Effects of Hydroxyl Groups in Organic Ammonium Counterions on the Properties of Fluorinated Surfactants
Zhang Tiantian, Zhou Hongyu, Wu Bowan, Xiao Jinxin
Longdong University—Fluobon Surfactant Engineering Technology Center, College of Chemistry and Chemical Engineering,Longdong University, China
Xing Hang
Beijing FLUOBON Surfactant Institute, China
Introduction
The molecular structure of ionic surfactants can be divided into two parts, namely the “surfactive ion” and the “counterion”. The surfactive ion contributes to the main function of a surfactant while the counterion can adjust the properties of a surfactant at different levels.The counterions of surfactants have been developed so far in diversity of types and structures, ranging from simple inorganic ions[1~3]to organic ions,[1,4~6]and from single charged ions to multiple charged ions with delicate structures.[7]

Among the known surfactants, the surface activity of fluorinated surfactants is outstanding. The unique properties arising from fluorination have made them irreplaceable in many application fields.[8~10]For ionic fluorinated surfactants,the counterions have affacted not only the solubility (e.g.Krafft point), but also the surface activity of the surfactant.Compared with the research work on hydrocarbon surfactants, the effects of organic counterions on the properties of fluorinated surfactants[11~14]are relatively less studied. It has shown that the effects of organic counterions are greatly larger than that of inorganic ones on the surface activity of fluorinated surfactants. For example, the tetraethylammonium ion has stronger adsorption ability onto perfluorooctanesulfonate micelles than lithium,[15,16]while the aqueous solution of tetrabutylammonium perfluorooctanoate even shows abnormal two cloudpoint phenomena.[17,18]As for fluorinated surfactants, the attention to organic counterions (mostly organic ammonium ions) has been mainly focused on the surfactant series of perfluorooctanesulfonates and perfluorooctanoates,including perfluorooctanoates with counterions such as tetraalkylammonium ions,[19~22]trialkylammonium ions,[23~25]dialkylammonium ions[25,26]and monoalkylammonium ions[25]and perfluorooctanesulfonates with counterions such as dialkylammonium ions[26]and trialkylammonium ions.[26]Compared with conventional inorganic counterions, fluorinated surfactants with organic counterions usually have higher surface activity, lower melting points (some of them are even roomtemperature ionic liquids)[25]and stronger tendency to form bilayer structures.[25]It is also shown that increasing the chain length of alkyl groups in counterion will facilitate the micellization of fluorinated surfactants.[25,26]
However, the effects of hydroxyl groups in organic ammonium counterions on the properties of fluorinated surfactants have not been studied in a systematical way so as to find the contribution solely of hydroxyl groups. Although fluorinated surfactants with mono-/di-/triethanolammonium ions as counterions have been reported previously,[27]such serial change in counterion structure is being added with the unit of CH2CH2OH successively, rather than simply respond to the effects of hydroxyl group. In this work, a series of counterion structures with 1, 2 or 3 hydroxyl groups being added to the basic structure of N+H(CH2CH3)3were studied(Scheme 1). The surface activity, foaming ability and the viscosity of corresponding fluorinated surfactants were measured thus to reflect the relationship between the number of hydroxyl groups in organic counterion and the performances of fluorinated surfactants. Herein, both perfluorooctanesulfonate and perfluorooctanoate were selected as surfactive ions, considering that the effects of counterions are expected to be closely related to their interactions with the headgroups from surfactive ions.
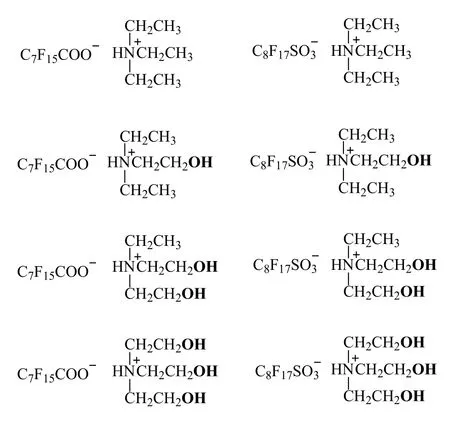
Scheme 1. Structure of fluorinated surfactants in this work
Experimental section
Materials
Perfluorooctanoic acid (C7F15COOH, 96%) was purchased from Acros. Perfluorooctanesulfonic acid(C8F17SO3H, 98%) was a product of Beijing FLUOBON Surfactant Institute which was synthesized according to the procedure in literature.[26]Anhydrous sodium carbonate (AR), triethylamine (AR) and triethanolamine(AR) were purchased from Sinopharm Chemical Reagent Beijing Co., Ltd. Diethanolamine (AR),diethylaminoethanol (AR) and bromoethane (AR)were purchased from Xiya Reagent. Ethanol (AR) was a product of Beijing Chemical Works. All aqueous solutions were prepared using deionized water.
Synthesis of N-ethyldiethanolamine
N-Ethyldiethanolamine was synthesized with the method from reference[28]in the following procedure:
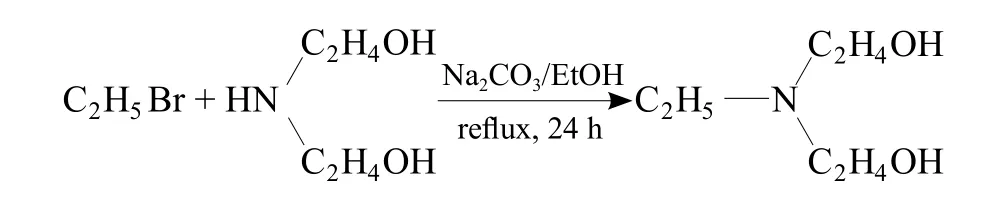
21.0 g diethanolamine (0.2 mmol), 27.6 g powdery anhydrous sodium carbonate (0.26 mmol) and 25.0 g bromoethane (0.23 mmol) were put into a 250 mL roundbottomed flask, and then 100 mL ethanol was added to the flask. A solid-liquid two-phase mixture was obtained after stirring at room temperature, in which the sodium carbonate had obviously turned to granules in appearance.The mixture was then heated to reflux and the reaction temperature was kept at 42 °C for 24 h. The granular solid gradually turned to slurry with the reaction proceeding and the reflux rate slowed down obviously. The mixture was then cooled to room temperature and treated with suction filtration to collect filtrate. The filter residue was washed with 100 mL ethanol, and the washing liquid was put together with the filtrate. Thus clear and colorless liquid was obtianed. Then the solvent and the unreacted bromoethane was removed by rotary evaporation. Keep it at 80°C for 30 min. Viscous colorless liquid together with white solid were obtained after cooling. Reduced pressure distillation was used to collect the cut fraction(13.3 Pa, 110~118 °C). No front cut fraction was found,neither after-cut was found after 130 °C. 17.60 g product of N-ethyldiethanolamine was obtained in appearance of viscous colorless transparent liquid (yield 74%).

