Neural Regeneration Research: Information for Authors
About the Journal
Founded in April 2006,Neural Regeneration Research (NRR; ISSN 1673-5374, eISSN 1876-7958) is an international, peer-reviewed, open-access journal (www.nrronline.org) published monthly, with its mission focused on reporting creative scientific advancements in all areas of neural regeneration. The journal is committed to publishing articles on original experimental and clinical research, technological advances and methods, expert reviews and perspective articles related to neural regeneration in the central and peripheral nervous systems.
NRRhas been indexed by Science Citation Index Expanded (SCIE; Impact Factor 1.769 in 2016), PubMed,SCOPUS and other major international indexing systems. We hope that,with your support,NRRwill become a highly recognized journal in the field of neural regeneration.
Aims and Scopes
NRRpublishes papers on a broad range of the latest advances in neural repair,protection, and regeneration on the pathobiology of injury to the nervous system.NRRis devoted to publishing articles regarding the diagnosis and treatment of the nervous system diseases (stroke, brain trauma, neurodegenerative diseases, neurodevelopmental diseases, spinal cord injuries, cranial nerve injuries, optic nerve injuries) and techniques that involve cell therapy,gene therapy, bioengineering, neuromodulation, pharmacotherapy, and neuroprosthetics, and articles addressing tissue engineering, neural plasticity,neuroimmunity, neuroinflammation,autophagy, exercise, brain health, and advanced brain technologies (brainlike organ, brain-like computer chip,optical genetics, single-unit recordings,neuroimaging, big data analytics) in thefield of neural regeneration.
The following types of articles are welcomed inNRR: Invited review articles of hotspot topics in neural rengeneration; clinical studies with registry number; basic research articles that include original data with DOI registry number; systematic review and evidence-based medicine articles; articles describing novel methods, software,databases or other tools; and negative studies.
NRRuses a rigorous and timely peer-review process that ensures the highest quality for publication. The journal ensures the accuracy, timeliness and completeness of the database’s content.NRRdedicated to disseminate each published article to achieve the largest display through the most influential international journal platform.
Readership
NRRbrings together the experts from the disciplines including neuroanatomy, biochemistry, biophysics,neuroimmunity, neuropathology,neuropharmacology, neurophysiology,neurosurgery, neurology, neurobiology, neuroimaging, neuroradiology and neurorehabilitation.
ing and Indexing
·Science Citation Index Expanded(SCIE)
·PubMed
·PubMed Central (PMC)
·BIOSIS previews (BP)
·SCOPUS
·Chemical Abstracts (CA)
·Excerpta Medica (EM)
·Index of Copurnicus (IC)
·OvidSP
·Chinese Science Citation Database(CSCD)
·Chinese Science and Technology Paper Citation Database (CSTPCD)
·DOAJ
Editorial Board
Editors-in-Chief
·Kwok-fai So, Member, Chinese Academy of Sciences; Fellow, National Academy of Inventors (USA); Director, GHM Institute of Neural Regeneration, Jinan University; Jessie Ho Professor in Neuroscience, Department of Ophthalmology of Li Ka Shing Faculty of Medicine, The University of Hong Kong; Chairman, Board of Directors of China Spinal Cord Injury Network;Director, Hong Kong Spinal Cord Injury Fund (HKSCI Fund).
·Xiao-ming Xu, Professor and Mari Hulman George Chair of Neurological Surgery, Scientific Director, Spinal Cord and Brain Injury Research Group, Indiana University School of Medicine, USA; Member, Scientific Review Group, National Institutes of Health.
Associate Editors
·Vance P. Lemmon, Walter G. Ross Distinguished Chair, Department of Developmental Neuroscience and Neurological Surgery, University of Miami, USA.
·George M. Smith, Professor, Department of Neuroscience, Shriners Hospitals Pediatric Research Center, Temple University, School of Medicine, USA.
Editorial Board Members
The editorial board led by Professor Kwok-fai So and Xiao-ming Xu comprises over a hundred members who are dedicated to developing a journal presenting outstanding peer-reviewed,evidence-based scholarly research in neural regeneration.
Recruitment of new editorial board members is ongoing.
Criteria for Publication
· The study presents the novel results of primary scientific research.
· Experiments, statistics, and other analyses are performed to a high technical standard and are described in sufficient detail.
