Investigation of Propoxylation and Ethoxylation of 2-Ethylhexanol in the Presence of an Alkaline and DMC Type Catalyst at Initial Stages of the Syntheses
Wieslaw Hreczuch, Karolina Dabrowska, Arkadiusz Chru?ciel
MEXEO, Institute of Technology, Poland
Katarzyna Materna, Agata Sznajdrowska
Institute of Chemical Technology and Engineering, Poznan University of Technology, Poland
Krystyna Czaja
Faculty of Chemistry, Opole University, Poland
Wanxu Wang, Qiuxiao Li, Yongqiang Sun
China Research Institute of Daily Chemical Industry, China
Introduction
Surfactants have been applied in many products of the chemical industry, such as detergents, paints, dyestuffs,paper coatings, inks, plastics and fibers, personal care and cosmetics, agrochemicals, pharmaceuticals, food processing,etc. In addition, they play an important role in the oil industry. Hence, it seems to be extremely important to search for the appropriate sources of raw materials for their production. Such proposals include 2-ethylhexyl alcohol.[1-3]The compound is available on the market at competitive prices, in comparison with the detergent range alcohols.The discussed 2-ethylhexanol (EH) derived surfactants are synthesized according to the general formula (1):

Where n is average ethoxylation grade, typically up to 15.
Generally, homogeneous and heterogeneous type catalysts are used in the alkoxylation process. The former is represented by KOH which is commonly applied in the synthesis and leads to an undesirable, wide range homolog distribution of the products generated. The latter can be represented by the dimetalcyanide (DMC) type catalyst, a recently developed coordinative type propoxylation catalyst,which also attracts attention in ethoxylation.[4,5]
The criteria for selecting an optimal catalyst include its kinetic performance in the studied synthesis, as well as composition and derived properties of the products obtained.
Kinetic models of polyaddition
The obtained polyaddition products are usually characterized by the average number of moles of the applied alkyloxirane (alkylene oxide) converted in the reaction per mole of the starter material. In fact, the synthesis follows a pattern of parallel, consecutive, second order reactions,leading to a polydispersed mixture of homologs within a certain range of polyaddition degrees around the average alkoxylation degree and some amount of the substrate usually remains unconverted. Generally, this is represented by the following set of kinetic Equations (2).
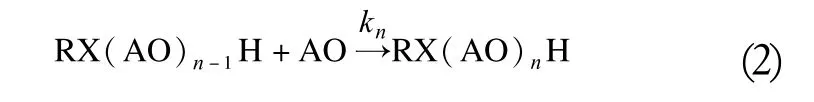
Where k0, k1, ..., kndenote rate constants for the successive steps of the process, and X stands for oxygen, in the ethoxylation of an alcohol or another intermediate group,such as amine, amide or carboxyl.
When the concentrations of the following homologs:RXH, RX(AO)H,..., RX(AO)nH are denoted as x0,x1, ..., xn, the alkyloxirane concentrations are described by xAOand the kinetic equations of the second order for the subsequent reactions are considered, then the reaction rates at the consecutive propagation steps can be expressed as follows (3) :
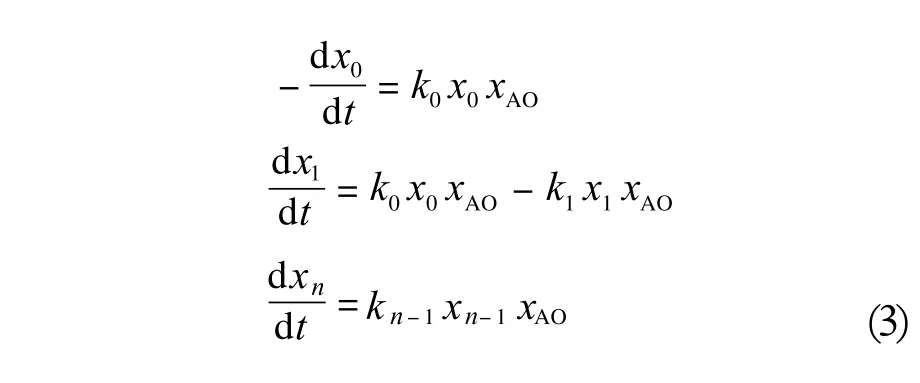
Dividing these equations by the reaction rate at the first addition step (dx0/dt) and defining the distribution coefficients cn as the ratio of the successive rate constants:c1= k1/k0, c2= k2/k0,..., cn= kn/k0provides the following set of Equations (4) :
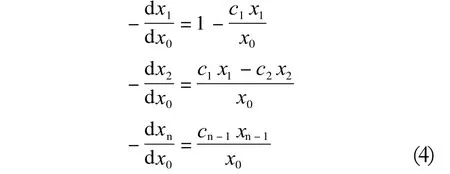
Where the concentration xn is expressed as functions of the content of the x0(ROH) reagent. The above set of equations can be solved numerically, e.g., using the numerical integration technique of the Runge-Kutta method,optimization algorithms of the Monte Carlo method and the simplex Nelder & Mead method.[6,7]
The Natta-Mantica Equation[8,9]can also be used for determination of the distribution coefficients(ci) (5):
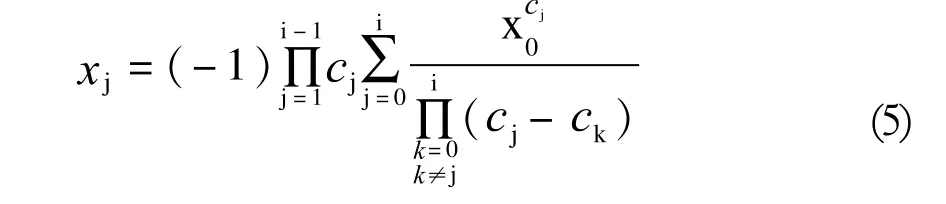
On the other hand, knowing an experimentally determined fractional content of an alkoxylation product(xi) and using the above Natta Mantica equation, one can compute the distribution constants (ci) and get results which are comparable to those of the analytical method. To that end, a dedicated computer software is required — and has also been developed and used by the present authors.[10]
An intermediate simplified model was also reported. The amount of unconverted substrate and average polyaddition degree enable determination of the so-called Weibull-Nycander-Gold coefficient (WNG). It shows the ratio of the addition rate constant at the first step of the reaction and the following propagation steps, which are presumed equivalent(6):
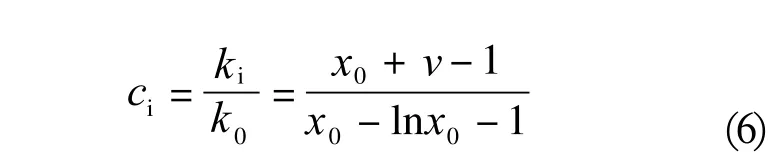
Where: kidenotes the reaction rate constant at the i-th addition step, whereas k1=k2=....kn, k0is the reaction rate constant at the first addition step, means average polyaddition degree and x0stands for the mole fraction of the unconverted substrate.
