Effect of process parameters on the recrystallization and size control of puerarin using the supercritical fuid antisolvent process
Ying Li,Yibin Yu,Hanbing Wang,*,Fengguang Zhao**
aDepartment of Pharmacy,School of Medicine,Shenzhen University,Shenzhen,China
bDivision of Life Science&Health,Graduate School at Shenzhen,Tsinghua University,Shenzhen,China
cSchool of Pharmacy,Shenyang Pharmaceutical University,Shenyang,China
dResearch Institute of Joincare Pharmaceutical Group Industry Co.Ltd,Shenzhen,China
Effect of process parameters on the recrystallization and size control of puerarin using the supercritical fuid antisolvent process
Ying Lia,b,Yibin Yuc,Hanbing Wangc,*,Fengguang Zhaod,**
aDepartment of Pharmacy,School of Medicine,Shenzhen University,Shenzhen,China
bDivision of Life Science&Health,Graduate School at Shenzhen,Tsinghua University,Shenzhen,China
cSchool of Pharmacy,Shenyang Pharmaceutical University,Shenyang,China
dResearch Institute of Joincare Pharmaceutical Group Industry Co.Ltd,Shenzhen,China
A R T I C L EI N F O
Article history:
Received 29 September 2015
Received in revised form 26
December 2015
Accepted 28 December 2015
Available online 26 January 2016
Puerarin
Microparticles
Supercritical fuid
GAS
Crystallization
Particle size
The purpose of this study was to investigate the infuence of the supercritical CO2processing on the particle size and morphology of puerarin crystals.The process parameters included solvents,temperature,pressures,antisolvent times,addition volumes,antisolvent addition rates and solute concentrations.After being processed,the dramatic reduction of the dimensions and the change of the crystal shape were noticed.Decreasing the antisolvent addition rate,increasing the temperature and the addition volume below 50 ml led to a decrease in size.The new crystal of puerarin generated at the optimal conditions was 30.34 μm. The solvent of methanol and the concentration of 60 mg/ml were found to determine the type and degree of crystallinity of particles.These results showed that this process has the potential to produce a drug recrystallization product with newly generated crystal forms and the size of drug particles could be controlled through the tuning of various experimental conditions.
?2016 The Authors.Production and hosting by Elsevier B.V.on behalf of Shenyang Pharmaceutical University.This is an open access article under the CC BY-NC-ND license
(http://creativecommons.org/licenses/by-nc-nd/4.0/).
1.Introduction
For most orally administered poorly-soluble compounds,the bio-absorption process is rate-limited by dissolution in gastrointestinal fuids;in the case of parenteral administration, the effective bio-availability of compounds is limited by solubility issues.As for the crystal drug,two key characteristics of crystalline solid dosage forms are crystal habit and the crystal size distribution[1].
The conventional techniques for reduction of particle size include mechanical comminution(through milling,crushing and grinding),lyophilization and recrystallization of the solute particles from solution(through solvent-antisolvent techniques,spray drying and freeze-drying).All these techniques suffer from one or more disadvantages,such as thermal and/ or chemical degradation,high solvent requirements or diffcult removal of solvent traces from the fnal product.Besides,the classical crystallization techniques usually lead to a mixture of polymorphs because of the multi-step process used.Therefore,there is increasing interest in developing technologies which,particularly in the case of pharmaceuticals,allow one to produce microparticles with controlled particle size distribution and product quality(crystallinity,purity,morphology) under mild and inert conditions.Since the mid-1980s,a new method of powder generation has appeared involving crystallization with supercritical fuids.CO2is the most widely used solvent and its innocuity and“green”characteristics make it the best candidate for the pharmaceutical industry.Supercritical fuid technology,particularly when using CO2,offers different possibilities to tackle the above-mentioned challenges[2,3].And it is also interesting to check if the supercritical crystallization(a single-step unit)may give different results.These include the processes called rapid expansion of supercritical solutions(RESS),precipitation with compressed antisolvent(PCA, sometimes referred to as SAS,i.e.,supercritical antisolvent process,or ASES,i.e.,aerosol spray extraction system)and gas antisolvent recrystallization(GAS)[4–7].In the GAS process, high pressure CO2is injected into the liquid phase solution, which causes a sharp reduction of the solute solubility in the expanded liquid phase.As a result,precipitation of the dissolved compound occurs.The potential advantages of the GAS recrystallization process lie in the possibility of obtaining micron and submicron particles with a narrow size distribution and lower residual solvent.By varying the process parameters,the particle size,size distribution and morphology can be“tuned”to produce a product with desirable qualities.This makes the GAS technique attractive for the micronization of highvalued products such as pharmaceuticals[8,9].
