Formaldehyde Donor Preservatives in Cosmetics
Xin Qu
ASI Shanghai Technical Center, Ashland Inc., China
Introduction
In China, demand for novel cosmetics is rising along with discretionary income. Marketers often respond to requests for novel products by incorporating more nutrient-rich substances and more active ingredients into finished formulations. The changing composition of cosmetic formulations in China and elsewhere, however,is raising new questions about how to best protect these cosmetic products from microbiological contamination.
Preservatives serve to control the growth of bacteria and to ensure that products remain essentially free of biological contaminants for a long time. A good preservative will also be non-toxic and non-irritant to humans, not react with other components in systems,remain stable in different pH and temperature ranges,and provide sufficient protection against microorganisms before the product expiration date.
More than ever before, consumers value natural and “green” preservation systems, yet these alternative systems have several disadvantages when compared to traditional chemical-synthesized preservatives. For example, batch-to-batch consistency is very difficult to accomplish in a truly natural product, yet consistency is what is required to assure a reliable, fully functional preservative system. Moreover, natural preservatives often have color and odor, and may add to the cost of formulations as formulators work to offset these challenges.[1]Another question formulators is whether or not natural preservatives will be safer than synthetic technologies. Studies conducted on natural preservatives suggest that antibacterial efficacy is less than required at times, and is typically formulation- dependent.[2]
Formaldehyde (FA) donor technologies have been widely used as cosmetic preservatives for decades.These preservatives are classified as safe and effective by the Cosmetic Ingredient Review when used within their permitted use levels.[3]Among these,imidazolidinyl urea was the first formaldehyde donor used as a preservative in cosmetics (beginning in 1973), and today is the second-most used preservative(second only to paraben). With recent restrictions and banning of some types of parabens by European Commission under the European cosmetic regulation,[4]formaldehyde donors are also under debate. In this review, we will discuss the application, regulatory developments and analytic methods for determining FA donor preservatives concentrations in cosmetics applications.
Formaldehyde
At high concentration, FA is known as a toxic substance to humans and the environment, and induces mutation in living organisms. European Commission Regulation (EU) No 605/2014 of 5 June 2014 (6th Adaption to Technical Progress (ATP) to the CLP Regulation) proposed a harmonized classification for FA as follows: Carcinogen category 1b, Mutagen category 2, Acute toxicity 3 (oral, dermal and inhalation), Skin corrosive category 1b and Skin sensitization category 1.The harmonized classification was intended to apply from 1 April, 2015, but was then extended to 1 January 2016 by the 7thATP.

FA is also considered as a colorless, natural metabolic intermediate, and produced by all living cells. Its natural concentration in the blood of humans and mammals is 3 mg/kg. In some cells of the human body,the concentration of FA can reach up to 6-12 mg/kg(Table 1).[5]FA is formed on Earth as a result of photochemical processes from the oxidation of methane in the atmosphere. It is broken down by sunlight and bacteria within a few hours and is quickly decomposed in the human metabolism.[6]In recent years, more people have questioned the safety of FA in cosmetics.These concerns often arise from unscientific, popular rhetoric published on the Internet or in mass market publications. FA and its solutions are rarely used in the cosmetic realm as preservatives. Instead, compounds,called FA donor, that slowly release FA have found widespread use as preservatives. This review places FA donor preservative technology into proper context.
