Study on the Effect of Surface Modi fi cation on the Properties of Bentonite Greases
(Department of Military Oil Application & Management Engineering, Logistical Engineering University of PLA, Chongqing 401311)
Study on the Effect of Surface Modi fi cation on the Properties of Bentonite Greases
Shi Chengfei; Guo Xiaochuan; Jiang Mingjun; He Yan
(Department of Military Oil Application & Management Engineering, Logistical Engineering University of PLA, Chongqing 401311)
In order to investigate the infuening factors of organic modifcation procedure and fnd out connections between organic modifcation and the properties of bentonite greases, organic montmorillonite (OMMT) thickeners with different surfactant dosages and constituents were synthesized through intercalation reaction between sodium montmorillonite (NaMMT) and quaternary ammonium surfactants in aqueous solvents. The lubricating greases were prepared with the resulting organoclays, while the penetration and oil separation of lubricating greases were evaluated, respectively. The surface modifcation process of montmorillonite (MMT) was analyzed and the thickening mechanism of OMMT was discussed in this study. The experimental results showed that, with an increasing amount of surfactant, the basal spacing between the clay platelets was increasing and the structure of modifer molecules layer in the interlayer was changing from lateral bilayer to paraffn-type bilayer. The optimal properties of lubricating greases were achieved, when the structure of surfactant molecules loaded in the interlayer was the paraffn-type monolayer, which meant that the dosage of modifer was equal to 120—140 mmol/(100g). Meanwhile, it was found that the thickening performance, colloid stability, anti-wear and friction-reducing performance of lubricating greases were improved, when the surfactants were mixed with octadecyl trimethyl ammonium chloride (OTAC) and hexadecyl trimethyl ammonium chloride (HTAC). And the optimum mole ratio of two surfactants is was 1:1.
organic montmorillonite; surface modifcation; thickening mechanism; bentonite greases
1 Introduction
To comply with the steadily growing awareness of ecological environment in legislation, a special focus of attention should be concentrated on the ecological impact of lubricating greases in many engineering felds[1]. Lubricating greases are semi-solid lubricating materials, resulting from the fne dispersion of thickeners in mineral or synthetic oils, which not only can play a superior role in the reduction of energy losses caused by friction in tribosystems of many machine elements but also can minimize the wear[1-2]. The conventional thickeners are metallic soaps prepared by saponifcation of fatty substances, including fatty acid soaps of lithium, sodium, aluminum and barium salts, which can cause direct or indirect environmental pollution[1,3-5]. Due to the improvement of public’s environmental consciousness and the guidance of the government policies, the market demand of eco-friendly lubricating grease has been increasing continuously. Being different from the conventional metallic soap thickeners, the non-soap product, such as organoclay, does not contain metallic elements, which is an eco-friendly thickener[3,5]. The use of organoclay as thickener in lubricating grease formulation provides the grease with excellent physical and chemical properties and extraordinary performance[6-7]. Meanwhile, the reserves of montmorillonite minerals in China rank second in the world, which would bring about convenient conditions for massive exploitation of this mineral. Compared with lithium greases, bentonite greases have more advantage in terms of its production cost.
In recent years, research and production of OMMTs have attracted great interest both in industry and academic circles, because these products are uninterruptedly experi-encing improvements in materials properties[8]. The major areas of applications of OMMTs involve the use of their rheological and adsorbent properties in various systems such as wastewater treatment, oil well drilling fuids, cosmetics, paint, personal care products, polymer-clay nanocomposites and hydrocarbon cracking catalyst[8-11]. Compared with the above applications, less attention is paid to using OMMT as grease thickener. And the researches related to bentonite grease are mainly focused on the infuencing factors of grease manufacturing, such as the compatibility between OMMTs thickener, base oil and polar activator[12-22], the post-treatment of bentonite grease[18-23], and the optimization of additives[17,24-28]. Mi Hongying[7,29-30]uses the low-priced oil-soluble urea-formaldehyde resin and phenol formaldehyde resin both with end group of octadecylamine as the organic modifier to prepare two bentonite thickeners, respectively. Two kinds of bentonite greases, which are made from two bentonite thickeners, both have broader temperature range of application, outstanding thermal oxidation stability, and water-resistant ability. Pogosian A K[31-32]had studied the impact of surfactant structure on the tribological properties of bentonite greases. The processes for organic modifcation of clay also have a strong infuence on the properties of bentonite greases. The previous studies, which are connected with the organic modifcation and the impact of organic modifcation on the properties of bentonite greases, are seldom reported.
The purpose of this article is to investigate the infuencing factors of organic modifcation procedure and fnd out connections between organic modifcation and the properties of bentonite greases. In this paper, OMMT thickeners were synthesized through exchanging interlayer cations of MMT with quaternary alkylammonium cations in aqueous solution. The lubricating greases were prepared with the resulting organoclays, while the penetration, oil separation, average wear scar diameter (WSD) and friction coefficient of lubricating greases were evaluated, respectively. Thickening mechanism of OMMT was discussed in this paper. And by the way of analyzing the basal spacing between the clay platelets, the relationship between the dosages of surfactants, the interlayer structure of OMMT and the performance of lubricating grease were also highlighted.
2 Experimental
2.1 Raw materials
The commercial CaMMT used in the present study was provided by the Zhejiang Fenghong New Material Co., Ltd. NaMMT was prepared in the laboratory by the reaction of CaMMT and Na2CO3. OTAC and HTAC were purchased from the Lvsen Chemical Reagent Co., Ltd., which were used as cationic surfactants. Naphthenic oil T110 was purchased from Nynas, Sweden. Ethanol (95%) was purchased from the Chengdu Kelong Chemical Co., Ltd. All chemical reagents were of analytically pure grade and used without further purifcation.
2.2 Experimental procedure
2.2.1 Preparation of NaMMT
In order to get excellent dispersibility in aqueous solution, Ca2+ions in the CaMMT interlayer should be displaced by Na+ions. Firstly, 20 g of dried CaMMT were dispersed in 200 mL of distilled water. Then 0.2 g of Na2CO3was added and the colloidal dispersion was then heated to 60 ℃ under continuous stirring for 30 min. Finally, the solid phase was separated by centrifugation. The resulting cake was dried at 80 ℃ for 12 h, and NaMMT powder was obtained via mechanical grinding with a mortar and sieving to a grain size of less than 200 meshes.
2.2.2 Preparation of OMMT
OMMT was synthesized by reacting NaMMT with a quaternary ammonium salt solution so as to alter the MMT surface from hydrophilic to hydrophobic. The typical preparation procedure was as follows: A certain amount of dried NaMMT was dispersed in distilled water by mechanical stirring. The mass ratio of NaMMT/water was 1:10. Subsequently, the surfactants were added into the suspension, and the intercalation reaction process was conducted under stirring at 80 ℃ for 60 min. The dosage of OTAC surfactants was equivalent to 100, 110, 120, 130, 140, 150, 160, 170, and 180 meq per 100 g of clay, respectively. Subsequently, the suspension was separated by vacuum fltration and washed three times with boiled distilled water. The flter cake was dried at 80 ℃ for 12 h, and the OMMT powder was obtained via mechanical grinding with a mortar and screening to a grain size of less than 200 meshes.
