Critical role of SDF-1/CXCR4 signaling pathway in stem cell homing in the deafened rat cochlea after acoustic trauma
Ali Asghar Peyvandi, Navid Ahmady Roozbahany,, Hassan Peyvandi,, Hojjat-Allah Abbaszadeh,, Niloofar Majdinasab,Mohammad Faridan, Somayeh Niknazar,
1 Hearing Disorders Research Center, Loghman Hakim Medical Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran
2 G. Raymond Chang School, Ryerson University, Toronto, Canada
3 Yale University, New Haven, CT, USA
4 Department of Biology and Anatomical Sciences, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran
5 Department of Occupational Health Engineering, School of Health, Loorestan University of Medical Sciences, Khorramabad, Iran
Introduction
Noise-induced hearing loss (NIHL) is a common occupational illness in industrialized countries (Nelson et al., 2005).It is a hearing impairment that results from damage to the hair cells in the inner ear (Chen and Fechter, 2003). Permanent hearing loss likely occurs when mammalian cochlear hair cells are not able to regenerate themselves (Matsui et al.,2005). There is no definitive treatment for irreversible damage to the auditory system. However, cell therapy has recently been identified as a promising approach to treat several diseases (Dunnett et al., 2001; Hussain and Theise, 2004;Hanna et al., 2007; Segers and Lee, 2008; Abbaszadeh et al.,2017). Stem cell therapy reportedly exhibits therapeutic effects on injury of the auditory system (Li et al., 2004). However, the mechanism by which the transplanted mesenchymal stem cells (MSCs) migrate to the injured organ remains unclear. There is strong evidence that MSCs have the ability to migrate to the injured tissue in myocardial ischemia (Barbash et al., 2003), brain stroke (Lu et al., 2001), spinal cord injury (Abbaszadeh et al., 2015), renal ischemia/reperfusion injury (Hagiwara et al., 2008), pulmonaryfibrosis (Ortiz et al., 2003) and liver injury (Chen et al., 2010; Xiao Ling et al.,2016). The primary factor that regulates cell mobilization is chemotactic signaling molecules called ‘chemokines’.MSCs express chemokine receptors on their cell surface, and cell migration is induced by chemotaxis signaling pathway(Wynn et al., 2004). SDF-1/CXCR4 signaling pathway has been considered as an important factor in the stem cell migration to the injured area (T?gel et al., 2005; Kitaori et al.,2009; Carbajal et al., 2010; Okada et al., 2016; Li et al., 2017;Liu et al., 2017; Park et al., 2017). SDF-1/CXCR4 interaction plays an important role in embryogenesis and angiogenesis(Murdoch, 2000). SDF-1 is a member of the CXC chemokine family and it is expressed by damaged cells in large quantities (Honczarenko et al., 2006). CXCR4, the family of G-protein receptor binding, is a known receptor for SDF-1 on the surface of stem cells (McGrath et al., 1999; Honczarenko et al., 2006). Recent studies have also found that the expression of SDF-1 is increased in the injured cochlea (Kilpatrick et al., 2011; Zhang et al., 2013). Migration of transplanted neural stem cells to the auditory nerve increases in rats subjected to an augmented acoustic environment (Zhang et al., 2013). Based on the above data, the present study was designed to investigate the role of SDF-1/CXCR4 signaling pathway as a migratory mechanism of stem cells in noise-induced cochlear injury.
Materials and Methods
Experimental animals
Thirty male Wistar rats, aged 2–3 months and weighing 180–250 g, were obtained from the Laboratory Animal Center of Shahid Beheshti University of Medical Sciences, Iran and included in this study. Animals were housed at constant temperature (21 ± 1°C), with 12/12-hour light/dark cycle.These rats were randomly divided into five groups (n= 6 per group): control, sham-operated, noise exposure, normal BMSCs (noise exposure followed by treatment with MSCs only) and AMD3100-treated BMSCs (noise exposure followed by AMD3100-treated BMSCs treatment) groups. The present study was approved by the Research Ethics Committee of Shahid Beheshti University of Medical Sciences (approval number: IR. SBMU. REC.1395.20) based on National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1985).
Cell isolation and culture
Rats were anesthetized using intraperitoneal (IP) injections of ketamine (90 mg/kg, Rotexmedica, Trittau, Germany)and xylazine (10 mg/kg, Alfasan Chemical Co, Woerden,Netherlands); and then bone marrow was aspirated from femur and tibia using a 22 gauge needle. Bone marrow-derived mesenchymal stem cells (BMSCs) were cultivated in Dulbecco’s modified Eagle’s medium (DMEM) supplementing with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin. Then cells were plated in 25 cm2flasks filling with 4–5 mL of DMEM culture medium, incubated in a humidified 5% CO2incubator at 37°C, and washed with PBS after 48 hours. Cell culture medium was changed every 2 to 3 days. All tests were performed on cells at passages 3 to 4. BMSCs were then incubated with CXCR4 antagonist AMD3100 (5 μg/mL, Sigma-Aldrich, St. Louis, MO, USA)in DMEM culture medium prior to use. Cultures without CXCR4 antagonist were considered as controls.