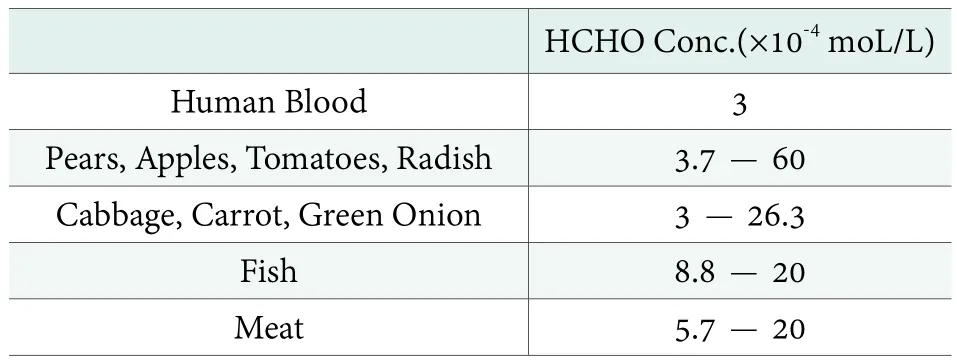
Table 1. FA Conc. in human blood and foods
Formaldehyde donors
As one of the preservation systems first used by humans, FA donors are highly effective broad-spectrum preservatives and have been used widely in industry and agriculture. FA is considered safe and effective at low concentration, or within the usage limit. Free FA releases very slowly from FA donor preservatives in cosmetic products, and efficiently kills bacteria and fungi in cosmetic products. The most common FA donor preservatives in the market are allantoin-based and hydantoin-based products, such as Imidazolidinyl Urea (Ashland Brand: Germall? 115), Diazolidinyl Urea (Ashland Brand: Germall? II) and DMDMH.The molecular structures of these preservatives are shown in Figure 1. Other preservatives, such as sodium hydroxymethylglycinate (Ashland Brand: Suttocide?A), 2-bromo-2-nitropropane-1, 3-diol (Bronopol), and Quaternium 15 (Dowacil 200) etc. are also available in the market. Our discussion will focus on Imidazolidinyl Urea and Diazolidinyl Urea, but most of measurements and applications apply to all of the above classes of preservatives.
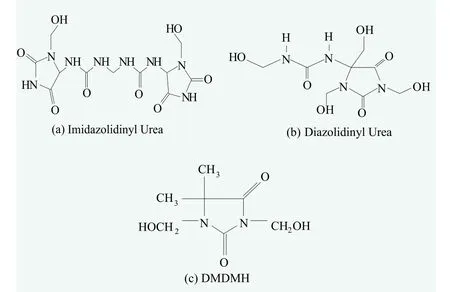
Figure 1. The structure of most common FA donor preservatives
FA donor will release free FA in aqueous solutions,whereby the majority of the released FA exists as methylene glycol (MG) in water; the others include a series of low molecular weight polyoxymethylene glycols.There are two equilibriums,[7]as shown in Figure 2. The first equilibrium is between methylene glycol (MG) and free FA. The equilibrium constant is about 2×103 at 25℃, which means 99.96% of MG and 0.04% free FA in aqueous solution.[8]European and other international bodies (SCCS 2012, ACC 2010, CIR 2012, OSHA 2010) have concluded that MG is an FA equivalent,consequently all recommendations on maximum use levels will include free FA and MG in the calculations.
The other equilibrium is between FA donor and free FA. If the donor has additional methylols, it can continue to release further free FA until the equilibrium has been established. The above equilibrium is greatly dependent on solution pH, temperature, and FA donor concentration. Changes in solution conditions will shift the equilibrium, as reported in literature.[9]Consumers tend to confuse FA donor with free FA and methylene glycol in cosmetic products,which are completely different substances, as demonstrated in various test methods and evaluations.A general misunderstanding of the composition of these different chemicals largely results in unnecessary concern about FA donor preservatives in the public domain.

Figure 2. Aqueous solution equilibrium of FA donors(K[H2O]25℃ = 2×103)
For FA donor, different counties and regions have defined the maximum use levels as shown in Table 2.
