Supersaturation induced by Itraconazole/Soluplus?micelles provided high GI absorption in vivo
Department of Pharmaceutics,Shenyang Pharmaceutical University,Wen Hua Road No.103,Shenyang 110016,China
Supersaturation induced by Itraconazole/Soluplus?micelles provided high GI absorption in vivo
Yue Zhong,Guanghui Jing,Bin Tian,Hao Huang,Yuanyuan Zhang, Jingxin Gou,Xing Tang,Haibing He*,Yanjiao Wang*
Department of Pharmaceutics,Shenyang Pharmaceutical University,Wen Hua Road No.103,Shenyang 110016,China
A R T I C L EI N F O
Article history:
Received 22 May 2015
Accepted 23 July 2015
Available online 2 September 2015
Solid dispersion
Itraconazole tablets
Hot melt extrusion
Supersaturation
Micelles
Bioavailability
To investigate the effect of supersaturation induced by micelle formation during dissolution on the bioavailability of itraconazole(ITZ)/Soluplus?solid dispersion.Solid dispersions prepared by hot melt extrusion(HME)were compressed into tablets directly with other excipients.Dissolution behavior of ITZ tablets was studied by dissolution testing and the morphology of micelles in dissolution media was studied using transmission electron microscopy(TEM).Drug transferring from stomach into intestine was simulated to obtain a supersaturated drug solution.Bioavailability studies were performed on the ITZ tablets and Sporanox?in beagle dogs.The morphology of micelles in the dissolution media was observed to be spherical in shape,with an average size smaller than 100 nm.The supersaturated solutions formed by Soluplus?micelles were stable and no precipitation took place over a period of 180 min.Compared with Sporanox?,ITZ tablets exhibited a 2.50-fold increase in the AUC(0–96)of ITZ and a 1.95-fold increase in its active metabolite hydroxyitraconazole(OHITZ)in the plasma of beagle dogs.The results obtained provided clear evidence that not only the increase in the dissolution rate in the stomach,but also the supersaturation produced by micelles in the small intestine may be of great assistance in the successful development of poorly water-soluble drugs.The micelles formed by Soluplus?enwrapped the molecular ITZ inside the core which promoted the amount of free drug in the intestinal cavity and carried ITZ through the aqueous boundary layer(ABL),resulting in high absorption by passive transportation across biological membranes.The uptake of intact micelles through pinocytosis together with the inhibition of P-glycoprotein-mediated drug effux in intestinal epithelia contributed to the absorption of ITZ in the gastrointestinal tract.These results indicate that HME with Soluplus?,which can induce supersaturation by micelleformation,may be of great assistance to the successful development of poorly watersoluble drugs.
?2016 The Authors.Production and hosting by Elsevier B.V.on behalf of Shenyang Pharmaceutical University.This is an open access article under the CC BY-NC-ND license
(http://creativecommons.org/licenses/by-nc-nd/4.0/).
1.Introduction
With an increasing number of active pharmaceutical ingredients(APIs)belonging to BCS II,aqueous solubility has become a critical issue since BCS II APIs must frst dissolve in the gastrointestinal fuid prior to being absorbed to exert a therapeutic effect.As a result,increasing the solubility of poorly watersoluble APIs is of major importance.
ITZ is a typical orally active,broad-spectrum,triazole antifungal drug belonging to BCS II and exhibits low oral bioavailability due to its poor aqueous solubility[1].The solubilities of ITZ in simulated gastric fuid(SGF)at pH 1.2,deionized water and simulated intestinal fuid(SIF)at pH 6.8 were about 3.9±0.7,0.002 and 0.003 μg/ml,respectively[2].As a result,the bioavailability of crystalline ITZ after oral administration is far below that of Sporanox?[3].Several investigations have reported that the poor bioavailability of ITZ was due to its poor solubility and rapid drug precipitation during dissolution[4,5]. As a result,enhancing the apparent solubility and dissolution rate of ITZ is necessary to improve its oral bioavailability.
Since drugs in a state of supersaturation are metastable,or kinetically soluble in solution at a concentration above their thermodynamic equilibrium solubility,formulation technologies that can induce supersaturation may be of great assistance to the successful development of poorly water-soluble drugs [6].Solid dispersion is one of the most promising strategies to improve solubility and bioavailability of poorly water-soluble drugs by dispersing the poorly soluble drugs as an amorphous state in the hydrophilic polymers[7].
Polymeric micelles are formed by self-assembly of amphiphilic polymers,with a hydrophobic core as a reservoir for lipophilic drugs[8].Depending on the physico-chemical properties of the drug as well as those of the polymer chains forming the micellar structure,the core of PMs is capable of solubilizing considerable amounts of drug molecules which otherwise would precipitate in the aqueous fuids of the GI tract.They can increase the bioavailability of poorly water-soluble drugs and provide an increasingly popular way to improve the oral absorption of poorly water-soluble APIs[9].
Tablets account for more than 80%of all dosage forms administered to humans.The principal reasons for their continued popularity include their ease of manufacture,convenience of dosing,and stability compared with other dosage forms[10]. The oral route is often preferred for drug administration,due to its acceptability and convenience.Also,it is much safer compared with other routes of administration.In this work,the main beneft of tableting was to protect the stability of the solid dispersions.The direct compression method was preferred due to its convenience and effciency,because no special manufacturing facilities or granulation processes were required for the preparation of tablets.These tablets,made by a low-cost direct compression method,can disintegrate rapidly when in contact with gastric fuid in the stomach and have suffcient mechanical integrity to withstand transportation and storage without any substantial structural damage.
The preparation of Sporanox?involved dissolving the drug and hydroxypropyl cellulose completely in a mixed solution of dichloromethane and denatured ethanol,and then spraying the solution onto the surface of sugar spheres in a fuidizedbed granulator.The pellets were then coated with polyethylene glycol 20,000 in methylene chloride and denatured ethanol solution.The ITZ in the fnal product was in an amorphous form to achieve the required oral bioavailability[3,11,12].