Flow cytometry
Markers of MSCs were analyzed using flow cytometry(Harting et al., 2008; Zhang and Chan, 2010). Passage 4 cells were detached by Trypsin/EDTA, washed twice in PBS, incubated with phycoerythrin (PE) and fluorescein isothiocyanate (FITC)-conjugated monoclonal rat anti-mouse CD44,CD45 CD73, CD90, and CD34 antibodies (Becton Dickinson, San Diego, CA, USA) for 40 minutes at 4°C in the dark.The labeled cells were analyzed using a FACS Caliber (Becton Dickinson, Franklin Lakes, NJ, USA) and Cell Quest software (BD Biosciences, San Jose, CA, USA). Rat anti-mouse IgG1 was used as a negative isotype control. Since the expression of cell surface marker CD184 (CXCR4) is critical for BMSCs homing, surface CXCR4 expression was assayed using flow cytometry on cultured cells (Bing et al., 2016).For CXCR4, passage 3 cells were stained with anti-CXCR4 antibody (Abcam, Cambridge, UK) or IgG isotype control,and the secondary antibody used was mouse anti-goat IgGFITC (Santa Cruz Biotechnology, Santa Cruz, CA, USA).
In vitro and in vivo cell migration assay
The Chemicon QCM? 96-well migration assay system with 8 μm pore size (Millipore, Billerica, MA, USA) was used to determine the migration ability of cells (Mirzamohammadi et al., 2015). BMSCs suspension (4 × 104cells /100 μL) was cultivated and treated with or without AMD3100 in the upper chamber. Various doses of recombinant rat SDF-1 (0,100 and 500 ng/mL; Enzo Life Sciences, Farmingdale, NY,USA) were added to the lower chamber according to the manufacturer’s instructions. Then, the plates were incubated for 24 hours at 37°C. Migratory cells were lysed and stained with CyQuant GR dye (Kit Components, (Part No. 90132)).Fluorescence of migrated cells was measured using a 480 nm/520 nmfilter set. Forin vivochemotaxis analysis, 1 × 105BMSCs were incubated with PBS (20 μL) or AMD3100 (5 μg/mL) for 30 minutes before injection and then perfused for 10 minutes at a rate of 2 μL/min into the inner ear (left)through the round window niche of rats. Hoechst 33342-labeled BMSCs were assayed under the fluorescence microscope (Nikon Inc., Melville, NY, USA).
Noise exposure protocol
Rats were subjected to DPOAE to assess their cochlear hearing function before noise exposure. The DPOAE test was administered to the control (n= 6) and sham-operated (n= 6)groups on days 0 (baseline audiometry) and 6 (with no noise exposure). The test was also administered to noise exposure (noise exposure without any treatment,n= 6), normal MSCs (noise exposure + treatment with normal BMSCs;n=3), and AMD3100-treated BMSCs (noise exposure + treatment with AMD3100-treated BMSCs;n= 3) on days 0 (before) and 6 (after) of the noise exposure protocol. DPOAEs(in dB SPL) were measured at stimulus frequencies of 2, 3,4, 5, 6, 7, 8, 9, and 10 kHz. Rats that had abnormal auditory function were excluded from the experiment. Rats with normal auditory function were exposed to 110 dB white noise,6 hours/day for 5 successive days in a glass chamber. Sound intensity was measured using a sound level meter (TES-1358 Sound Analyze, Taiwan).
Auditory assay
The DPOAE test was performed in a sound-attenuated chamber (San Diego Instruments, San Diego, CA, USA)covered with sound-absorbing foam. Before each recording session, rats were anesthetized using intraperitoneal injections of ketamine (90 mg/kg) and xylazine (10mg/kg). In all animals, probe was placed in the left ear canal. DPOAEs were recorded with the Neuro-Audio (NEUROSOFT, Russia) System. DPOAEs were generated by simultaneously presenting two sinusoids differing in frequency (f1 and f2)(Lonsbury-Martin and Martin, 1990).