With the cicoefficient, the corresponding fractional content model can be generated according to the following WNG Equation (7):
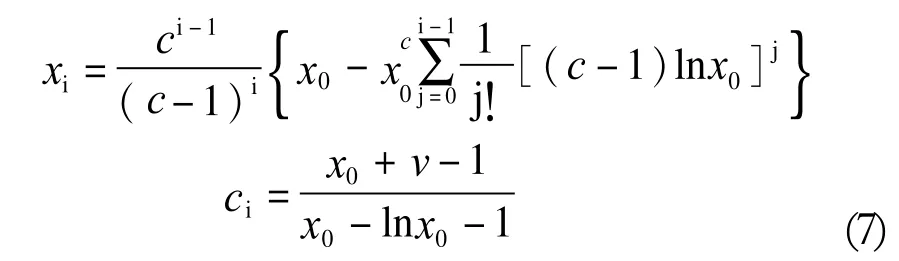
The most general kinetic model reflecting the Poisson type distribution was described by Flory[11,12], with the assumption that an equivalent reaction rate occurs at the first and each of the following propagation steps (k0=k1=k2......kn)(8):

The WNG distribution is much closer to that determined experimentally, in comparison with the Flory model.Moreover, the higher the value of CWNG=k/k0or ci=ki/k0or ci(NM),the broader the resulting homolog distribution, which is also shown in Figure 1.
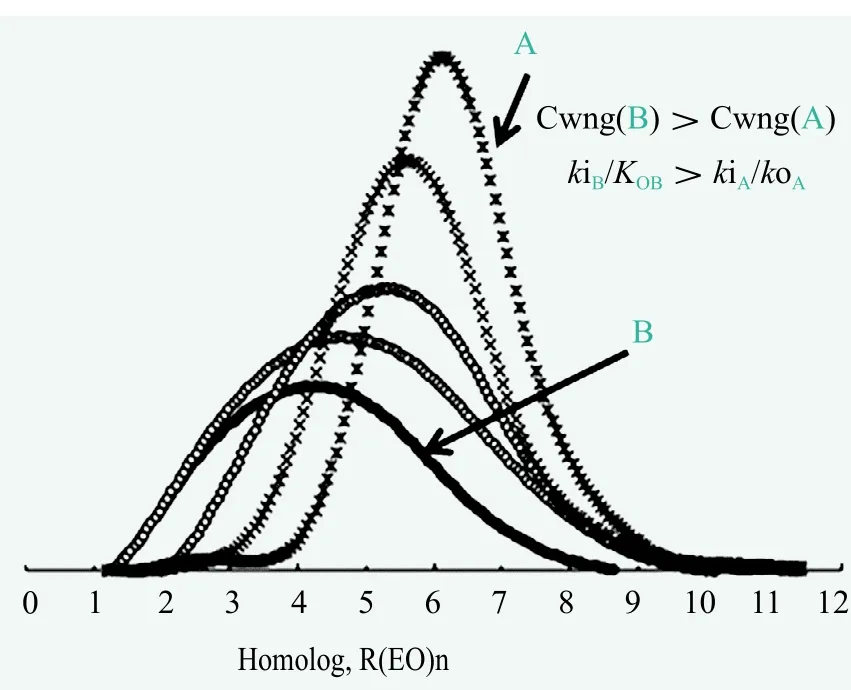
Figure 1. Illustration of the influence of the CWNG coefficient on the homolog distribution in polyaddition products
Assuming accurate quantitative analysis results, the cicoefficients of the NM model reflect exactly the kinetic parameters (kik0); this has to take place in order to fix the results determined experimentally. The other theoretical model of WNG is also useful in practice for quantifying any existing difference in homolog distributions among the products compared, by contrasting the calculated CWNGvalues.
Oxiranes readily react with diverse compounds by ring opening. Their typical reactions are those with nucleophiles,proceeding via the SN1 or SN2 mechanism both in acidic (weak nucleophiles: water, alcohols) and alkaline media (strong nucleophiles: OH—, RO—, NH3, RNH2, RR'NH, etc.). The general reaction pattern is as follows (9):
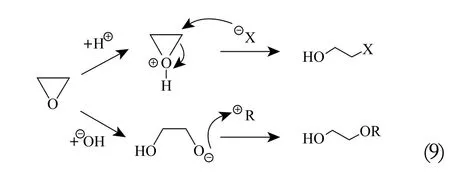
The alkaline mechanism leads to the broadening of the obtained homolog distribution in the product mixture,as described by the Weibull-Tornquist effect,[13-16]through the semi-crown complexation of the alkaline cation by the growing oligo-ether chains Figure 2.
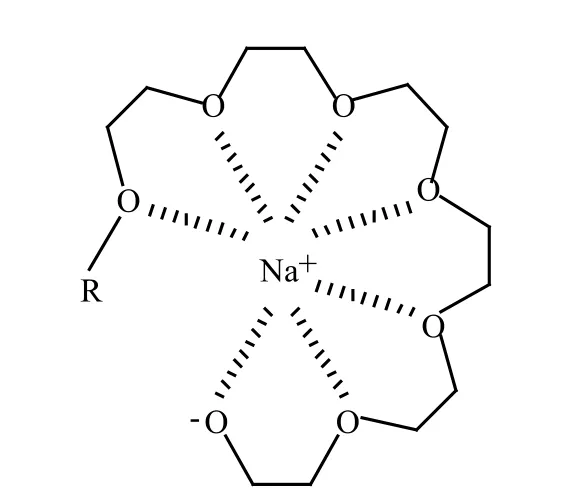
Figure 2. Semi-crown complex of an alkaline metal active center
Loosening the transient alcoholate-metal cation bond facilitates the connection with the next alkoxy-mere by decreasing the energetic barrier. It was further proved by calculating the distribution coefficients ci=ki/k0, that this implies increased reaction rate constants at the following addition steps until i=6, resulting in a broader distribution of the products formed.[10]The effect is blocked by bivalent or trivalent cations of the catalysts, yielding a narrower homolog mixture, that can also be attributed to the bivalent zinc active center of the complex DMC type catalyst.
