Changes in energetic profile of pregnant ewes in relation with the composition of the fetal fluids
?
Changes in energetic profile of pregnant ewes in relation with the composition of the fetal fluids
Samia Haffaf1*, Bouabdellah Benallou21Institute of Agro-Veterinary Sciences, University of Batna, Batna, Algeria
2Institute of Veterinary Sciences, University of Tiaret, Tiaret, Algeria
Faunal research http://dx.doi.org/10.1016/j.apjtb.2015.11.005
Tel: +213 772750269
E-mail: samiahafaf@yahoo.fr
Peer review under responsibility of Hainan Medical University. The journal implements double-blind peer review practiced by specially invited international editorial board members.
2221-1691/Copyright?2016 Hainan Medical University. Production and hosting by Elsevier B.V. This is an open access article under the CC BY-NC-ND license (http:// creativecommons.org/licenses/by-nc-nd/4.0/).
ARTICLE INFO
Article history:
Received 8 Oct 2015
Received in revised form 20 Oct,
2nd revised form 2 Nov, 3rd revised form 5 Nov 2015
Accepted 12 Nov 2015
Available online 31 Dec 2015
Keywords:
Energetic profile Fetal fluid
Ewes
Pregnancy
ABSTRACT
Objective: To evaluate the energetic profile of fetal fluids and to make comparisons of the concentrations of the constituents present with those in the maternal plasma.
Methods: A study was conducted in 102 gravid sheep uteri. The four stages of gestation as Stage I (0-60 days), Stage II (61-90 days), Stage III (91-120 days) and Stage IV (121-145 days) were identified based on the crown anus length of the embryo/fetus. The amniotic and allantoic fluids collected from the gravid uteri of each group were subjected to biochemical analysis of glucose, cholesterol and triglyceride.
Results: The levels of glucose and triglyceride in maternal plasma were lower (P<0.05) on late pregnancy as well as in amniotic and allantoic fluids. No significant variation (P>0.05) of plasma cholesterol levels was detected between the sampling periods. Contrariwise, cholesterol concentrations of fetal fluids were higher in Stages III and IV of pregnancy when compared with the Stages I and II.
Conclusions: The influence of pregnancy on the biochemical composition of fetal fluids was statistically significant.
1. Introduction
During pregnancy, the growing fetus principally takes nutrient supplies from the mother depending on uterine blood flow. Indeed, fetal and placental weights and uterine blood flow are highly correlative [1,2]. Fetal fluids are important for physiologic exchanges between fetal and maternal tissues, so they are necessary for the efficient handling of fetal waste products and preventing mechanical shock to the developing fetus during entire gestation [3-5].
A high relative rate of growth demands a highly specific pattern of substrate and metabolic intermediates for ordered development of cells, tissues and organs[6]. Thus, the quality of fetal nutrition is most important early in gestation [6,7].
The concentration of components in amniotic and allantoic compartments is affected by the exchange through the placenta, metabolic yields of the fetus, fetal urine formation and fluid run through the urachus or urethra and fetal secretions from lung and salivary glands. However, amniotic and allantoic fluids differ substantially in composition than that of fetal urine[8]. Amniotic fluid composition reflects the physiological status during fetal development and it may be used to detect potential pathological conditions [9,10]. Amniotic fluid contains large amounts of proteins and metabolites produced by the amnion epithelial cells, fetal tissues, fetal excretions and placental tissues[11,12].
Studying the composition of maternal plasma and fetal fluids provides useful information about the requirements for fetus, fetal growth and maturation. It is observed that several circulatory and transport properties of the ewe's placenta are as the same as those of the human placenta and as such, for that, the pregnant sheep helps greatly in giving an outstanding model to study the growth of fetus[13].
Glucose is indispensable for the fetus development since it is considered mainly as a source of energy. The sheep fetus uses this substance as a chief metabolite and because of the speedy development of the fetus, the energy necessities of the ewe augment throughout late pregnancy [14].
Studies on sheep also paid much attention to the change that occursinthecompositionoffetalfluidinearlypregnancy[15].This change is a reflection to changing metabolic and transport activity as well as modification in the relative involvement of the fetal and placental tissues to the amniotic and allantoic compartments[16].
In understanding fetal metabolism and identifying pathological conditions throughout pregnancy, a broad knowledge of amniotic fluid is required [17].
The current investigation was conducted to evaluate the energetic profile of the fetal fluids and maternal plasma throughout different stages of pregnancy in ewes.