The OMMT powder, which was modifed by surfactants mixed with OTAC and HTAC at a proper ratio, was also prepared by the same procedure mentioned above.
2.2.3 Preparation of lubricating greases
The lubricating greases were prepared using the OMMT thickeners, which were synthesized according to the procedure shown above, the base oil (T110) and the polar activator (ethanol). The preparation procedure was as follows: At frst, a 90% ratio of T110 was put into a stirred batch reactor. Then a 10% ratio of OMMT was slowly added to the base oil in the vessel under vigorous stirring to obtain a homogenous mixture. When the base oil and the thickener were uniformly mixed, the polar activator, which was equal to 40% of the thickener, was dropwise injected into the mixture to ensure the thorough dispersion of the thickener in the base oil. The dispersion process was accomplished by mechanical shear for 30 min. Finally, the bentonite greases were obtained after being homogenized in a three-roller mill for three times.
2.3 Methods of analysis
Infrared spectra of the samples were measured with a Perkin Elmer Spectrum 577 FT-IR spectrometer equipped with a Universal Attenuated Total Refection (ATR) accessory in the range of 400—4 000 cm-1at a resolution of 4 cm-1. The powder samples were crushed and blended with potassium bromide (KBr).
The XRD analyses of powder samples were conducted on a Shimadzu XRD-6100 X-ray diffractometer using Cu Kα radiation (λ=0.154 18 nm) with a scan speed of 2(°)/min. The applied voltage and current value were 40 kV and 30 mA, respectively.
The SEM photographs were recorded with a Hitachi S-4800 microscope, using an accelerating voltage of 10.0 kV. For the preparation of the SEM samples, approximate 0.01 g of the dried powder samples were placed into the standard Al mounts and then coated with a thin conductive layer of gold. The soap fber was extracted from the lubricating grease by the Soxhlet extractor.
2.4 Penetration tests
The thickening performance of OMMT thickener was evaluated by determining the penetration of lubricating grease. The penetration was measured according to the national standard GB/T 269, which was similar to ASTM D217. Triplicate measurements were made and the average value was reported.
2.5 Oil separation tests
Colloid stability of lubricating grease was evaluated by the oil separation. The oil separation (pressure method) was measured according to the national standard GB/T 392. Duplicate measurements were made and the average value was reported.
2.6 Friction and wear tests
The friction-reduction and anti-wear properties of the grease were evaluated by a MMW-1 four-ball friction tester according to the standard method SH/T 0204—1992. Duplicate measurements were made and the average value was reported.
3 Results and Discussion
3.1 Results of FTIR analyses
The full FTIR spectra of OTCA surfactant, CaMMT and OMMT modifed by OTCA were recorded in the range of 400—4 000 cm-1as illustrated in Figure 1.
The spectrum of CaMMT shows the characteristic absorption bands of unmodifed MMT. The asymmetric stretching vibration of the structural -OH groups of CaMMT at 3 623 cm-1, the symmetric stretching and in-plane bending vibration of the free -OH groups of interlayer water molecules at 3 436 cm-1and 1 640 cm-1correspond to the surface property changes from the hydrophilic state to the hydrophobic state[8-9]. Compared with the absorption band of CaMMT, the absorption band at 1 632 cm-1of the OMMT sample, corresponding to the bending vibration of interlayer water molecules of MMT, weakens and shifts slightly to the lower wavenumber. The intensity of the absorption bands at 3 623 cm-1and 3 436 cm-1is decreased. This phenomenon indicates that the interlayer water content is reduced. By means of organic modification, the surface property of MMT is changed from hydrophilic to hydrophobic.
Meanwhile, the characteristic absorption bands of asymmetric and symmetric stretching vibration of the -CH2groups at around 2 919 cm-1and 2 850 cm-1and the absorption bands at 1 468 cm-1and 720 cm-1which are at-tributed to the in-plane and out-of-plane bending vibration of -CH2groups are shown in the spectra of the OMMT sample. These new characteristic absorption bands also denote that the MMT is modifed successfully.
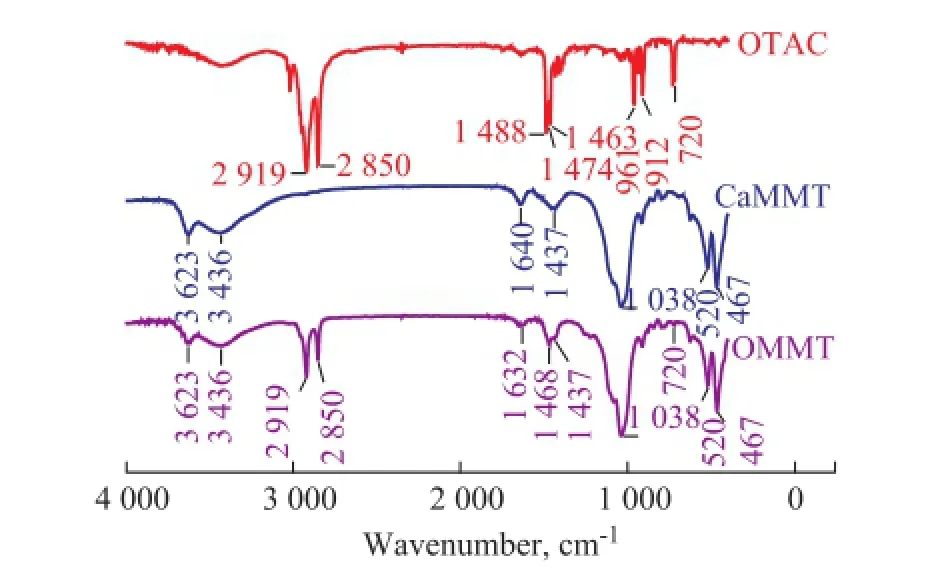
Figure 1 FI-IR spectra of OTAC, CaMMT and OMMT modi fi ed by OTCA
3.2 Results of XRD analyses
To obtain additional evidence to the FTIR analyses for the intercalation of surfactant into the interlayer of MMT, the XRD patterns of OMMT, CaMMT and NaMMT are shown in Figure 2.