Despite the homolog distribution of the target polyaddition products, the applied catalyst is During alkoxylation, diols can be formed as by-products by the catalytic hydration of alkylene oxide (10):
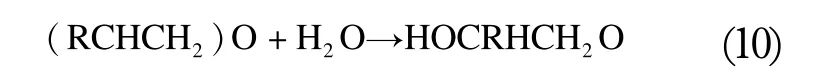
Where, R denotes Hor CH3.
Further propagation generates dialkylene glycol,trialkylene glycol and higher polymers.
When alkylene oxide is heated to about 150-300 ℃ in the presence of a catalyst, it can isomerize to form acetaldehyde(11):
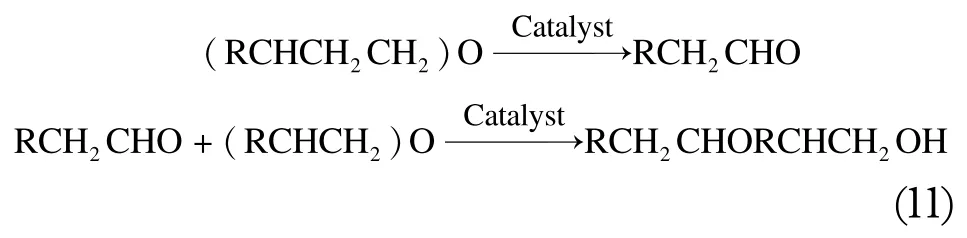
Moreover, it is a well known fact that, by eliminating the proton from the methyl group, methyloxirane can be converted into an allyl alcohol (12):
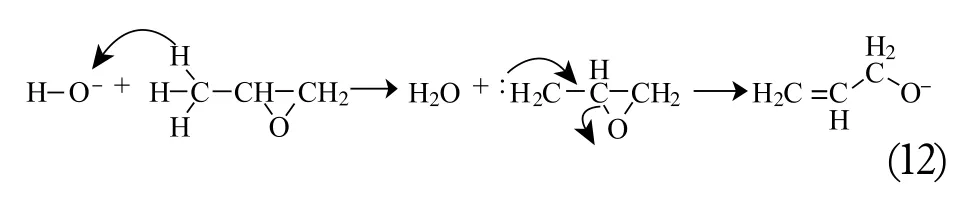
Which is also capable of polyether chain propagation.
The reactions described above affect the selectivity of the discussed polyaddition process as well as the quality and properties of the obtained products.
A substantial difference appears in the structures of the symmetric oxirane and non-symmetric methyloxirane,which leads to isomerisation and steric hindrance effects during propagation. Sometimes, the methyl-branched structure and the formed secondary hydroxyl groups of poly-propoxy chains are responsible for their limited reactivity with comparison with the primary and linear polyethers formed by the oxirane addition.Therefore, ethoxylation and propoxylation should be considered separately, especially when they are applied in a production process one by one.
The aim of this work is to compare the activity of a conventional alkaline (KOH) and a DMC type catalyst in the propoxylation and ethoxylation of 2-ethylhexanol.Moreover, the kinetics and selectivity at the initial stage of the syntheses were investigated. These parameters are important for the following propagation steps and properties of the final products.
Experimental section
Materials
2-ethylhexanol was commercially produced by the K?dzierzyn Nitrogen Works, Azoty Group, K?dzierzyn-Ko?le, Poland. Ethylene oxide was obtained from PKN Orlen, P?ock, Poland. Propylene oxide was manufactured by PCC Rokita S.A., Brzeg Dolny, Poland. KOH, 85% pure,was purchased from Brenntag, K?dzierzyn-Ko?le, Poland.The applied DMC type catalyst[4]was manufactured by MEXEO, K?dzierzyn-Ko?le, Poland. NM-CAD, the computer program used for the calculation of kinetic parameters in polyaddition reactions, was developed by MEXEO.
Methods
Preparation of the DMC catalyst. K3[Co(CN)6] (7.5 g)was dissolved in a round-bottom flask, filled with a mixture of distilled water (40 mL) and tBuOH (50 mL) (Solution A). Solution B was prepared by dissolving ZnCl2(75 g) in a mixture of water (75 mL) and tBuOH (2 mL). Solution B was added to Solution A over 30 min with stirring using a mechanical stirrer. After combining them, stirring was continued for another 15min. 200 mL of a 1% aqueous solution of acrylic acid polyamide, a flocculent which enables a precipitate to be separated from aqueous solutions, was added to the prepared reaction mixture and stirred for 10 min more. Precipitation of a virtually amorphous precipitate of zinc hexacyanocobaltate(III) followed. The mixture was centrifuged and the resulting catalyst cake was dissolved in a mixture of tBuOH (130 mL) and 1% aqueous solution of the flocculent (55 mL). The reaction mixture was stirred vigorously for 15 min. The described operation of rinsing and filtration was performed once more and the obtained catalyst solid was dried to a constant weight at 50 ℃.[4,5]
Polyaddition of methyloxirane/oxirane was carried out by means of a 1-L laboratory autoclave equipped with a programmable logic control system (PLCS)controlling its operation, as well as mechanical stirrer,heating jacket and cooling coil. The reactor was charged with an initiator and catalyst and dehydration followed through nitrogen purge over 30 min, at 130 ℃. The syntheses were performed through a one-step drop of the monomer into the starter material at a 1:1 molar ratio. In this case, the desired amount of the monomer was introduced at a time into a mixture of the starter with the catalyst and the reaction was continued at the desired temperature until overpressure in the reactor dropped to a constant level. Changes in overpressure reflected the kinetics of the monomer conversion.