2. Materials and methods
A total of 102 female genitalia from pregnant ewes were collected aimlessly from local slaughterhouse. The uteri were immediately collected after slaughter, washed and brought to the laboratory in ice. Uteri were opened along the dorsal curvatures and maternal caruncles were separated gently from fetal cotyledons. The intact amnion and allantois along with embryo or fetus were separated. Allantoic and amniotic sacs were punctured and 10 mL of allantoic and amniotic fluids were aspirated from each sac. About 10 mL sterile syringes and needles were used for collection of these fluids. Samples were stored in labeled plastic tubes and frozen at?20°C until biochemical analysis. The samples of jugular blood were gathered from ewes in sterilized glass tubes. In an icebox, these tubes were put and taken to the laboratory. These samples were dealt with under specific conditions and they were centrifuged at 4000 r/min for 3 min. On the other hand, the plasma was separated and stored at?20°C for further analysis. The embryo/fetuses were removed from the uterus and the crown anus length of the fetus from the vertex of skull to the anus was measured using a measuring tape. The stages of gestation and age of the fetus were determined by applying the Keller formula: L = X (X + 3.5) [18], where X denotes the developmental age in months and L is the crownrump length (cm). Based on the age of the fetus, the course of gestation was divided into four stages as: Stage I (0-60 days), Stage II (61-90 days), Stage III (91-120 days) and Stage IV (121-145 days). The samples of plasma and fetal fluid were made under analysis for some biochemical metabolites (glucose, cholesterol and triglyceride) using commercially available kits by photometer method on an autoanalyzer Selectra junior.
The values of mean±SD for concentrations of various biochemicalcomponentsoffetalfluidsandplasmawerecomputed. To highlight the impact of gestational age variation in concentrationsofvariousbiochemicalconstituentsoffetalfluidsandplasma, the data were directed to One-way ANOVA. Tukey's multiple comparisontestwasconductedtotestsignificancebetweenmeans. If P<0.05, it was tended to consider the differences as significant.
3. Results
The mean values and SD for some biochemical metabolites levels of fetal fluids and the maternal plasma at different stages of gestation in ewes were presented in Table 1.
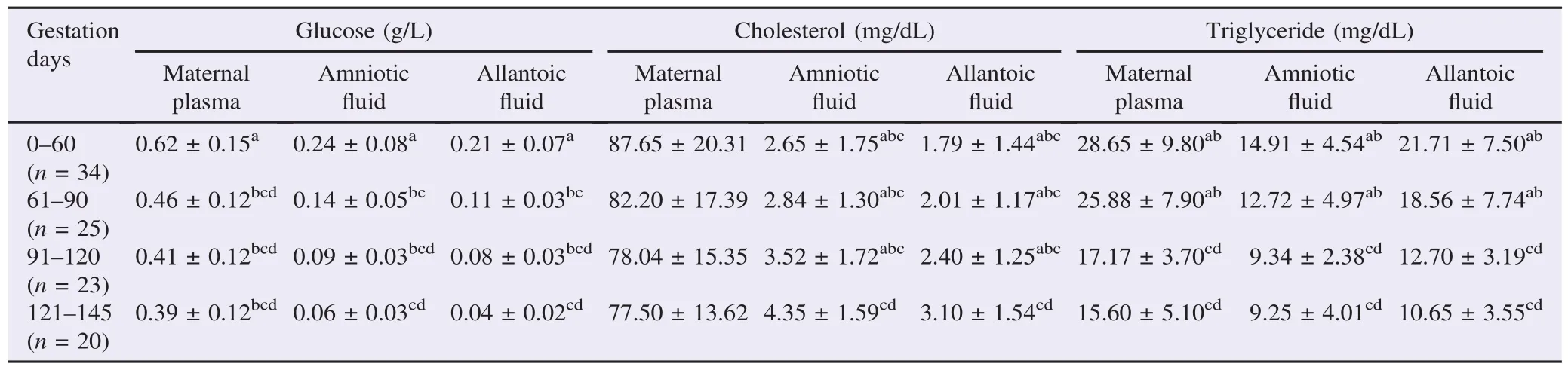
Table 1 Levels of metabolite constituents of fetal fluids and maternal plasma during different stages of pregnancy in ewes.
There was a marked decrease in the levels of glucose and triglyceride in maternal plasma with advancement of pregnancy as well as in amniotic and allantoic fluids. No significant variation (P>0.05) of plasma cholesterol levels was detected between the sampling periods. Contrariwise, cholesterol concentrations of fetal fluids were higher in Stages III and IV of pregnancy in comparison with the Stages I and II.