The frst objective of this study was to investigate the stability and dissolution behavior of ITZ tablets.The second objective was to investigate the supersaturation induced by the micelles in simulated gastrointestinal fuid tract which produced an increase in the extent and routes of absorption that contributed to the high bioavailability of ITZ compared with Sporanox?.
2.Materials and methods
2.1.Materials
ITZ(purity 99.4%)was purchased from Shandong Shouguang Pharmaceutical Company(Shandong,China).Soluplus?was kindly provided by BASF(Shanghai,China).Sporanox?capsules(lot number:120221224)were obtained from Xian Janssen Pharmaceutical Ltd.(Xian,China).ITZ and the active metabolite,OH-ITZ(purity more than 99%),were purchased from J&K Scientifc Ltd.(Shanghai,China)and used as reference substances.All reagents were of analytical or chromatographic grade.The beagle dogs used forin vivoevaluation were purchased from the Military Medical Science Research Institute (Beijing,China).The experimental protocol was evaluated and approved by the University Ethics Committee for the use of experimental animals and conformed to the Guide for Care and Use of Laboratory Animals.
2.2.Sample preparation
2.2.1.Preparation of the tablets
The ITZ tablets were prepared by hot melt extrusion(HME)with direct compression.ITZ/Soluplus?solid dispersions were prepared by HME using a co-rotating twin-screw extruder(Coperion Keya Co.,China).Physical mixtures were mixed uniformly in a mixer prior to the extrusion.The temperatures of four zones were set at 140,160,160,and 160°C,separately.Feeding and extrusion rates were both carried out at 3.5 Hz.The drug content in the solid dispersions was 40%(w/w).
The milled extrudates and other excipients were placed in a mixing machine to obtain a homogeneous powder.Theamounts of the extrudates,microcrystalline cellulose(MCC), croscarmellose sodium,talc and magnesium stearate were 50%, 42.5%,5%,2%and 0.5%,respectively.The mixed powders were placed in the hopper of the rotary tablet machine and then compressed into tablets.The hardness of the tablets was between 100 and 120 N to meet the demands of transportation and storage conditions throughout the whole experiment.Each of the tablets contained approximately 100 mg ITZ.
2.3.Characterization in vitro
2.3.1.Differential scanning calorimetry
Differential scanning calorimetry(DSC)measurements were performed using a DSC 1 instrument(Mettler Toledo,Switzerland)equipped with a refrigerated cooling system.Each sample (approximately 4 mg,accurately weighed)was placed in hermetically sealed aluminum pans and then heated over a temperature range of 20–200°C at a heating rate of 10°C/min in an atmosphere of nitrogen.A pin hole was made in the lid to allow the escape of moisture.
2.3.2.HPLC analysis for ITZ
An HPLC method was used to quantifying ITZ.Data were collected and integrated using EZChrom Elite?software.The column was Thermo?Hyprsil BDS 3 μm C18,100×4.6 mm (Thermo?,USA).An isocratic solvent system consisting of 40:60 (v/v)acetonitrile–deionized water containing 0.02 mol/l tetrabutylammonium hydrogen sulfate was used at a fow rate of 1 ml/min.The injection volume is 10 μl.The peaks were detected at a wavelength of 225 nm(λmax).The retention time of ITZ was approximately 8 min.The HPLC assay for related substances was performed according to the USP35.
2.3.3.Dissolution testing of tablets
Dissolution tests were performed under non-sink conditions according to the USP 35 method 2 using a ZRS-8G dissolution test system(Tianda Tianfa,China).In order to investigate the dissolution of the tablets,each of the tablets(equivalent to 100 mg ITZ)was directly added to a series of different dissolution media at a temperature of 37°C with a paddle rotation speed of 75 rpm.Then,six-milliliter samples were removed and immediately replaced with fresh dissolution medium at 5,10, 20,30,45,and 60 min.Samples were passed through 0.22 μm Millipore flter membranes.After discarding the frst 2 ml,the fltrates were analyzed using HPLC.Analyses were carried out in triplicate.
2.3.4.Determination of critical micelle concentration
The critical micelle concentration(CMC)was determined by a fuorescence probe technique using pyrene as a fuorescence probe.
To prepare sample solutions,100 μl pyrene(approximately 1×10?3mol/l)in acetone were added to each of a series of 10 ml volumetric fasks and acetone was subsequently removed by evaporation under nitrogen gas fow,followed by vacuum drying for 2 h to evaporate the residual acetone at room temperature.The fnal concentration of pyrene in the solution was adjusted to 1.0×10?7M.Ten milliliter samples of a series of different concentrations of the polymer solution(0.05,0.1,0.5,1, 5,10,50,100 mg/l)were added to the volumetric fasks.The combined solution of pyrene and polymer was sonicated for 30 min and allowed to reach equilibrium by cooling overnight at room temperature before measurement.Fluorescence excitation spectra were recorded at an emission wavelength of 390 nm.The emission and excitation wavelengths were from 350 nm to 300 nm in steps of 1 nm using a Microplate Reader (BIO-RAD 680,Bio-Rad Laboratories,Inc,USA).CMC was measured from the onset of a rise in the intensity ratio of peaks at 338 nm to peaks at 335 nm in the fuorescence spectra of pyrene plotted versus the logarithm of polymer concentration.
2.3.5.Gel Permeation Chromatography analysis
The splitting of the polymer chains was measured by gel permeation chromatography(GPC)analysis.Number average molecular weight(Mn),weight average molecular weight(Mw) and polydispersity index(PDI=Mw/Mn)of Soluplus?and Soluplus?extrudates were measured using Waters 1515 GPC with three Styragel columns(Waters Corp;105,104,and 103) in tandem and a 2414 differential refractive index detector.DMF containing 0.1 M LiBr was selected as the eluent at a fow rate of 1.0 ml/min at 35°C.The sample concentrations were approximately 2 mg/ml.
