Effects of aging on working memory performance and prefrontal cortex activity: A time‐resolved spectroscopy study
Jie Shi, Wenjing Zhou, Tongchao Geng, Huancong Zuo, Masahiro Tanida, Kaoru Sakatani(?)
?
Effects of aging on working memory performance and prefrontal cortex activity: A time‐resolved spectroscopy study
Jie Shi1, Wenjing Zhou1, Tongchao Geng2, Huancong Zuo1, Masahiro Tanida3, Kaoru Sakatani4(?)
1Department of Neurosurgery, Tsinghua University Yuquan Hospital, Beijing 100040, China
2Department of Neurology, Tsinghua University Yuquan Hospital, Beijing 100040, China
3Shiseido Research Center, Shin‐Yokohama 224‐8558, Japan
4NEWCAT Research Institute, Nihon University College of Engineering, Fukushima 963‐8642, Japan
ARTICLE INFO
Received: 6 January 2016
Revised: 18 February 2016
Accepted: 19 February 2016
? The authors 2016. This article is published with open access at www.TNCjournal.com
KEYWORDS
aging;
working memory;
prefrontal cortex;
time‐resolved spectroscopy
ABSTRACT
Objective: This study aimed to employ time‐resolved spectroscopy (TRS) to explore age‐related differences in prefrontal cortex (PFC) activity while subjects performed a working memory task.
Methods: We employed TRS to measure PFC activity in ten healthy younger and ten healthy older subjects while they performed a working memory (WM) task. All subjects performed the Sternberg test (ST) in which the memory‐set size varied between one and six digits. Using TRS, we recorded changes in cerebral blood oxygenation as a measure of changes in PFC activity during the task. In order to identify left/right asymmetry of PFC activity during the working memory task, we calculated the laterality score, i.e., Δoxy‐Hb (right Δoxy‐Hb—left Δoxy‐Hb); positive values indicate greater activity in the right PFC, while negative values indicate greater activity in the left PFC. Results: During the ST, statistical analyses showed no significant differences between the younger and older groups in accuracy for low memory‐load and high memory‐load. In high memory‐load tasks, however, older subjects were slower than younger subjects (P < 0.05). We found that the younger group showed right lateral responses with a stronger right than left activation in the frontal pole, whereas the older group showed bilateral responses (P < 0.05).
Conclusions: The present results are consistent with the hemispheric asymmetry reduction in older adults (HAROLD) model; working memory tasks cause asymmetrical PFC activation in younger adults, while older adults tend to show reduced hemispheric lateralization.
Citation Shi J, Zhou WJ, Geng TC, Zuo HC, Tanida M, Sakatani K. Effects of aging on working memory performance and prefrontal cortex activity: A time‐resolved spectroscopy study. Transl. Neurosci. Clin. 2016, 2(1): 3–7.
? Corresponding author: Kaoru Sakatani, E-mail: sakatani.kaoru@nihon-u.ac.jp Supported by the Strategic Research Foundation Grant-aided Project for Private Universities (No. S1411017) and a Grant-in-Aid for Exploratory Research (No. 25560356) from the Ministry of Education, Culture, Sports, Sciences, and Technology of Japan.
1 Introduction
Near‐infrared spectroscopy (NIRS), a noninvasive optical method for brain function imaging, measures changes of oxygenated hemoglobin (oxy‐Hb) and deoxygenated hemoglobin (deoxy‐Hb) concentration in cerebral tissue. The basis of measuring neural activity with NIRS relies on neurovascular coupling. NIRS presents several advantages such as the resistance to motion artifacts, a natural environment without restraint or sedation, and a relatively high temporal resolution. NIRS has been widely applied for researchand clinical purposes in various areas. Time‐resolved spectroscopy (TRS) is a recently developed NIRS system that can detect quantitative hemodynamics and absolute values of cerebral blood volume (CBV).