Preparation of fluorinated surfactants with different counterions
The fluorinated surfactants with corresponding counterions were prepared by neutralizing perfluorooctanoic acid or perfluorooctanesulfonic acid with triethylamine,diethylaminoethanol, N-ethyldiethanolamine and triethanolamine, respectively. First, quantitative perfluorinated acid was put into a small beaker. Small amount of water was added and a turbid suspension was obtained under magnetic stirring. With the dropwise addition of another aqueous solution in which organic amine had already been diluted or dispersed,the undissolved perfluorinated acid became gradually dissolved during neutralization with pH detection by Delta320 pH meter (Metler-Toledo) under magnetic stirring. Finally, until the pH was 7~8 which was no longer changed for at least 15 minutes, the aqueous solution thus obtained was then carefully transferred(together with washing liquo) and brought to volume in a volumetric flask as the stock solution of corresponding fluorinated surfactant.
Methods
Measurement of surface activity of surfactants. The surface tension was measured by drop volume method[29]at (25.0 ± 0.1) °C. The critical micelle concentration (cmc)and the surface tension at cmc (γcmc) were obtained from the break points of γ-lgc curve. The maximum amount adsorbed (Γmax) and the minimum molecular area (Amin)in the surface adsorption layer were estimated using Gibbs equation.[29]
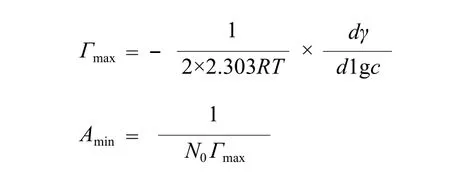
where c is the concentration of surfactant, N0is the Avogadro constant, R is the gas constant, and T is the thermodynamic temperature.
Measurement of foam expansion raio and 25%drainage time.The foam expansion ratio (α) was measured in the following steps:[30]3 mL aqueous solution was put into a 100 mL measuring cylinder with stopper.Close the stopper and shake violently until the height of foam was no longer increased. The foam expansion ratio(α) was defined as α = V/3, where V (expressed in mL) is the final volume of foam.
25% drainage time was defined by the time elapsed when the volume of drained liquid was equal to 25%of the volume of liquid before foaming.[30]Prepartory work: 0.75 g deionized water was put into the measuring cylinder with stopper, then the measuring cylinder was put onto a level table. The measuring cylinder was marked with a line along the liquid level to denote the volume of 0.75 mL. Determination: 3 mL aqueous solution was put into the marked measuring cylinder. Close the stopper and shake violently until the height of foam was no longer increased. Start the timer immediately after shaking, put the measuring cylinder onto a level table rapidly, then open the stopper and record the time when the drained liquid reaches the marked line (i.e. 25% drainage time).
Measurement of viscosity of surfactant solutions.The viscosity of the aqueous solution was measured on a Ubbelohde viscometer at (25.0 ± 0.1) °C. It was expressed in relative viscosity(ηr), as defined below, where η is the viscosity of surfactant solution and η0is the viscosity of pure solvent (water).

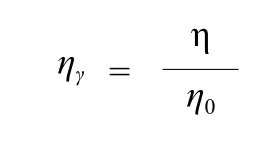
Results and discussion
Effects of counterions on the surface activity of surfactants
Figure 1 shows the variation of surface tension (γ) with the concentration (c) of perfluorooctanoates (Figure 1a) and perfluorooctanesulfonates (Figure 1b) at 25 °C. No minima were observed in the surface tension curves (γ-lgc) of these surfactants,indicative of no surface active impurities.[29]The values of cmc,γcmc, Γmaxand Aminobtained from Figure 1 are listed in Table 1.