2.3.6.Transmission electron microscopy
The morphology of the micelles in simulate gastrointestinal fuid was studied using transmission electron microscopy(TEM). After passing through a 0.22 μm Millipore flter,a drop of micelle solution was immediately spread on a copper grid for about 20 min and the excess liquid was removed with flter paper. Two percent aqueous phosphotungstic acid was then dropped onto the grid for negative staining for 2 min.Samples were dried under room temperature for 6 hours and viewed using a JEM-2100 TEM(JEOL,Tokyo,Japan).
2.3.7.Predicting the precipitation of ITZ upon entry in the
small intestine
To simulate the transfer of ITZ out of the stomach into the intestine,a transfer model was employed[13].A solution of the drug in hydrochloric acid at pH=1.2 was continuously pumped into a phosphate buffer at pH=6.8,and the drug precipitation in the acceptor medium was examined using concentration–time measurements.A peristaltic pump(BT100-2J,Longer Pump,China)was used to transfer the donor phase (hydrochloric acid at pH=1.2 containing dissolved drug)into a dissolution vessel containing 500 ml phosphate buffer at pH=6.8 as the acceptor phase.The transfer rate(3.0 ml/min) represented the physiological fow rate out of the stomach.The acceptor phase medium was maintained at 37°C.To examine the infuence of intestinal motility on drug precipitation,a paddle rotation speed of 75 rpm was used in the acceptor phase. Samples(1.5 ml)were withdrawn at predetermined intervals (5,10,15,20,25,30,35,40,45,50,55,60,70,80,90,105,120, 135,150,165 and 180 min)during transportation.These samples were passed through 0.22 μm Millipore flters and then analyzed by HPLC.All tests were performed in triplicate,and data are presented as the mean±S.D.
2.4.In vivo studies
2.4.1.Animal experiments
Six male beagle dogs(10±0.5 kg)were kept in an air-conditioned experimental animal room at a temperature of 22±2°C and a relative humidity of 50±10%.Access to water and laboratory chow was provided ad libitum.Dogs were allowed to acclimatize for at least 7 days and then fasted,but allowed free access to water for 12 hours prior to the experiment.Dogs received two different treatments with ITZ tablets and Sporanox?at 2-week intervals in a randomized two-way crossover study. Blood samples(400 μl)were collected and transferred into heparinized glass tubes at 0.5,0.45,1,2,4,6,8,10,12,24,36,48,60, 72 and 96 hours after administration.Blood samples were centrifuged(4000 rpm,10 min,4°C)and the plasma samples were stored at?80°C until analysis.
2.4.2.Analysis of ITZ in plasma
The samples for UPLC-ESI-MS/MS analysis were prepared by liquid–liquid extraction.One hundred microliters of plasma sample,50 μl of internal standard(loratadine 50 ng/ml in methanol)and 100 μl of methanol were added into each centrifuge tube.After vortex-mixing for 1 min,50 μl of potassium dihydrogen phosphate buffer(pH=8.4)and 2 ml of tert-butyl methyl ether were added and vortex-mixed for 10 min to extract ITZ.After centrifuging at 4000 rpm for 10 min,the organic layer was transferred to another tube and evaporated under a light stream of nitrogen at 40°C.This extraction was repeated,and the organic layer residues were reconstituted in 100 μl methanol before vortexing for 10 min and centrifuging at 12,000 rpm for 10 min.
Chromatography was performed on an ACQUITYTM UPLC system(Waters Corp.,Milford,MA,USA)with a column temperature of 40°C and an autosampler temperature of 10°C. Samples(5 μl)were eluted with a mobile phase consisting of acetonitrile and 0.1%(w/w)methanolic acid solution(50:50,v/v) from an ACQUITY UPLCTM BEH C18 column(50 mm×2.1 mm i.d.,1.7 μm;Waters Corp.,Milford,MA,USA).Validation of the analytical methods for both ITZ and OH-ITZ under the selected conditions showed that the chosen method was of acceptable precision and accuracy with a linear response of 10–1000 ng/ml.Individual plasma concentrations versus time data for ITZ and OH-ITZ were analyzed using DAS 2.0 software.
3.Results
3.1.Evaluation of tablet properties
3.1.1.Stability of amorphous ITZ
Fig.1 shows the DSC spectra of the ITZ,Sporanox?and ITZ tablets.Pure itraconazole showed an endothermic peak at 173°C.Sporanox?showed two endothermic peaks at 59°C and 186°C,corresponding to the melting point of PEG and itraconazole,respectively[14],while ITZ tablets showed no melting endotherms,indicating that amorphous ITZ was obtained.
3.1.2.Dissolution properties
The dissolution profles of ITZ tablets and Sporanox?in different dissolution media are shown in Fig.2.The dissolution media were hydrochloric acid at pH 1.2(USP 35),acetate buffer at pH 4.5(USP 35),phosphate buffer at pH 6.8(USP 35)and SGFsp, respectively.The composition of SGFspdissolution medium, simulating gastric conditions in the fasted state was 2 g NaCl, 3.2 g pepsin and 7 ml HCl in 1000 ml water,and the medium had a pH of about 1.2.Result(Fig.2)shows that the dissolution rates and extents of the ITZ tablets were much higher compared to Sporanox?.For tablets dissolved in hydrochloric acid at pH 1.2,the percentage release of ITZ was approximately 98%in 10 min while the corresponding result for Sporanox?was only about 14%,indicating that ITZ in the tabletsdissolved immediately when they came into contact with the hydrochloric acid at pH 1.2.A similar dissolution behavior of ITZ tablets was obtained in the experiment conducted in the SGFsp.The maximal dissolution extent of ITZ tablets in acetate buffer and phosphate buffer was 78.66%and 71.71%,respectively.However,the maximal dissolution extent of Sporanox?was 4.90%and 5.36%,which were much lower than that of ITZ tablets.
