Natural antibacterial remedy for respiratory tract infections
?
Natural antibacterial remedy for respiratory tract infections
Reham F. El-Kased*
Department of Microbiology and Immunology, Faculty of Pharmacy, the British University in Egypt, Cairo, Egypt
Entomological research http://dx.doi.org/10.1016/j.apjtb.2015.12.002
Tel: +20 100 500 5081
E-mail: reham.kased@bue.edu.eg
Peer review under responsibility of Hainan Medical University. The journal implements double-blind peer review practiced by specially invited international editorial board members.
2221-1691/Copyright?2016 Hainan Medical University. Production and hosting by Elsevier B.V. This is an open access article under the CC BY-NC-ND license (http:// creativecommons.org/licenses/by-nc-nd/4.0/).
ARTICLE INFO
Article history:
Received 3 Jul 2015
Received in revised form 14 Jul, 2nd revised form 16 Jul, 3rd revised form 27 Nov 2015
Accepted 1 Dec 2015
Available online 13 Jan 2016
Keywords:
Klebsiella pneumonia
Staphylococcus aureus
Streptococcus pyogenes
Pseudomonas aeruginosa
Streptococcus pneumonia
Honey
MIC
MBC
Antibacterial activity
ABSTRACT
Objective: To evaluate the antibacterial activity of Egyptian honey against bacteria causing respiratory tract infections.
Methods: Sputum and throat swab specimens were used, from which five bacterial species were isolated, namely, Klebsiella pneumonia, Staphylococcus aureus, Streptococcus pyogenes, Pseudomonas aeruginosa and Streptococcus pneumonia were isolated, identified and grown on suitable media for further identification or confirmation. Different concentrations (100%, 75% and 25%) of honey and simulated honey solution were used for activity assay and estimation of minimum inhibitory concentration and minimum bactericidal concentration.
Results: All the tested bacterial isolates were completely susceptible to the 75% concentrations of honey and to the 100% concentration of the simulated honey solution. This may be due to the high osmotic pressure exerted by the high sugar content in both honey samples. Moderate susceptibility of the isolated bacteria to honey at 100% v/v concentration, and resistance to honey at 25% concentration and the 75% and 25% concentrations of simulated honey solution, indicated the presence of other antimicrobial components responsible for the activity other than the osmotic pressure. Therefore, it was suggested that honey showed distinguished antibacterial activities against the most common bacteria causing respiratory infections with varied sensitivity.
Conclusions: Honey, a non-toxic, nutritious, safe for human consumption and cheap natural antibacterial agent, should be globalized.
1. Introduction
Therespiratory tract begins fromthe larynx andconsists of the oropharynxandnasopharynxinadditiontothesinuses,themiddle ear and finally extends to the lungs. Infection of the respiratory tract isoneofthe commonestillnessin thegeneral populationand results in significant morbidity[1]. Over 50 million deaths around the world are caused by respiratory tract infections, which are the main cause for clinic visits and antibiotics prescription. Poor immunity and malnutrition are the main causes for the high incidence of respiratory tract infections.
The increase of quality of life over the past 50 years is mainly due to the use of antibiotics as antimicrobial chemotherapy. However, antibiotic-resistant bacteria have become a challenging public health problem worldwide[2]. The reason may be due to the side effects accompanying antibiotics systemic administration, such as hypersensitivity reactions, kidney problems, liver problems and gastrointestinal upset.
Natural health remedies and supplements are undergoing extensive studies to overcome such bacterial resistance to antibiotics and to offer alternative natural antimicrobial agents with least adverse effects on human body[3,4].
Honey was first used as a food source since ancient times and then became an effective natural cure for certain infections, such as some respiratory diseases and for the healing of skin burns and wounds[5]. The therapeutic property of honey has received well recognition from the medical field [6]. The antimicrobial potency and medical applications of honey are tremendous as it has demonstrated inhibitory effects against a number of pathogenic bacteria[7].