Adopting a GAS process to recrystallize pharmaceutical compounds will provide highly versatile methodology to generate new polymorphs of drugs.Many researchers have employed the GAS process for micronization and recrystallization of various pharmaceutical substances[10–12].They have concentrated on the size reduction of pharmaceutical compounds and they observed changes in the external shape and size distribution of the resulting particles.The diversity of experimental parameters of the GAS process can vary the conditions for nucleation and crystal growth steps in a wide range.It is possible to produce drug particles with different crystalline arrangements but identical chemical compositions.Such behavior is called polymorphism,meaning the ability of any compound to crystallize into more than one distinct crystalline state[13].This can be important to the quality of a given product.In the pharmaceutical feld,an active substance may exhibit different activities and shelf life depending on the polymorph.Properties such as solubility,dissolution rate,density,physical stability and melting point change depending on the type of crystalline forms. Therefore,the various polymorphs of a given pharmaceutical compound will exhibit different drug release characteristics and biological activity.
With the application of modern isolation technology and high throughput biological screen capability,more and more natural compounds with biological activity are being isolated and identifed.However,many of these compounds with potent activityin vitrofail to generate good activityin vivoowing to their poor water-solubility,poor permeability and/or poor stability[14].Puerarin(Pur)was such a drug,chemically designated as 8-C-β-D-Glucopyranosyl-4′,7-hydroxy-Isofavone,and was one of the main active constituents ofPueraria lobata(wild.)ohwi, a famous Chinese traditional medicine.Pur has many types of benefcial effects on cardiovascular,neurological and hyperglycemic disorders.However,its poor solubility in water limits its absorptionin vivo[15–17].Solubilizer is often added to the injection formulation used clinically to increase its solubility.Research on the polymorphism of Pur was quite few.There was only related literature which reported that Pur did have various crystalline forms depending on experimental conditions adopted in the conventional solution crystallization techniques[18].The objective of this research was to investigate the feasibility of the GAS recrystallization technique to generate small particles of Pur with a high degree of purity and narrow size distribution.By surveying the production of different crystalline forms of Pur using the GAS process,we focused on how the solid-state properties of the recrystallized particles including particle size,morphology and crystal form varied with the process parameters such as the antisolvent addition rates,the pressures,the addition volumes,the concentrations,the antisolvent times,the types of solvent and temperatures.And we hope to fnd new polymorphs with better physico-chemical properties such as higher solubilities,dissolution rate and smaller particle size.The newly generated polymorph of Pur could be used for the injection or oral formulation with better bioactivity.So,here we recrystallized Pur by using CO2as an antisolvent and examined the characteristics of the GAS processed crystals by using various analytical instruments.
2.Materials and methods
2.1.Materials
Absolute ethanol,acetone and methanol,analytical grade,purity of 99.7%,were bought from Guangzhou Chemical Reagent 2 Factory(Guangzhou,China).Pur,purity of 99.9%,was obtained from Beijing Union Pharmaceutical Factory(Beijing, China).Liquid CO2,instrument grade,purity of 99.5%,was purchased from Zhonghong Industrial Gas(Shenzhen)Co.Ltd. (Shenzhen,China).