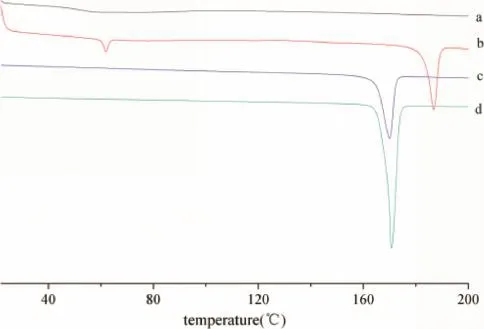
Fig.1–DSCs of(a)Itraconazole Tablets(6 months at 40°C/RH75%),(b)Sporanox?(12 months at 25°C/RH60%),(c)Physical mixtures(same proportion as tablets),(d)Crystalline Itraconazole.
It was concluded that itraconazole can be largely solubilized by Soluplus?in these three media,the order of the solubilization capacity for these media was:hydrochloric acid at pH 1.2>acetate buffer at pH 4.5>phosphate buffer at pH 6.8.
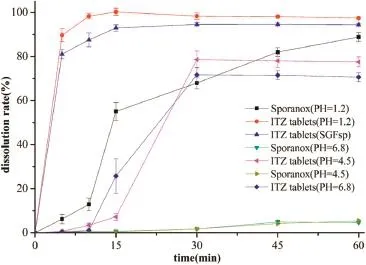
Fig.2–Comparison of drug release profles between ITZ tablets and Sporanox?in different dissolution media.
3.1.3.Evaluation of micelle properties
The presence and the morphology of the micelles in SGFspand SIF(USP 35)were examined by TEM.As can be seen from the TEM analysis,the micelles were spherical in shape with uniform smooth surfaces.It has been reported that the mean particle size of blank Soluplus?micelles is about 65 nm[15].According to Fig.3,it can be seen that the size of the micelles was approximately 70 nm in the simulated gastric fuids,while the size of micelles was only 50 nm in the SIF.It can be concluded that the hydrophilic domain(polyethylene glycol)of Soluplus?served as a fexible outer shell of the core–shell structure in aqueous solution while the hydrophobic domain (polyvinyl caprolactam)formed the solid inner core,which would entrap the hydrophobic drug in its interior.
3.2.Critical micelle concentration of Soluplus?
Fig.4 shows the fuorescence excitation spectra of pyrene at various concentrations of Soluplus?and Soluplus?extrudates. The fuorescence intensity increased with the increased polymer concentration,which indicates self-assembly of Soluplus?in water[16,17].The CMC was presented as the intensity ratio of I338/I335versus the logC of Soluplus?for the pyrene excitation spectra,as shown above.The critical micelle concentration values of Soluplus?and Soluplus?extrudates were calculated to be 1.93 mg/L and 4.24 mg/l at 37°C,respectively.It is speculated that the difference in CMC values between Soluplus?and Soluplus?extrudates was due to partial splitting of chains.
The splitting of the polymer chains was measured by gel permeation chromatography(GPC)analysis.Results showed that the polydispersity indexes(PDI=Mw/Mn)for blank Soluplus?and blank Soluplus?extrudates were 1.442 and 1.802,respectively,and the retention times were 23.337 min and 23.400 min, respectively.As expected,the overall results of the GPC data demonstrated that trace amounts of chains were split during the extrusion process.
Fig.5 shows the formation of micelles by ITZ/Soluplus?solid dispersion and ITZ/Soluplus?physical mixers.Lower CMC values are generally due to the presence of highly hydrophobic domains in the micelle core which denote greater stability of the micelles[18].In this study,the low CMC value(4.24 mg/l) of Soluplus?extrudates demonstrated that Soluplus?through the HME process was able to form stable micelle systems.
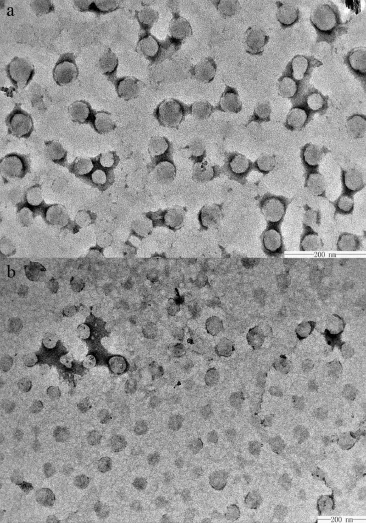
Fig.3–TEM images of ITZ Soluplus?micelles in the SGFsp(a)and SIF(b).
3.3.Transfer experiments of ITZ
In vitrodrug precipitation under pH=6.8 phosphate buffer conditions was examined since the possibility of drug precipitation upon entry into the small intestine may also affect the amount of drug available for absorption through the intestinal mucosa [19].Fig.6 shows the concentration–time profle of ITZtransferred from hydrochloric acid at pH=1.2 to a phosphate buffer at pH=6.8.
It can be seen that the maximum concentration observed in phosphate buffer at pH=6.8 during the experiment and its correspondingTmaxwas about 50 min.Soluplus?kept drug from precipitating in the phosphate buffer at pH=6.8 for at least 180 min,while supersaturation and then little precipitation of ITZ were observed for Sporanox?.The maximum concentrations in the acceptor phase media were 15.60 μg/ml and 11.44 μg/ml,respectively,while the fnal equilibrium concentrations were 13.94 μg/ml and 7.32 μg/ml,respectively.
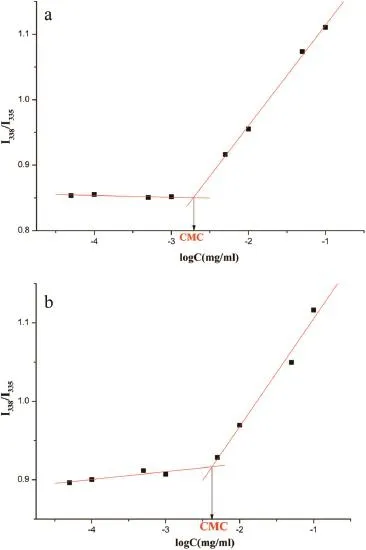
Fig.4–Fluorescence intensity(I338/I335)ratio of pyrene as a function of the logarithm of the Soluplus?concentration. (a)Soluplus?(b)Soluplus?extrudates.