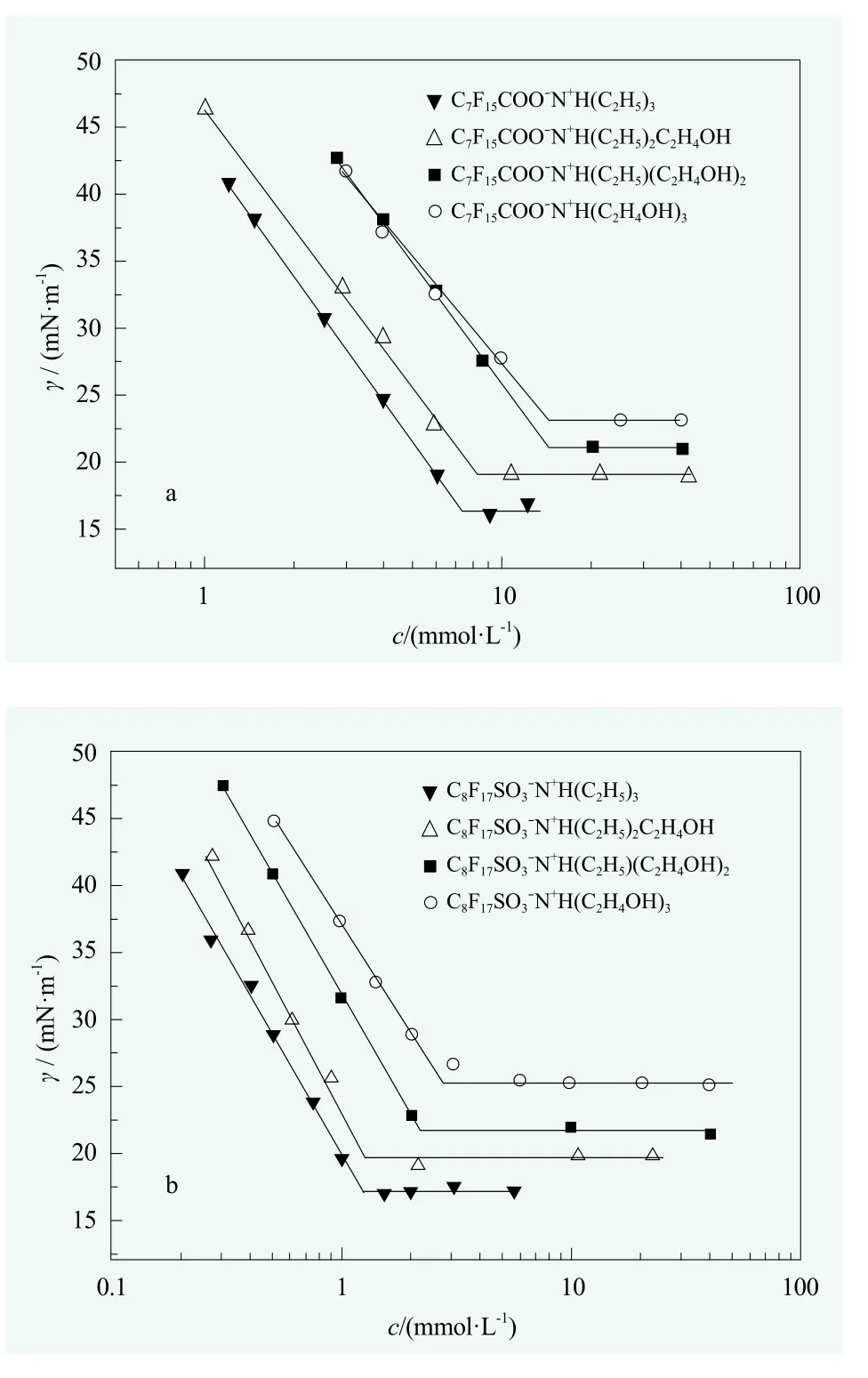
Figure 1. Surface tension (γ) vs the concentration (c) for the aqueous solutions of surfactants with different counterions at 25 °C; a. Perfluorooctanoate surfactants;b. Perfluorooctanesulfonate surfactants
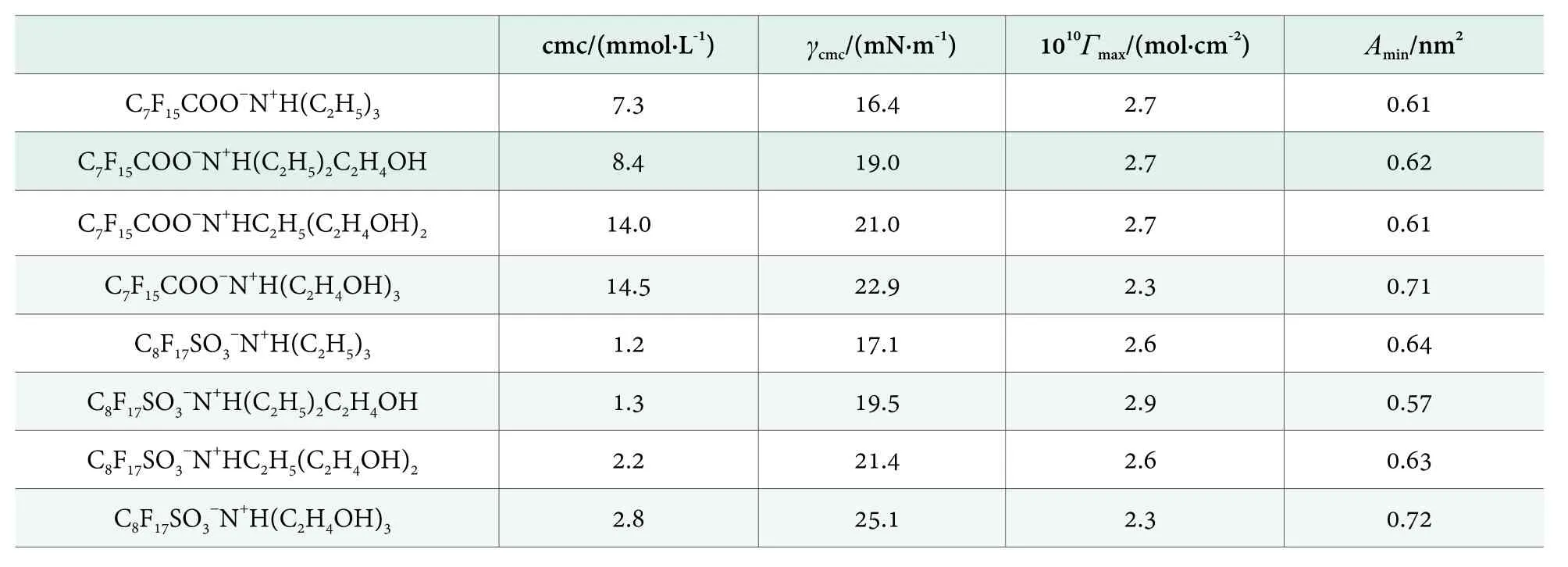
Table 1. The cmc, γcmc, Γmax and Amin for perfluorooctanoate surfactants and perfluorooctanesulfonate surfactants at 25 °C
As shown in Table 1, both cmc and γcmcwere increased with the increasing number of hydroxyl groups in counterion structure, no matter the surfactive ion was perfluorooctanoate or perfluorooctanesulfonate. In other words, introducing hydroxyl group to the alkyl groups in counterion structure would do harm to the surface activity. It was consistent with the rule reported for cationic hydrocarbon surfactants: It is known that the cmc and γcmcare also increased with the hydrophilicity of counterions for quaternary ammonium surfactants with counterions of HCOO?, CH3COO?and CH3CH(OH)COO?.[4]
SinceΓmaxholds a reciprocal relationship with Amin, herein only the results of Aminwere selected for discussion. As shown in Table 1, in the series of C8F17SO3?N+H(C2H4OH)x(C2H5)3-x,the Amindecreased at first and then increased when the number of hydroxyl groups (x) was increased from 0, 1, 2 to 3; however,in the series of C7F15COO?N+H(C2H4OH)x(C2H5)3-x, the Aminwas almost identical for x = 0, 1 and 2 and then remarkably increased when x = 3. It could be explained by the combined influences from the following three factors:
(i) Hydrogen bond. When the H atom from the hydroxyl group in counterion got close enough to the O atom from the headgroup in surfactive ion, a hydrogen bond would form.Such hydrogen bonding would draw the headgroups closer to each other and thus lead to smaller Amin.