· Results reported have not been published elsewhere.
· Conclusions are presented in an appropriate fashion and are supported by the data.
· The research meets all applicable standards for the ethics of experimentation and research integrity.
· The article adheres to appropriate reporting guidelines and community standards for data availability.
· The article is presented in an intelligible fashion and is written in American English.
Rigorous Peer Review
Referees ofNRRare asked to evaluate the manuscript of technically sound.Judgments about the significance of any particular paper are made after publication by the readership, who are the most qualified to determine what is of interest to them.
Editorial Process
Each submission toNRRpasses through a quality control check and peer-review evaluation process before receiving a decision. The initial inhouse quality control check deals with issues such as plagiarism check, competing interests, ethical requirements for studies involving human participants or animals,financial disclosures,full compliance withNRR’s data availability policyetc. Submissions may be returned to authors for queries, and will not be assigned to our Editorial Board or peer reviewers until they pass the quality control check.
Peer Review Process
Once the manuscript has passed quality control check, it is assigned to the strict double-blinded peer review process for a decision, either to accept, revise, or reject the article. Before manuscripts are sent for review, invited peer reviewers are confirmed regarding their availability, conflicts of interest with the manuscript, their agreements to have their names and comments published afterwords. A peer review report together with the reviewer’s name, if permitted, will be posted at the end of the article. Only 15–20%of over 2,000 manuscripts submitted per year are published inNRR. Most manuscripts will be evaluated by 3–5 external reviewers. Average time from the submission to the first editorial decision is 1 month. The review time could be shortened to 7 days for the paper with sophisticated review comments from other recognized journals in the field. According to these comments, the academic editors will make a decision as to accept, reject, request a revision or send to another peer review. Authors who receive a decision of Minor Revision or Major Revision have 21 days to resubmit the revised manuscript.
If you are submitting a revised manuscript, the following items with your revised submission are required:
· Response to reviewers form: Address the specific points made by each reviewer.
· Revised manuscript (traced copy):Include a traced copy of your manuscript file showing the changes you have made on the original submission.
· Revised manuscript (clean copy):Upload a clean copy of your revised manuscript that does not show your changes.
Reviewer Recognition
The quality ofNRRdepends on the effort that is generously contributed by our reviewers who have dedicated their expertise and time helping to ensure we publish great science. Special thanks go out toNRR’s 2017 outstanding reviewers (Additionalfile 1) who have provided an extraordinary number of thoughtful reviews and reviewed four or more manuscripts in 2017.
NRRprovides a certification of review for those who provide more than 100 words of general comments and complete the peer-review process within 14 days.
We encourage the reviewers to share and discuss their review comments on Publons (www.publons.com).NRRwill also give credit to registered reviewers on Publons.
Submission Guidelines
Manuscripts should be submitted online at http://www.editorialmanager.com/nrr/.
Initial Submission
Cover letter
Authors are required to declare the following statements, including:
· The manuscript is original, has not been submitted to or is not under consideration by another publication and has not been previously published in any language or any form, including electronic.
· All authors approve thefinal version of the manuscript and of its submission toNRR.
· Recommending 3–5 scientists as peer reviewers for your manuscript, including their contact information. The suggested reviewers should be in the samefield of your study, and from different institutions and have not copublished articles previously.· Once submitted toNRR, the manuscript should not be submitted to other journals within 1 month, whether it is undergoing or awaiting the paper review process.
Update your ORCID record after publication
The first author or the corresponding author should provide ORCID upon manuscript submission.NRRalso helps authors to register a unique ORCID identifier to uniquely identify author’s publication.
Transfer of copyright agreement
Once your paper is successfully submitted, publishing agreements should be signed by all authors and uploaded to the editorial system.
Article Type
·Invited perspectives:Authors with outstanding achievements in thefield from international renowned laboratories are invited to write a short paper that has not been previously published,introducing their scientific hypothesis, specific animal models or patients as participants, a novel technique or method, materials, or cell type. The invited perspectives should introduce compelling new stories about how scientists or laboratories yielded their striking thoughts or achievements,rather than simply describe their research progress in the field of neural regeneration. These papers will provide readers with novel thoughts and insights.
Submit an outline for your paper before writing, and the manuscript within 2 months after permitted byNRReditorial board.