GPC analyses were performed using a GPC apparatus from Shimadzu, equipped with two LC-20AD pumps with a gradient, degassing unit Model DGU-20A3 and a fraction collector Model FRC-10A. Separation was carried out on the Shodex GPC column KF-805L. The flow rate of the eluent(tetrahydrofurane) was 1 cm3·min-1at a temperature of 40 ℃.The polymers’ molecular weights were found by calibration performed for polymethacrylate standards with narrowrange distributions of molecular weights (Sigma-Aldrich).The results were processed using the Shimadzu LCSolution software for determination of the molecular weights of the analyzed systems, their dispersity and purity.
Gas chromatography (GC) was applied for analyzing the products’ compositions, using a gas chromatograph from Hewlett-Packard with flame ionization detector,equipped with a CHROMA computer integrator and a DB-5HT capillary column with the parameters 0.53 mm×15 m×0.1 μm. The analysis conditions: the column temperature programmed by 1min isotherm with an initial rate of 8 ℃ · min-1in the range of 50-340 ℃, the injector temperature was 340 ℃, and detector temperature was 350 ℃. The carrier gas (helium) flow rate was 2 mL · min-1.Interpretation of the results was performed using the internal standardization methodology.
Results and discussion
The experimental products examined in this work were manufactured by monopropoxylation of 2-ethylhexanol followed by its monoethoxylation. PO conversion was investigated in the presence of KOH and DMC catalysts.One mole of PO per one mole of 2-EH and catalyst was added to the autoclave feed at an ambient temperature. Then the reaction temperature was gradually increased. In both cases, initiations of the syntheses were observed at ca. 120℃ and 100 ℃ with KOH and DMC catalyst, respectively.From that moment on, overpressure began to decrease. The comparison of the initiation temperature and the kinetics of conversion during monopropoxylation of EH in the presence of KOH and DMC as the catalysts, where the catalyst concentration was 0.3% and 0.05% per product weight,respectively, is shown in Figure 3.
The time of reduction of overpressure reflected the average rate of PO conversion. The amount of PO introduced into the reactor was consumed within 120min and 10min in the presence of KOH and DMC catalyst, respectively. Hence,the DMC catalyst’s activity was much higher, despite its significantly lower concentration in the reaction medium.
The obtained products were further subjected to ethoxylation by 1 mole of EO per mole of EH-PO using the catalyst residue remaining in the starter. Dynamic trends from the synthesis are compared in Figure 4.
The same initiation temperature was determined at c.-a.130℃ for both catalysts and the time of EO conversion was 80 and 10min, with KOH and DMC catalyst, respectively.
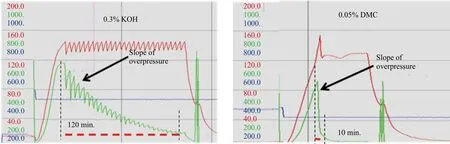
Figure 3. The initiation temperature and the kinetics of conversion during monopropoxylation of EH in the presence of 0.3% KOH and 0.05% DMC as the catalysts (the red line depicts the reactor temperature, the green line – the overpressure, the blue line – the weight of PO in the feeding vessel)
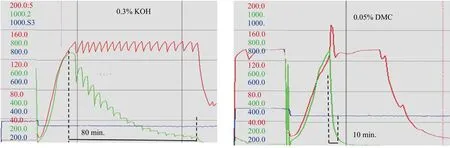
Figure 4. The initiation temperature for monoethoxylation of EH-PO in the presence of 0.3 % KOH and 0.05% DMC as the catalysts (the red line depicts the reactor temperature, green line - the overpressure, blue line - weight of PO in the feeding vessel)
The differences in the kinetics of the compared PO conversion in the presence of KOH and DMC type catalyst can be quantified by calculating the average reaction rate factor Rp, expressed as g of PO consumed per minute, per g of catalyst and per g of starter, respectively Equation (13):
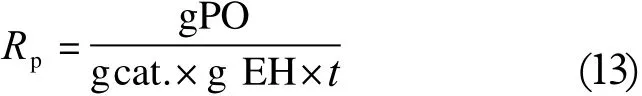
A comparison of the calculated average reaction rate factors is shown in Table 1.
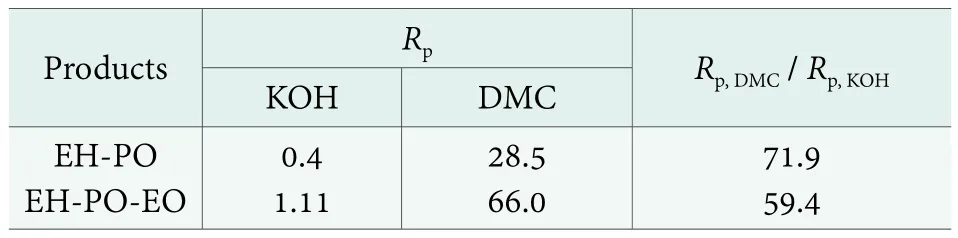
Table 1. Average reaction rate factor Rp for the EH-PO and EH-PO-EO products
A significant kinetic advantage of the DMC type catalyst over the compared alkaline catalyst was found in both of the cases shown in Table 1. Conversion was 60 to 70 times as fast in the presence of the DMC type catalyst, in comparison with KOH.
The following systems investigated represent a model where one mole of oxirane or methyloxirane reacted directly with one mole of 2-ethylhexanol and the reaction products marked as EH-PO and EH-EO, respectively, were formed.Alkylene oxide was introduced into the reactor at the adjusted reaction temperature. This way, the influence of the catalyst’s concentration and the reaction temperature on the synthesis performance was examined. The obtained results are shown in Figure 5 and Figure 6.