2.2.Experimental procedure
A schematic diagram of the GAS apparatus is shown in a previous paper[19].The unit consists of three sections:a CO2supplying system controlled by Isco Series D pump power controller(Teledyne Isco,Inc.,Lincoln,NE),a crystallizing chamber (Thar Designs Inc.,Pittsburgh,PA),and a depressurizing section.The temperature of the vessel was maintained in an Athena heater(Model 2000-B,Athena Controls,Inc.,Plymouth Meeting, PA).In the batch GAS step confguration,the precipitation unit was initially loaded with a volume of Pur solution.Then CO2was added until the fnal pressure was reached.The rate of CO2addition is 75 ml/min.The volume of the precipitation vessel was 250 ml.The vessel was flled with CO2at the desired temperature and left for 3 h without any agitation.A pure constant CO2fow rate of 25 ml/min was then maintained in order to completely remove the residual solvent.After this washing step which lasted for approximately 90 min,the autoclave was depressurized for 30 min at the experimental temperature.Finally, the dry solid powder was collected for off-line analysis.
GAS experiments were carried out with the following experimental conditions range:recrystallization temperature of 30–43°C,recrystallization pressure of 8–14 MPa,Pur solute concentration of 60–120 mg/ml,solution addition volume of 10–100 ml,antisolvent time of 1–6 h,type of solvent of absolute methanol,absolute ethanol and acetone,antisolvent addition rate of 25–105 ml/min.
2.3.Powder morphology
Samples of the processed powder were observed by scanning electron microscopy(SEM;Jeol JSM-6460LV,Japan).Powder samples were manually dispersed on an aluminum stub with a thin self-adhered carbon flm.The samples were coated with a thin layer of gold using an ion sputter under 0.5 mbar argon atmosphere(at room temperature for 90 s,at an accelerating voltage of 20 kV,working distance of 15 mm,and at 1000,3000 and 5000 magnifcation).
2.4.Particle size distribution
Particle size distribution(PSD)was measured by laser diffraction using Master size 2000 laser particle analyzer(Malvern Instruments Ltd.,Malvern,UK).The method used for analysis was dry method.
3.Results and discussion
The detailed operating conditions and the particle size results of the GAS recrystallization experimental runs are reported in Table 1.The recrystallization of Pur was performed by changing several experimental variables of the GAS process as shown in the above range.Of all the variables,the type of solvent most strongly affected the crystal form and morphology.The temperature,addition volume and antisolvent addition rate most strongly affected the particle size.The reproducibility of the experimental results had been duly checked.A good reproducibility of the obtained particle size and size distribution was observed.In general,average measured yields from experiments were determined to be above 90%.The trends in particle size and shape from manipulation of these variables were discussed below accordingly.
3.1.Effect of the recrystallization pressure
Several experiments were conducted in the pressure range of 8–14 MPa,with the other operating conditions maintaining at the temperature of 38°C,antisolvent addition rate of 75 ml/ min,Pur concentration of 100 mg/ml,solvent of absolute ethanol,antisolvent time of 3 h and addition volume of 50 ml. When the pressure was increased from 8 MPa to 14 MPa,the particle size of Pur is increased(Fig.1A).It was reported that when pressure was high,it was in favor of nucleation,which created a lot of nucleus and thus we obtained crystals with smaller size[13].When the pressure was low,the solvent was not fully expanded,more solvent existed between particles,and the crystal’s growth time was prolonged and larger particleswere obtained.In our investigation,the higher the pressure, the stronger the dissolving abilities of supercritical CO2to ethanol and thus the higher degree of supersaturation.A large amount of micronuclei is precipitated instantaneously,and conglutination between particles may lead to the increased particle size.The variation of pressure is directly related to the rate of nucleation and crystal growth and may result in a different crystal molecular arrangement.Yet in our research,the pressure appeared to have little effect on the particle shape of Pur, which all exhibited as needle-like crystals as shown in Fig.2. When the pressure was increased to 14 MPa,the increased solubility of Pur in the supercritical solution and the decreased supersaturation lead to more needle crystals and the particle size is increased accordingly.

Table 1–Process parameters and particle size result for Pur crystals prepared by GAS.

