rev,55: 44-52.
Hinneburg I,Dorman H J D,Hiltunen R.2006.Antioxidant activities of extracts from selected culinary herbs and spices.Food Chem,97: 122-129.
Hlebowicz J,Darwiche G,Bj?rgell O,et al.2007.Effect of cinnamon on postprandial blood glucose,gastric emptying,and satiety in healthy subjects.Am J Clin Nutr,85(6): 1552-1556.
Huang D,Ou B,Prior R L.2005.The chemistry behind antioxidant capacity assays.J Agric Food Chem,53(6): 1841-1856.
Konczak I,Zabaras D,Dunstan M,et al.2010.Antioxidant capacity and phenolic compounds in commercially grown native Australian herbs and spices.Food Chem,122: 260-266.
Lu M,Yuan B,Zeng M M,et al.2011.Antioxidant capacity and major phenolic compounds of spices commonly consumed in China.Food Res Int,44: 530-536.
Mata A T,Proen?a C,Ferreira A R,et al.2007.Antioxidant and antiacetylcholinesterase activities of five plants used as Portuguese food spices.Food Chem,103: 778-786.
Martinez-Tome M,Jimenez A M,Ruggieri S,et al.2001.Antioxidant properties of Mediterranean spices compared with common food additives.J Food Protect,64: 1412-1419.
Ou B,Huang D,Hampsch-Woodili M,et al.2003.When the east meets west: the relation yin-yang and antioxidation-oxidation.FASEB J,17: 127-129.
Re R,Pellegrini N,Proteggente A,et al.1999.Antioxidant activity applying an improved ABTS radical cation decolorization assay.Free Radical Bio Med,26: 1231-1237.
Roginsky V,Lissi E A.2005.Review of methods to determine chainbreaking antioxidant activity in food.Food Chem,92: 235-254.
Rice-Evans C A,Miller N J,Paganga G.1996.Structure-antioxidant activity relationships of flavonoids and phenolic acids.Free Radical Bio Med,20(7): 933-956.
Scalbert A,Manach C,Morand C,et al.2005.Dietary polyphenol and the prevention of diseases.Crit Rev Food Sci Nutr,45: 287-306.
Srinivasan K,Viswanad B,Asrat L,et al.2005.Combination of highfat diet-fed and low-dose streptozotocin-treated rat: a model for type 2 diabetes and pharmacological screening.Pharmacol Res,52: 313-320.
Tapsell L C,Hemphill I,Cobiac L,et al.2006.Health benefits of herbs and spices: the past,the present,the future.Med J Australia,185: 4-24.Wojdy?o A,Oszmiański J,Czemerys R.2007.Antioxidant activity and phenolic compounds in 32 selected herbs.Food Chem,105: 940-949.
 Journal of Northeast Agricultural University(English Edition)2015年1期
Journal of Northeast Agricultural University(English Edition)2015年1期
- Journal of Northeast Agricultural University(English Edition)的其它文章
- Investigation and Study on Employment Status of Migrant Workers in Heilongjiang Province
- Farmers' Perception and Awareness and Factors Affecting Awareness of Farmers Regarding Crop Insurance as a Risk Coping Mechanism Evidence from Pakistan
- Suggestions on Forest Ecological Compensation
—Taking Mudanjiang City as an Example - Journal of Northeast Agricultural University (English Edition)
- Revelation of Rural System of Medical Services in BRICS to China
- Fault Line Selection Method Considering Grounding Fault Angle for Distribution Network