3.4.In vivo performance
Fig.7 shows the plasma concentration–time curves of ITZ and its primary active metabolite,OH-ITZ in beagle dogs,after oral administration of ITZ tablets and Sporanox?in a randomized two-way crossover study.The ITZ tablets increased the relative bioavailability of ITZ and OH-ITZ signifcantly compared with Sporanox?.The pharmacokinetic parameters of ITZ and OH-ITZ were listed in Table 1.There was a 1.50-fold increase in the AUC(0–t)of ITZ and a 0.95-fold increase in its active metabolite,OH-ITZ,compared with Sporanox?in plasma according to the bioequivalence results calculated using DAS 2.0 software.For ITZ,the meanCmaxwas 1.33-times higher than that of Sporanox?while the meanTmaxwas 1.5 hours earlier and,for OH-ITZ,the meanCmaxwas 1.28-times higher and the meanTmaxwas 0.6 hour earlier than that of Sporanox?.The confdence intervals of the equivalence analysis(ABE):[1-2α]were 141.3%–332.2%and 86.6%–323.2%for ITZ and OH-ITZ,respectively.The available data demonstrated that the two dosage forms(the ITZ tablet and Sporanox?)were not bioequivalent and the ITZ tablet was outside of the BE range(80.0%–120.0%).
4.Discussion
4.1.Characterization of ITZ in preparations
The purpose of HME was to obtain an amorphous state of API that dispersed homogeneously in the amphiphilic carrier.The lack of a melting peak in the DSC of the milled powders of ITZ tablets indicated that the drug in the ITZ tablets was present in an amorphous state rather than a crystalline form under stress conditions(40°C/75%RH)for 6 months.It can be concluded that the amorphous state dispersion in the tablets was well protected during storage,which would ensure the maintenance of the dissolution profles and the therapeutic effect. Also,the presence of crystalline ITZ in the DSC of Sporanox?confrmed that the method used in the present study had an advantage in stability of amorphous state over the cosolvent evaporation method which was used in the Sporanox?preparation.
4.2.Effects of Soluplus?on dissolution and
supersaturation under usual dosing conditions(100 mg)
The proposed drug solution upon coating(cosolvent evaporation)and controlled drying of the coated beads produced an amorphous form of drug in HPMC that allows quick dissolution of the drug from Sporanox?upon reaching the stomach [3,11].However,the dissolution rate of Sporanox?was far lower than that of ITZ tablets in the frst 5 min.It can be concluded that,in a fasted state,the pellets would pass into the duodenum without being fully dissolved.According to the dissolution curve,it can be seen that the drug could not continue to dissolve from the pellets,since the dissolution of ITZ in Sporanox?in acetate buffer at pH=4.5 and phosphate buffer at pH=6.8 was much lower than that of the tablets,and the changes in conditions and pH may lead to precipitation of ITZ.It can be inferred that it was one of the reasons for the higher oral bioavailability of the tablets compared with Sporanox?.
So,it can be presumed that with the absorption of ITZ in the GI tract,the drug continues to be released from the micelles during the whole absorption period.Results showed that Soluplus?acted as a supersaturation inducer in an attempt to prevent drug precipitation upon GI fuid dilution and change in pH values in the gastrointestinal tract.Once the tablet comes in contact with gastric juice,the drug is completely dissolved in the frst 5 min.As a result,ITZ existed as the molecular state and ionic state of ITZ dissolved in gastric juice as well as themolecular ITZ that entrapped in the micelles.When passing into the small intestine,the drug released from micelles continuously can be absorbed immediately by the gastrointestinal tract.
It has been reported that nonionic surfactants possess a greater ability to dissolve poorly water-soluble drugs[20].In addition,the strength of the intermolecular interactions between ITZ and polymeric stabilizers largely determines the extent of supersaturation in aqueous media and the stability of the supersaturated solutions[4].In this study,Soluplus?served not only as a functional carrier but also as an inhibitor of ITZ molecular precipitation due to the self-assembly ability of Soluplus?which would enwrap the drug inside the core of the micelles and prevent the drug from precipitating during absorption.The solubilization system is thermodynamically stable,and a CMC value less than 135 mg/ml should be regarded as a guide due to the fact that lower CMC values mean greater resistance to the effects of dilution and,therefore,greater stability[21].In recent years,researchers have shown that the solubilizing agent was evenly distributed in polymeric micelles.Solubilization of the micelles is a dynamic equilibrium procedure,constantly involving disintegration and reforming[22].It appears that the excellent apparent solubility property was a result of the formation of the polymeric micelles and the amphiphilic nature of Soluplus?.
Since supersaturation is known to be a metastable state[23], the apparent solubility of supersaturated Soluplus?micelle solutions was 81.55 μg/ml in acetate buffer at pH=4.5 and 74.40 μg/ml in phosphate buffer at pH=6.8.Furthermore,the micelles could keep the drug from precipitating for at least 4 hours.In general,the gastric pH in young,healthy,fasting humans who have taken 200 ml water varies within the range pH 1.5–5[24].The high apparent solubility indicates that the dissolved drug could be kept in the micelles and prevented from precipitating without limitation of pH values under normal dosing conditions.The presence of pepsin did not affect the rate and extent of dissolution of the tablets due to the fact that the dissolution curve for SGFspis similar to that of hydrochloric acid at pH 1.2.Therefore,the achlorhydric population with a gastric pH>5 or even higher may obtain a much better therapeutic effect compared with Sporanox?.It would,therefore,behighly desirable to have pharmaceutical formulation which can be administrated to a patient,independently of the food intake. However,further research is needed to confrm this.