Several researches reported that honey has an inhibitory effect over 60 species of bacteria including aerobes, anaerobes, Gram-positives and Gram-negatives [8]. Other studies showed that honey has broad antibacterial, anti-inflammatory and antioxidant effects and plays a role in boosting the body immune system. Honey is also characterized by its least adverse effects on human body [9].
A different study reported the antifungal action of honey against some yeasts infections and species of Aspergillus and Penicillium [8], in addition to the common dermatophytes [10].
Honey not only serves as a cheap antimicrobial agent but also a full nutritional source. It consists of carbohydrates (fructose, glucose), amino acids, minerals (calcium, sodium, phosphorus, magnesium, silicon, iron, manganese, copper), organic acids, water, vitamins (A, B complex, C, D, E), enzymes (invertase, amylase), and antioxidants (pinocembrin, ascorbic acid, catalase, selenium)[11]. Phenolic acids and flavonoids were found to play an important role in the therapeutic capacity of honey[9].
This study aims to evaluate the antibacterial activity of Egyptian honey against bacteria causing respiratory tract infections.
2. Materials and methods
2.1. Bacterial strains
Sputum and throat swab specimens were used, from which five bacterial species were isolated, recognized with respiratory tract infections at a local diagnostic lab. Five bacterial species were isolated. For complete identification of the isolated bacteria, the samples were inoculated on blood agar, chocolate agar, MacConkey agar and cetrimide agar (Oxoid UK), and the plates were incubated at 37°C for 24-48 h. Identification of the growing microorganisms was done by colony morphology. Pure colonies were sub-cultured on blood agar, nutrient agar and chocolate agar media. Further identification or confirmation was carried out using biochemical tests as recommended by Cheesebrough[12].
2.2. Preparation of test samples
Crude honey obtained from Sinai, Egypt was used as concentrated solution. For the diluted crude solution, 50 mL sterile volumetric flask was used, where the required amount of crude honey was added, then the volume was completed with sterile distilled water to make solutions of 75% and 25% dilutions.
For preparing the simulated honey solution, 38.4 g of fructose, 30.3 g of glucose, 1.3 g of sucrose, 8.6 g of maltose and 1.4 g of maltodextrin were dissolved in 17.2 mL of distilled water.
A dilution series with simulated honey concentrations (75% and 25%) together with the diluted crude honey solutions (75% and 25%), in addition to the undiluted solutions of both the crude honey and the simulated honey, were used for the activity assays. Control plates of nutrient agar with no honey were made in duplicate and included in each susceptibility assay to confirm the viability and density of the cultures.
2.3. Antibacterial activity assay
Mueller-Hinton agar (Oxoid UK) was prepared for Pseudomonas aeruginosa (P. aeruginosa), Staphylococcus aureus (S. aureus) and Klebsiella pneumonia (K. pneumonia), and blood agar was prepared for Streptococcus pyogenes (S. pyogenes) and Streptococcus pneumoniae (S. pneumoniae). An autoclave at 120°C was used to sterilize all media. Thirty milliliters of the agar media with the respective inoculated strains of bacteria were transferred aseptically in each sterilized Petri plate. All plates were left at room temperature to solidify. Wells of 6 mm diameter were made in the agar using a sterile cork borer. The test sample was placed in each respective well using sterile droppers. Antibacterial assay plates were incubated at 37°C for 48 h. The exact procedure was also done in control plates, but instead the wells were filled with sterile distilled water for negative control and the standard antibiotics disc of 6 mm diameter imipenem (30 mg/ disc, Oxoid UK) was used as a positive control for antibacterial activity. The plates were incubated at 37°C for 48 h. After incubation, clear area around the wells indicated the inhibition zones, which were measured in millimeters by caliper in order to evaluate the degree of susceptibility of the test organisms and labeled‘sensitive’or‘resistant’was compared to the standard antibiotics. All experiments were done in a duplicate manner.
2.4. Determination of minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of the agents
MIC was employed to determine minimum concentration of honey which will inhibit growth of the isolated microorganisms. The MIC was carried out using the Mueller-Hinton broth dilution method in serial dilution preparations [13]. A dilution schedule of MIC, growth visibility and non-growth tubes were registered then proceeding with the MBC test[14].