The typical XRD refection of CaMMT related to the basal spacing between clay platelets appears at 2θ=5.80° (d001=1.52 nm). During the sodium modifcation of CaMMT, the calcium cations with the bilayer hydrated water molecules are displaced by small-sized sodium cations with the monolayer hydrated with water molecules. As a result of the replacement, the basal spacing between clay platelets reduces. As shown in Figure 2, the refection shifts to a higher angle (2θ=6.98°), corresponding tod001=1.26 nm, indicating that the sodium modifcation of CaMMT is successful. After intercalation with the surfactant OTCA, this peak disappears and moves to lower angle (2θ=4.36°), and the corresponding interlayer spacing of OMMT is 2.03 nm. The expansion of the interlayer spacing indicates that the surfactants are intercalated in the platelets of NaMMT, which are not just adsorbed on the clay surface.
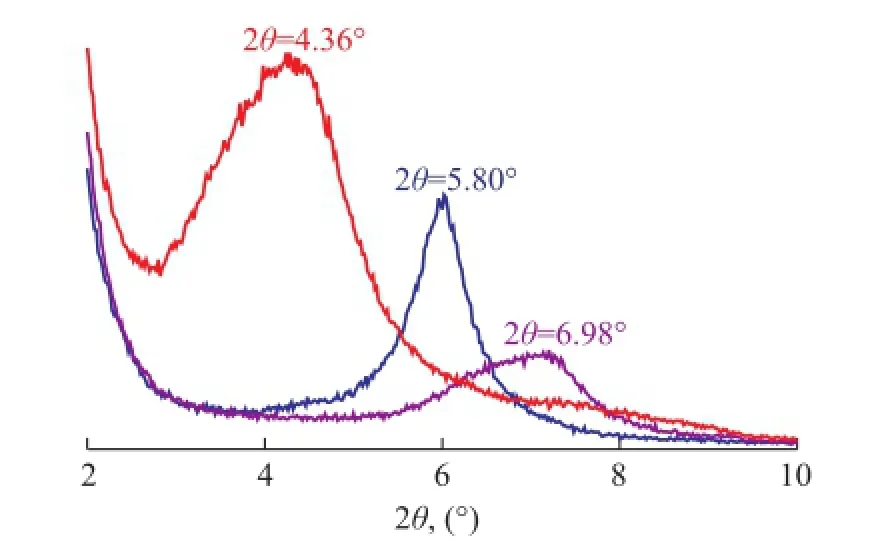
Figure 2 XRD patterns of CaMMT, NaMMT andOMMT modi fi ed by OTCA
3.3 Results of SEM analyses
The scanning electron microscope (SEM) is an effcient tool for investigating the morphology of powder samples and can provide a lot of valuable information at the micro-scale level. Figure 3 shows the SEM images of the MMT and OMMT.
The SEM photograph of MMT demonstrates a lot of villous clumps with irregular shape. The clay platelets with slightly wavy lattice fringes were stacked together in a disordered pattern to form agglomerates. However, the surface morphology of the clay particles changed signifcantly after being modifed with OTCA. The physical appearance of clay particles took the shape of fakes. Small and well-separated particles were also observed. After intercalation, the surfactant molecules loaded inthe interlayer could reduce the surface energy of MMT, which could improve the dispersion of the clay particles and lead to their changes in physical appearance. These changes in the SEM photographs indicated that the intercalation was accomplished.

Figure 3 SEM photos of CaMMT and OMMT modi fi ed by OTCA
3.4 Process for modi fi cation of bentonite
There are two processes to prepare the OMMT including sodium modifcation and organic modifcation, as shown in Figure 4.
In order to obtain excellent dispersibility in aqueous solution, the CaMMT needs to be modified by sodium salt in aqueous solution, and the process is presented in Figure 4 (a)—4 (c). After being subject to hydration and mechanical shearing, water molecules introduced into the interlayer area could increase the basal spacing of MMT. Under the impact of diffusive force and electrostatic force, the hydrated Na+ions would enter the interlayers and replace the hydrated Ca2+ions, as shown in Figure 4 (b) and 4 (c). After the ion exchange, NaMMT was then obtained.
The organic modifcation of NaMMT, as shown in Figure 4 (c) to 4 (d), could change the surface character of MMT from hydrophilic to hydrophobic, which would enable the organoclays to disperse in the base oil. When cationic surfactants were added into the NaMMT suspension, the surfactant molecules were in a state of micelle in aqueous solution (with nonpolar alkyl side pointing to the interior, and the hydrophilic cation end pointing to the external). The intercalation of surfactants was conducted under appropriate conditions. The quaternary ammonium salts, which were driven by various chemical interactions, viz.: the hydrogen bond, the acid base reactions, the charge transfer, and the van der Waals force, were adsorbed on the interlayers and surfaces of clays[33]. When the cation exchange reaction was over, the OMMT was prepared thereby.
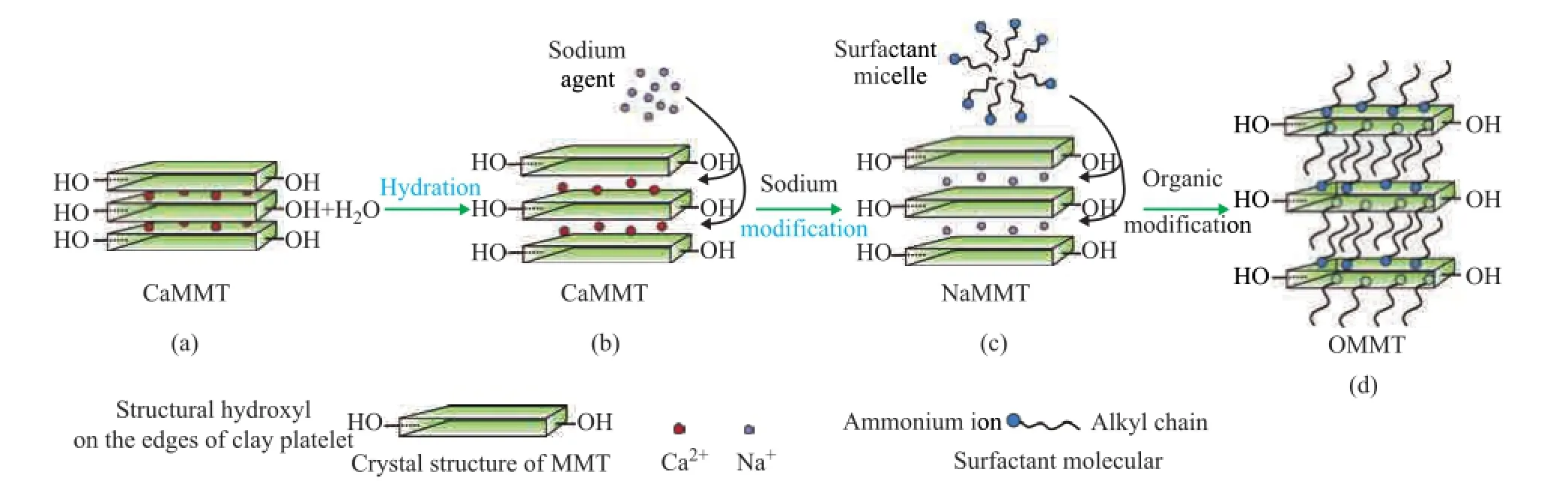
Figure 4 Sodium transition and organic modi fi cation process of MMT
3.5 Thickening mechanism of OMMT
The preparation of bentonite grease is essentially divided into two processes including the dispersion and gelation, as shown in Figure 5.