Hematoxylin-eosin staining of the cochlear tissue
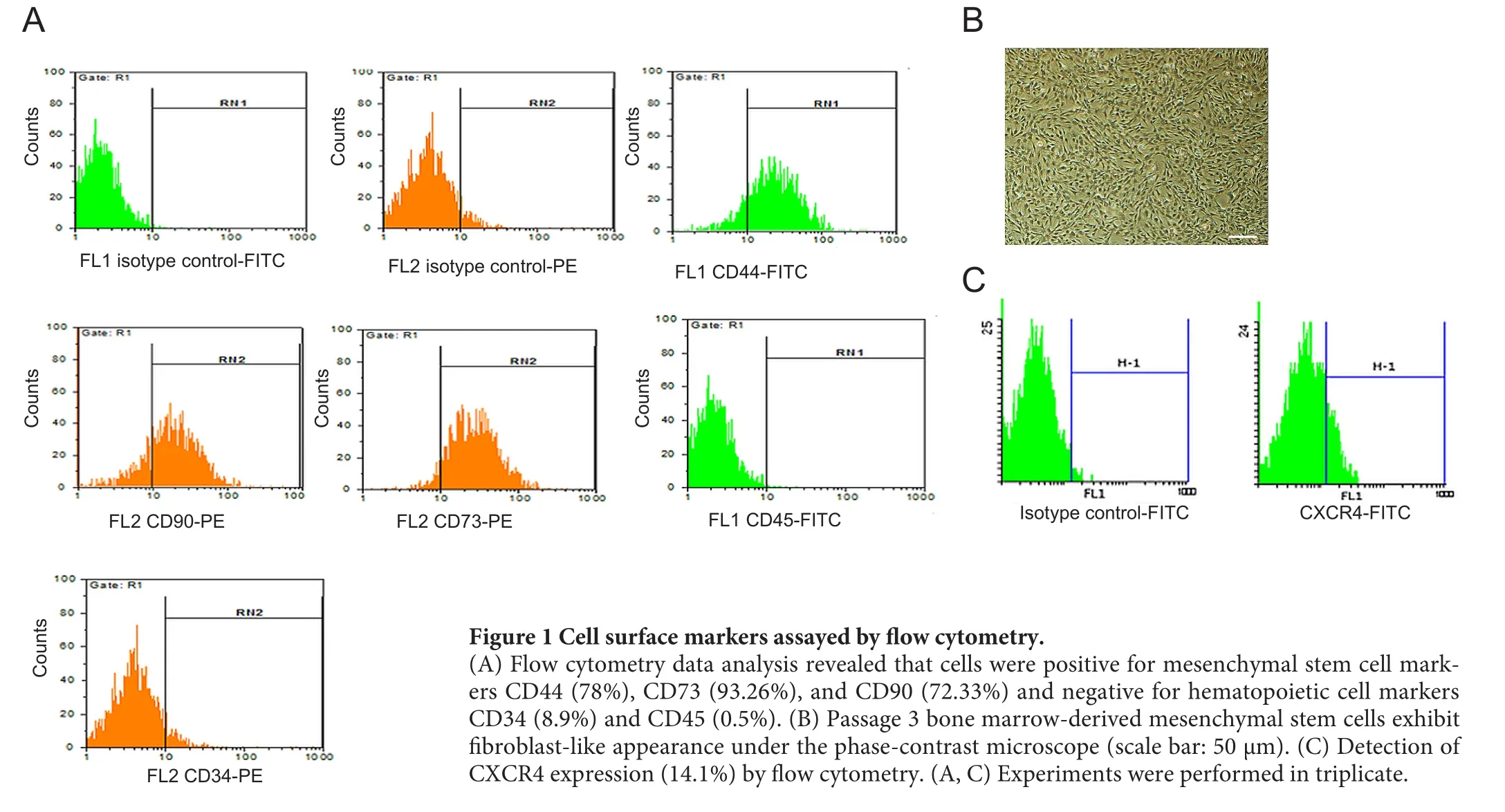
Figure 1 Cell surface markers assayed by flow cytometry.
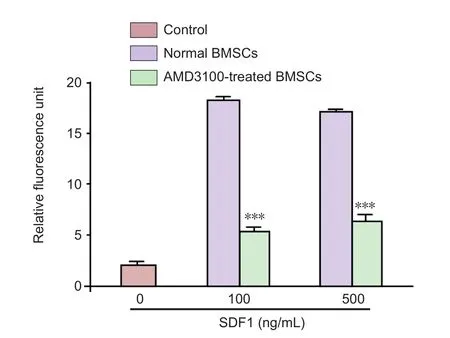
Figure 2 Effect of chemotaxis inhibitor (AMD3100) on in vitro migration of BMSCs.
After decapitation, the left cochlea was dissected in icecold 10 mM phosphate-buffered saline (PBS). Round window and oval window of the cochlea were opened by afine needle, and then 4% paraformaldehyde was perfused. The cochlea was fixed in 4% paraformaldehyde overnight and then decalcified in 5% ethylenediaminetetraacetic acid (pH 7.4) containing 5% sucrose for 15 days and then embedded in paraffin. Next, 5 μm-thick sections were cut on a sliding microtome and stored in a cryoprotectant solution. For the staining procedure, paraffin-embedded blocks were deparaffi nized and rehydrated; and sections were stained later with hematoxylin-eosin (Sigma-Aldrich, St. Louis, MO, USA).