MBC was measured from the broth dilution tests using decreasing concentrations of honey and simulated honey solution by sub-culturing to antibiotic-free Mueller-Hinton agar from tubes showing no visible growth and also from the two dilution suspensions preceding the MIC dilution. A standard inoculum of the microorganism was added to an equal volume of each concentration. Mueller-Hinton broth tubes were prepared once with the crude honey and a second time with simulated honey. All Mueller-Hinton broth tubes were incubated at 35°C for 24 h. The experiment was done in a duplicate manner for each of the five microorganisms. Results were registered to compare with the media control Mueller-Hinton broth tubes prepared as follows; one broth tube containing the test bacteria, the second broth tube containing the standard antibiotics and the third broth tube containing sterile distilled water. The dilution of product that produced no growth was recorded as the MBC.
3. Results
A total of 90 samples were collected from sputum (n = 55) and throat swab (n = 35). The isolated bacterial species were identified and confirmed as: 26 of K. pneumoniae (28.9%), 19 ofS. pneumonia (21.1%), 18 of S. aureus (20.0%), 16 of S. pyogenes (17.8%) and 11 of P. aeruginosa (12.2%).
Table 1 shows a relative susceptibility pattern of the isolated bacterial microorganisms to the test samples. The highest inhibition zone (20.0 mm) was recorded from 75% diluted crude honey solution followed by 100% crude honey sample (15.3 mm) against S. pneumonia. On the other hand, 100% simulated honey solution showed lower inhibition zone (6.5 mm) where the 25% crude honey sample showed the least inhibition zone against S. pneumonia. It was found that the 75% simulated honey solution showed the least inhibition zone (4.0 mm) followed by the 25% simulated honey solution which did not show any activity in well diffusion method when tested against the same bacteria. In the same manner, higher inhibition zones were recorded from 75% diluted crude honey solution followed by 100% and 25% of the crude honey sample against the other four microorganisms, while again the 100% simulated honey solution showed lower inhibition zones followed by the 75% simulated honey solution which showed the least inhibition zones against S. aureus, P. aeruginosa, S. pyogenes and K. pneumonia, respectively, followed by the 25% simulated honey solution which did not show any activity.