(ii) The hydrophobicity of counterion. The hydrophobicity of counterion was reduced by hydroxyl group thus the tendency of counterion for participation in surface adsorption layer would be weakened. As a result, the less electrostatic screening upon headgroups would lead to larger Amin.
(iii) Steric effect. The counterion containing more hydroxyl groups will be more bulky in size. Additionally, it also means that the charge density of counterion will be reduced at the same time (resulting in less electrostatic screening upon headgroups). Both considerations would lead to larger Amin.
In the case of C8F17SO3?N+H(C2H4OH)x(C2H5)3-x, the headgroup —SO3?had three O atoms, which was in favor of hydrogen bonding. Therefore, if one hydroxyl group was introduced into the counterion structure (i.e. x from 0 to 1),factor (i) was dominated and thus the Amin became smaller;If the number of hydroxyl group kept on increasing, factors (ii)and (iii) would become increasingly overwhelming, thus Aminwas then increased when x was changed from 1 to 3.
In contrast, as for C7F15COO?N+H(C2H4OH)x(C2H5)3-x,the headgroup —COO?had only two O atoms, thus less probability of hydrogen bond formation was expected. In addtion, hydrogen bond has directionality. The headgroup—SO3?was tetrahedral whereas —COO?was planar. The geometry of the latter would be relatively more confined in hydrogen bonding with the H from counterion hydroxyl group. Therefore, as a combination of the three factors (i),(ii) and (iii), no change of Aminwas found when introducing 1 or 2 hydroxyl groups in counterion, and Amindid not begin to increase unless the third hydroxyl group was added when factors (ii) and (iii) became increasingly dominated.
In one word, C7F15COO?with carboxylate headgroup was inferior to C8F17SO3?in their abilities of forming hydrogen bond with N+H(C2H4OH)x(C2H5)3-xcounterions,thus during the increment of OH in counterion structure no signs of closer packing had ever shown.
Song et al.[28]have reported the surface activity of cationic hydrocarbon surfactants, C18H37N+(C2H4OH)x(C2H5)3?xBr?(x = 0, 1, 2, 3), in which the effects of the number of hydroxyl groups in the headgroups have been revealed. The Aminkeeps on decreasing remarkably owing to hydrogen bonding because the hydroxyl groups are located in the headgroup and thus directly in action.[28]Here, as mentioned above, the hydrogen bonding was also taken into account as a factor in lowering Aminin the present work, although the behavior of hydroxyl groups being in counterion was much more complex.
The differences between perfluorooctanoate and perfluorooctanesulfonate with the same counterion
The surface activity was compared between the perfluorooctanoate (C7F15COO?) and the perfluorooctanesulfonate(C8F17SO3?) with the same counterion. According to Table 1, for the same counterion, perfluorooctanesulfonates exhibited much smaller cmc than those of perfluorooctanoates, however, the γcmcof perfluorooctanesulfonates were larger than that of perfluorooctanoates. It is generally known that the longer hydrophobic tail is, the smaller cmc of a surfactant will be. C8F17SO3?has one more CF2in its hydrophobic tail and consequently exhibits stronger micellization than C7F15COO?, as shown by the cmc values in Table 1.However, the γcmcof perfluorooctanesulfonates were larger than that of perfluorooctanoates (Table 1), which could be ascribed to the difference in charge density of headgroups.Quantum chemical calculation on these two surfactive ions had shown that the negative charge of sulfonic headgroup (?0.526) was smaller than that of carboxyl headgroup (?0.773),[26]thus the headgroup of C8F17SO3?would exhibit smaller binding ability to counterions than the headgroup of C7F15COO?, leading to higher water penetration in surface adsorption layer and larger γcmc.
Effects of counterions on the foaming properties and viscosity of surfactants
Foam expansion ratio.The effects of counterions on the foam expansion ratio, 25% drainage time and solution viscosity for perfluorooctanoates and perfluorooctanesulfonates are shown in Figures 2, 3 and 4, respectively. It should be noted that, the maximum volume scale for measuring the foam volume was 100 mL,thus the upper limit of measuring foam expansion ratio was 33.3. Since the main purpose of the following work was to investigate the change of foaming performances before and after cmc, it didn’t disturb us in discussing the relative rules of counterion effect on foaming. On the other hand, the ordered structure within the surfactant solutions at high concentrations and related properties were left in future studies.
The influencing factors upon the foaming of a surfactant solution include the surface tension, the rigidity and elasticity of liquid membrane, electrostatic repulsion,viscosity, etc.[31]As shown in Figures 2a and 2b, with the increasing number of hydroxyl groups in counterion,completely different trends of foam expansion ratio (α) vs concentration were observed for perfluorooctanoates and perfluorooctanesulfonates.