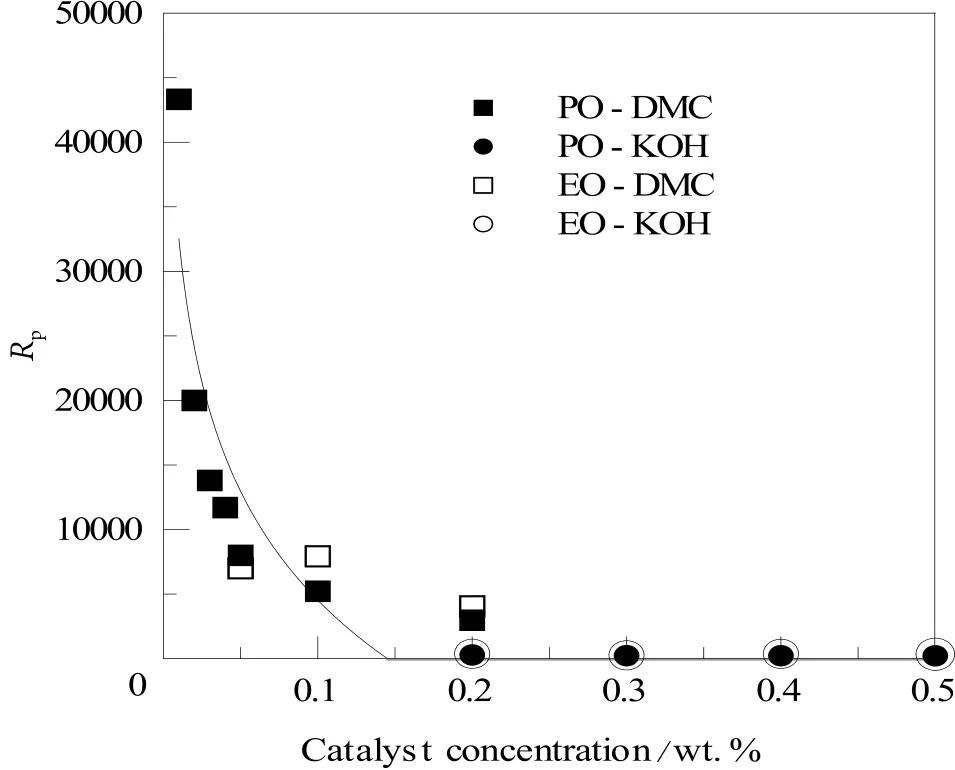
Figure 5. The reaction rate factor Rp depending on the catalyst type and its concentration, at the initial stage of the reaction(starter/monomer=1 mole/1 mole)
The influence of the catalyst type investigated in this work and its concentration on the reaction rate Rp, is presented in Figure 5. It can be observed that the DMC catalyst’s activity decreases along the increase in its concentration while, with KOH, kinetics seems stable, although at a relatively low Rpvalue.
Further research was performed to investigate the influence of the reaction temperature on the synthesis performance at selected concentrations for both catalysts.The obtained results are presented in Figure 6.
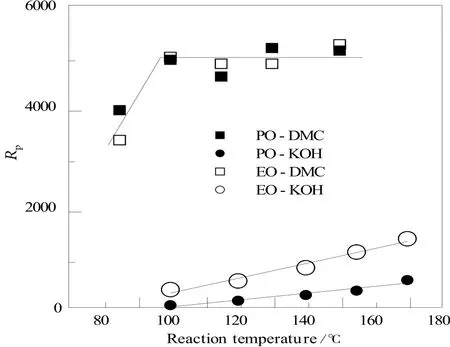
Figure 6. The influence of reaction temperature on reaction rates of propoxylation and ethoxylation for both catalyst types at the initial stage of the reaction (starter/monomer = 1 mole/1 mole), at 0.3% of KOH and 0.05% DMC, respectively
It was observed that, after initiating the reaction between 80℃ and 100℃, the kinetics of EO and PO addition seemed weakly influenced by a further increase in the reaction temperature with the DMC catalyst, even though the values of Rpwere relatively high. In the case of KOH, starting from 100℃, the conversion speeded-up more significantly in the case of ethoxylation as compared with propoxylation.
Such significant differences between the activities of the compared catalysts, expressed by the values of Rpfactors, can be accounted for by different reaction mechanisms, while the DMC catalyst coordinates the ring opening attack at alkylene oxide of very high reactivity in the addition reaction, as compared with that of SN2 nucleophilic substitution, creating an active center at the less reactive alcoholate anion, further activated by the increase in temperature. Obviously, oxirane exhibits higher activity than does methyloxirane because of the lower energy barrier of the epoxy-ring opening in this case. It should be further stated that the apparent decrease in the activity of the DMC catalyst connected with its increased concentration and stable reactivity along the increased temperature may only prove that the reaction progress criterion was shifted from its kinetic phase to the diffusion rate of gaseous alkyloxirane into the liquid reaction medium.This means that the reaction rate is faster than dissolution of the gaseous reagents in the liquid reaction medium. As the result, the reaction rate with the DMC catalyst is fast enough to convert instantly all the available monomer, just dissolved.Consequently, the apparent decrease in the Rpfactor can only be attributed to the result of mathematical calculation, in fact.Therefore, a more efficient mass transfer reactor-absorber system should be used for investigating the reaction kinetics in this case.
The influence of the catalyst concentration at a constant reaction temperature (130℃) and that of the reaction temperature at a constant catalyst concentration (KOH, 0.3%and DMC, 0.05%) on the obtained homolog distributions, as reflected by the CWNGdistribution coefficients, is presented in Figure 7.
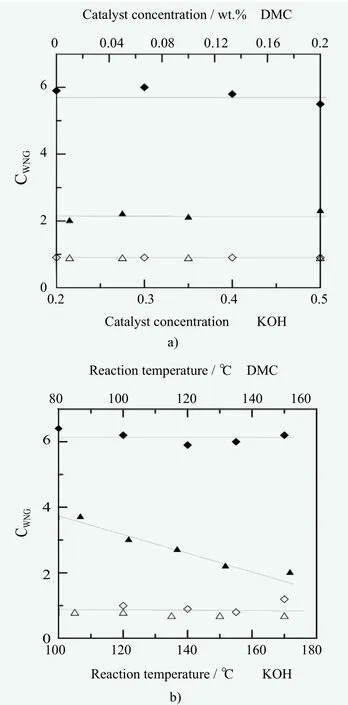
Figure 7. Comparison of the CWNG=k/k0 coefficients for EH-PO, marked by the symbols Δ? and EHEO denoted as ▲?, obtained in the presence of KOH (diamond) and DMC catalyst (triangle) in relationship to a) catalysts concentrations and b)reaction temperatures, respectively

The obtained homolog distributions depend on the applied adduct (PO or EO) and catalyst (KOH or DMC).No influence of the reaction temperature or the applied catalyst concentration was noted during propoxylation. It appears, that the steric hindrance effect of the branched structure of methyloxirane and the generated second order hydroxyl groups as the synthesis active center prevail over the influence of the reaction mechanism, conditioned by the investigated catalyst type and reaction temperature, which play a dominant role in ethoxylation.