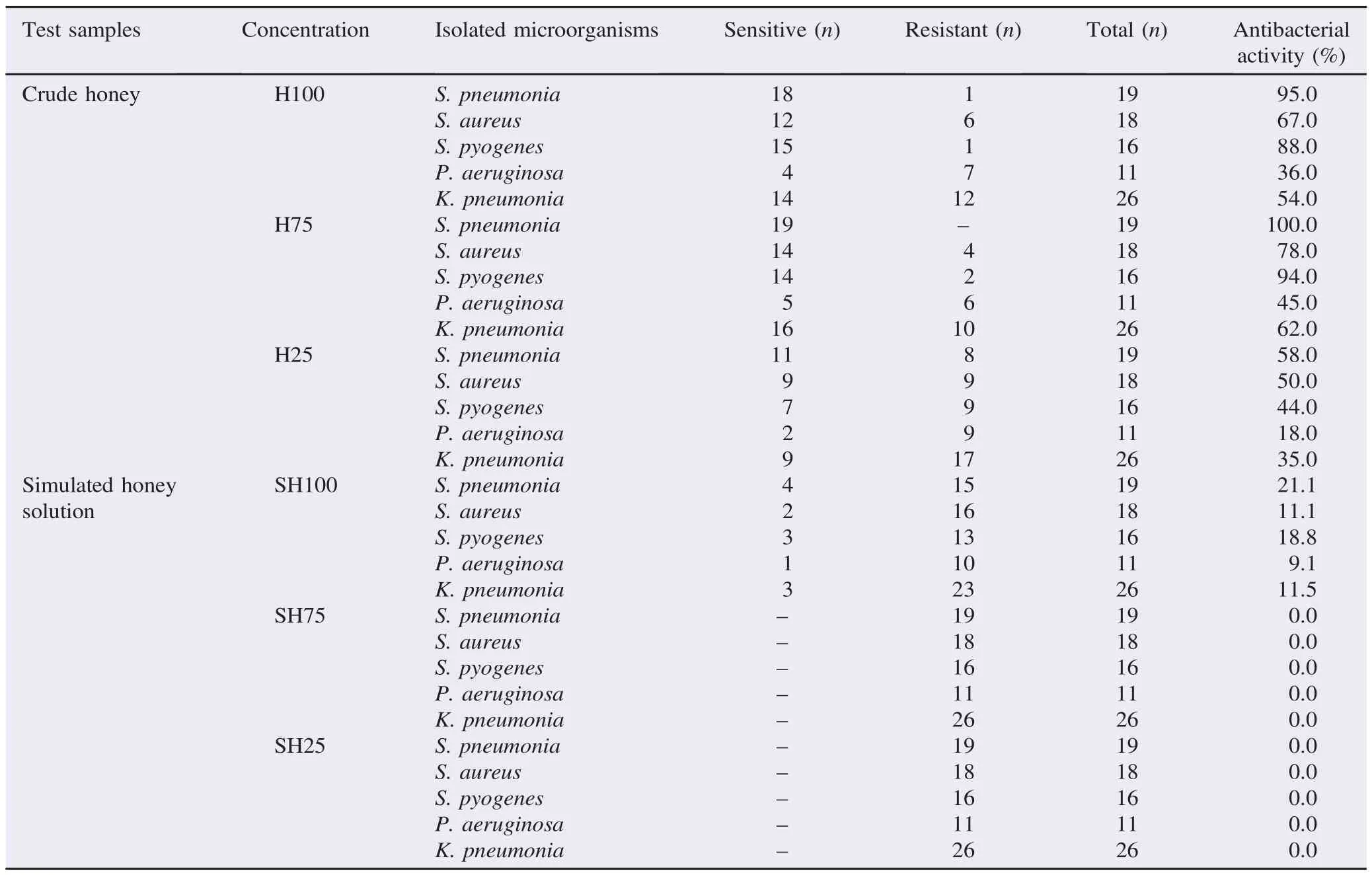
Table 1 The antibacterial activity represented by zones of inhibition of crude honey samples and simulated honey solutions against the isolated microorganisms.
The MIC of crude honey and simulated honey against the isolated bacteria was present in Table 2. Turbid (growth) and clear (no growth) tubes were identified comparing to the turbidity of the positive control. The negative growth was observed in crude honey at 100% and 75% concentrations against all tested microorganisms while growth was observed at 25% concentration. The negative growth was observed in simulated honey solution only at the highest concentration (100%) and only against S. pneumonia, S. pyogenes and P. aeruginosa. Growth was always observed at lower concentrations of simulated honey solution against all isolated bacteria (Table 2).
The MBC of different concentrations of crude honey and simulated honey solution against the isolated bacteria were present in Table 2. Mueller-Hinton agar dishes streaked from no growth (negative growth) tubes showed no colonies while streaked from positive tubes (growth) showed colonies of the isolated bacteria. The result of MBC of the crude honey concentrations and simulated honey concentrations against the isolated bacteria was exactly similar to the respective MIC value.
It can be seen that the growth of all five bacterial isolates was inhibited by crude honey at 75%-100% concentration. On the contrary, the bacterial isolates were inhibited only by 100% concentration of the simulated honey solution.
As could be seen, the difference between the antibacterial activities of the different dilutions of crude honey and the same dilutions of the simulated honey solution indicated the presence of other antibacterial components in crude honey other than the osmotic effect.