In order to obtain suffcient dispersion, a combination of mechanical shear and chemical polar activators is necessary for the dispersion process[34]. Base oils can infltrate into the interlayer area of OMMT under mechanical shear. The basal spacing of clays is increasing and a part of aggregated clay platelets are separated[34-35], as shown in Figure 5 (a) and 5 (b). Polar activators, including acetone, methanol, ethanol and propylene carbonate, are dispersion promoters. Owing to the small-sized molecules, polar activators can easily overcome the steric hindrance of the surfactants and permeate into the interlayer area of clays. The polar activators, which are adsorbed on the surface of clays by the dipole-dipole interaction, can reduce the attractive forces between the platelets and allow mechanical shear to easily separate the clay platelets[34], as shown in Figure 5 (c).
Gelation is described as the mechanism of hydrogen bonding between dispersed clay platelets to create a gel network[13,35]. In the dispersion process, a part of aggregated clay platelets are separated, and the hydrogen bonded clay platelets are formed by hydroxyl groups on the edges of the individual clay platelets. Since the hy-droxyl groups on the edges of the individual clay platelets are rigid and the number of structural hydroxyl groups is inadequate, the structural strength of the hydrogen bond network between the platelets is weak. In order to obtain excellent gelation, polar activators are usually employed. Polar activators, which contain a small amount of water, are not only the dispersion promoters but also the excellent hydrogen bond formers[34]. The polar functional groups in activators, such as hydroxyl, amino and carboxyl species, can form hydrogen bond bridges with nearby platelets and connect the platelets “hand in hand”, as shown in Figure 5 (d). After adding polar activators, the crosslinking degree of the gel network is increasing and the structural strength of the hydrogen bond network is promoted.
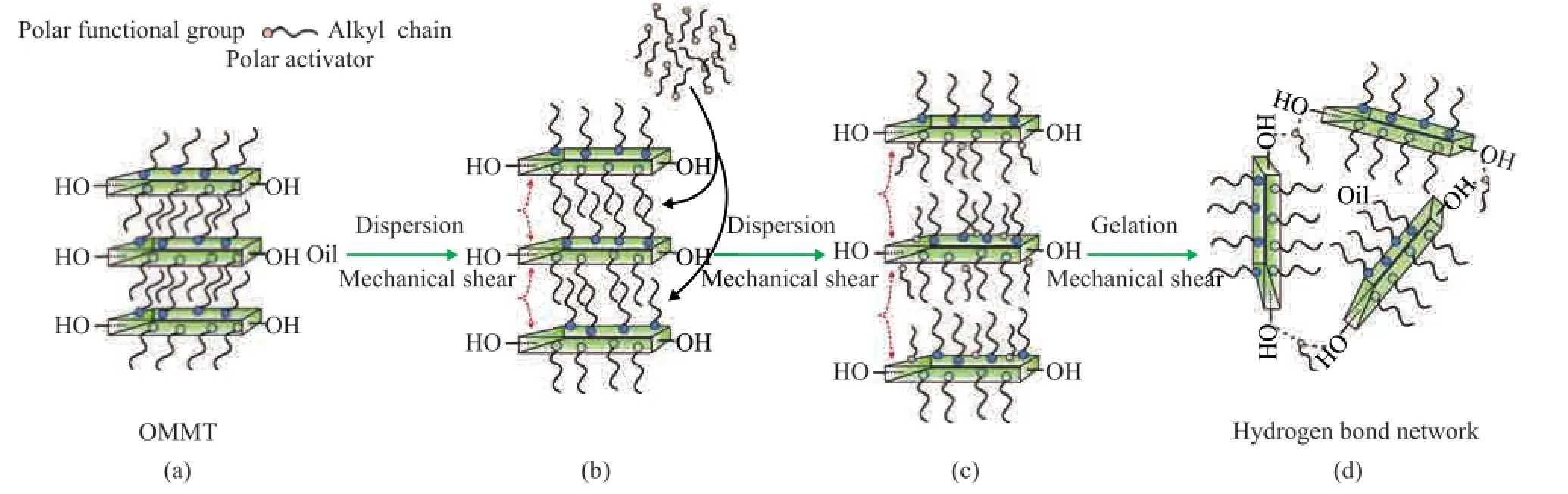
Figure 5 Process for preparation of bentonite grease
The SEM image of the OMMT obtained through reflux extraction of lubricating grease by means of the Soxhlet extractor is shown in Figure 6. Compared with the morphology of OMMT powder in Figure 4 (b), it can be seen that clay platelets in the lubricating grease were fully dispersed. And the platelet-to-platelet hydrogen bond connection can be obviously seen, as shown in Figure 6 (a), (b) and (c).
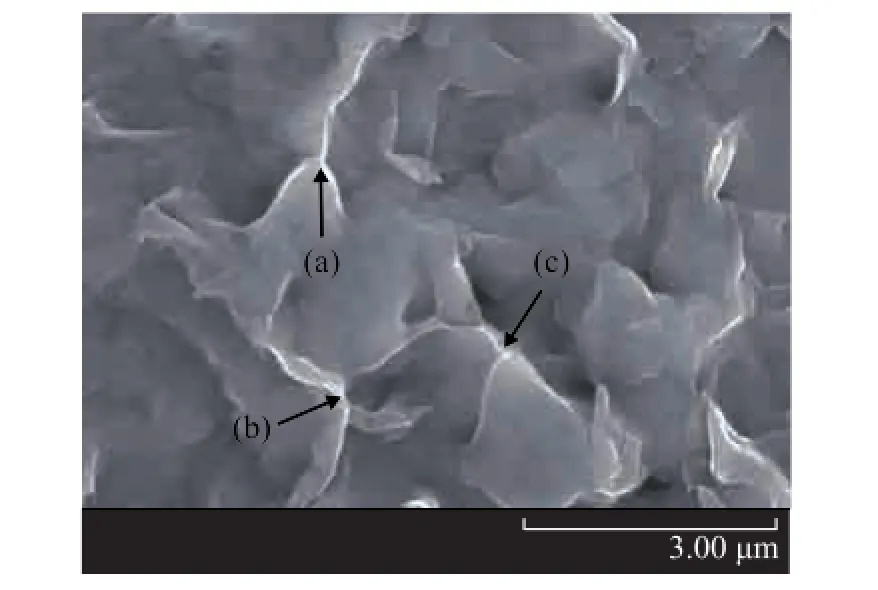
Figure 6 SEM photo of OMMT extracted from lubricating grease
3.6 Effect of surfactant dosage on the properties of bentonite greases
To study the infuence of the dosage of surfactants on the properties of bentonite greases, lubricating greases with different surfactant contents were prepared. The dosage of OTAC surfactants was equivalent to 100, 110, 120, 130, 140, 150, 160, 170, and 180 meq per 100 g of clay, respectively.