For the EH-EO products, the observed differences in the calculated values of CWNGcoefficients are quite significant,taking into account the very initial stage of propagation.The applied DMC type catalyst gives narrower homolog distributions in comparison with KOH, as reflected by the lower values of CWNGwhere the ratio of reaction rate constants at the propagation steps to that at the first addition step is relatively lower. In consequence, the DMC based catalysis yields also a relatively higher conversion of the starter in the obtained products. Furthermore, the increased reaction temperature brings an additional narrowing effect when the DMC catalyst is used for ethoxylation, which is not observed with KOH. This seems an interesting issue, derived from the substantial differences in the reaction mechanisms.Its explanation will be the subject of further research.
Initially, gas chromatography was selected for determining the obtained product content of a reasonably low molecular weight (weight balance average Mw<200).
The issued chromatograms are shown in Figure 8.
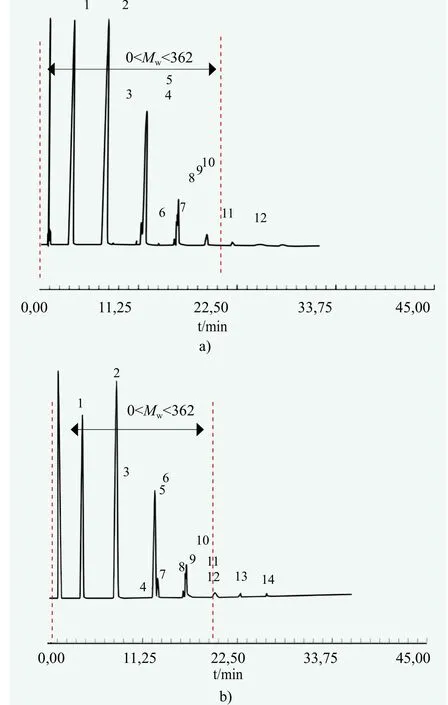
Figure 8. Gas chromatograms for the EH-PO products in the presence of a) KOH and b) DMC catalyst, respectively
State-of-the-art knowledge indicates that addition of methyloxirane generates two isomeric forms, resulting in the formation of a first-order or a second-order hydroxyl group at the end of the polyether chain. Both isomer homologs can have the same number of mers so their peaks overlap or appear very close each other in the gas chromatograms.Additionally, polyglycols of the same number of mers are formed as by-products, which appear very close. For the sake of simplicity, the peak areas which are very close each other,presumably of the same number of mers, were additively unified as a single homolog in the calculations. The fractional contents, as quantified from the chromatograms in Figure 8,and the kinetic parameters, as calculated from the fractional contents, are shown in Table 2.
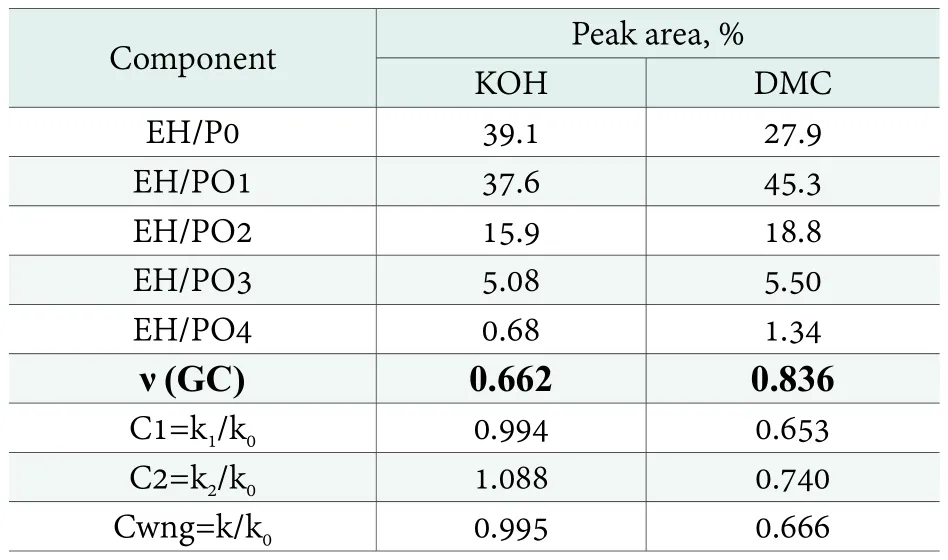
Table 2. Peak areas for monopropoxylates of ethylhexanol (EH-PO,Figure 8) and the derived kinetic parameters
Quite a similar situation occurs when monoethoxylation of 2-ethylhexanol is compared (Figure 9, Table 3).