Fig.1–Effect of various conditions on the particle size of puerarin in GAS process.Pressures(A),temperatures(B), concentrations(C),antisolvent times(D),addition volumes(E),antisolvent addition rates(F)and solvents(G)were compared.
3.2.Effect of the recrystallization temperature
Experiments were performed at fxed conditions as mentioned above while varying the temperatures only.As illustrated in Fig.1B,an increase in the process temperature(33,38,and 43°C)for the recrystallization process resulted in a direct decrease of the Pur particle size and a narrowing of the size distribution.The particle size is similar at 38°C and 43°C, but it is increased tremendously at 33°C.Temperature has two different effects on the particle size.On the one hand, higher temperature promotes the mass transfer between solvent and supercritical CO2which favor the formation of high supersaturation and smaller particles are prepared.On the other hand,higher temperature leads to the reduction of CO2density and the reduction of the dissolving ability for solvent.Thus supersaturation is decreased and bigger particles are formed. High temperature would also accelerate the molecular motion that favors the particles aggregation.Finally,the above positive and negative impacts determine the formation of particles[20].Besides,the crystallization temperature has practically no effect on the crystal habit of Pur within the temperature range investigated.Pur crystal particles prepared at different temperatures are mainly characterized by prismatic crystals with needle-like structures which are almost the same(Fig.3).
3.3.Effect of the Pur solution concentration
We varied the concentrations of Pur ethanol solution from 60 to 120 mg/ml while maintaining other process parameters.The results showed that an increase in Pur concentration gave a decrease in particle size,then a little increase at Pur concentrations above 100 mg/ml(Fig.1C).This could be explained by a combined effect of two factors in terms of nucleation and growth of particles.It is commonly admitted that increasing the solute concentration results in larger particles and enlarged PSD.At higher solute concentrations,precipitation of the solute occurs earlier in time during the expansion process, resulting in increased time for crystal growth.At lower solute concentrations,precipitation of the solute is reached later during the expansion process;hence,nucleation is the prevailing mechanism giving smaller particles[8].In our research we noted that the smallest particle size was got in the middle of the concentration range.Firstly,at concentrations below 100 mg/ml, supersaturation is increased with the increase of the solute concentration,and the primary nuclei size would diminish to the smallest at 100 mg/ml;then,when the concentration was above 100 mg/ml,high solute concentrations produced more aggregated particles.The higher the nuclei concentration,the higher the interaction between particles formed.Particles agglomerate at high Pur concentrations and PSD is broadened.DifferentPur concentrations in the GAS process produced particles with similar morphology(Fig.4).Low solute concentrations produce long prism crystals not very uniformly while high concentrations favor the uniform particle appearances and the length of prisms shortens.Because of dilute solutions,low supersaturation is produced and less particles are formed.The growth mode of particles dominates and long prism-like structure is obtained.

Fig.3–SEM micrographs of Pur precipitated with different temperature:(A)33°C,(B)38°C and(C)43°C.
3.4.Effect of the antisolvent time

Fig.4–SEM micrographs of Pur precipitated with different Pur concentration:(A)60 mg/ml,(B)80 mg/ml,(C)100 mg/ml and (D)120 mg/ml.
It was reported that in the GAS process the solute crystallized more often in a supercritical mixture of CO2and solvent.The antisolvent time in this mixture may induce transformations as dissolution–recrystallization,solid/solid transition, etc.Therefore,the antisolvent time of the powder in crystallization vessel may be an important parameter for themorphology of the powder fnally obtained(polymorphism, aggregation...)[21].In our research,the results of particle size showed that too short and too long antisolvent time all led to the increase of particle size(Fig.1D).The extension of antisolvent time has a negative infuence on the decrease of particle size when a certain time has reached.3 h is enough for the shortening of experiment time.Yet the antisolvent time has no effect on the particle morphology(Fig.5).

Fig.5–SEM micrographs of Pur precipitated with different residence time:(A)1 h,(B)3 h and(C)6 h.