Western blot analysis
After decapitation of the animals, the bulla was quickly removed and placed in ice-cold 10 mM PBS. The bony capsule and the lateral wall of the cochlea were removed. Cochlea sample included the organ of corti, and the modiolus was dissected. Specimen was homogenized in a RIPA lysis buffer (sc-24948, Santa Cruz Biotechnology). Homogenate was centrifuged, and supernatant solution was collected.Total protein amount was determined according to the Bradford method (Bradford, 1976) using bovine serum albumin as a protein concentration standard. Protein was separated using SDS gel and transferred into a nitrocellulose membrane. After blocking, the membrane was incubated with primary antibodies for rabbit polyclonal to SDF-1 (AB 25117, 1:1,000; Abcam, Cambridge, UK) and B-actin(AB, ab8227, 1:2,000; Abcam) (as an internal control) at 4°C overnight. After rinsing to eliminate unbound primary antibody, the membrane was incubated with horseradish peroxidase-conjugated anti-rabbit IgG secondary antibody(AB, 6721, 1:2,000; Abcam) for 2 hours at room temperature. The protein bands were visualized using an enhanced chemiluminescence kit (Amersham Pharmacia Biotech Inc.,Piscataway, NJ, USA) and quantified by TotalLab software(Wales, UK) (Niknazar et al., 2016, 2017).
Hoechst labeling
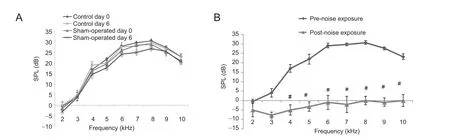
Figure 3 Auditory function of rats assessed by DPOAE assay.
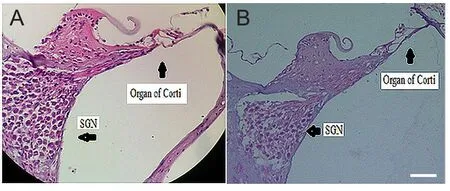
Figure 4 Effect of noise exposure on the structure of rat cochlea.

Figure 6 Hoechst 33342-labeled BMSCs migration to the endolymph of injured cochlea.
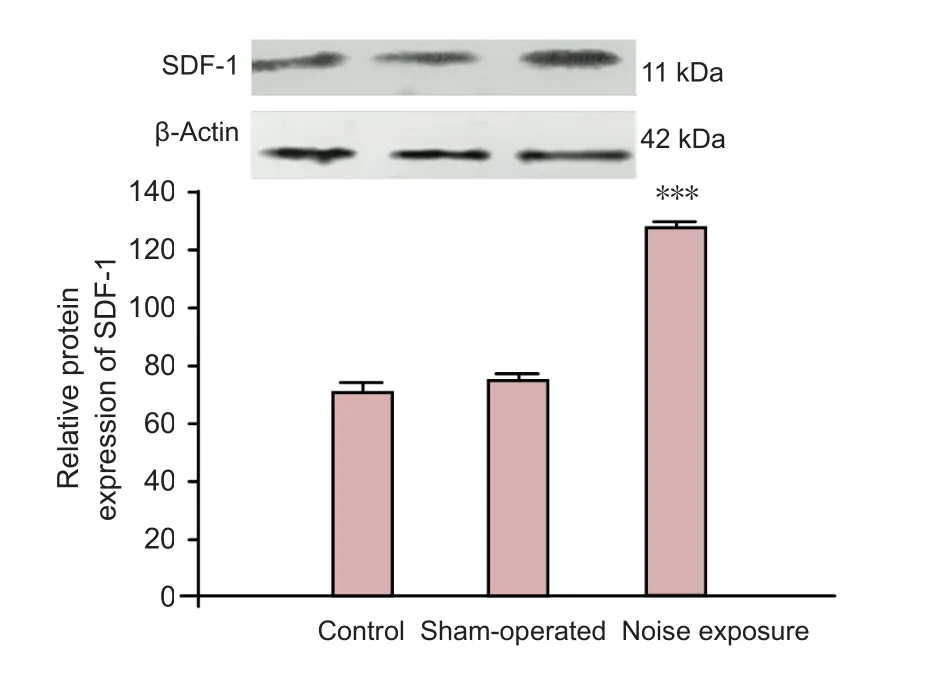
Figure 5 Effects of noise exposure on SDF-1 protein expression in the cochlea of rats.
To evaluate BMSCs migration to the endolymph of cochlea, Hoechst 33342-labled BMSCs were injected into the perilymph through the round window niche. Deaf rats were divided into two groups, which were transplanted with Hoechst 33342-labeled untreated BMSCs (1 × 105) (n= 3) and AMD3100-treated BMSCs (1 × 105) (n=3). Animals were decapitated and the cochleae were removed 24 hours after BMSCs transplantation. The cochleae werefixed through intrascalar perfusion of 4% paraformaldehyde and then decalcified in 5% ethylenediaminetetraacetic acid (pH 7.4) containing 5% sucrose for 15 days. Next, the cochlea was embedded in paraffin and then cut into 5 μm-thick sections. The number of Hoechst (Hoechst 33342; Thermo Fisher Scientific, Waltham, MA, USA) -labeled BMSCs in the cochlea was assayed using a fluorescence microscope(Nikon Inc., Melville, NY, USA). Data were analyzed using ImageJ software (NIH, Bethesda, MD, USA).