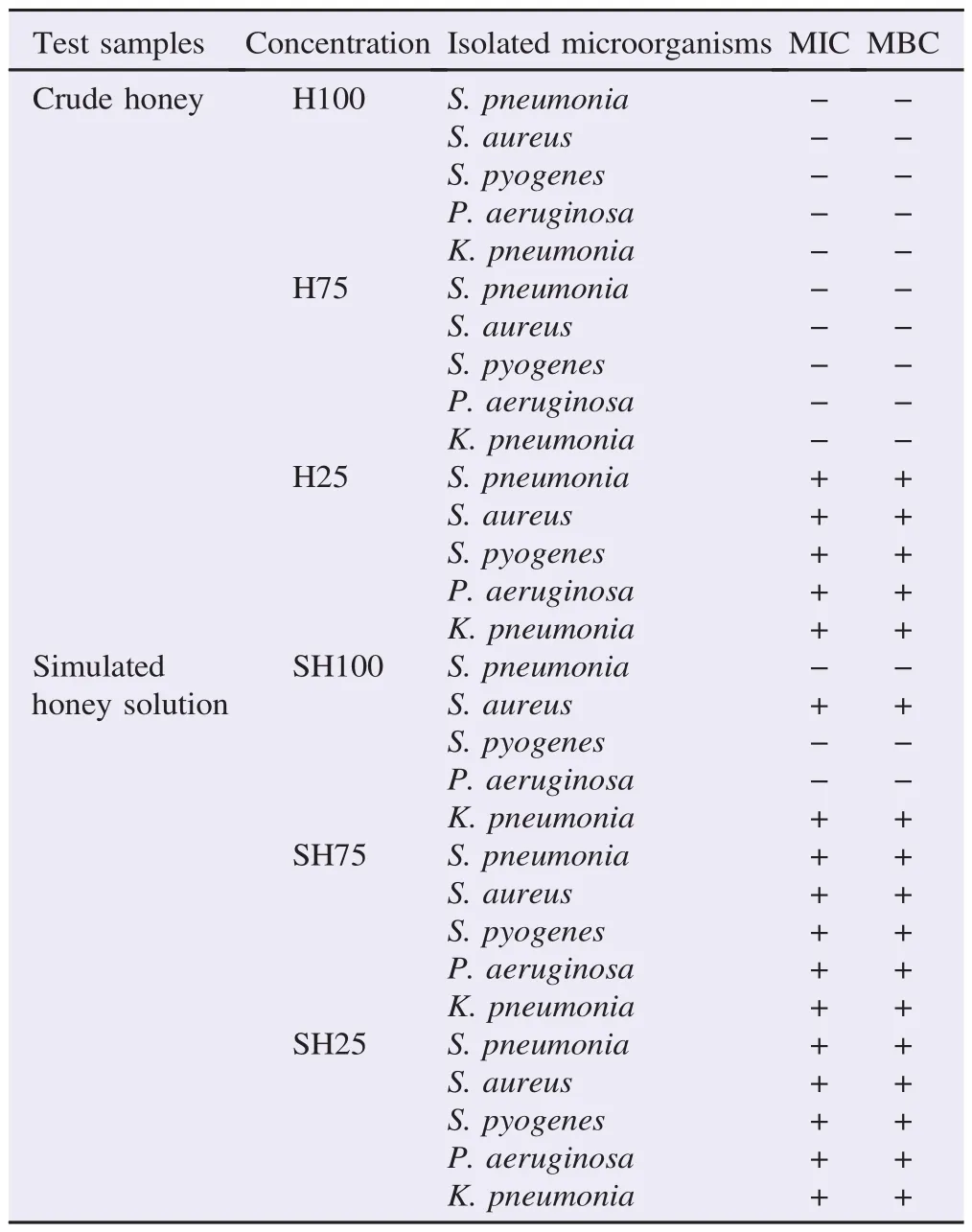
Table 2 MIC and MBC of different concentrations of crude honey and simulated honey solutions against isolated microorganisms.
4. Discussion
More recently, honey has been reported to have an inhibitory effect to around 60 species of bacteria including aerobes and anaerobes, Gram-positives and Gram-negatives [8]. The current prevalence of antibiotic-resistant microbial species has led to a re-evaluation of the therapeutic use of natural remedies, including honey [15].
Recent researches showed the antibacterial activity of honey against pathogenic bacteria of the gastrointestinal tract, urinary tract, wound and burn infections.