As shown in Figure 2a, the changing rule of foam expansion ratio (α) for perfluorooctanoates was consistent with that of the minimum molecular area in surface adsorption layer (Amin): The foaming abilities of C7F15COO?N+H(C2H4OH)x(C2H5)3-xwere similar when x = 0, 1, 2, but the foaming ability was relatively weak when x = 3 (i.e., higher surfactant concentration was required for equal foam expansion). It suggested that,as for the perfluorooctanoates under investigation, the compactness of surface molecular packing was more important among the factors.
The compact packing of surface molecules means that the liquid membrane might possess better strength and higher elasticity:[31]Firstly, the hydration layer of the ionic headgroups (together with that of counterions) from the close packed molecules could enhance the strength of liquid membrane, and close packing could also induce thick diffused electric double layer at both side of the liquid membrane, which could hinder the liquid membrane from thinning through electrostatic repulsion and thus prevent foam destruction. Secondly, smaller Aminmeans a larger differential surface pressure during the increase of surface area (i.e. better liquid membrane elasticity), which facilitates in driving the adsorbed surfactant molecules in the vicinity to transfer to the deformation area in surface adsorption layer, and such transfer would carry the water molecules beneath the surface to recover the thickness of the thinned liquid membrane.[31]These were important for the number increase or volume expansion of bubbles at the beginning stage when foams were rapidly forming.
Among the perfluorooctanesulfonates, peculiar behaviors were observed for C8F17SO3?N+H(C2H5)3and C8F17SO3
?N+H(C2H5)2C2H4OH, of which the foam expansion first increased and then decreased with the increase of concentration (Figure 2b). Such peculiar behaviors were owing to the significant increase of viscosity when exceeding a certain concentration. It was evidenced by the phenomena of “pulling thread” between the glass rod and the mouth of container (Figure 2c) for high concentrations of solutions of C8F17SO3?N+H(C2H5)3(10, 20, 30 mmol/L) and C8F17SO3?N+H(C2H5)2C2H4OH(10, 20, 30, 40 mmol/L) as dipping a small amount of solution on the glass rod. Combined with the results of viscosity (Figure 4), it was found that, the concentration when the foam expansion ratio began to drop was almost identical with the turing points where the viscosity began to increase (Figures 2 and 4). Additionally, the slightly rising of α for the solution of 40 mmol/L C8F17SO3?N+H(C2H5)3(Figure 2) exactly accompanied with the falling of viscosity for the same solution (Figure 4). All these results had shown the influences of viscosity on foam expansion,as determined by the feature of foam-shaking method:On the one hand, high viscosity means more difficulty for air bubbles to enter into the viscous liquid during shaking; On the other hand, high viscosity means that a part of energy will be depleted as heat by internal friction thus unfavorable to the increase of surface engery under the same mechanical shaking. Therefore, it was observed that the apparent foaming ability was decreased with the increase of viscosity.
It was also observed in Figures 2a-2b that, for other systems with viscosity close to water, the concentration of perfluorooctanesulfonates when started foaming was significantly lower than that of perfluorooctanoates,which was consistent with the smaller cmc of the former.Moreover, the turning points after the abrupt increase of foam expansion were approximately near cmc, which was consistent with the general rule of foaming: The foaming ability was the best at the concentrations near cmc.[31]
For further study on the effects of hydroxyl group in counterion, it was necessary to make a detailed comparison between the perfluorooctanesulfonates without hydroxyl group and with only one hydroxyl group in the counterion structure (i.e. N+H(C2H5)3and N+H(C2H5)2C2H4OH).These two almost have identical cmc values (Table 1). As shown in Figure 2b, in the region of low concentration, the foaming ability of C8F17SO3?N+H(C2H5)3was significantly better than that of C8F17SO3?N+H(C2H5)2C2H4OH because of the lower surface tension of the former (their γcmcwere 17.1 mN/m and 19.5 mN/m, respectively) which means easier foaming under the same mechanical shaking. However, the maximum foam expansion ratio of C8F17SO3?N+H(C2H5)3was remarkably lower than the maximum foam expansion ratio of C8F17SO3?N+H(C2H5)2C2H4OH (Figure 2b), despite the fact that the latter solution at corresponding concentration was much more viscous. It could be ascribed to the more compact packing of surfactant molecules at surface (smaller Amin)for the latter.
The foam stability and viscosity of surfactant solutions. The foam stability can be reflected by the rate of drainage from the foam. In the investigated concentration range, as shown in Figure 3, after a certain concentration the 25% drainage time of C8F17SO3?N+H(C2H5)3began to sharply increase at first and then decreased. Meanwhile,the 25% drainage time of C8F17SO3?N+H(C2H5)2C2H4OH showed abrupt increase after a certain concentration. In contrast, the 25% drainage time for other systems was very short all the time. A combination of Figures 3 and 4 showed that, the rate of foam drainage was well consistent with the results of solution viscosity.