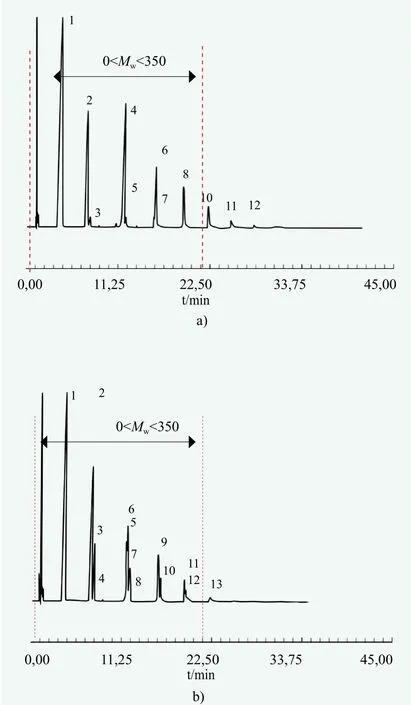
Figure 9. Gas chromatograms for the EH-EO products in the presence of a) KOH and b) DMC catalyst, respectively
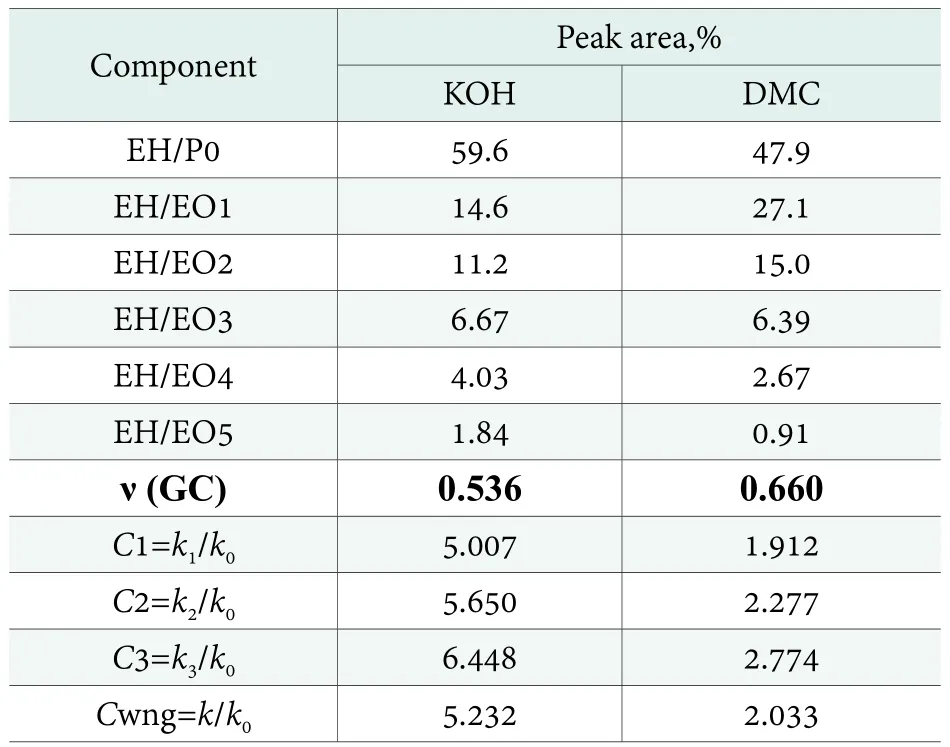
Table 3. Peak area for ethoxylation of EH (EH-EO, Fig.9) and the derived kinetic parameters
The average polyaddition degrees (ν) for the products from Table 2 and 3 should be theoretically equal to Table 1, as calculated from the synthesis wt. balance. The values calculated based on the GC results at the level below ν=1,reflect the reaction’s lower selectivity, in addition to a possible analytical error. As the result, the GC results discussed above,describe only part of the reaction product. Consequently, the KOH catalyst seems to be less selective in comparison with DMC, while the calculated average oligoaddition degrees are lower in that case. The substantially lower average polyaddition degrees determined by GC, in comparison with that expected from the synthesis weight balance,prompted additional analyses by the GPC technique,allowing determination of the product contents over a much wider range of molecular masses. The obtained products’chromatograms are shown in Figure 10 and 11.
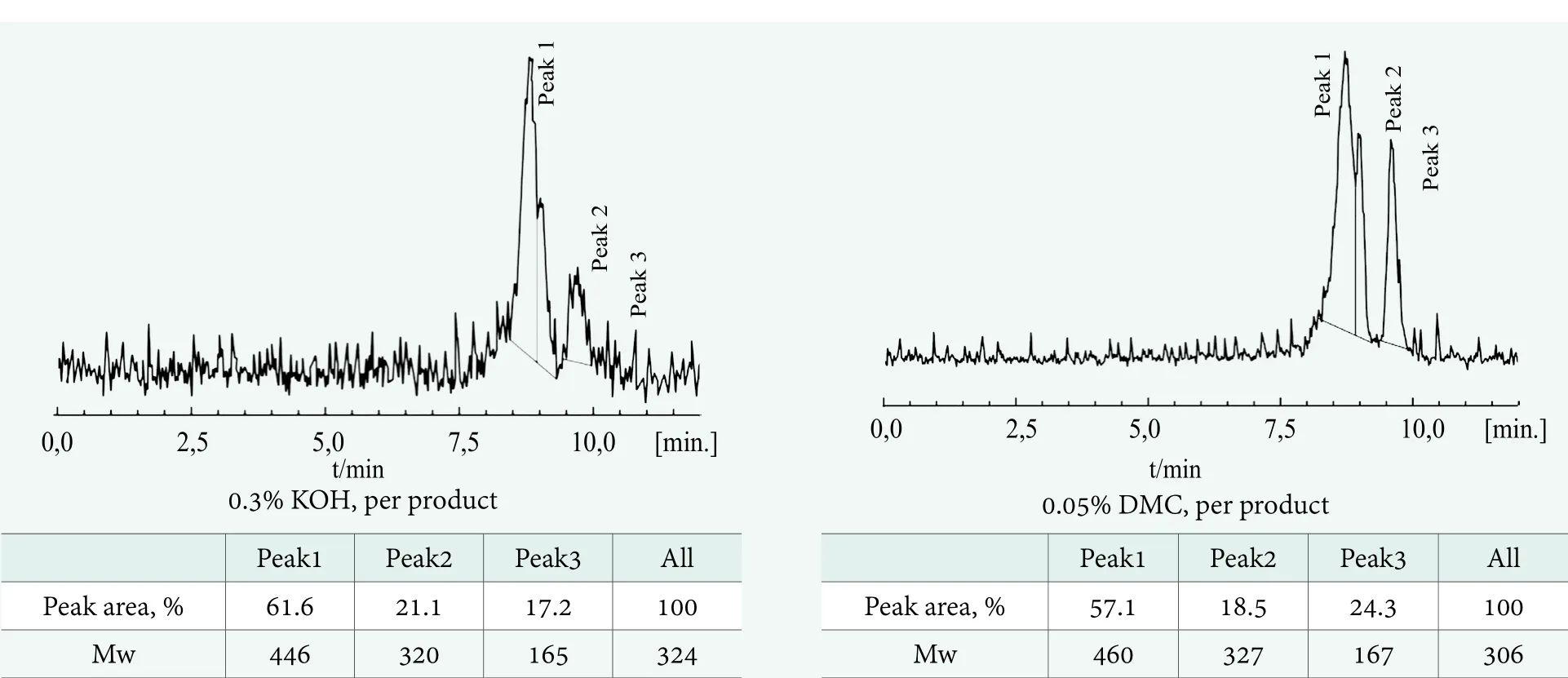
Figure 10. Comparison of GPC chromatograms obtained for the EH-PO1 products in the presence of KOH and DMC catalyst, respectively (Mw - molecular weight)
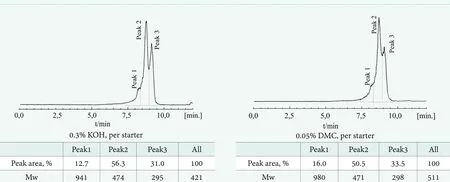
Figure 11. Comparison of GPC chromatograms obtained for the EH-EO1 products in the presence of KOH and DMC catalyst, respectively
Figure 10 and 11 show that additional products or,rather, by-products of much higher molecular weights were found using the GPC technique within the range from up to 460 and 960 Da in the case of polypropoxylates and polyethoxylates, respectively.