4. Discussion
In this study, it is observed that there is a significant decrease of glucose concentration in advanced pregnancy in fetal fluids. These results were mainly in agreement with former results in ewes and cattle[19-21], but they were different from those reported in sheep[22]. In late pregnancy, the glucose level in the allantoic fluid was notably lower (P<0.01) and this finding is different from other reports [23]. During all the probation period, the level of glucose in maternal plasma was significantly higher than that of fetal fluids. Indeed, with advancing pregnancy, the glucose values in maternal plasma were decreased. This result was mainly in agreement with those reported in sheep [24-26]. They concluded that fetal intake of glucose due to the development of the fetal swallowing reflex may be the cause behind decreasing glucose levels in gestation.
There were no significant differences of cholesterol concentration in maternal plasma between the sampling periods. Many studies reported that with advancing pregnancy in ewes, a decreasing trend of the serum cholesterol level was marked. During all stages of pregnancy, the cholesterol level in maternal plasma was significantly higher in comparison to that of fetal fluids.
In the late stages of gestation, the cholesterol concentrations in fetal fluids were considerably higher than in earlier gestation. The marked increase in cholesterol level with advancement of pregnancy in amniotic and allantoic fluids meets the need for the synthesis of progesterone [27].
With advancing pregnancy, there was a slow decrease in the levels of triglycerides in fetal fluids and maternal plasma. This finding is similar to results in goats [28]. Triglycerides are the store of lipids of animals in the plasma where the body uses them, mainly as fuel. When energy necessities increase, mainly in advanced pregnancy, the ewes go to consume the plasma lipids[29].
To conclude with, the concentrations of glucose, cholesterol and triglycerides in fetal fluids and maternal plasma of pregnant ewes were changed with advancing gestation stages. Based upon the evaluation of energetic profile, it may be possible to detect the early aberrations in metabolism and thereby, appropriatecorrections could be made to overcome the metabolic disturbances during pregnancy.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgments
The authors would like to express their special thanks to the staff members of M'sila abattoirs for all sorts of assistance.
References
[1] Banan Khojasteh SM, Khadjeh GH, Ranjbar R, Salehi M. Studies on biochemical constituents of goat allantoic fluid during different stages of gestation. Egypt J Sheep Goat Sci 2011; 6(1): 1-5.
[2] Dunlap KA, Brown JD, Keith AB, Satterfield MC. Factors controlling nutrient availability to the developing fetus in ruminants. J Anim Sci Biotechnol 2015; 6: 16.
[3] Kamath-Rayne BD, Smith HC, Muglia LJ, Morrow AL. Amniotic fluid: the use of high-dimensional biology to understand fetal wellbeing. Reprod Sci 2014; 21(1): 6-19.
[4] Sheppard SJ, Khalil RA. Risk factors and mediators of the vascular dysfunction associated with hypertension in pregnancy. Cardiovasc Hematol Disord Drug Targets 2010; 10(1): 33-52.
[5] Vonnahme KA, Lemley CO, Caton JS, Meyer AM. Impacts of maternal nutrition on vascularity of nutrient transferring tissues during gestation and lactation. Nutrients 2015; 7: 3497-523.
[6] Dunford LJ, Sinclair KD, Kwong WY, Sturrock C, Clifford BL, Giles TC, et al. Maternal protein-energy malnutrition during early pregnancy in sheep impacts the fetal ornithine cycle to reduce fetal kidney microvascular development. FASEB J 2014; 28(11): 4880-92.
[7] Verbeek E, Oliver MH, Waas JR, McLeay LM, Blache D, Matthews LR. Reduced cortisol and metabolic responses of thin ewes to an acute cold challenge in mid-pregnancy: implications for animal physiology and welfare. PLoS One 2012; 7(5): e37315.
[8] Khadjeh GH, Ranjbar R, Salehi M, Banankhojasteh SM. Biochemical evaluation of amniotic fluid during different stages of gestation in the goat. Iran J Vet Res 2007; 8: 266-9.
[9] Bazer FW, Song G, Kim J, Dunlap KA, Satterfield MC, Johnson GA, et al. Uterine biology in pigs and sheep. J Anim Sci Biotechnol 2012; 3: 23.
[10] Pelizzo G, Ballico M, Mimmi MC, Peir`o JL, Marotta M, Federico C, et al. Metabolomic profile of amniotic fluid to evaluate lung maturity: the diaphragmatic hernia lamb model. Multidiscip Respir Med 2014; 9(1): 54.
[11] Fanos V, Atzori L, Makarenko K, Melis GB, Ferrazzi E. Metabolomics application in maternal-fetal medicine. Biomed Res Int 2013; 2013: 720514.
[12] Shi L, Mao C, Zeng F, Zhang L, Xu Z. Central angiotensin I increases swallowing activity and oxytocin release in the near-term ovine fetus. Neuroendocrinology 2012; 95: 248-56.
[13] Spencer TE. Biological roles of uterine glands in pregnancy. Semin Reprod Med 2014; 32(5): 346-57.