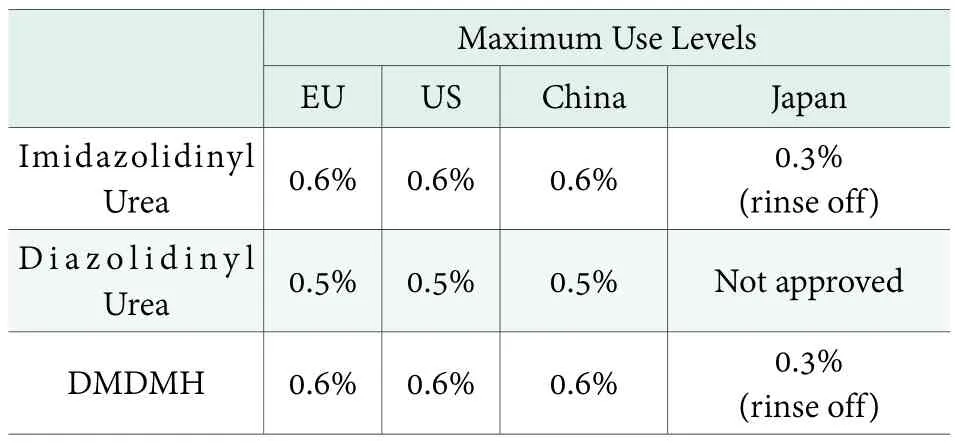
Table 2. Use levels of FA Donor Preservatives in different countries and regions
Determination of free FA concentration
Free FA released by FA donor preservatives has equilibrium with methylene glycol in aqueous solutions.The consideration is determining what type of FA (i.e.,free, available, total) is being measured, and how the equilibrium is affected by the experiment, particularly by sample preparation. The FA determination methods could be divided into two categories: methods that perturb equilibrium (chemical, HPLC, and titration),and a method that does not perturb equilibrium(C-13 NMR method). In the first category,equilibrium will be disturbed by adding specific reagents, followed by procedures such as dilution. These methods could quantify the total amount of released free FA in the system, which is a good evaluation of longterm preservative efficacy. The C-13 NMR method can determine the true free FA at the desired concentration, pH, and temperature without disturbing the equilibrium. This method will reflect the free FA concentration under real conditions, and the results could illustrate the safety of FA donor preservatives in cosmetics.
(I) Methods that perturb equilibrium

The chemical methods for FA analysis are batch type analysis consisting of derivatization of “available” FA by reagents such as acetylacetone (Nash reagent) and 1, 3-dihydroxynaphthalene (DHN). In addition to the initial free FA, this method also derivatizes the bound FA that is available to be unbound. The derivatization of the unbound, free FA forces the equilibrium to be reestablished, that is, additional available bound FA is freed and subsequently derivatized. The most common method involves acetylacetone (AA) as the derivatizing reagent, which reacts to form a yellow-colored lutidine compound. The amount of lutidine is determined by measuring the UV spectrum and quantified by comparison with FA external standards.
Currently, two methods, European (EEC), and Japanese methods are used in practice. The difference between them is in the temperature at which the derivatization is performed and the duration of the reaction. The Japanese method indicates 40℃ for 30 minutes while European requires 60℃ for 10 minutes.The two methods give similar values for total FA, with those of the Japanese method being slightly lower, as shown in Table 3. Of the 43% theoretical total FA of Diazolidinyl Urea, only 20.6% is available as measured by the Nash reagent method; whereas, with 23.2%theoretical FA of Imidazolidinyl Urea, only 9.4% is available and measured by the Nash Reagent Method.
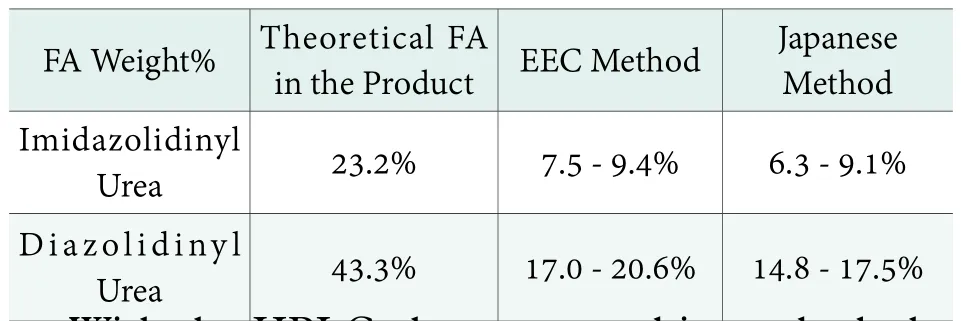
Table 3. Mass fraction of FA found based on solid product
With the HPLC chromatographic method, the free FA in the product will separate from all other components, including FA released during the analysis,and be derivatized, detected, and quantified in postcolumn derivatization[10]. This method has been adopted by the European Commission (EC), the administrative arm of the European Union (EU). However, this method still has several serious shortcomings because it does not consider the effects of sample preparation on the equilibrium, such as the effect of the chromatographic mobile phase. None of these methods could give the accurate results of free FA amount due to a disturbance of the equilibrium that result from testing.