It was interesting to be noted that, the viscosity of C8F17SO3
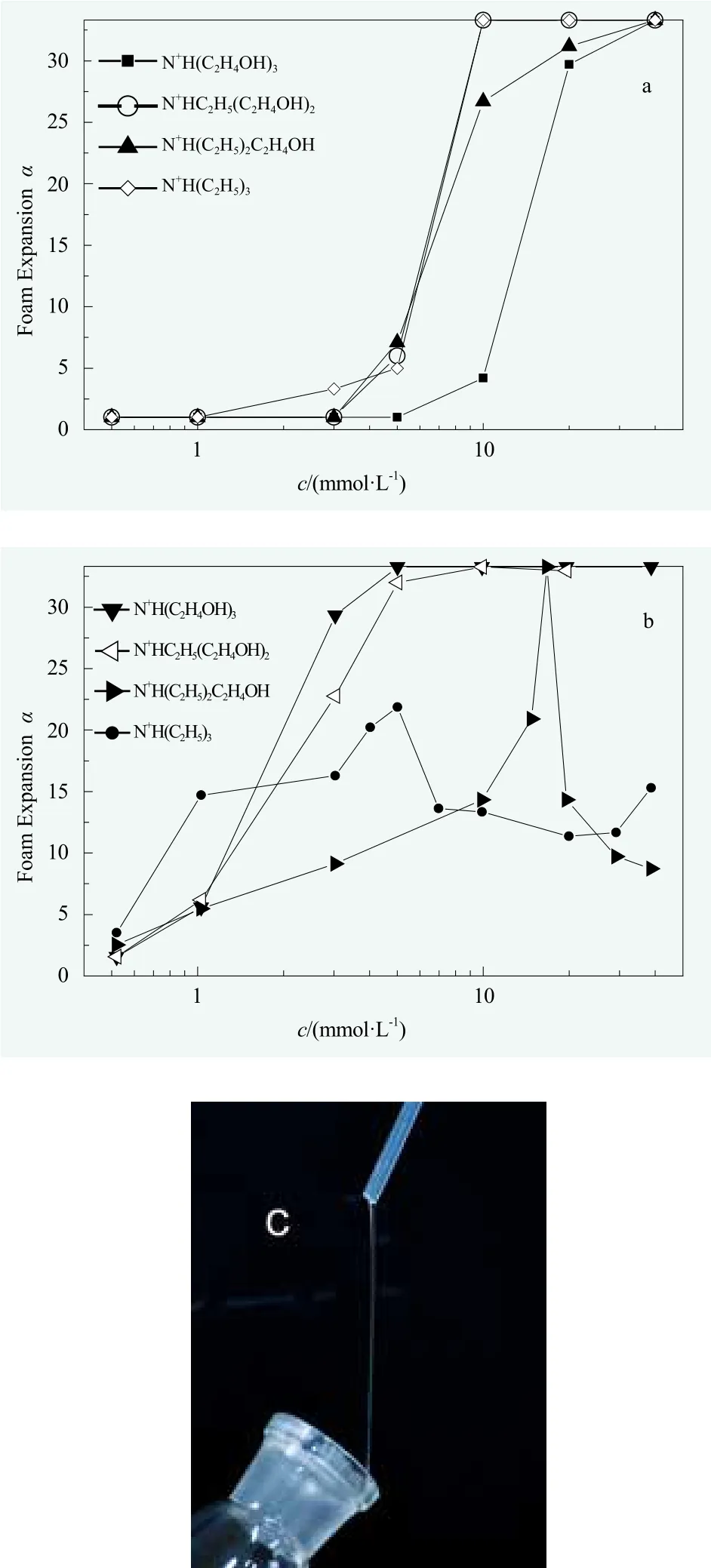
Figure 2. The foam expansion ratio (α) vs the concentration (c)for perfluorooctanoates (a) and perfluorooctanesulfonates (b)with different counterions; (c) The viscous phenomena for the solutions of C8F17SO3?N+H(C2H5)3 (10, 20, 30 mmol/L) and C8F17SO3?N+H(C2H5)2C2H4OH (10, 20, 30, 40 mmol/L)
?N+H(C2H5)3and C8F17SO3?N+H(C2H5)2C2H4OH exhibited completely different behaviors (Figure 4), although they had almost the same cmc and their counterions were only differed by one hydroxyl group. After a certain concentration, the viscosity of C8F17SO3?N+H(C2H5)3began to remarkably increase and then sharply decreased(The phenomena of “pulling thread” as shown in Figure 2c became increasingly obvious for the solutions of 10, 20 and 30 mmol/L, but it became dilute again for the solution of 40 mmol/L). In contrast, the viscosity of C8F17SO3?N+H(C2H5)2C2H4OH was continuously increasing after a certain concentration (The phenomena of “pulling thread” became increasingly obvious for the solutions of 10, 20, 30 and 40 mmol/L). Because the concentration had already been far beyond cmc, such viscosity change for the two systems should be related to the ordered structure in solution. We speculated that the increase of viscosity could be possibly due to the tangling or crosslinking of wormlike micelles (the feature of non-Newtonian fluid was already observable by Ubbelohde viscometer: For the systems with relative large viscosity, the viscosity of solution became gradually lower with the increase of test number (the lowering extent of viscosity measured after 5 times was approximately 2%~4% in total)). As for the solution of 40 mmol/L C8F17SO3?N+H(C2H5)3appearing dilute again, there was possibly structure conversion for the surfactant aggregates in solution. Individual work will be needed for further studies on these solutions of fluorinated surfacants at high concentrations, where the effects of counterions containing hydroxyl group on the ordered structure in solution might be very complex.
Conclusions
The effects of hydroxyl groups in organic ammonium counterions on the properties of fluorinated surfactants were studied by measuring the surface tension, foam expansion ratio,25% drainage time and viscosity. It could be concluded that:
1) The cmc and γcmcwere both increased with the number of hydroxyl groups in counterion.
2) For the same counterion, perfluorooctanesulfonates exhibited smaller cmc than those of perfluorooctanoates,however, the γcmcof perfluorooctanesulfonates were larger than that of perfluorooctanoates probably due to the higher charge density for the headgroup of perfluorooctanoates.
3) As the number of hydroxyl groups (x) in counterion(N+H(C2H4OH)x(C2H5)3-x) increased, perfluorooctanoate and perfluorooctanesulfonate surfactants exhibited different trends of Aminbecause the sulfonate headgroup migh be easier in hydrogen bonding with the hydroxyl group from counterion.
4) The variation of foam expansion ratio for C7F15COO?N+H(C2H4OH)x(C2H5)3-xwas consistent with that of Amin.In general, for the surfactant systems with viscosity close to water (excluding C8F17SO3?N+H(C2H5)3and C8F17SO3?N+H(C2H5)2C2H4OH which were exceptional systems because of the abnormal viscosity), the concentration of perfluorooctanesulfonates when started foaming was significantly lower than that of perfluorooctanoates, which was consistent with their smaller cmc.
5) The variation of foam stability was consistent with that of solution viscosity.