3.6.1 The thickening performance and colloidal stability of bentonite greases
The penetration and oil separation of bentonite greases were evaluated, with the results shown in Figure 7. It can be observed that when the dosage of surfactants was less than 120 mmol/(100 g), the penetration and oil separation of bentonite greases decreased gradually with an increasing dosage. Penetration, which is the depth expressed in tenths of millimeters after a standard cone assembly sinks into the grease, is an index for determining the consistency of lubricating greases. The decrease in penetration means that the ability of lubricating grease to resist deformation under external force is improved. In other words, the structural strength of the bentonite grease gel network is improved. The oil separation of lubricating greases refers to the trend of base oil to be separated from gel network of grease under a constant pressure, which is one of the indicators to evaluate the colloidal stability of grease. The decrease in oil separation indicates that the adsorption capacity of grease gel network for base oil is improved. In this range, the thickening performance and colloid stabil-ity of lubricating greases are improved. And the minimum values of penetration and oil separation were obtained at a surfactant dosage of 120—140 mmol/(100 g). However, when the dosage was more than 140 mmol/(100 g), the penetration value and oil separation of bentonite greases increased rapidly with an increasing amount of surfactant. The experimental results showed that the optimum dosage of modifer was equal to 120—140 mmol/(100 g). Excessive and insuffcient amount of surfactants both had negative effects on the thickening performance and colloidal stability of lubricating greases.
In order to further explain the above phenomenon, the effects of surfactant dosage on the interlayer structure of OMMT were investigated. The XRD patterns of OMMT modified by different surfactant contents are shown in Figure 8.

Figure 7 Effect of surfactant dosage on the thickening performance and colloidal stability of bentonite greases
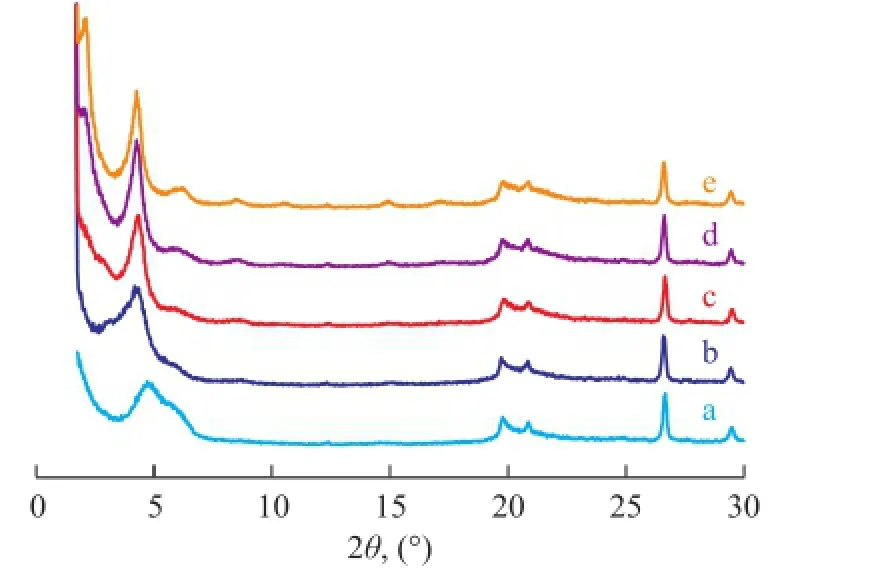
Figure 8 XRD patterns of OMMT modi fi ed by different surfactant dosages
It can be seen from Figure 8 that the refection around 4.82° shifted to lower angles with an increasing amount of surfactant, and a new peak was found at around 2.10° when the dosage of surfactant was more than 140 mmol/(100 g). The experimental results indicated that the basal spacing between the clay platelets was expanding with an increasing amount of surfactant, and when the surfactant content exceeded a certain amount, the structure of modifer molecules located in the clay interlayers was changed.
According to previous studies, the organic chains have been long supposed to lie either parallel to the silicate layer, forming mono-or bi-layers or, depending on the packing density and the chain length, to move away from the surface, forming mono-or even bi-molecular tilted“paraffinic” arrangement[36-38], as shown in Figure 9. The different structure types can easily be detected by their basal spacing[38]. The monolayer has a basal spacing of 1.36 nm and the bilayer has a spacing of 1.77 nm[38-39]. With a further increasing quantity of surfactant, the amine chains must rise up on the edge to accommodate the crowd situation[39], and the structure of paraffintype monolayer is formed, as shown in Figure 9 (c). The basal spacing of around 2.00 nm is observed for this situation[38-39]. Further crowding must gradually force the amine molecules to form a paraffn-type bilayer structure, accounting for the rapid increase of basal spacing.
The relationship between the dosage of surfactants and the interlayer structure of OMMT is shown in Table 1. The experimental results indicate that the structure of surfactant molecules located in the interlayer hadchanged from the lateral bilayer to the paraffin-type bilayer with an increasing amount of surfactant. When the dosage of surfactant was less than 120 mmol/(100 g), the organic chains would lay parallel to the silicate layer and the organic modification of layered silicates was inadequate. Since organic cations could lower the surface energy of the clay surface and improve the wetting situation with the base oil[40], inadequate organic modification of layered silicates could lead to inadequate dispersion in the base oil, and would result in poor performance of bentonite grease. The optimum dosage of modifer was equal to 120—140 mmol/(100 g). In this dosage range, the structure of surfactant molecules would transform into the paraffn-type monolayer, and this orientation should theoretically provide space for about 25% more surfactant molecules[39]. Correspondingly, the properties of lubricating greases could be improved. However, when the dosage of surfactant was more than 120 mmol/ (100 g) to reach 160 or 180 mmol/(100 g), the structure of paraffn-type bilayer would be formed and the organic modifcation of clay was excessive. More modifers were adsorbed on the crystal edges[39], which could shield the structural hydroxyl groups on the edges of the clay platelets so that the gelation process was blocked. As a result, the performance of lubricating grease would decline.
The analysis results indicate that the properties of bentonite greases are closely related to the interlayer structure ofOMMT. And by the way of analyzing the interlayer structure of OMMT, a preliminary conclusion on whether or not the OMMT is appropriate for preparation of bentonite grease can be drawn.