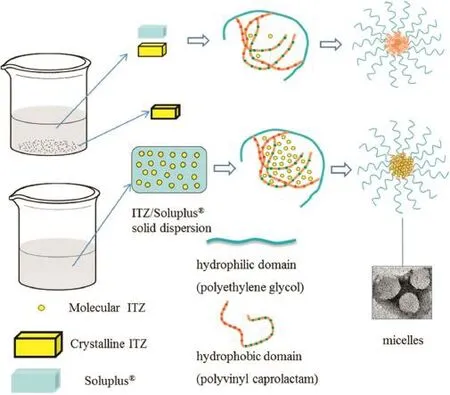
Fig.5–Different dissolution processes between ITZ/Soluplus?solid dispersion and physical mixers.
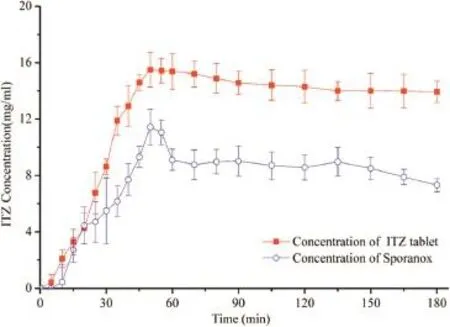
Fig.6–Measured ITZ concentration of ITZ tablets and Sporanox?in phosphate buffer at pH=6.8(n=3±SD) using a paddle speed of 75 rpm at a transfer rate 3 ml/min.
The apparent solubility of the physical mixture(ITZ/ Soluplus=40/60)was 4.74 μg/ml compared with that of crystalline ITZ,4.92 μg/ml,in hydrochloric acid pH=1.2(USP 35)after shaking for 72 h at a temperature of 37°C.So,this means that,ITZ can barely be solubilized by Soluplus?in its crystalline state.The different solubility properties between the physical mixtures and solid dispersions were mainly due to the fact that the crystalline state needed to overcome crystal lattice energy upon dissolution.The crystalline nature of ITZ can be disrupted by solid-state dispersion of the drug into water soluble carrier molecules which replace the drug molecule in the crystal lattice.This results in lower lattice tensile strength and particles with lower lattice tensile strength are easier to dissolve.Therefore,the amorphous state of the drug through HME in the solid solution played a crucial role in obtaining the supersaturated micelle solutions.
4.3.In vivo implications of pharmacokinetic data
Comparative statistical analysis showed that the ITZ tablets were not bioequivalent to Sporanox?.The ITZ tablets have been shown to achieve better bioavailability than Sporanox?in beagle dog studies.It is also worth noting that theTmaxof ITZ tablets was earlier than that of Sporanox?,mainly due to the rapid and complete dissolution of the tablets in the stomach and the supersaturation state maintained in the intestinal cavity.
The permeability of biological membranes is a key determinant of drug pharmacokinetics.Fig.8 shows how micelles can permeate the intestinal membrane.The transportation of a drug across the intestinal membrane is a complex transfer process involving several mechanisms:(1)transcellular permeation through the epithelial cells that consists of passive transcellular and carrier-mediated permeation;(2)paracellular permeation passing between the cells which can pass through the tight junction channels between adjacent epithelial cells and;(3)uptake of intact micelles through pinocytosis which refers to transport into a cell[25].
Both passive transcellular and carrier-mediated processes coexist and contribute to ITZ transport activities across biological membranes.Passive diffusion through the lipid bilayer portion of a biological membrane is suggested to be the dominant route.The ABL is adjacent to a membrane that can be a permeation barrier for highly lipophilic drugs.Due to the hydrophilicity of PEG cores that remained outside the assembled micelles,drug molecules inside the micelles are carried through the ABL covering on the small intestinal villi,arriving at the surface of the microvilli.Here,drugs are gradually released from micelles and pass through the microvilli into the cells of the epithelial mucosa of the small intestine(the micelle shell remains in the intestinal cavity).The oral administration of ITZ is also complicated by the multidrug transporter P-gp in the small intestine since ITZ is a known substrate of the P-gp effux system.However,the increased dissolution rate and solubility provided a higher luminal concentration that could more effciently saturate the intestinal effux and/or metabolism of ITZ.In addition,it has been reported that Soluplus?has a negligible impact on P-gp-mediated drug effux[18,26].These two key factors markedly promoted the absorption of ITZin vivo.
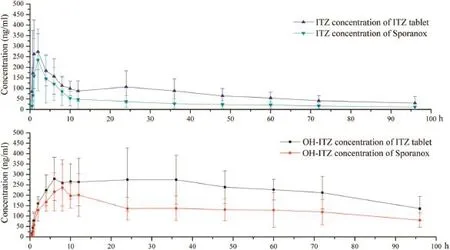
Fig.7–Plasma concentration–time profles of(a)ITZ and(b)OH-ITZ after oral administration of self-made tablets and Sporanox?to male beagle dogs.(mean±SD,n=6).
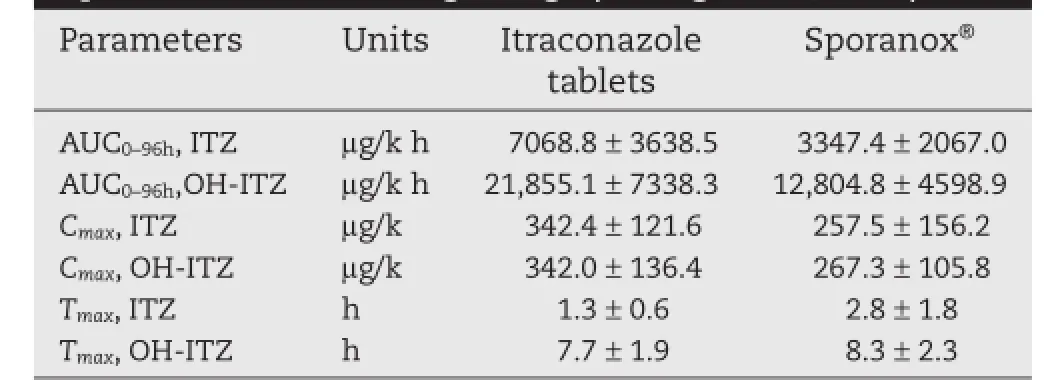
Table 1–Pharmacokinetic parameters of ITZ and OHITZ after oral administration of Itraconazole tablets and Sporanox?in male beagle dogs(average±SD,n=6).