3.5.Effect of the addition volume
Experiments were performed at fxed conditions as mentioned above while varying the addition volume in the precipitation vessel between 10,25,50 and 100 ml.The effect of this process parameter was not investigated in previous similar researches.From our results,it should be noted that the initial addition of volume in the precipitation vessel does have effect on the particle size of Pur crystals.When the solution was less than 50 ml in volume,the larger the volume, the smaller the particle size(Fig.1E).The PSD is narrowest at the lowest volume of 10 ml,but the size is biggest,a really strange phenomenon.When the solution volume was 100 ml, the largest particle size and the widest PSD of Pur crystals are obtained.The volume expansion rate is high when the initial solution volume is small.Thus a large number of particles were precipitated and collided into bigger particles while the PSD was uniform.When the volume was 100 ml,the precipitation vessel was relatively overloaded,and the expansion rate of volume was pretty low.Few particles were produced and the growth dominated over nucleation,so the largest particles were got.The SEM picture indicated that there was no difference between particles morphology at different solution volumes (Fig.6),but it was clear that the particle size distribution went to a relatively wide range.Because a high solution volume implicated a lower supersaturation and bigger particles were produced,some small ones were also produced.And thus a wide distribution was achieved.
3.6.Effect of the antisolvent addition rate
First,let us focus on the experiment runs numbered 17,9 and 18 in Table 1,which have been performed at increasing values of the antisolvent addition rate.Pur crystal microparticles formed at 25 ml/min and 75 ml/min had similar size,while particles generated at 105 ml/min had much bigger ones(Fig.1F). The various process parameters of the GAS process such as the type of solvent,crystallizing temperature,and antisolvent injection rate clearly affected the solid-state characteristics of particular hydrophobic drug particles[22].The magnitude of the supersaturation level is a strong function of the applied volumetric expansion rate.A faster rate of antisolvent addition will generate higher levels of supersaturation,thus,higher rates of nucleation,and consequently,a larger number of smaller size particles with narrow particle size distribution.Oppositely,low addition rates caused both nucleation and crystal growth.The competition between the nucleation and growth dynamics of the GAS process needs to be considered to explain the bigger particles formed at lower pressure addition rates[23]. Our contrary experimental results with GAS micronization of Pur particles indicated that during the rapid pressure increasing process at 105 ml/min of antisolvent addition rate,the solution may achieve supersaturation locally and some crystal nuclei were formed frstly,thus the particle size distribution was uneven and bigger particles were obtained since the consumption of the solute mainly depended on the growth of the nuclei.SEM photomicrograph showed that the crystal habit changed a little bit at different antisolvent addition rates(Fig.7). The slowest rate resulted in regular short prisms and the fastestrate produced both prism-like and needle-like crystal morphology with obvious increase in the particle size.The two kinds of particles morphology produced at the highest rate of pressure stemmed from the local expansion in the solution and different growth rates of the crystal surfaces of a single crystal [19].To go into more details,a remarkable reduction of the crystal dimensions with a restricted particle size distribution was obtained at 25 and 75 ml/min of antisolvent addition rates, with 75 ml/min seemingly being the most effcient rate to micronize this drug as a whole.

Fig.6–SEM micrographs of Pur precipitated with different addition volume:(A)10 ml,(B)25 ml,(C)50 ml and(D)100 ml.
3.7.Effect of the solvent
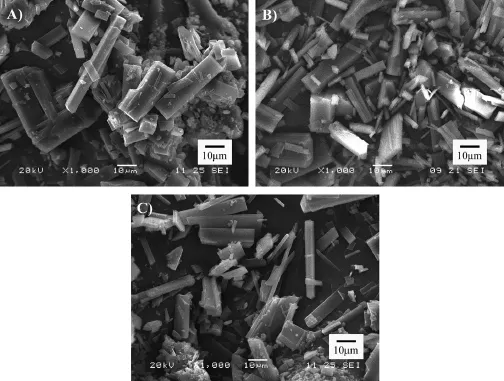
Fig.7–SEM micrographs of Pur precipitated with different antisolvent addition rate:(A)25 ml/min,(B)75 ml/min and(C) 105 ml/min.