The MIC and MBC are easy and simple methods for the determination of inhibitory doses of an antibacterial agent for particular bacteria. Honey showed successful growth inhibition rates of dangerous bacteria such as Escherichia coli, S. aureus, Salmonella, Shigella, and Vibrio cholera[6]and even better than several known antibiotics. Honey showed successful growth inhibition rates of pathogenic microorganisms isolated from urine samples of patients with urinary tract infections [16].
In this study respiratory tract infection was chosen because of its high prevalence among diseases especially in children and it is one of the diseases directly correlated with industrialization and air pollution.
S. aureus causes pneumonia as a secondary disease to viral infections, which is found to be highly associated with a number of complications. P. aeruginosa causes mostly nosocomial infections, while K. pneumonia more commonly affects debilitated individuals with malnutrition [17]. Acute S. pyogenes infections commonly take the form of pharyngitis, scarlet fever (rash), impetigo, cellulitis, or erysipelas. Invasive infections can result in necrotizing fasciitis, myositis and streptococcal toxic shock syndrome, while S. pneumoniae causes pneumonia and meningitis [18,19].
On oral administration of honey,first it acts topically on the upper respiratory tract, then it acts on the lower respiratory tract as well after blood absorption.
In this study, K. pneumoniae, was the predominant organism isolated from the clinical specimens which is in consistency with several other researches such as a study conducted by Sikarwar and Batra who stated that out of the 59 clinical isolates they used [20], 20 were K. pneumoniae which had the highest percentage of the isolates. Ophori and Wemabu [21], in a second study, identified bacterial isolates including H. influenzae, K. pneumoniae, S. pneumoniae, Moraxella catarrhalis and S. pyogenes. However, H. influenzae had the highest percentage prevalence of 20.8%, followed by K. pneumoniae (19.2%).
Honey is characterized by high sugar content and low water concentrations. In this study, a simulated sugar solution was done to mimic the property of high sugar concentration in honey which leads to the high osmolarity that produces antimicrobial action.
The antimicrobial activity of this solution was compared with that of honey using the same microorganisms.
All the tested bacterial isolates were completely susceptible to the 75% concentrations of honey and to the 100% concentration of the simulated honey solution. This may be attributed to the high osmotic pressure exerted by the high sugar content in both the crude honey and the simulated honey solution.
They were also moderately susceptibility of the isolated bacteria to honey at 100% v/v concentration, but showed resistance to honey at 25% concentration and the 75% and 25% concentrations of simulated honey solution, which indicated the presence of other antimicrobial components responsible for the activity other than the osmotic pressure. This is in agreement with a study by Chute et al. which stated that dilution of 20% did not show any zones of inhibition for S. aureus and the same for higher concentrations of Klebsiella as well [22].
Ghori and Ahmad[23], and Kawaii et al.[24]reported that the 100% concentrated solution of crude honey samples inhibited the growth of all the bacterial isolates.
The increased activity of honey on limited dilution is due to the presence of glucose oxidase enzyme, which on dilution of honey, releases hydrogen peroxide. This enzyme activity increases by a factor of 2500-50000 on dilution of honey, therefore giving a“sustained-release”antiseptic at a level which is antibacterial but not tissue destructive. Adcock proved that both hydrogen peroxide and the antibacterial activity of honey were destroyed by exposure to light [25].
White et al. found that the enzyme is practically inactive in concentrated honey [26], and it gives rise to hydrogen peroxide only when the honey is diluted. Furthermore, unlike other antiseptics it has no harmful effects on tissues due to the slow enzymatic production of hydrogen peroxide.
The involvement of hydrogen peroxide in the antibacterial activity of diluted honey is also supported by the finding that when the catalase enzyme in the human body depletes the hydrogen peroxide, the non peroxide factors play their role. Non peroxide factors include bee defensins, methylglyoxal and other phytochemical factors, which are complex phenols and organicacids often referred to as“flavonoids”. Dilution, heat or light do not affect these complex chemicals. To confirm the presence of these antibacterial factors, honey is treated with catalase enzyme to remove the hydrogen peroxide activity [3]. S. pneumonia is becoming continuously more resistant to antibiotics [22]. In this study, it was found to be the most susceptible bacterial species for honey, showing 100% susceptibility.