[14] Firat A, Ozpinar A. Metabolic profile of pre-pregnancy, pregnancy and early lactation in multiple lambing Sakiz ewes. 1. Changes in plasma glucose, 3-hydroxybutyrate and cortisol levels. Ann Nutr Metab 2002; 46: 57-61.
[15] Li N, Wells DN, Peterson AJ, Lee RS. Perturbations in the biochemical composition of fetal fluids are apparent in surviving bovine somatic cell nuclear transfer pregnancies in the first half of gestation. Biol Reprod 2005; 73: 139-48.
[16] Tabatabaei S, Mamoei M. Changes in the biochemical composition of fetal fluids and maternal blood serum during different days of gestation in cattle. Comp Clin Pathol 2012; 21: 1005-12.
[17] Prestes NC, Chalhoub MC, Lopes MD, Takahira RK. Amniocentesis and biochemical evaluation of amniotic fluid in ewes at 70, 100 and 145 days of pregnancy. Small Rumin Res 2001; 39: 277-81.
[18] Arthur GH, Noakes DE, Pearson H. Veterinary reproduction and obstetrics. 5th ed. London: Bailli`ere Tindall; 1982.
[19] Keller-Wood M, Feng X, Wood CE, Richards E, Anthony RV, Dahl GE, et al. Elevated maternal cortisol leads to relative maternal hyperglycemia and increased stillbirth in ovine pregnancy. Am J Physiol Regul Integr Comp Physiol 2014; 307(4): R405-13.
[20] Khatun A, Wani GM, Bhat JIA, Choudhury AR, Khan MZ. Biochemical indices in sheep during different stages of pregnancy. Asian J Anim Vet Adv 2011; 6(2): 175-81.
[21] Murugavel K, Abdul Salam D, Barathiraja S, Thanislass J, Antoine D, Raju MS. Biochemical constituents of amniotic and allantoic fluids in chital deer (Axis axis). Indian J Anim Reprod 2014; 35(2): 31-3.
[22] Tangalakis K, Moritz K, Shandley L, Wintour EM. Effect of maternal glucocorticoid treatment on ovine fetal fluids at 0.6 gestation. Reprod Fertil Dev 1995; 7: 1595-8.
[23] Anitha A, Thangavel A. Biochemical profile of ovine amniotic and allantoic fluids. Tamilnadu J Vet Anim Sci 2011; 7(6): 262-7.
[24] Haffaf S, Chachoua I, Mamache B, Djaalab I. [Changes in biochemical profile during pregnancy and after parturition in Ouled Djellal ewes]. Renc Rech Rum 2012; 19: 365. French.
[25] McCoard S, Sales F, Wards N, Sciascia Q, Oliver M, Koolaard J, et al. Parenteral administration of twin-bearing ewes with L-arginine enhances the birth weight and brown fat stores in sheep. Springerplus 2013; 2: 684.
[26] Smith NA, McAuliffe FM, Quinn K, Lonergan P, Evans AC. The negative effects of a short period of maternal undernutrition at conception on the glucose-insulin system of offspring in sheep. Anim Reprod Sci 2010; 121(1-2): 94-100.
[27] Olfati A, Moghaddam G, Kor NM, Bakhtiari M. The relationship between progesterone and biochemical constituents of amniotic fluid with placenta traits in Iranian crossbred ewes (Arkhar-Merino×Ghezel). Asian Pac J Trop Med 2014; 7: S162-6.
[28] Tabatabaei S. Gestational variations in the biochemical composition of the fetal fluids and maternal blood serum in goat. Comp Clin Pathol 2012; 21: 1305-12.
[29] Ozegbe PC. Comparative biochemical assessment of the amniotic fluid and maternal plasma of pregnant rabbits. Vet Arhiv 2005; 75(5): 431-7.
*Corresponding author:Haffaf Samia, Institute of Agro-Veterinary Sciences, University of Batna, Batna, Algeria.
 Asian Pacific Journal of Tropical Biomedicine2016年3期
Asian Pacific Journal of Tropical Biomedicine2016年3期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Cyclical mastalgia: Prevalence and associated determinants in Hamadan City, Iran
- Natural antibacterial remedy for respiratory tract infections
- Bee pollen extract of Malaysian stingless bee enhances the effect of cisplatin on breast cancer cell lines
- In vitro antihistamine-releasing activity of a peptide derived from wasp venom of Vespa orientalis
- The inhibition of Typhonium flagelliforme Lodd. Blume leaf extract on COX-2 expression of WiDr colon cancer cells
- Evaluations of cytotoxicity of Smilax myosotiflora and its effects on sexual hormone levels and testicular histology in male rats