Statistical analysis
All data are expressed as the mean ± SEM and were statistically analyzed with GraphPad Prism version 5.04 Windows(GraphPad Software, San Diego, CA, USA). Statistical significance was determined by the Student’st-test or one-way analysis of variance followed by Tukey’s test. A level ofP<0.05 was considered statistically significant.
Results
Characterization of BMSCs
Cell surface markers were assayed using flow cytometry.Results analysis revealed that cells were positive for CD44,CD73, and CD90, while they were negative for CD34 and CD45. In addition, 14.1% passage 3 BMSCs expressed surface CXCR4 (Figure 1).
In vitro BMSCs migration
Migration of AMD3100-treated BMSCs in response to SDF-1 at different concentrations was assayed. AMD3100-treated BMSCs revealed a significantly lower migration capacity in response to SDF-1 (100 and 500 ng/mL) after 24 hours compared to normal BMSCs (P< 0.001) (Figure 2).
Cochlear hearing function assessment
DPOAEs were measured to assess cochlear function. DPOAE test results showed that there was no significant difference in DPOAE responses at any frequency between the control and sham-operated groups on days 0 and 6 (Figure 3A). In the noise exposure group, DPOAE responses were significantly decreased on day 6 than those on day 0 (Figure 3B).
Pathological changes in cochlear tissue
Hematoxylin-eosin staining of the cochlear tissue sections revealed an irregular arrangement of the auditory hair cells and degeneration of spiral ganglion neurons in the cochlea of rats (n= 3) (Figure 4B) following chronic exposure to 110 dB white noise compared to control rats (n= 3) (Figure 4A).
SDF-1 protein expression
SDF-1 protein expression in normal (n= 6), sham-operated(n= 6), and noise induced ears (n= 6) was determined using western blot assay. Antibodies against SDF-1 detected a band on the blot at about 11 kDa. Western blot assay results revealed that SDF-1 protein expression was significantly increased in the cochlea of rats in the noise exposure group compared to the control group (P< 0.001). These results confirmed that SDF-1 protein expression was increased following cochlear injury (Figure 5).
In vivo BMSCs migration to the injured cochlea
To evaluate the migration of BMSCs to cochlear endolymph,Hoechst 33342-labeled BMSCs were injected through the round window into noise-induced deaf rats. About 24 hours after BMSCs transplantation, rats were decapitated. The number of Hoechst-stained BMSCs per cochlear section was counted, and three sections per animal were used in each group. The number of transplanted BMSCs was significantly lower in the CXCR4-treated group than in the normal BMSCs group (n= 3) (P< 0.001; Figure 6).
Discussion
In this study, we investigated the mechanism by which the SDF-1/ CXCR4 signaling pathway regulates the homing of MSCs in a rat model of noise-induced hearing loss. We used CXCR4 antagonist to inhibit CXCR4 activity on the surface of BMSCs to influence the homing ability of BMSCs to the damaged site of cochlea. For this purpose, adult male rats subjected to the chronic white noise exposure were used,and then electrophysiological and molecular alterations of the cochlea were evaluated. DPOAE responses were attenuated after noise exposure. SDF-1 expression was increased in the cochlea of rats in the noise exposure group. Normal and AMD3100-treated BMSCs were injected into the perilymph through the round window. A smaller number of transplanted BMSCs (~5%) was observed in the cochlear endolymph in the AMD3100-treated MSCs group than in the normal MSCs group.
BMSCs can produce several molecules that provide further guidance for effective delivery and homing of injected cells to the injured tissue and contribute to tissue repair (Augello et al., 2010; Méndez-Ferrer et al., 2010). It is demonstrated that the expression of CXCR4 and this ligand SDF-1 in BMSCs support their survival and proliferation through the autocrine-paracrine loop (Nagasawa et al., 1996; Kortesidis et al., 2005). SDF-1 is a chemotactic factor produced by various pathological conditions. In the present study, chronic exposure to white noise increased the SDF-1 protein expression in the cochlear tissue of rats. This is consistent withfindings from previous studies demonstrating SDF-1 upregulation in the auditory nerve following spiral ganglion neuron degeneration due to ouabain application in mice (Guyon and Nahon, 2007; Tan et al., 2008; Kilpatrick et al., 2011) and acoustical trauma in rats (Zhang et al., 2013). Several factors including cytokines, integrins, and chemokines associated with their receptors are involved in the migration of BMSCs(Li et al., 2012; Sohni and Verfaillie, 2013; Esfahani et al.,2015; Kamiya, 2015). CXCR4 is a chemokine receptor specific to SDF-1α, which is normally expressed on the surface of BMSCs (Liu et al., 2011; Yu et al., 2015). SDF-1/CXCR4 system plays an important role in stem cells mobilization to the damaged area. Previous studies have demonstrated that SDF-1/CXCR4 pathway has been upregulated in numerous injured organs and tissues of the experimental models such as spinal cord injury, ischemic brain damage, myocardial infarction, burn wounds, acute pancreatitis, and acute kidney injury (Marquez-Curtis and Janowska-Wieczorek, 2013).Recently, several researches have shown that AMD3100 can inhibit CXCR4 activity. There is evidence that in renal ischemia, migration of MSCs to ischemic tissue was mainly dependent on CXCR4 and completely inhibited by the CXCR4 antagonist AMD3100 (Liu et al., 2013a, b). Li et al. (2015)found that SDF-1/CXCR4 signaling pathway has a critical role in the mobilization of transplanted BMSCs to the lesion site following spinal cord injury. They indicated that CXCR4 expression was enhanced by erythropoietin, but attenuated significantly after AMD3100 treatment.