Although GPC is not a very quantitative determination in terms of molecular weight content, the products’ average molecular masses can reasonably be interpreted qualitatively.Presumably, they represent the polymers described by Equation (10) and, to some extent, the side products described in Equations (11,12). It is worth mentioning that their total contribution seems higher than that of the target polyadducts of 2-ethylhexanol. This can be explained by a much higher polymerization rate, with two active centers per molecule, in comparison with that of the polyaddition reaction. Such a low selectivity of polyaddition, especially in the presence of KOH, was not reported before for relatively low molecular weights. However, it has not been expected and not detectable by the GC technique, commonly applied in this case.
Conclusions
It was found that the investigated DMC type catalyst is significantly more active in propoxylation and ethoxylation of ethylhexanol, in comparison with KOH. Additionally,it generates a more narrow homolog distribution and more selective products. However, in spite of the target polyaddition, both of the compared catalysts promote parallel polymerization consuming a significant part of the converted alkylene oxides.
Acknowledgment
This work was supported by the National Centre for Research and Development (NCBR): Applied Research Program 2012-2015 (PBS 1), Project No. PBS1/A5/28/2012(EH-SURF).
[1] ECOSURFTM EH. Specialty Surfactants. The DOW Chemical Company 2009, 119-02244-0309 AMS.
[2] BERGSTROM K. An Alkoxylate Mixture and Its Use As A Cleaning Agent for Hard Surfaces. Patent EP 1 519 907 B1, 2003.
[3] HRECZUCH W. et. al. The Method to Obtain Nonionic Surfactant of A Steric Amphiphilic Structure. Patent PL 215601,2011.
[4] CHRU?CIEL A; HRECZUCH W; WOJDY?A H; CZAJA K; JANIK J; DOMARECKA D; DOMARECKI W. The Composition and Method to Obtain An Oxyalkylation Catalyst.Patent Application PL 398518, 2012.
[5] CHRUSCIEL A; HRECZUCH W; JANIK J; CZAJA K;DZIUBEK K; FLISAK Z; SWINAREW A. Characterization of A Double Metal Cyanide (DMC)-Type Catalyst in the Polyoxypropylation Process: Effects of Catalyst Concentration.Ind. Eng. Chem. Res. 2014, 53, 6636-6646.
[6] SZYMANOWSKI J; ATAMA?CZUK B; SZEWCZYK H.Molar Mass Distribution of the Polyoxyethylene Derivatives of Alcohols, Alkylphenols and Alkylamines. J. Chem. Tech.Biotechnol 1987, 40, 1-10.
[7] KHORZOUGHI M; BEIRANVAND M; RASAEI M.Investigation of A New Multiphase ow Choke Correlation by Linear and Non-linear Optimization Methods and Monte Carlo Sampling. J. Petrol Explor. Prod. Technol 2013, 3, 279–285.
[8] NATTA G; MANTICA E. The Distribution of Products in a Series of Consecutive Competitive Reactions., J. Am. Chem. Soc 1952, 74, 3152-3156.
[9] POSKROBKO H; POSKROBKO J; HRECZUCH W;SOBCZY?SKA A; SZYMANOWSKI J. Oxyethylation and Oxypropylation of Alcohols of Low Relative Molecular Mass in the Presence of Amine-type Catalysts. J. Chem. Technol.Biotechnol 2000, 75, 547-552.
[10] NM-CAD proprietary software, developed by MEXEO,K?dzierzyn-Ko?le, Poland.
[11] FLORY P I. Molecular Size Distribution in Ethylene Oxide Polymers. J. Am. Oil Soc. 1940, 62, 1561-1565.
[12] TATIANA S. ARTURI, NOEMI E. ZARITZKY, and EDGARDO M. CONTRERAS, Simple High-Performance Liquid Chromatography-Ultraviolet Method to Quantify the Molecular Size Distribution of Nonylphenol Ethoxylates., Ind.Eng. Chem. Res., Ind. Eng. Chem. Res 2014, 53, 1327-1333.
[13] WEIBULL B; TORNQUIST J. Intern. Kongr. für Grenzflachenaktive Stoffe. Zurich 1972.
[14] RUPP M; RUBACK W; KLEMM E. Alcohol Ethoxylation Kinetics: Proton Transfer Influence on Product Distribution in Microchannels. Chem. Eng. Process 2013, 74, 187-192.
[15] HRECZUCH W; SZYMANOWSKI J. Synthesis of Surfactants with Narrow Range Distribution of Polyoxyethylene Chain J.Am. Oil. Chem. Soc. 1996, 73:73-78.
[16] MATERNA K; TIC W J; SOKOLOWSKI A; HRECZUCH W.C12 Hydroxyester Ethoxylates as Nonionic Surfactants. Centr Eur. J. Chem. 2011, 9, 300-307.
 China Detergent & Cosmetics2016年1期
China Detergent & Cosmetics2016年1期
- China Detergent & Cosmetics的其它文章
- Application of Sensory Evaluation in the Concealer Product Research
- Formaldehyde Donor Preservatives in Cosmetics
- Analysis and Suggestions on Patent Application Status for Traditional Chinese Medicine Cosmetics
- Standardization of Surfactant and Detergent Industry in China
- Current Status of Global Ban on Cosmetic Tests on Animals
- Kitchen Cleaners in China