Working memory (WM) tasks are associated with asymmetrical activation in the prefrontal cortex (PFC) of younger adults, while older adults tend to exhibit reduced hemispheric lateralization (i.e., reduced asymmetry). This phenomenon is called hemispheric asymmetry reduction in older adults (HAROLD); prefrontal activity during cognitive performances tends to be less lateralized in older adults than in younger adults[1]. Employing two‐channel TRS, Sakatani et al.[2]recently reported that the reduction of asymmetry could occur in individuals less than fifty years old. These results are consistent with results obtained by PET[3]. Notably, two‐channel TRS is more compact and less expensive than PET or fMRI. Additionally, TRS measurements can be made in a more natural environment where head restraint is not required. If the results obtained by TRS are reproducible, it could be a useful tool for cognitive dysfunction screening tests.
In the present study, we investigated whether asymmetry reduction occurring in individuals less than fifty years old is also detectable with NIRS in Chinese subjects living in China. We measured evoked cerebral blood oxygenation (CBO) changes in the PFC during performance of the Sternberg test (ST) with TRS and evaluated the relation between changes in oxy‐Hb and reaction time and accuracy in the test.
2 Materials and methods
2.1 Subjects
We studied ten young Chinese women (mean age of 21.7 ± 0.9 years old) and ten middle‐aged Chinese women (mean age of 47.2 ± 1.3 years old). All subjects were healthy, right‐handed (according to Edinburgh Handedness Inventory), and had normal or corrected‐to‐normal vision. The two groups were education‐matched. All subjects provided written informed consent, as required by the Human Subjects Committee of Yuquan Hospital of Tsinghua University School of Medicine in Beijing.
2.2 Working memory task
As a working memory task, we employed a modified ST that was previously used in our NIRS experiments[2]. In the ST, subjects were asked to remember one digit and six digits in turns. There were eight 1‐digit trials and eight 6‐digit trials. Each trial began with the presentation of one digit or a set of six digits to be encoded for 1 second on a CRT. A blank display was then inserted for 2 seconds, followed by the test digit until a response was obtained (up to 2 seconds). Subjects were required to press the right button if they thought the test digit was contained within the encoded stimulus and to press the left one if it was not.
2.3 TRS measurements
We measured CBO in the bilateral PFC with a two‐channel NIRS monitor which uses time‐resolved reflectance spectroscopy (TRS‐20, Hamamatsu Photonics K.K., Hamamatsu, Japan)[4]. The NIRS probes were set symmetrically on the forehead so that the midpoint between the emission and detection probes was 3 cm above the centers of the upper edges of the bilateral orbital sockets. The distance between the emitter and the detector was 4 cm. MRI confirmed that the emitter‐detector was located over the dorsolateral and frontopolar areas of the PFC[5]. The concentrations of oxy‐Hb, deoxy‐Hb, and total Hb (t‐Hb = oxy‐Hb + deoxy‐Hb) were expressed in μM.
2.4 Data analysis
We monitored Hb concentration changes in the bilateral PFC and calculated average values every second during: (1) baseline conditions for 60 seconds; (2) the ST for 60‐80 seconds (the period varied according to each subject’s reaction time); and (3) the recovery for 60 seconds. To analyze PFC activity in response to WM performance, we calculated changes in oxy‐Hb concentration during the ST. The mean baseline values (measured during the last 30 seconds) were subtracted from the mean activation values (during the first 60 seconds under task performance).
In order to evaluate laterality of PFC activity, we calculated the laterality score of Δoxy‐Hb (i.e., right Δoxy‐Hb – left Δoxy‐Hb); positive values indicate rightdominant activity while negative values indicate left dominant activity. We used Pearson’s correlation for analysis of the laterality score and reaction time, and we used Spearman’s rank correlation for analysis of the psychological tests.
3 Results
Most subjects in each group showed a typical oxygenation pattern: oxy‐Hb increases and deoxy‐Hb decreases during the task period. However, two subjects in the young group and two subjects in the middle‐aged group showed a deactivation response of oxy‐Hb during the ST task, i.e., a negative Δoxy‐Hb and/or positive Δdeoxy‐Hb. Since the mechanism underlying this inverse oxygenation response has not been clarified, the data from these four subjects were excluded from analysis. Another subject’s data in the middle‐aged group were also excluded from analysis because of a failed response in the ST task. Therefore, the data of eight subjects in the young group and seven subjects in the middle‐aged group were included in the analysis.