Our data revealed that expression of SDF-1, which was induced in the injured cochlea, can promote BMSCs migration to this area. AMD3100, as a SDF-1/CXCR4 signaling pathway antagonist, significantly attenuated BMSCs mobilization to the endolymph of injured cochlea, which confirms that interaction of production of SDF-1 in the cochlea with CXCR4 expression in BMSCs has a crucial role in BMSCs migration towards the noise-induced damaged cochlea in rats.
In conclusion, migration and homing of transplanted stem cells is an essential mechanism for cell delivery to the damaged target area. The presentfindings demonstrated that SDF-1/CXCR4 signaling pathway plays an important role in the migration of transplanted cells to the injured cochlea following NIHL. Thus, the activation of SDF-1/CXCR4 signaling pathway may be used as a cell-based therapy approach for hearing recovery after acoustic trauma.
Author contributions:All authors contributed to conception and design of this study, performed the experiments, participated in data collection and analysis, contributed to preparation of manuscript, and approved thefinal version of the paper.
Conflicts of interest:The authors report no biomedicalfinancial interests or potential conflicts of interest.
Financial support: This work was supported by the Hearing Disorders Research Center of Shahid Beheshti University of Medical Sciences,which played no role in the study other than to provide funding.
Research ethics:The present study has been approved by the Research Ethics Committee of Shahid Beheshti University of Medical Sciences (approval number: IR. SBMU. REC.1395.20) based on National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1985).
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under identical terms.
Open peer reviewer:Matta Reddy Alavala, Adikavi Nannaya University, India.
Abbaszadeh HA, Tiraihi T, Noori-Zadeh A, Delshad AR, Sadeghizade M, Taheri T (2015) Human ciliary neurotrophic factor—overexpressing stable bone marrow stromal cells in the treatment of a rat model of traumatic spinal cord injury. Cytotherapy 17:912-921.
Abbaszadeh HA, Tiraihi T, Sadeghi Y, Delshad AR, Sadeghizadeh M,Taheri T, Noori-Zadeh A (2017) Decrease in cavity size and oligodendrocyte cell death using neurosphere-derived oligodendrocyte-like cells in spinal cord contusion model. Iran Biomed J.
Augello A, Kurth TB, De Bari C (2010) Mesenchymal stem cells: a perspective from in vitro cultures to in vivo migration and niches.Eur Cell Mater 20:33.
Barbash IM, Chouraqui P, Baron J, Feinberg MS, Etzion S, Tessone A, Miller L, Guetta E, Zipori D, Kedes LH (2003) Systemic delivery of bone marrow—derived mesenchymal stem cells to the infarcted myocardium. Circulation 108:863-868.
Bing W, Pang X, Qu Q, Bai X, Yang W, Bi Y, Bi X (2016) Simvastatin improves the homing of BMSCs via the PI3K/AKT/miR-9 pathway. J Cell Mol Med 20:949-961.
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248-254.
Carbajal KS, Schaumburg C, Strieter R, Kane J, Lane TE (2010) Migration of engrafted neural stem cells is mediated by CXCL12 signaling through CXCR4 in a viral model of multiple sclerosis. Proc Natl Acad Sci U S A 107:11068-11073.
Chen G-D, Fechter LD (2003) The relationship between noise-induced hearing loss and hair cell loss in rats. Hear Res 177:81-90.
Chen Y, Xiang LX, Shao JZ, Pan RL, Wang YX, Dong XJ, Zhang GR(2010) Recruitment of endogenous bone marrow mesenchymal stem cells towards injured liver. J Cell Mol Med 14:1494-1508.
Dunnett SB, Bj?rklund A, Lindvall O (2001) Cell therapy in Parkinson’s disease—stop or go? Nat Rev Neurosci 2:365-369.
Esfahani M, Karimi F, Afshar S, Niknazar S, Sohrabi S, NajafiR (2015)Prolyl hydroxylase inhibitors act as agents to enhance the efficiency of cell therapy. Expert Opin Biol Ther 15:1739-1755.
Guyon A, Nahon J-L (2007) Multiple actions of the chemokine stromal cell-derived factor-1α on neuronal activity. J Mol Endocrinol 38:365-376.
Hagiwara M, Shen B, Chao L, Chao J (2008) Kallikrein-modified mesenchymal stem cell implantation provides enhanced protection against acute ischemic kidney injury by inhibiting apoptosis and inflammation. Hum Gene Ther 19:807-819.