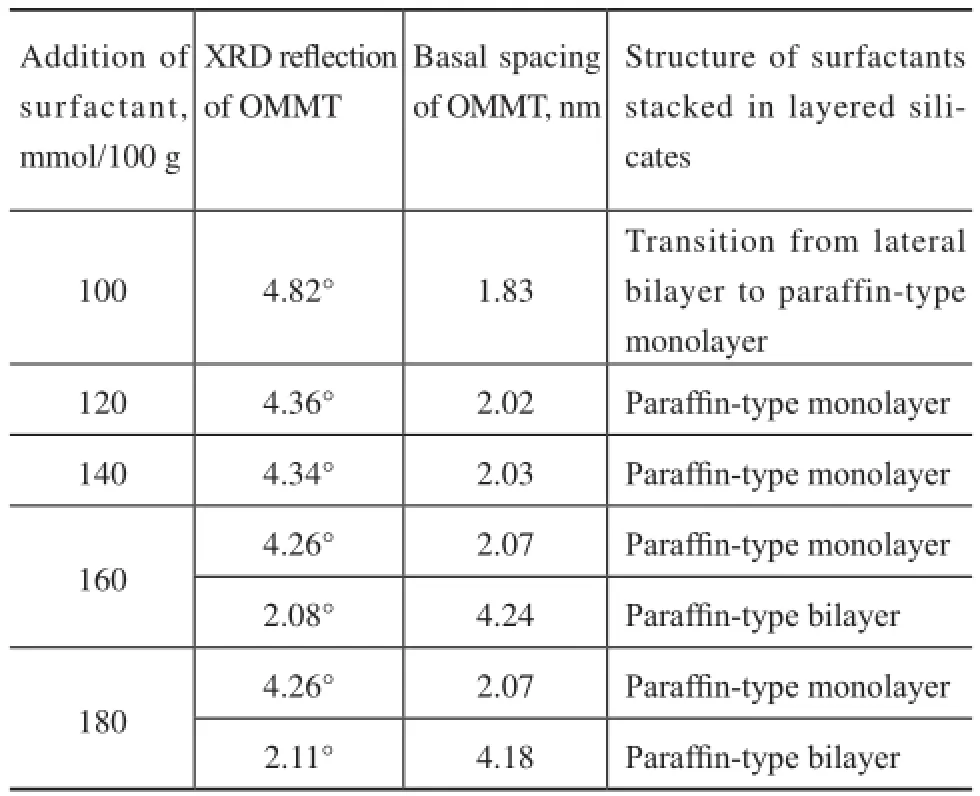
Table 1 Relationship between the dosage of surfactants and the interlayer structure of OMMT
3.6.2 Lubricating performance of bentonite greases
The average wear scar diameter and friction coeffcient of bentonite greases were evaluated, with the results shown in Figure 10. It can be observed that with an increasing amount of surfactant the friction coeffcient increased at frst and then decreased. However, an opposite trend was found in the WSD values of bentonite greases. The experimental results showed that the bentonite greases, which were modifed with 120—140 mmol/(100 g) of surfactant dosage, demonstrated a preferable anti-wear performance and a poor friction-reducing performance.
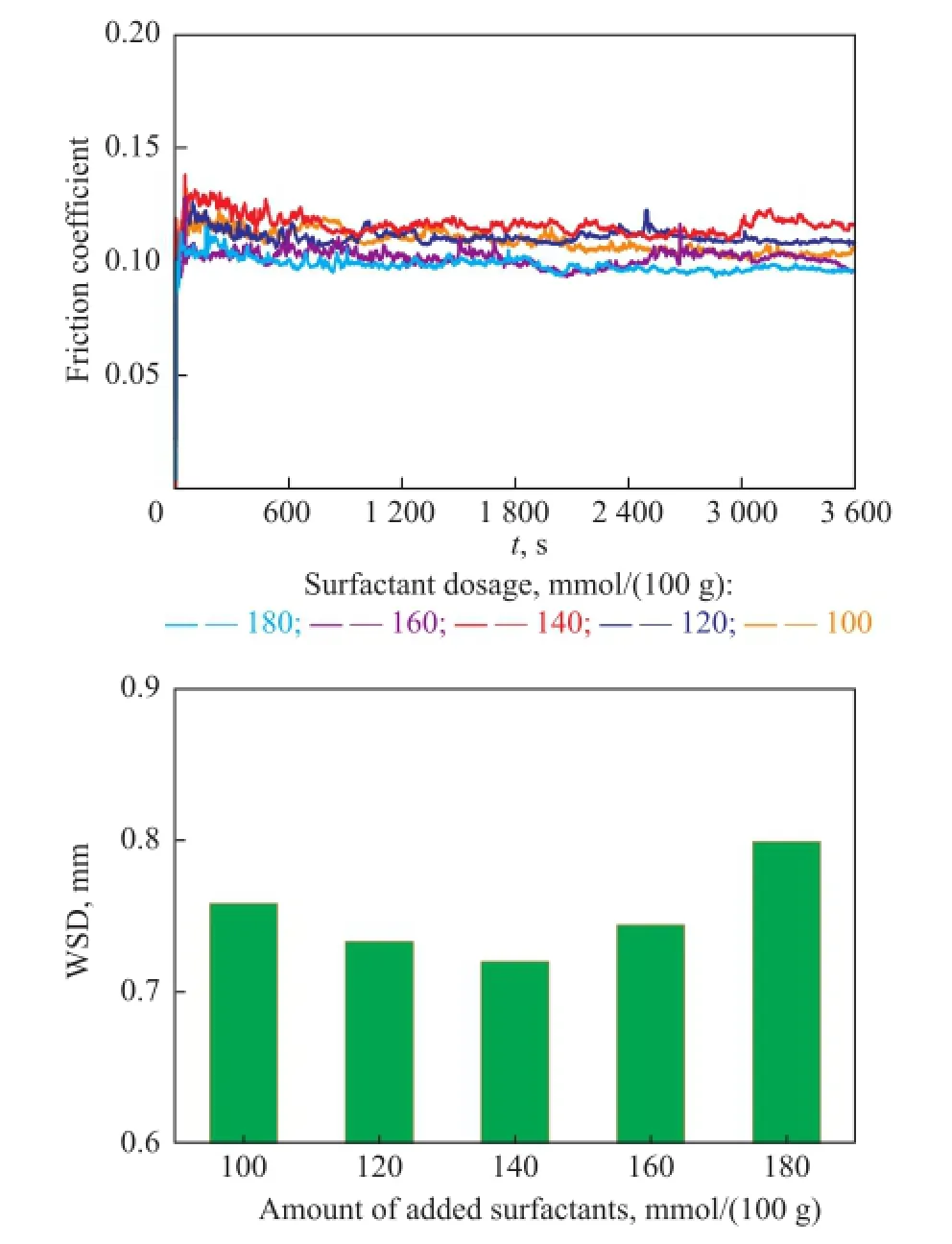
Figure 10 Effect of surfactant dosages on the frictionreducing and anti-wear performance of bentonite greases
3.7 Effect of composite surfactants on the properties of bentonite greases
In order to investigate the effect of composite surfactants on the properties of greases, lubricating greases with different composite surfactants were prepared. The compos-ite surfactants were composed of OTAC and HTAC, and the amount of surfactants was equal to 120 mmol/(100 g). The mole fraction of HTAC was 0, 25%, 50%, 75%, and 100%, respectively.