Table 1 summarizes the ST task performance of the young and middle‐aged groups. There was no significant difference in accuracy between the two groups. However, the middle‐aged group exhibited a significantly slower reaction time in high memory‐load tasks than the young group (P < 0.05).
TRS allowed for the measurement of absolute oxy‐and deoxy‐haemoglobin baseline concentrations. Table 2 shows the absolute concentrations of hemoglobin during the 30‐second baseline before the working memory task in the right and left PFC of both groups.

Table 1 Task performance of young and middle‐aged groups in the Sternberg test
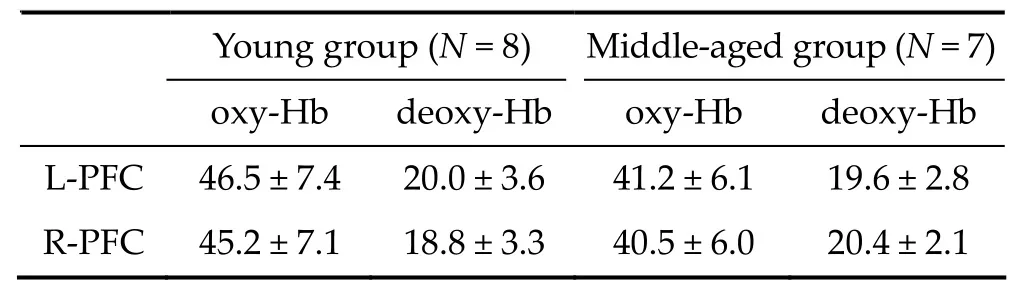
Table 2 Baseline concentration (μM) of hemoglobin in the right (R‐PFC) and left prefrontal cortex (L‐PFC) in the young and middle‐aged groups
TRS demonstrated increases of oxy‐Hb associated with a decrease of deoxy‐Hb during the performance of the ST in the bilateral PFC of both groups. Figure 1 shows the average changes in NIRS parameters in the young and middle‐aged groups. Interestingly, the young group exhibited a right‐dominant increase of oxy‐Hb, whereas the middle‐aged group showed similar increases of oxy‐Hb in the bilateral PFC. There was a significant difference in the laterality score of Δoxy‐Hb between the young (0.22 ± 0.38) and middle‐aged (–0.16 ±–0.22) groups (P < 0.05).
Table 3 summarizes Δoxy‐Hb in bilateral PFC and the laterality score in the two groups. There was no significant difference in Δoxy‐Hb between the left and right side of the two groups. However, there was a significant difference in the laterality score of Δoxy‐Hb between the young (0.22 ± 0.41) and middle‐aged(–0.16 ± 0.23) groups (P < 0.05).

Table 3 Changes of oxy‐Hb and laterality score in the right (R) and left (L) prefrontal cortex in the young and middle‐aged groups
4 Conclusions
We found differences between the young and middle‐aged groups in PFC activation patterns that were induced by the ST; the middle‐aged group showed bilateral activation, while the young group exhibited right‐dominant activation. These findings observed in Chinese women are consistent with the results obtained in a study on Japanese women[2].
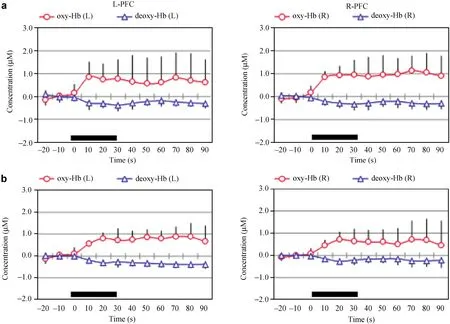
Figure 1 Changes of NIRS parameters during the Sternberg test in young (a) and middle‐aged groups (b). Red and blue lines indicate Δoxy‐Hb and Δdeoxy‐Hb, respectively. Thick bars indicate task periods.