In summary, based on the factors of hydrogen bond,hydrophilicity and steric effect of counterion, the change of the number of hydroxyl groups could influence the viscosity and the parameters of surface activity of fluorinated surfactants (Amin, γcmcand cmc), meanwhile, the viscosity and the parameters of surface activity could decide the foaming performances of fluorinated surfactants. Such theoretical studies on the counterion structure for fluorinated surfactants may be important to enhance the application performance, improve water solubility, and even build inert ionic liquids, etc. Currently, the PFOS and PFOA are in the process of being phased out, so the focus of future research on organic counterions will surely be transferred to other fluorinated surfactants for seeking new replacement products.[32,33]
Acknowledgements
This work was supported by the Applied Chemistry Key Subject of Gansu Province (No. GSACKS20130113)and Youth Science and Technology Innovation Project of Longdong University (No.XYZK1512, No.XYZK1511).
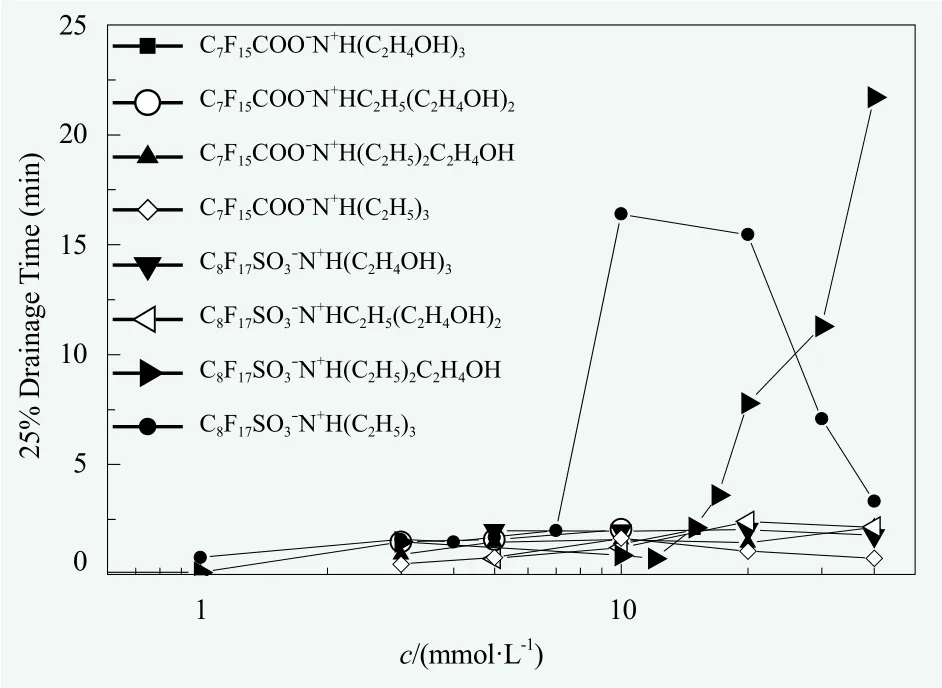
Figure 3. The 25% drainage time vs the concentration(c) for perfluorooctanoate and perfluorooctanesulfonatesurfactants with different counterions
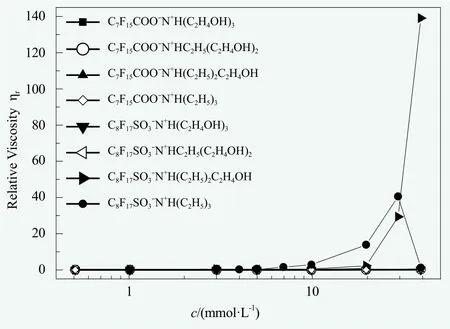
Figure 4. The relative viscosity (ηr) vs the concentration(c) for perfluorooctanoate and perfluorooctanesulfonatesurfactants with different counterions
[1] Tang Weiyue; Geng Tao; Jiang Yajie; et al. Properties of several didecyl quaternary ammonium salts with novel counterions. Fine Chemicals 2015, 32 (10), 1106-1111.
[2] Liu Xuefeng; Chen Hui; Tian Feifei; et al. Effect of counter ions on self-assembly of ionic liquid 1-(2-hydroxyethyl)-3-dodecylimidazolium chloride in aqueous solutions. Chinese Journal of Applied Chemistry 2013, 30 (4), 431-435.
[3] Dong Shuli; Li Xin; Xu Guiying. Study on cationic fluorocarbon surfactants I. Growth and properties of micelles in aqueous solution. Acta Chimica Sinica 2006, (20), 2051-2056.
[4] Xu Baocai; Zhang Guiju; Han Fu; et al. DMC and quaternary ammonium surfactants with new counter anion types. Fine Chemicals 2011, 28 (9), 839-842.
[5] Cui Hui; Tu Yan; Shang Yazhuo; et al. Syntheses and aggregation behaviors of ionic liquid surfactants 1-butyl-3-methylimidazolium alkyl sulfate. Chemistry 2017, 80 (7), 672-678.
[6] Yan Huicheng; Li Qiuxiao; Geng Tao; et al. Properties of the quaternary ammonium salts with novel counterions. Journal of Surfactants and Detergents 2012, 15, 593—599.
[7] Masuda J.; Nakahara H.; Karasawa S.; et al. Solution properties of perfluorinated anionic surfactants with divalent counterion of separate electric charges. Langmuir 2007, 23(17), 8778-8783.
[8] Xiao Jinxin; Zhao Zhenguo. Application principle of surfactants. Beijing: Chemical Industry Press, 2015.
[9] Xiao Jinxin; Jiang Hong. Fluorocarbon surfactants. China Surfactant Detergent & Cosmetics 2001, 31 (5), 24-27.
[10] Kissa E. Fluorinated surfactants and repellents. New York:Marcel Dekker, 2001.
[11] Caboi F.; Chittofrati A.; Lazzari P.; et al. Counterion effect on the phase behavior of perfluoropolyether carboxylates:micelles and liquid crystals in water. Colloids and Surfaces A:Physicochemical and Engineering Aspects 1999, 160 (1), 47-56.