(II)Method that not perturb equilibrium
C-13 NMR is first used to study the equilibrium of FA solution in 1994.[11]It is the only method available that does not disturb the equilibrium, and therefore the only method to detect the accurate amount of free FA and MG in cosmetics products.[12]The amount of methylene glycol was determined by Ashland using this method, and the free FA amount was calculated according to the chemical equilibrium (Methylene Glycol amount ×0.04%) in Figure 2. The results are shown in Table 4. In cosmetic products, the equilibrium between MG and free FA will be affected by pH,temperature etc.; the 0.04% ratio thus only gives a rough estimation. Tallon et al. recently used C-13 NMR to study the pH & temperature influences on the stability of FA donor in cosmetic products. An increase in temperature (25℃ to 60℃) and pH (5.5 to 8.5) resulted in an increase of Methylene Glycol and subsequently free FA, but the level of free FA in imidazolidinyl urea solutions was the lowest. After one month of heat-aging at 25℃, 40℃ and 60℃, the free FA level in moisturizing lotion slightly declined, while DMDM Hydantoin increased over time, suggesting it was less stable in the facial cleanser.
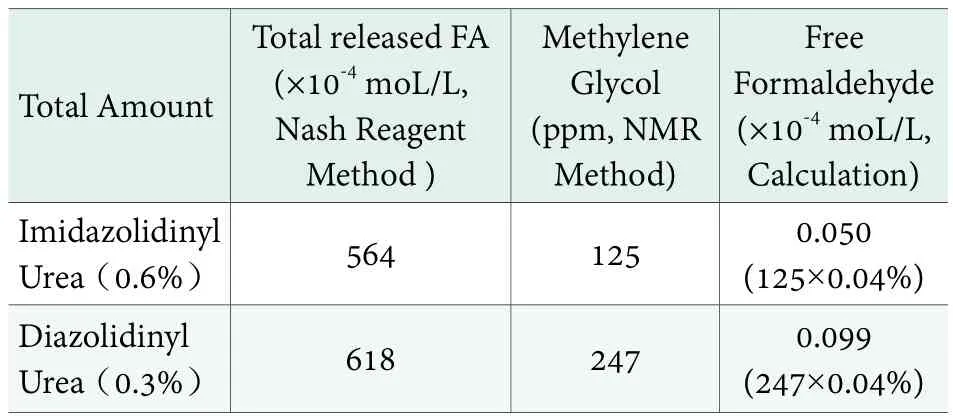
Table 4. Determination of free FA concentration in finished formulations (Non-ionic Moisturizing lotion,pH5.9, 25℃)
In real cosmetic systems, the MG is in liquid form,while the free FA amount is extremely low (below 0.2ppm) as shown in Table 4. Human exposure to FA in gaseous form predominantly occurs via inhalation.Lefebvre[13]measured consumer exposure to FA from personal care products (PCP)containing FA donor preservatives. The data concluded that inhalation exposure to FA emissions from the use of PCP containing FA donors represents an insignificant fraction of the total human FA exposure from other sources and therefore poses no or negligible risk to human health.