3.7.1 Thickening performance and colloidal stability of bentonite greases
The penetration and oil separation of bentonite greases were evaluated, with the results presented in Figure 11. It can be seen that the penetration and oil separation of lubricating greases modifed by OTAC were less than that of lubricating greases modifed by HTAC. One important thing to note is that the thickening performance and colloidal stability of lubricating greases were improved, when the surfactants were mixed with OTAC and HTAC. And the optimum mole ratio of two surfactants was equal to 1:1. The experimental results indicated that the modifcation effect of composite surfactants with different carbon atoms in the alkylammonium chain was better than that of surfactants with a single carbon chain length.
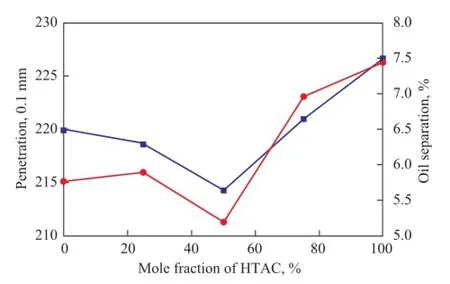
Figure 11 Effect of composite surfactants on the thickening performance and colloidal stability of bentonite greases
3.7.2 Lubricating performance of bentonite greases
The average wear scar diameter and friction coeffcient of bentonite greases were evaluated, with the results shown in Figure 12. It can be seen that the friction-reducing and anti-wear performance of lubricating greases was improved, when the surfactants were composed of 50% of OTAC and 50% of HTAC. The experimental results indicated that using composite surfactants with different carbon atoms in the alkylammonium chain could improve the friction-reducing and anti-wear performance of bentonite greases.
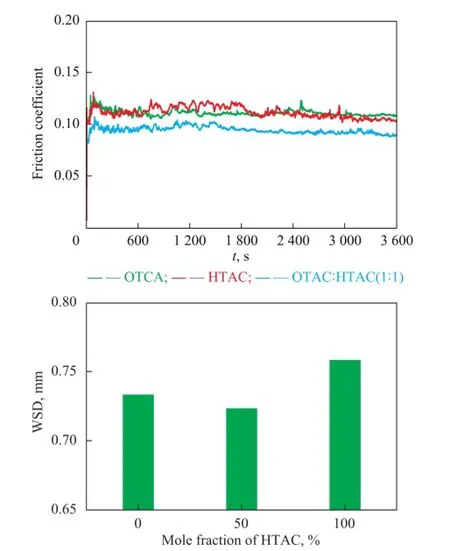
Figure 12 Effect of surfactant dosages on the frictionreducing and anti-wear performance of bentonite greases
4 Conclusions
In order to investigate the influencing factors of organic modification procedure and find out the connection between organic modification, the interlayer structure of OMMT and the performance of lubricating grease, the OMMT thickeners with different surfactant dosages and constituents were synthesized. The lubricating greases were prepared with the resulting organoclays, and the penetration and oil separation of lubricating greases were evaluated, respectively. The experimental results showed that with an increasing amount of surfactant, the basal spacing between the clay platelets increased also and the structure of modifier molecules located in the interlayer shifted from the lateral bilayer to the paraffn-type bilayer. The optimal properties of lubricating greases were realized, when the structure of the surfactant molecules loaded in the interlayer was the paraffn-type monolayer, while the dosage of modifer was equal to 120—140 mmol/(100 g).
The analysis results indicated that the properties of bentonite greases were closely related to the interlayer structure of OMMT. And by analyzing the interlayer structureof OMMT, a preliminary conclusion on whether or not the OMMT is appropriate for preparation of bentonite grease can be drawn. Meanwhile, it was found that the thickening performance, colloidal stability, and the antiwear and friction-reducing performance of lubricating greases were improved, when the surfactants were mixed with different carbon atoms in the alkylammonium chain. And the optimum mole ratio of OTAC and HTAC should be equal to 1:1.
Acknowledgements: This work was financially supported by the Chongqing Construction Project of Innovation Teams in Colleges and Universities-Petroleum Products Application Engineering and Technology (Project No. KJTD201342) and the Chongqing Project of Innovation Research by Postgraduates (Project No. CYB16130).
Reference
[1] Fiedler M, Sanchez R, Kuhn E, et al. Infuence of oil polarity and material combination on the tribological response of greases formulated with biodegradable oils and bentonite and highly dispersed silica acid[J]. Lubrication Science, 2013, 25(6): 397-412
[2] Chtourou M, Frikha M H, Trabelsi M. Modified Tunisian smectite clays used in the formulation of high performance lubricating greases[J]. Applied Clay Science, 2006, 32(3): 210-216
[3] Liu Wei, Zhang Tiansheng. The present research situation and development prospect of environmentally friendly greases[J]. Lubricating Oil, 2005, 20(2): 26-29 (in Chinese)
[4] Zhang S. Green tribology: Fundamentals and future development[J]. Friction, 2013, 1(2): 186-194
[5] Florea O, Luca M, Steliean C. Ecological lubricating greases[J]. Tribology in Industry, 2004, 26(1): 52-57
[6] Chen T, Xia Y, Liu Z, et al. Preparation and tribological properties of attapulgite–bentonite clay base grease[J]. Industrial Lubrication and Tribology, 2014, 66(4): 538-544
[7] Mi Hongying, Guo Xiaochuan, Yang Tingdong, et al. The development of a new bentonite grease[J]. Acta Petrolei Sinica (Petroleum Processing Section), 2009, 25(S1): 26-31 (in Chinese)
[8] Zhang Z, Liao L, Xia Z. Ultrasound-assisted preparation and characterization of anionic surfactant modified montmorillonites[J]. Applied Clay Science, 2010, 50(4): 576-581
[9] Sarier N, Onder E, Ersoy S. The modifcation of Na-montmorillonite by salts of fatty acids: An easy intercalation process[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2010, 371(1): 40-49
[10] Kango S, Kalia S, Celli A, et al. Surface modifcation of inorganic nanoparticles for development of organic-inorganic nanocomposites—A review[J]. Progress in Polymer Science, 2013, 38(8): 1232-1261
[11] Patel H A, Somani R S, Bajaj H C, et al. Nanoclays for polymer nanocomposites, paints, inks, greases and cosmetics formulations, drug delivery vehicle and waste water treatment[J]. Bulletin of Materials Science, 2006, 29(2): 133-145