[12] Sulak K.; Wolszczak M.; Chittofrati A.; et al. Aggregation of perfluoropolyether carboxylic salts in aqueous solutions.Fluorescence probe study. Journal of Physical Chemistry B 2005, 109 (2), 799-803.
[13] Szajdzinska-Pietek E.; Sulak K.; Dragutan I.; et al. ESR study of aqueous micellar solutions of perfluoropolyether surfactants with the use of fluorinated spin probes. Journal of Colloid and Interface Science 2007, 312 (2), 405-412.
[14] Guo W.; Brown T. A.; Fung B. M. Micelles and aggregates of fluorinated surfactants. Journal of Physical Chemistry 1991, 95 (4), 1829-1836.
[15] Bossev D. P.; Matsumoto M.; Nakahara M. 1H and 19F NMR study of the counterion effect on the micellar structures formed by tetraethylammonium and lithium perfluorooctylsulfonates. 1. Neat systems. Journal of Physical Chemistry B 1999, 103 (39), 8251-8258.
[16] Bossev D. P.; Matsumoto M.; Sato T.; et al. 1H and 19F NMR study of the counterion effect on the micellar structures formed by tetraethylammonium and lithium perfluorooctylsulfonates. 2. Mixed systems. Journal of Physical Chemistry B 1999, 103 (39), 8259-8266.
[17] Yan P.; Huang J.; Lu R. C.; et al. Two cloud-point phenomena in tetrabutylammonium perfluorooctanoate aqueous solutions:Anomalous temperature-induced phase and structure transitions.Journal of Physical Chemistry B 2005, 109 (11), 5237-5242.
[18] Yang L. K.; Zhao K. S.; Xiao J. X. Study of tetrabutylammonium perfluorooctanoate aqueous solutions with two cloud points by dielectric relaxation spectroscopy. Langmuir 2006, 22 (21), 8655-8662.
[19] Jin Chen; Yan Peng; Wang Chen; et al. Effect of counterious on fluorinated surfactants 1. Surface activity and micellization.Acta Chimica Sinica 2005, 63(4), 279-282, 256.
[20] Wang C.; Yan P.; Xing H.; et al. Thermodynamics of aggregation of ammonium/ tetraalkylammonium perfluorooctanoates: Effect of counterions. Journal of Chemical & Engineering Data 2010, 55 (5), 1994-1999.
[21] Xing H.; Yan P.; Xiao J. X. Unusual location of the pyrene probe solubilized in the micellar solutions of tetraalkylammonium perfluorooctanoates. Soft Matter 2013, 9 (4), 1164-1171.
[22] Xing H.; Lin S. S.; Lu R. C.; et al. NMR investigation on micellization of ammonium/ tetraalkylammonium perfluorooctanoates. Colloids and Surfaces A: Physicochemical and Engineering Aspects 2008, 318 (1—3), 199-205.
[23] Zhou Hongtao; Xing Hang; Gou Zhiming; et al. Surface activity, wetting and foaming properties of trialkylammonium perfluorooctanoat.China Surfactant Detergent & Cosmetics 2010, 40 (5), 320-325.
[24] Zhou Hongtao; Gao Antao; Xing Hang; et al. Abnormal surface-active behavior of perfluorooctanoates induced by salt.Acta Chimica Sinica 2011, 69 (9), 1035-1040.
[25] Pottage M. J.; Greaves T. L.; Garvey C. J.; et al. The effects of alkylammonium counterions on the aggregation of fluorinated surfactants and surfactant ionic liquids. Journal of Colloid and Interface Science 2016, 475, 72-81.
[26] Gao A. T.; Xing H.; Zhou H. T.; et al. Effects of counterion structure on the surface activities of anionic fluorinated surfactants whose counterions are organic ammonium ions.Colloids and Surfaces A: Physicochemical and Engineering Aspects 2014, 459, 31-38.
[27] Zhou Hongtao; Pan Duoqiang; Xing Hang; et al. Study on properties of ethanol ammonium saltsof perfluorooctanoate and perfluorooctylsulfonate surfactants. Chemical Research and Application 2010, 22 (1), 59-66.
[28] Song B.; Shang S.; Song Z. Solution behavior and solid phase transitions of quaternary ammonium surfactants with head groups decorated by hydroxyl groups. Journal of Colloid and Interface Science 2012, 382 (1), 53-60.
[29] Zhu Buyao; Zhao Zhenguo. Fundamentals of interface chemistry. Beijing: Chemical Industry Press, 1996, 26-30.
[30] Xiao Jinxin; Gao Zhan; Wang Minghao; et al. Laboratory method for evaluating the performance of aqueous filmforming foam fire-extinguishing agent. Chemical Research and Application 2008, 20 (5), 569-572.
[31] Zhao Guoxi; Zhu Buyao. Principles of surfactant action.Beijing: China Light Industry Press, 2003, 535-548.
[32] Florindo C.; Tome L. C.; Marrucho I. M. Thermodynamic study of aggregation of cholinium perfluoroalkanoate ionic liquids. Journal of Chemical and Engineering Data 2016,61(12), 3979-3988.
[33] Yake A.; Corder, T.; Moloy K.; et al. Fluorinated pyridinium and ammonium cationic surfactants. Journal of Fluorine Chemistry 2016, 187, 46-55.
 China Detergent & Cosmetics2017年4期
China Detergent & Cosmetics2017年4期
- China Detergent & Cosmetics的其它文章
- Influence of Shaving Behavior on Skin PhysioIogicaI Properties and Sensory Discomfort of Chinese Men
- Dispersion and Stability of Physical Sunscreen Agents in Liquid Medium
- Application of Dodecyl Dimethyl benzyl A mmonium Chloride in bacteriostatic Laundry Detergent
- China National Standard
——Determination of Chloroacetic Acid (Chloroacetate) Contents in Surfactants(GB/T28193-2011) - International Training Program Brings Closer Relationship Between China and Developing Countries
- Meeting Review: 2017 CCIA Annual Meeting in Nanjing