Conclusions
In summary, FA donor preservatives have been used safely in cosmetics for decades, and their efficacy has been widely demonstrated.At the concentrations used in cosmetics, FD preservatives have been deemed safe (i.e., non-toxic,non-carcinogenic, non-mutagenic, and not a concern for systemic toxicity). By using C-13 NMR methods to detect the free FA amount, and analyze the equilibrium in systems, we can conclude that free FA is far less than the maximum regulated use level in cosmetic products- when FA donor preservative is added within regulated levels. FA donor preservatives can prevent decay in cosmetic products while remaining safe to consumers.The mechanism of cosmetic product preservation by FA donors is not the same as those by FA itself, and accordingly, the concentrations of FA in cosmetic products preserved with FA donors cause no safety concerns.
Acknowledgements
The author would like to acknowledge Dr. Karen Winkowski and Louise Olivier of Ashland Specialty Ingredients for their valuable suggestions and discussions of this article.
[1] T. Branna. Preservative Market Update.Happi 2008, 5,109-116.
[2] K. Roden. Natural Preservatives: Myth or Magic?. Personal Care Asia Pacific 2008. 11,39-43.
[3] M. Lefebvre; W. J. A. Meuling; R. Engel,et al.. Consumer Inhalation Exposure to Formaldehyde from the Use of Personal Care Products/Cosmetics. Regulatory Toxicology and Pharmacology 2012,63,171—176.
[4] EU Cosmetic Regulation (EC) No 1223/2009 (Amended),No 1003/2014, and No 1004/2014.
[5] H. A. Heck; M. Casanova.The Implausibility of Leukemia Induction by Formaldehyde: A Critical Review of the Biological Evidence on Distant-Site Toxicity. Regulatory Toxicology and Pharmacology 2004, 40,92-106.
[6] V. J. Feron; et al. Aldehydes: Occurrence, Carcinogenic Potential, Mechanism of Action and Risk Assessment.Mutation Research 1990, 259, 363-385.
[7] H. R. Gerberich; G. C. Seaman.Formaldehyde. Kirk-Othmer Encyclopedia of Chemical Technology 1994, 11, 929-951.
[8] J. Winkleman; M. Vookwinde; M. Ottens et al.. Kinetics and Chemical Equilibrium of the Hydration of Formaldehyde.Chem. Engin. Sci. 2002, 57,4067-76.
[9] M. Tallon; K. Winkowski; A. Wingenfeld. Free Formaldehyde Preservative Stability in Personal Care. Cosmetics &Toiletries 2015, 9,31-37.
[10] K. Kijima; M. Takeda;et al. A Study on Release of Formaldehyde From Its-Donor Type Preservatives.Analytical Sciences 1991,7.
[11] I. Hahnenstein; H. Hasse; C. G. Kreiter. 1H-and 13C-NMRSpectroscopic Study of Chemical Equilibria in Solutions of Formaldehyde in Water, Deuterium Oxide, and Methanol.Ind. Eng. Chem. Res. 1994, 33 (4),1022—1029.
[12] M. Tallon; JJ Merianos; S. Subramanian. Non-Destructive Method for Determining the Actual Concentration of Free Formaldehyde in Personal Care Formulations Containing Formaldehyde-Donors. S?FW 2009,135,22-32.
[13] M. A. Lefebvre;W. J. A. Meuling; R. Engel et al.. Consumer Inhalation Exposure to Formaldehyde from the Use of Personal Care Products/Cosmetics. Regulatory Toxicology and Pharmacology 2012 ,63,171-176.
 China Detergent & Cosmetics2016年1期
China Detergent & Cosmetics2016年1期
- China Detergent & Cosmetics的其它文章
- Application of Sensory Evaluation in the Concealer Product Research
- Investigation of Propoxylation and Ethoxylation of 2-Ethylhexanol in the Presence of an Alkaline and DMC Type Catalyst at Initial Stages of the Syntheses
- Analysis and Suggestions on Patent Application Status for Traditional Chinese Medicine Cosmetics
- Standardization of Surfactant and Detergent Industry in China
- Current Status of Global Ban on Cosmetic Tests on Animals
- Kitchen Cleaners in China