The clearing of infection seen with honey indicates more than just antibacterial properties. Recent research showed that honey, at concentrations as low as 0.1%, enhances the proliferation of peripheral blood B-lymphocytes and T-lymphocytes in cell culture, and also phagocytes are activated by honey at concentrations as low as 0.1%[27]. In addition, 1% honey solution also enhances monocytes in cell culture to release cytokines, interleukin-1, interleukin-6, and tumor necrosis factor-alpha. It was clearly shown in these researches that honey has several actions on the immune system which all activated the immune response to infection [28].
Moreover, the glucose content of honey and the low acidic pH (between pH 3 and pH 4) may contribute in stimulating the action of macrophages in bacterial destruction[29].
This study showed that crude honey has potent antimicrobial activity against the most common bacteria causing respiratory tract infections with a relatively higher sensitivity against S. pneumonia and S. pyogenes followed by S. aureus, K. pneumonia and finally P. aeruginosa. Common bacterial infections are getting more and more resistant and less susceptible to antibiotics due to increasing misuse of drugs. Therefore the use of honey as a non-toxic and cheap natural antibacterial agent should be globalized after being subjected to pharmaceutical standardization and further clinical trials.
Conflict of interest statement
I declare that I have no conflict of interest.
References
[1] Adeshina GO, Mshelia BM, Onaolapo JA. Antibacterial susceptibility of Klebsiella pneumoniae isolated from respiratory tract infections to honey and lemon. Annu Res Rev Biol 2014; 4(4): 625-37.
[2] Odonkor ST, Addo KK. Bacteria resistance to antibiotics: recent trends and challenges. Int J Biol Med Res 2011; 2(4): 1204-10.
[3] Aurongzeb M, Azim MK. Antimicrobial properties of natural honey: a review of literature. Pak J Biochem Mol Biol 2011; 44: 118-24.
[4] Mandal S, DebMandal M, Pal NK, Saha K. Antibacterial activity of honey against clinical isolates of Escherichia coli, Pseudomonas aeruginosa, and Salmonella enterica serovar Thypi. Asian Pac J Trop Med 2010; 3: 961-4.
[5] Basualdo C, Sgroy V, Finola MS, Marioli JM. Comparison of the antibacterial activity of honey from different provenance against bacteria usually isolated from skin wounds. Vet Microbiol 2007; 124: 375-81.
[6] Boukra?a L. Honey in traditional and modern medicine. Boca Raton: CRC Press; 2013, p. 13-20.
[7] Bizerra FC, Da Silva PI Jr, Hayashi MA. Exploring the antibacterial properties of honey and its potential. Front Microbiol 2012; 3: 398.
[8] Mandal MD, Mandal S. Honey: its medicinal property and antibacterial activity. Asian Pac J Trop Biomed 2011; 1(2): 154-60.
[9] Ewnetu Y, Lemma W, Birhane N. Antibacterial effects of Apis mellifera and stingless bees honeys on susceptible and resistant strains of Escherichia coli, Staphylococcus aureus and Klebsiella pneumoniae in Gondar, Northwest Ethiopia. BMC Complement Altern Med 2013; 13: 269.
[10] Brady NF, Molan PC, Harfoot CG. The sensitivity of dermatophytes to the antimicrobial activity of manuka honey and other honey. Pharm Pharmacol Commun 1996; 2(10): 471-3.
[11] Bogdanov S, Jurendic T, Sieber R, Gallmann P. Honey for nutrition and health: a review. J Am Coll Nutr 2008; 27: 677-89.
[12] Cheesebrough M. Medical laboratory manual for tropical countries. Cambridge: Cambridge University Press; 2010.
[13] National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility test for bacteria that grow aerobically. Approved standard. NCCLS document M7-A5. 5th ed. Wayne:NationalCommitteeforClinicalLaboratoryStandards;2000.