[12] Huang Weicong, Xiao Rui, He Jingquan, et al. Development of excellent high and low temperature properties of bentonite grease[J]. Lubrication Engineering, 2012, 37(8): 112-115
[13] He Yifeng, Sun Hongwei, Duan Qinghua. Investigation on mechanical stability of organobentonite grease[J]. Acta Petrolei Sinica (Petroleum Processing Section), 2014, 30(6): 1027-1033 (in Chinese)
[14] Wang Jing, Guo Xiaochuan, Zhong Yuanli, et al. Preparation of ultrafne powder bentonite as thickening agent by wet-milling[J]. Petroleum Processing and Petrochemicals, 2014, 45(11): 95-99 (in Chinese)
[16] Wang Jing, Guo Xiaochuan, He Yan, et al. Impact of base oil on the performance of ultrafne bentonite grease[J]. Petroleum Processing and Petrochemicals, 2015, 46(7): 83-88
[17] Wang Xin, Cheng Jinshan, Zhang Guangliao, et al. Effect of bentonite type on performance of grease[J]. Petroleum Processing and Petrochemicals, 2015, 47(1): 67-70 (in Chinese)
[18] Chen Xinguo, Zeng Hui, Ji Hongbing. Study on thermal stability and oxidation resistance of bentonite grease[J]. Lubrication Engineering, 2011, 36(2): 111-113
[19] Li Su. The factors of affection on the consistency of bentonite grease[J]. Lubricating Oil, 2008, 23(2): 43-45
[20] Li Yunkang, Chen Defang, Wang Zhong, et al. Rheological properties of bentonite grease[J]. Journal of Xian Jiaotong University, 2000, 34(10): 108-110 (in Chinese)
[21] Kang Jian, Zhao Yuzhen. Influencing factors of bentonite grease manufacturing[J]. Synthetic Lubricants, 2010, 37(1): 26-28 (in Chinese)
[22] Zhang Donghao, Jiang Mingjun, Guo Xiaochuan, et al. Study on properties of an organic bentonite grease[J]. Journal of Logistical Engineering University, 2014, 30(6): 38-43 (in Chinese)
[23] Martín-Alfonso J E, Valencia C, Franco J M. Compositionproperty relationship of gel-like dispersions based on organo-bentonite, recycled polypropylene and mineral oil for lubricant purposes[J]. Applied Clay Science, 2014, 87(1): 265-271
[24] Wang Jing, Guo Xiaochuan, Guo Wanqing, et al. Balance between consistency and mechanical stability of bentonite greases[J]. Lubrication Engineering, 2015, 40(10): 45-48
[26] Czarny R, Paszkowski M. The infuence of graphite solid additives, MoS2and PTFE on changes in shear stress values in lubricating greases[J]. Journal of Synthetic Lubrication, 2007, 24(1): 19-29
[27] Wang Z, Xia Y, Liu Z. Study on the sensitivity of solid lubricating additives to attapulgite clay base grease[J]. Tribology Letters, 2011, 42(2): 141-148
[28] Wang Z, Xia Y, Liu Z. Comparative study of the tribological properties of ionic liquids as additives of the attapulgite and bentonite greases[J]. Lubrication Science, 2012, 24(4): 174-187
[29] Song Hongbin, Feng Legang, Xu Jing. Application of additives in bentonite grease[J]. Lubricating Oil, 2011, 26(4): 33-36 (in Chinese)
[30] Fan X, Xia Y, Wang L, et al. Multilayer graphene as a lubricating additive in bentonite grease[J]. Tribology Letters, 2014, 55(3): 455-464
[31] Mi Hongying, Guo Xiaochuan, Yang Tingdong. Study on the shearing stability of bentonite grease[J]. Lubrication Engineering, 2007, 32(6): 109-111
[32] Mi Hongying, Guo Xiaochuan, Yang Tingdong, et al. Study on the synthesis of grease with organo-bentonite modifed by urea formaldehyde resin using octadecylamine as chain terminator[J]. Lubrication Engineering, 2007, 32(4): 156-158
[33] Pogosian A K, Martirosyan T R. Impact of surfactant structure on the tribological properties of bentonite-based greases[J]. Journal of Tribology, 2007, 129(4): 920-922
[34] Pogosyan A K, Martirosyan T R. Tribological properties of bentonite thickener-containing greases[J]. Journal of Friction and Wear, 2008, 29(3): 205-209
[35] Bergaya F, Lagaly G. Surface modification of clay minerals[J]. Applied Clay Science, 2001, 19(1): 1-3
[36] Magauran E D, Chiavoni A, Reichert W W, et al. Studies of the behavior of dispersed organoclays in grease systems[J]. NLGI Spokesman, 1986, 50(3): 94-102
[37] Kieke M, Kyriakopoulos C, Maas J. Application studies of self activating organoclay grease gellants[J]. NLGI Spokesman, 1988, 52(3): 93-101
[38] Alexandre M, Dubois P. Polymer-layered silicate nanocomposites: preparation, properties and uses of a new class of materials[J]. Materials Science and Engineering: R: Reports, 2000, 28(1): 1-63
[39] Lee S M, Tiwari D. Organo and inorgano-organo-modified clays in the remediation of aqueous solutions: an overview[J]. Applied Clay Science, 2012, 59(5): 84-102
[40] Lagaly G. Characterization of clays by organic compounds[J]. Clay Miner, 1981, 16(1): 1-21
[41] Jordan J W, Hook B J, Finlayson C M. The organophilic bentonites. II. Organic liquid gels[J]. The Journal of Physical Chemistry, 1950, 54(8): 1196-1208
[42] Pavlidou S, Papaspyrides C D. A review on polymerlayered silicate nanocomposites[J]. Progress in Polymer Science, 2008, 33(12): 1119-1198
Received date: 2016-06-01; Accepted date: 2016-07-23.
Professor Guo Xiaochuan, E-mail: 1418718262@qq.com.
- 中國(guó)煉油與石油化工的其它文章
- Methodology for Design of Reactive Distillation Column and Kinetics for Isoamylene Etheri fi cation Catalysed by Amberlyst 35
- Optimization and Control of Extractive Distillation with Heat Integration for Separating Benzene/Cyclohexane Mixtures
- Molecular Simulation of Adsorption of Quinoline Homologues on FAU Zeolite
- Improvement of Magnetic Field on Tribological Properties of Lubricating Oils with Zinc Butyloctyldithiophosphate
- Study on Tribology Performance of Different Lubricant Additives under Atmospheric Pressure and High Vacuum Conditions
- Investigation on Microcrystalline Wax Doped Asphalts by Temperature Control Mode of Atomic Force Microscopy