The effect of solvent on determining the crystal growth mechanism in liquid crystallization is a subject of extensive research. The degree of crystallinity of the formed particles isimportant for processing pharmaceuticals,as it sheds some light on the role of the solvent during particle growth.To investigate the infuence of the solvent on Pur crystallinity,the effect of the organic solvents,methanol and acetone were studied in addition to ethanol.The results showed that compared with other process parameters,the type of solvent had a dramatic effect on particle size,the external shape and internal characteristics of crystals as reported in other researches [6,24].The particle size was smallest with ethanol as solvent and the size was biggest with methanol as solvent(Fig.1G). This result encouraged investigating the infuence of solvents on the physicochemical properties of pharmaceutical compounds in our future studies.
The precipitation of Pur from ethanol and methanol gave a very light,voluminous powder,as typical of CO2-precipitated samples showing long prisms and needle morphology in SEM pictures(Fig.8A and B)which was consistent with the research of Moneghini et al.[25],whilst the gross morphology of sample treated with acetone was less voluminous and characterized by the presence of long needles with brushes(Fig.8C). The variation of solvent to acetone resulted in a modifcation of the crystal habit of Pur.But whatever the solvent used,Pur produced by GAS was all a crystalline product,contrary to what happened in the case of SEDS(Solution Enhanced Dispersion by Supercritical Fluid)method where amorphous microparticles have been obtained[26].Though the external shape of Pur crystals was different in acetone,the crystal pattern was the same with Pur obtained in ethanol which was identifed and published in another paper by the confrmation of a series of characteristic analysis[27].It was known that inclusion of solvent molecules in the solid lattice and/or interaction of the solvent with the growing crystal surface may drastically change crystal morphology.Therefore,the results obtained in these experiments were not surprising even if it was rather diffcult to explain and predict them.

Fig.8–SEM micrographs of Pur precipitated with different solvent:(A)Methanol,(B)Ethanol and(C)Acetone.

Fig.9–SEM micrograph of commercial Pur.
Generally,Pur recrystallization by GAS in this experiment all reproducibly yielded a crystalline product at each condition.At most conditions,Pur produced was in the same crystal form(named crystal form II),while with 60 mg/ml as the Pur concentration(crystal form III)and with methanol as solvent (crystal form IV),Pur in two new crystal forms was achieved. The confrmation of the new crystal forms was published in another paper[27].The GAS processed crystals in three crystal forms all showed more ordered appearances with clean surfaces and sharp angles compared with the unprocessed particles (Fig.9)and there was only one pure crystal produced at eachexperimental condition.Various general studies on the recrystallization of drug by GAS method also indicated a change in particle morphology[28,29].Besides the newly generated crystal form,the effects of various process conditions on Pur crystals were mainly investigated.From the particle size results, the optimized process conditions for Pur in crystal form II were: pressure of 10 MPa,temperature of 38°C,antisolvent addition rate of 75 ml/min,Pur concentration of 100 mg/ml, antisolvent time of 3 h,solvent of ethanol and addition volume of 50 ml.Under these conditions,Pur crystals with the average size of 30.34 μm were obtained.Compared with the commercial Pur sized 45.34 μm,Pur recrystallized by GAS in crystal form II reduced the particle size dramatically,thus this may facilitate its dissolution rate and further enhance the bioavailability which should be confrmed in future research.
4.Conclusion
In this study,the GAS crystallization technique has been applied to the preparation of Pur.To sum up,it was clear that several parameters had an infuence on the size and morphology of the crystals obtained and it was demonstrated that the particles’mean diameter and size distribution can be strongly controlled through the manipulation of the process parameters in GAS process.Of all process parameters,a good choice of the solute concentration and the solvent may lead to new pure polymorphs,while temperature,addition volume,and antisolvent addition rate had many infuences on the particle size and size distribution.It must be pointed out that the analysis of morphology and the size of the drug showed a dramatic change of the crystals after treatment with supercritical fuids,indicating the formation of a light voluminous powder made of small particles having needle-like and prism habit. The GAS recrystallization process may provide an entirely different concept and kinetics for nucleation and molecular arrangement.Therefore,adopting a GAS process to recrystallize pharmaceutical compounds will provide a highly versatile methodology to generate new polymorphs of the drug in addition to conventional crystallization techniques and provide the potential for better therapeutic effcacy of the drug because of the newly generated crystal form.