[14] National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved Standard. NCCLS document M7-A2. 2nd ed. Villanova: National Committee for Clinical Laboratory Standards; 1990.
[15] Rayes AAH. Assessment of the anti-bacterial activity of different types of natural and commercial honeys on bacteria isolated from wounds and burns in Saudi Arabia. Acad Arena 2013; 5(10): 15-21.
[16] Yassin NA. Honey as therapeutic agent for infectious diseases. Diag Ther Stud 2013; 2(3): 43-50.
[17] Kumar V, Abbas AK, Fausto N, Aster JC. Robbins & Cotran pathologic basis of disease. 8th ed. Philadelphia: Saunders Elsevier; 2009.
[18] Wong SSY, Yuen KY. Streptococcus pyogenes and re-emergence of scarlet fever as a public health problem. Emerg Microbes Infect 2012; 1: e2.
[19] Chen Y, Deng W, Wang SM, Mo QM, Jia H, Wang Q, et al. Burden of pneumonia and meningitis caused by Streptococcus pneumoniae in China among children under 5 years of age: a systematic literature review. PLoS One 2011; 6(11): e27333.
[20] Sikarwar AS, Batra HV. Challenge to healthcare: multidrug resistance in Klebsiella pneumoniae. Int Conf Food Eng Biotechnol 2011; 9: 130-4.
[21] Ophori EA, Wemabu EC. Antimicrobial activity of propolis extract on bacteria isolated from nasopharynx of patients with upper respiratory tract infection admitted to Central Hospital, Benin City, Nigeria. Afr J Microbiol Res 2010; 4(16): 1719-23.
[22] Chute RK, Deogade NG, Kawale M. Antimicrobial activity of Indian honey against clinical isolates. Asiatic J Biotech Res 2010; 1: 35-8.
[23] Ghori I, Ahmad SS. Antibacterial activities of honey, sandal oil and black pepper. Pak J Bot 2009; 41(1): 461-6.
[24] Kawaii S, Tomono Y, Katase E, Ogawa K, Yano M, Koizumi M, et al. Quantitative study of flavonoids in leaves of citrus plants. J Agric Food Chem 2000; 48: 3865-71.
[25] Adcock D. The effect of catalase on the inhibine and peroxide values of various honeys. J Apic Res 1962; 1: 38-40.
[26] White JW Jr, Subers MH, Schepartz AI. The identification of inhibine, the antibacterial factor in honey, as hydrogen peroxide and its origin in a honey glucose-oxidase system. Biochim Biophys Acta 1963; 73: 57-70.
[27] Eteraf-Oskouei T, Najafi M. Traditional and modern uses of natural honey in human diseases: a review. Iran J Basic Med Sci 2013; 16: 731-42.
[28] Fukuda M, Kobayashi K, Hirono Y, Miyagawa M, Ishida T, Ejiogu EC, et al. Jungle honey enhances immune function and antitumor activity. Evid Based Complement Altern Med 2011; 2011: 908743.
[29] Ryan GB, Majno G. Acute inflammation. A review. Am J Pathol 1977; 86(1): 183-276.
*Corresponding author:Reham F. El-Kased, Department of Microbiology and Immunology, Faculty of Pharmacy, the British University in Egypt, Cairo, Egypt.
 Asian Pacific Journal of Tropical Biomedicine2016年3期
Asian Pacific Journal of Tropical Biomedicine2016年3期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Cyclical mastalgia: Prevalence and associated determinants in Hamadan City, Iran
- Bee pollen extract of Malaysian stingless bee enhances the effect of cisplatin on breast cancer cell lines
- In vitro antihistamine-releasing activity of a peptide derived from wasp venom of Vespa orientalis
- Changes in energetic profile of pregnant ewes in relation with the composition of the fetal fluids
- The inhibition of Typhonium flagelliforme Lodd. Blume leaf extract on COX-2 expression of WiDr colon cancer cells
- Evaluations of cytotoxicity of Smilax myosotiflora and its effects on sexual hormone levels and testicular histology in male rats