It has been reported that working memory tasks cause asymmetrical PFC activation in younger adults, while older adults tend to exhibit a reduction in hemispheric lateralization (i.e., reduced asymmetry); the reduction of asymmetry in PFC activity during aging is known as HAROLD[1]. This reduction of hemispheric asymmetry in older adults can also be explained by the compensation‐related utilization of neural circuits hypothesis (CRUNCH) model[6]. Interestingly, right‐lateralized PFC activity is associated with a more successful cognitive performance[7]. Recently, employing TRS, Sakatani et al. demonstrated that administration of Ginkgo biloba leaves improved working memory function in a group of middle‐aged subjects. This was concomitant with changes of the PFC activation pattern from bilateral activation to a right‐dominant activation pattern, which resembles the pattern observed in young subjects[2]. Additionally, there were significant positive correlations between the changes of reaction time and the laterality index (i.e., the more the laterality index increased, the shorter the reaction time), suggesting that Ginkgo biloba might improve working memory function in middle‐aged subjects by counteracting the occurrence of HAROLD.
Finally, potential limitations of the present NIRS study should be noted. For example, it has been demonstrated that the scalp related hemodynamic changes locked into the functional activation tasks[8]. This suggests that extracranial blood flow might affect the present results, although the extracranial blood flow cannot explain the present NIRS data completely since oxy‐Hb increased in the bilateral PFC during the cognitive task consistently.
Additionally, it was reported that CO2‐reactivity declined significantly from the 4thto the 5thdecades in women, but not in men[9]. Such age‐dependentdifferences in CO2‐reactivity might also affect the present results. Further studies are necessary to clarify these issues.
Acknowledgments
It was supported through grants from Iing Co., Ltd. (Tokyo, Japan), Alpha Electron Co., Ltd. (Fukushima, Japan), NJI Co., Ltd. (Fukushima, Japan), and the Southern Tohoku General Hospital (Fukushima, Japan).
Conflicts of interest
The authors have no financial interests to disclose regarding the article.
References
[1] Cabeza R. Hemispheric asymmetry reduction in older adults: The HAROLD model. Psychol Aging 2002, 17(1): 85–100.
[2] Sakatani K, Tanida M, Hirao N, Takemura N. Ginkobiloba extract improves working memory performance in middleaged women: Role of asymmetry of prefrontal cortex activity during a working memory task. Adv Exp Med Biol 2014, 812: 295–301.
[3] Dixit NK, Gerton BK, Kohn P, Meyer-Lindenberg A, Berman KF. Age-related changes in rCBF activation during an N-back working memory paradigm occur prior to age 50. NeuroImage 2000, 11(5 Suppl): S94.
[4] Oda M, Yamashita Y, Nakano T, Suzuki A, Shimizu K, Hirano I, Shimomura F, Ohmae E, Suzuki T, Tsuchiya Y. Near-infrared time-resolved spectroscopy system for tissue oxygenation monitor. SPIE 2000, 4160: 204–210.
[5] Tanida M, Katsuyama M, Sakatani K. Relation between mental stress-induced prefrontal cortex activity and skin conditions: A near-infrared spectroscopy study. Brain Res 2007, 1184: 210–216.
[6] Berlingeri M, Danelli L, Bottini G, Sberna M, Paulesu E. Reassessing the HAROLD model: Is the hemispheric asymmetry reduction in older adults: A special case of compensatory-related utilisation of neural circuits? Exp Brain Res 2013, 224(3): 393–410.
[7] Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends Cogn Sci 2004, 8(4): 170–177.
[8] Kirilina E, Jelzow A, Heine A, Niessing M, Wabnitz H, Brühl R, Ittermann B, Jacobs AM, Tachtsidis I. The physiological origin of task-evoked systemic artefacts in functional near infrared spectroscopy. NeuroImage 2012, 61(1): 70–81.
[9] Kastrup A, Dichgans J, Niemeier M, Schabet M. Changes of cerebrovascular CO2reactivity during normal aging. Stroke 1998, 29(7): 1311–1314.
 Translational Neuroscience and Clinics2016年1期
Translational Neuroscience and Clinics2016年1期
- Translational Neuroscience and Clinics的其它文章
- Malignant transformation and treatment of cystic mixed germ cell tumor
- Surgical complications secondary to decompressive craniectomy for patients with severe head trauma
- A new drainage tube device
- An association between the location of white matter changes and the behavioral and psychological symptoms of dementia in Alzheimer’s disease patients
- Effects of regional cerebral blood flow perfusion on learning and memory function and its molecular mechanism in rats
- Repairing skull defects in children with nano-hap/collagen composites: A clinical report of thirteen cases