Acknowledgements
This work was supported by the Basic Research Program from Science,Industry,Trade and Information Technology CommissionofShenzhenMunicipality(Grantno. JCYJ20130402145002398)and National Natural Science Foundation of China(Grant no.81102824).
R E F E R E N C E S
[
1]Perrut M,Jung J,Leboeuf F.Enhancement of dissolution rate of poorly-soluble active ingredients by supercritical fuid processes.Part I:micronization of neat particles.Int J Pharm 2005;288:3–10.
[2]Pasquali I,Bettini R,Giordano F.Supercritical fuid technologies:An innovative approach for manipulating the solid-state of pharmaceuticals.Adv Drug Deliver Rev 2008;60:399–410.
[3]Yadav AV,Yadav VB.Pharmaceutical application of supercritical fuid technique:An overview.J Pharm Res 2009;2:31–44.
[4]Chingunpitak J,Puttipipatkhachorn S.Micronization of dihydroartemisinin by rapid expansion of supercritical solutions.Drug Dev Ind Pharm 2008;34:609–617.
[5]Kim MS,Jin SJ,Kim JS,et al.Preparation,characterization and in vivo evaluation of amorphous atorvastatin calcium nanoparticles using supercritical antisolvent(SAS)process. Eur J Pharm Biopharm 2008;69:454–465.
[6]Kim YH,Shin KS.Supercritical fuid-micronized ipratropium bromide for pulmonary drug delivery.Powder Technol 2008;182:25–32.
[7]Kim YH,Sioutas C,Shing KS.Infuence of stabilizers on the physicochemical characteristics of inhaled insulin powders produced by supercritical antisolvent process.Pharm Res 2009;26:61–71.
[8]Bakhbakhi Y,Charpentier PA,Rohani S.Experimental study of the GAS process for producing microparticles of beclomethasone-17,21-dipropionate suitable for pulmonary delivery.Int J Pharm 2006;309:71–80.
[9]Okamoto H,Danjo K.Application of supercritical fuid to preparation of powders of high-molecular weight drugs for inhalation.Adv Drug Deliver Rev 2008;60:433–446.
[10]Mochizuke S,Teramoto A,Yamashita F,et al.Size-controlled recrystallization of fullerene by gas-antisolvent process.J Mater Sci 2010;45:1588–1594.
[11]Bouchard A,Jovanovic N,Hofand GW,et al.Ways of manipulating the polymorphism of glycine during supercritical fuid crystallization.J Supercit Fluids 2008;44:422–432.
[12]Moribe K,Tozuka Y,Yamamoto K.Supercritical carbon dioxide processing of active pharmaceutical ingredients for polymorphic control and for complex formation.Adv Drug Deliver Rev 2008;60:328–338.
[13]Chen KX,Zhang XY,Pan J.Recrystallization of andrographolide using the supercritical fuid antisolvent process.J Crystal Growth 2005;274:226–232.
[14]Ruan LP,Chen S,Yu BY,et al.Prediction of human absorption of natural compounds by the non-everted rat intestinal sac model.Eur J Med Chem 2006;41:605–610.
[15]Wang J,Ji M,Hua WY,et al.Development of puerarin.Prog Pharm Sci 2003;27:70–73.
[16]Li Y,Pan WS,Chen SL,et al.Pharmacokinetic,tissue distribution,and excretion of puerarin and puerarinphospholipid complex in rats.Drug Dev Ind Pharm 2006;32:413–422.
[17]Li Y,Pan WS,Chen SL,et al.Studies on preparation of puerarin phytosomes and their solid dispersions.Chin Pharm J 2006;37:695–697.