The uptake of micelles in the intestine and the extent of drug absorption increased with the reduction of particle size [27].It has been reported that size is an important parameter controlling the internalization of nanoparticles into epithelia of the GI tract,and nanoparticles less than 100 nm in diameter have been detected in the intestinal mucosa and gutassociated lymphoid tissues and 500 nm particles have been found in the intestinal tissues,while particles larger than 1 μm in diameter and have been found only in the Peyer’s patches [28,29].In this study,the size of the drug-loaded micelles with Soluplus?was found to be about 70 nm(smaller than 100 nm) and have met the requirement of uptake by gastrointestinal tissue to achieve a better drug absorption[17].
All of the results obtained confrmed the advantage of micelles formed by Soluplus?,contributing to the high bioavailability of ITZ.The pharmacokinetic parameters in beagle dogs are a strong evidence to support the viewpoints that were discussed earlier:micelles formed by Soluplus?in the gastrointestinal tract maintained supersaturation of ITZ and contributed to the absorption of ITZin vivo.
In addition,before micelle formation in the gastrointestinal tract,extrudates were well protected in the tablets as a solid solution during long-term storage.Since drug absorption in the intestine lasts for only a few hours,therefore,unlike other conventional polymeric micelle solutions,there is no need to take the long-term storage stability of micelle solutions into consideration.
In summary,this marked improvement in the pharmacokinetic parameters of ITZ and OH-ITZ in the tablets after oral administration could be explained by a combination of the following effects:(1)the amorphous state ITZ dispersed homogeneously in the tablets increases the dissolution rate and extent of ITZ,(2)Soluplus?micelles possess a signifcantly low CMC;thus,Soluplus?micelles remain intact even when suffering from severe dilution and pH change in the gastrointestinal tract[30].Soluplus?also acts as a stabilizer of supersaturation upon drug release and prevents precipitation in the intestine,(3)the hydrophilic shell of micelles is an advantage for passing through the ABL,(4)reduced elimination of ITZ due to the inhibitory effect of Soluplus?on intestinal P-gp drug effux,(5)the micelles(smaller than 100 nm)seem to produce an effcient interfacial interaction with the cell membrane,such as phagocytosis and pinocytosis,(6)as a nanosized drug delivery system,Soluplus?micelles reduce the uptake by the mononuclear phagocyte system(MPS),allowing longer circulation in the body[31];

Fig.8–Representation of how micelles can permeate the intestinal membrane.
5.Conclusions
This study demonstrates that the self-assembly of Soluplus?in aqueous solutions as well as the micelles formed in the gastrointestinal tract by solid dispersions through HME represent a promising vehicle for the oral delivery of poorly watersoluble compounds.Supersaturation maintained by the micelles increases the concentration in the intestinal cavity.Drugs in the micelles are carried through the ABL,contributing to the absorption by passive transportation across biological membranes,allowing the uptake of intact micelles through pinocytosis and the presence of Soluplus?inhibit the drug effux by P-gp in the intestinal tract.This formulation increases the bioavailability in the gastrointestinal tract and leads to higher concentrations in the plasma which is a great advantage compared with Sporanox?.
Acknowledgement
This work was fnancially supported by the National Natural Science Foundation of China(NSFC 81102399).
Dr.David B Jack is gratefully thanked for correcting the English of the manuscript.
R E F E R E N C E S
[1]Grant SM,Clissold SP.Itraconazole:a review of its pharmacodynamic and pharmacokinetic properties,and therapeutic use in superfcial and systemic mycoses.Drugs 1989;37:310–344.
[2]Ghazal HS,Dyas AM,Ford JL,et al.In vitroevaluation of the dissolution behaviour of itraconazole in bio-relevant media. Int J Pharm 2009;366:117–123.
[3]Zhang K,Yu H,Luo Q,et al.Increased dissolution and oral absorption of itraconazole/Soluplus extrudate compared with itraconazole nanosuspension.Eur J Pharm Biopharm 2013;85:1285–1292.
[4]Miller DA,DiNunzio JC,Yang W,et al.Enhancedin vivoabsorption of itraconazole via stabilization of supersaturation following acidic-to-neutral pH transition. Drug Dev Ind Pharm 2008;34:890–902.
[5]Taupitz T,Dressman JB,Buchanan CM,et al.Cyclodextrinwater soluble polymer ternary complexes enhance the solubility and dissolution behaviour of poorly soluble drugs. Case example:Itraconazole.Eur J Pharm Biopharm 2013;83:378–387.
[6]Takano R,Takata N,Saito R,et al.Quantitative analysis of the effect of supersaturation onin vivodrug absorption.Mol Pharm 2010;7:1431–1440.
[7]Vasconcelos T,Sarmento B,Costa P.Solid dispersions as strategy to improve oral bioavailability of poor water soluble drugs.Drug Discov Today 2007;12:1068–1075.
[8]Lawrence MJ.Surfactant systems:their use in drug delivery. Chem Soc Rev 1994;23:417–424.
[9]Gaucher G,Satturwar P,Jones MC,et al.Polymeric micelles for oral drug delivery.Eur J Pharm Biopharm 2010;76:147–158.
[10]Jivraj M,Martini LG,Thomson CM.An overview of the different excipients useful for the direct compression of tablets.Pharm Sci Technol Today 2000;3:58–63.
[11]Remenar JF,Morissette SL,Peterson ML,et al.Crystal engineering of novel cocrystals of a triazole drug with 1, 4-dicarboxylic acids.J Am Chem Soc 2003;125:8456–8457.