Hanna J, Wernig M, Markoulaki S, Sun C-W, Meissner A, Cassady JP, Beard C, Brambrink T, Wu L-C, Townes TM (2007) Treatment of sickle cell anemia mouse model with iPS cells generated from autologous skin. Science 318:1920-1923.
Harting M, Jimenez F, Pati S, Baumgartner J, Cox C (2008) Immunophenotype characterization of rat mesenchymal stromal cells. Cytotherapy 10:243-253.
Honczarenko M, Le Y, Swierkowski M, Ghiran I, Glodek AM, Silberstein LE (2006) Human bone marrow stromal cells express a distinct set of biologically functional chemokine receptors. Stem Cells 24:1030-1041.
Hussain MA, Theise ND (2004) Stem-cell therapy for diabetes mellitus. Lancet 364:203-205.
Kamiya K (2015) Inner ear cell therapy targeting hereditary deafness by activation of stem cell homing factors. Front Pharmacol 6:2.
Kilpatrick LA, Zhu J, Lee FS, Lang H (2011) Role of stromal cell-derived factor-1 expression in the injured mouse auditory nerve.Otolaryngol Head Neck Surg 145:1007-1015.
Kitaori T, Ito H, Schwarz EM, Tsutsumi R, Yoshitomi H, Oishi S, Nakano M, Fujii N, Nagasawa T, Nakamura T (2009) Stromal cell-derived factor 1/CXCR4 signaling is critical for the recruitment of mesenchymal stem cells to the fracture site during skeletal repair in a mouse model. Arthritis Rheum 60:813-823.
Kortesidis A, Zannettino A, Isenmann S, Shi S, Lapidot T, Gronthos S (2005) Stromal-derived factor-1 promotes the growth, survival,and development of human bone marrow stromal stem cells. Blood 105:3793-3801.
Li H, Corrales CE, Edge A, Heller S (2004) Stem cells as therapy for hearing loss. Trends Mol Med 10:309-315.
Li J, Guo W, Xiong M, Han H, Chen J, Mao D, Tang B, Yu H, Zeng Y (2015) Effect of SDF-1/CXCR4 axis on the migration of transplanted bone mesenchymal stem cells mobilized by erythropoietin toward lesion sites following spinal cord injury. Int J Mol Med 36:1205-1214.
Li L, Yang M, Wang C, Zhao Q, Liu J, Zhan C, Liu Z, Li X, Wang W,Yang X (2012) Effects of cytokines and chemokines on migration of mesenchymal stem cells following spinal cord injury. Neural Regen Res 7:1106.
Li M, Sun X, Ma L, Jin L, Zhang W, Xiao M, Yu Q (2017) SDF-1/CXCR4 axis induces human dental pulp stem cell migration through FAK/PI3K/Akt and GSK3B/B-catenin pathways. Sci Rep 7:40161.
Liu JM, Zhao K, Du LX, Zhou Y, Long XH, Chen XY, Liu ZL (2017)AMD3100 inhibits the migration and differentiation of neural stem cells after spinal cord injury. Sci Rep 7:64.
Liu L, Yu Q, Lin J, Lai X, Cao W, Du K, Wang Y, Wu K, Hu Y, Zhang L, Xiao H, Duan Y, Huang H (2011) Hypoxia-inducible factor-1α is essential for hypoxia-induced mesenchymal stem cell mobilization into the peripheral blood. Stem Cells Dev 20:1961-1971.
Liu N, Patzak A, Zhang J (2013a) CXCR4-overexpressing bone marrow-derived mesenchymal stem cells improve repair of acute kidney injury. Am J Physiol Renal Physiol 305:F1064-1073.
Liu N, Tian J, Cheng J, Zhang J (2013b) Migration of CXCR4 genemodified bone marrow-derived mesenchymal stem cells to the acute injured kidney. J Cell Biochem 114:2677-2689.
Lonsbury-Martin BL, Martin GK (1990) The clinical utility of distortion-product otoacoustic emissions. Ear Hear 11:144-154.
Lu D, Mahmood A, Wang L, Li Y, Lu M, Chopp M (2001) Adult bone marrow stromal cells administered intravenously to rats after traumatic brain injury migrate into brain and improve neurological outcome. Neuroreport 12:559-563.
Marquez-Curtis LA, Janowska-Wieczorek A (2013) Enhancing the migration ability of mesenchymal stromal cells by targeting the SDF-1/CXCR4 axis. Biomed Res Int 2013:561098.
Matsui JI, Parker MA, Ryals BM, Cotanche DA (2005) Regeneration and replacement in the vertebrate inner ear. Drug Discov Today 10:1307-1312.
McGrath KE, Koniski AD, Maltby KM, McGann JK, Palis J (1999)Embryonic expression and function of the chemokine SDF-1 and its receptor, CXCR4. Dev Biol 213:442-456.
Méndez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, MacArthur BD, Lira SA, Scadden DT, Ma’ayan A, Enikolopov GN, Frenette PS (2010) Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 466:829-834.