[18]Pan J,Zhang Y,Yuan CX.Studies on the polymorphism of puerarin.Chin J Pharm Anal 2004;24:119–122.
[19]Yeo SD,Kim MS,Lee JC.Recrystallization of sulfathiazole and chlorpropamide using the supercritical fuid antisolvent process.J Supercrit Fluids 2003;25:143–154.
[20]Wang HY,Zhang LZ,Zhang MH.Supercritical antisolvent and the application in pharmaceuticals.Chem Ind Eng Prog 2004;23:705–709.
[21]Fages J,Lochard H,Letourneau JJ,et al.Particle generation for pharmaceutical applications using supercritical fuid technology.Powder Tech 2004;141:219–226.
[22]Park SJ,Yeo SD.Recrystallization of caffeine using gas antisolvent process.J Supercrit Fluids 2008;47:85–92.
[23]Zhao HW,Liang Y.Elementary studies of gas antisolvent crystallization technique.Heilongjiang Med J 2003;16:452–454.
[24]Park SJ,Yeo SD.Antisolvent crystallization of sulfa drugs and the effect of process parameters.Sep Sci Technol 2007;42:2645–2660.
[25]Moneghini M,Kikic I,Voinovich D,et al.Study of solid state of carbamazepine after processing with gas anti-solvent technique.Eur J Pharm Biopharm 2003;56:281–289.
[26]Li Y,Yang DJ,Chen SL,et al.Comparative physicochemical characterization of phospholipids complex of puerarin formulated by conventional and supercritical methods. Pharm Res 2008;25:563–577.
[27]Li Y,Yang DJ,Zhou W,et al.Recrystallization of puerarin using the supercritical fuid antisolvent process.J Crystal Growth 2012;340:142–148.
[28]Sanganwara GP,Sathiqari S,Babu RJ,et al.Simultaneous production and co-mixing of microparticles of nevirapine with excipients by supercritical antisolvent method for dissolution enhancement.Eur J Pharm Sci 2010;39:164–174.
[29]Kim MS,Lee S,Park JS,et al.Micronization of cilostazol using supercritical antisolvent process:effect of process parameters.Powder Technol 2007;177:64–70.
*< class="emphasis_italic">Corresponding author.
.Shenyang Pharmaceutical University,No.103,Wenhua Road,Shenyang 110016,China.Tel.:+86 755 23986313; 15840481348.
E-mail address:yingliwhb@163.com(H.Wang).
**Corresponding author.Research Institute of Joincare Pharmaceutical Group Industry Co.Ltd,No.17 Lang Shan Road,North Area of High-Tech Park,Nanshan District,Shenzhen 518057,China.Tel.:+86 755 86252038;13825202338;fax:+86 755 86252165.
E-mail address:zhaofengguang@joincare.com(F.Zhao).
Peer review under responsibility of Shenyang Pharmaceutical University.
http://dx.doi.org/10.1016/j.ajps.2015.12.001
1818-0876/?2016 The Authors.Production and hosting by Elsevier B.V.on behalf of Shenyang Pharmaceutical University.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
 Asian Journal of Pharmacentical Sciences2016年2期
Asian Journal of Pharmacentical Sciences2016年2期
- Asian Journal of Pharmacentical Sciences的其它文章
- Preparation and characterization of solidifed oleanolic acid–phospholipid complex aiming to improve the dissolution of oleanolic acid
- Development of a topical ointment of betamethasone dipropionate loaded nanostructured lipid carrier
- Supersaturation induced by Itraconazole/Soluplus?micelles provided high GI absorption in vivo
- Quality assessment of Chrysanthemum indicum Flower by simultaneous quantifcation of six major ingredients using a single reference standard combined with HPLC fngerprint analysis
- Rapid and sensitive analysis of melatonin by LC-MS/MS and its application to pharmacokinetic study in dogs
- The 1st Euro-Mediterranean Workshop:Natural Products in Health and Diseases:Cairo,Egypt, March 2,2015