[12]Gilis PMV.Antifungal compositions with improved bioavailability,U.S.5,633,0155;1997.
[13]Kostewicz ES,Wunderlich M,Brauns U,et al.Predicting the precipitation of poorly soluble weak bases upon entry in the small intestine.J Pharm Pharmacol 2004;56:43–51.
[14]Kapsi SG,Ayres JW.Processing factors in development of solid solution formulation of itraconazole for enhancement of drug dissolution and bioavailability.Int J Pharm 2001;2229:193–203.
[15]Yu H,Xia D,Zhu Q,et al.Supersaturated polymeric micelles for oral cyclosporine A delivery.Eur J Pharm Biopharm 2013;85:1325–1336.
[16]Dahmani FZ,Yang H,Zhou J,et al.Enhanced oral bioavailability of paclitaxel in pluronic/LHR mixed polymeric micelles:preparation,in vitroandin vivoevaluation.Eur J Pharm Sci 2012;47:179–189.
[17]Jeong YI,Kim DH,Chung CW,et al.Doxorubicinincorporated polymeric micelles composed of dextran-bpoly(DL-lactide-co-glycolide)copolymer.Int J Nanomedicine 2011;6:1415–1427.
[18]Francis MF,Cristea M,Winnik FM.Polymeric micelles for oral drug delivery:why and how.Pure Appl Chem 2004;76:1321–1335.
[19]Mathot F,Van Beijsterveldt L,Préat V,et al.Intestinal uptake and biodistribution of novel polymeric micelles after oral administration.J Control Release 2006;111:47–55.
[20]Lu Y,Park K.Polymeric micelles and alternative nanonized delivery vehicles for poorly soluble drugs.Int J Pharm 2013;453:198–214.
[21]Rege BD,Kao JPY,Polli JE.Effects of nonionic surfactants on membrane transporters in Caco-2 cell monolayers.Eur J Pharm Sci 2002;16:237–246.
[22]Patist A,Kanicky JR,Shukla PK,et al.Importance of micellar kinetics in relation to technological processes.J Colloid Interface Sci 2002;245:1–15.
[23]Brouwers J,Brewster ME,Augustijns P.Supersaturating drug delivery systems:the answer to solubility-limited oral bioavailability?J Pharm Sci 2009;98:2549–2572.
[24]Kalantzi L,Goumas K,Kalioras V,et al.Characterization of the human upper gastrointestinal contents under conditions simulating bioavailability/bioequivalence studies. Pharm Res 2006;23:165–176.
[25]Sugano K,Kansy M,Artursson P,et al.Coexistence of passive and carrier-mediated processes in drug transport. Nat Rev Drug Discov 2010;9:597–614.
[26]Linn M,Collnot EM,Djuric D,et al.Soluplus?as an effective absorption enhancer of poorly soluble drugsin vitroandin vivo.Eur J Pharm Sci 2012;45:336–343.
[27]Florence AT,Hussain N.Transcytosis of nanoparticle and dendrimer delivery systems:evolving vistas.Adv Drug Deliv Rev 2001;50:69–89.
[28]Desai MP,Labhasetwar V,Walter E,et al.The mechanism of uptake of biodegradable microparticles in Caco-2 cells is size dependent.Pharm Res 1997;14:1568–1573.
[29]Desai MP,Labhasetwar V,Amidon GL,et al.Gastrointestinal uptake of biodegradable microparticles:effect of particle size.Pharm Res 1996;13:1838–1845.
[30]Gong J,Chen M,Zheng Y,et al.Polymeric micelles drug delivery system in oncology.J Control Release 2012;159:312–323.
[31]Carstens MG,Rijcken CJF,van Nostrum CF,et al. Pharmaceutical micelles:combining longevity,stability,and stimuli sensitivity.In:Multifunctional pharmaceutical nanocarriers,vol.4.New York:Springer;2008. p.263–308.
Abbreviations:ITZ,Itraconazole;HME,Hot melt extrusion;P-gp,P-glycoprotein;DSC,differential scanning calorimetry;CMC,critical micelle concentration;GPC,Gel Permeation Chromatography;OH-ITZ,hydroxyitraconazole;ABL,aqueous boundary layer;APIs,active pharmaceutical ingredients;SGF,simulated gastric fuid;SIF,simulated intestinal fuid;MCC,microcrystalline cellulose;GI,Gastrointestinal.
*< class="emphasis_italic">Corresponding authors.
s.Department of Pharmaceutics,Shenyang Pharmaceutical University,Wen Hua Road No.103,Shenyang 110016, China.Tel.:+86 24 23986343;fax:+86 24 23911736.
E-mail addresses:emily_hehaibing@hotmail.com(H.He);tanglab@126.com(Y.Wang). Peer review under responsibility of Shenyang Pharmaceutical University.
http://dx.doi.org/10.1016/j.ajps.2015.07.001
1818-0876/?2016 The Authors.Production and hosting by Elsevier B.V.on behalf of Shenyang Pharmaceutical University.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
 Asian Journal of Pharmacentical Sciences2016年2期
Asian Journal of Pharmacentical Sciences2016年2期
- Asian Journal of Pharmacentical Sciences的其它文章
- Preparation and characterization of solidifed oleanolic acid–phospholipid complex aiming to improve the dissolution of oleanolic acid
- Development of a topical ointment of betamethasone dipropionate loaded nanostructured lipid carrier
- Quality assessment of Chrysanthemum indicum Flower by simultaneous quantifcation of six major ingredients using a single reference standard combined with HPLC fngerprint analysis
- Rapid and sensitive analysis of melatonin by LC-MS/MS and its application to pharmacokinetic study in dogs
- Effect of process parameters on the recrystallization and size control of puerarin using the supercritical fuid antisolvent process
- The 1st Euro-Mediterranean Workshop:Natural Products in Health and Diseases:Cairo,Egypt, March 2,2015