Mirzamohammadi S, Aali E, Najafi R, Kamarul T, Mehrabani M,Aminzadeh A, SharifiAM (2015) Effect of 17B-estradiol on mediators involved in mesenchymal stromal cell trafficking in cell therapy of diabetes. Cytotherapy 17:46-57.
Murdoch C (2000) CXCR4: chemokine receptor extraordinaire. Immunol Rev 177:175-184.
Nagasawa T, Hirota S, Tachibana K, Takakura N, Nishikawa S, Kitamura Y, Yoshida N, Kikutani H, Kishimoto T (1996) Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature 382:635-638.
Nelson DI, Nelson RY, Concha-Barrientos M, Fingerhut M (2005)The global burden of occupational noise-induced hearing loss. Am J Ind Med 48:446-458.
Niknazar S, Nahavandi A, Peyvandi AA, Peyvandi H, Mehrjerdi FZ, Karimi M (2017) Effect of maternal stress prior to conception on hippocampal BDNF signaling in rat offspring. Mol Neurobiol 54:6436-6445.
Niknazar S, Nahavandi A, Peyvandi AA, Peyvandi H, Akhtari AS,Karimi M (2016) Comparison of the adulthood chronic stress effect on hippocampal BDNF signaling in male and female rats. Mol Neurobiol 53:4026-4033.
Okada K, Kawao N, Yano M, Tamura Y, Kurashimo S, Okumoto K,Kojima K, Kaji H (2016) Stromal cell-derived factor-1 mediates changes of bone marrow stem cells during the bone repair process.Am J Physiol Endocrinol Metab 310:E15-23.
Ortiz LA, Gambelli F, McBride C, Gaupp D, Baddoo M, Kaminski N,Phinney DG (2003) Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates itsfibrotic effects. Proc Natl Acad Sci U S A 100:8407-8411.
Park S, Jang H, Kim BS, Hwang C, Jeong GS, Park Y (2017) Directional migration of mesenchymal stem cells under an SDF-1α gradient on a microfluidic device. PLoS One 12:e0184595.
Segers VF, Lee RT (2008) Stem-cell therapy for cardiac disease. Nature 451:937-942.
Sohni A, Verfaillie CM (2013) Mesenchymal stem cells migration homing and tracking. Stem Cells Int 2013:130763.
Tan BTG, Lee MM, Ruan R (2008) Bone marrow-derived cells that home to acoustic deafened cochlea preserved their hematopoietic identity. J Comp Neurol 509:167-179.
T?gel F, Isaac J, Hu Z, Weiss K, Westenfelder C (2005) Renal SDF-1 signals mobilization and homing of CXCR4-positive cells to the kidney after ischemic injury. Kidney Int 67:1772-1784.
Wynn RF, Hart CA, Corradi-Perini C, O’Neill L, Evans CA, Wraith JE, Fairbairn LJ, Bellantuonoi(2004) A small proportion of mesenchymal stem cells strongly expresses functionally active CXCR4 receptor capable of promoting migration to bone marrow. Blood 104:2643-2645.
Xiao Ling K, Peng L, Jian Feng Z, Wei C, Wei Yan Y, Nan S, Cheng Qi G, Zhi Wei W (2016) Stromal derived factor-1/CXCR4 axis involved in bone marrow mesenchymal stem cells recruitment to injured liver. Stem Cells Int 2016:8906945.
Yu Q, Lin J, Wang Y, Xuan X (2015) SDF-1 [alpha]/CXCR4 axis mediates the migration of mesenchymal stem cells to the hypoxic-ischemic brain lesion in a rat model. Cell J 16:440.
Zhang L, Chan C (2010) Isolation and enrichment of rat mesenchymal stem cells (MSCs) and separation of single-colony derived MSCs. J Vis Exp pii: 1852.
Zhang PZ, He Y, Jiang XW, Chen FQ, Chen Y, Xue T, Zhou K, Li X,Wang Y, Wu YX, Mi WJ, Qiu JH (2013) Up-regulation of stromal cell-derived factor-1 enhances migration of transplanted neural stem cells to injury region following degeneration of spiral ganglion neurons in the adult rat inner ear. Neurosci Lett 534:101-106.
- 中國神經(jīng)再生研究(英文版)的其它文章
- Neural Regeneration Research: Information for Authors
- Injury of the Papez circuit in a patient with traumatic spinal cord injury and concomitant mild traumatic brain injury
- Low-frequency pulsed electromagneticfield pretreated bone marrow-derived mesenchymal stem cells promote the regeneration of crush-injured rat mental nerve
- PTEN knockdown with the Y444F mutant AAV2 vector promotes axonal regeneration in the adult optic nerve
- Neuroprotective mechanisms of rutin for spinal cord injury through anti-oxidation and anti-inflammation and inhibition of p38 mitogen activated protein kinase pathway
- Does combined therapy of curcumin and epigallocatechin gallate have a synergistic neuroprotective effect against spinal cord injury?