Word limit: 1,500-3,000 words, 1–2 authors, 10 references maximum, no abstract, 1figure required, and 2 published pages maximum.
·Invited Reviews:Invited reviews are of great interest in neural regeneration.
Word limit: 6,000 words minimum including the abstract, but excluding references, tables and figures, 8–10 published pages
·Research Article:NRRwill consider manuscripts on any clinical or basic science with creative results that are relevant to intervention, repair, pro-tection, and regeneration after nerve injury.
Word limit: 4,000–6,000 words, 8–10 published pages.
·Imaging in Neural Regeneration:We will consider photographic, radiographic, or artistic images that have exceptional visual impact and have relevance to neurologic recovery.
Word limit: 1,500–2,000 words, 2 published pages maximum, no abstract, 2–3 figures required to display the relationships among histology of nerve repair, nerve regeneration, and function.
·Letters to the Editor:Brief communications and case reports should offer an important new observation and not simply review the literature. In rare instances, we will consider case reports for this article type, but only if the topic is extraordinarily novel.
Templates of Invited Reviews, Invited Perspectives, Research Articles, and Imaging in Neural Regeneration are shown in Additionalfiles 2–4.
Reporting Guidance
Manuscripts should be prepared in accordance with ICMJE Recommendation for the Conduct, Reporting,Editing, and Publication of Scholarly work in Medical Journals. The following guidelines should be followed when writing an article:
· ARRIVE guideline (Animal Research:Reporting ofIn VivoExperiments): The ARRIVE guidelines are recommended forin vivoexperiments in animals. See https://www.nc3rs.org.uk/arrive-guidelines for more information.
·CONSORT statement (Consolidated Standards of Reporting Trials):The CONSORT statement is recommended for reporting randomized controlled trials. The authors are encouraged to complete 25-item checklist in their cover letter. In addition, the registration identification number and protocol version of the randomized controlled trial is listed at the end of the Abstract.
See http://www.consort-statement.org/ for more information.
·STARD statement (STAndards for the Reporting of Diagnostic accuracystudies):This checklist is recommended for reporting diagnostic accuracy studies. See http://www.stard-statement.org/ for more information.
·STREGA statement (STrengthening the REporting of Genetic Associations):These guidelines are recommended for the reporting of genetic association studies. See http://www.strega-statement.org/ for more information.
·STROBE statement (STrengthening the Reporting of OBservational studies in Epidemiology):These guidelines are recommended for the reporting of observational studies in epidemiology).See http://www.strobe-statement.org/for more information.
·TREND statement (Transparent Reporting of Evaluations with Nonrandomized Designs):These guidelines are recommended for the reporting of non-randomized evaluations of behavioral and public health interventions.See http://www.cdc.gov/trendstatement/ for more information.
·MOOSE checklist (a Reporting Checklist for Authors, Editors, and Reviewers of Meta-analyses of Observational Studies):This checklist is recommended for meta-analyses of observational studies.
See https://www.editorial-manager.com/jognn/account/MOOSE.pdf for more information.
·PRISMA statement (Preferred Reporting Items for Systematic Reviews and Meta-Analyses):The PRISMA Statement is recommended for the reporting of systematic evaluation and meta-analysis articles.
See http://www.prisma-statement.org/ for more information.
·Equator Network (standards for Enhancing the QUAlity and Transparency Of health Research):This organization works to improve the reliability and value of reports on medical research studies by promoting transparent and accurate reporting of research studies.
See http://www.equator-network.org/ for more information.
Acceptance and Publication
After acceptance, the authors are free inquiry regarding the progress of their manuscript online using the account number assigned to the corresponding author at any time. Generally,manuscripts will be published within 3–6 months after acceptance, authors could apply for express publication within 2–3 months after revision if their publications meet the following conditions:
· Major grant-funded research;
· Previous reviewed by prestigious journals;
· Basic research with open data registered in public data repository;
· Prospective clinical trials with register number;
· Fast publications with significant innovations and impact.
Ethical Guidance
According to ICMJE recommendations, the authors should follow all ethical principles for medical research involving humans and experimental animals.
Requirements for ethical issues related to medical research
· Medical researchers should abide by the relevant principles for medical research involving human participants,conscientiously accept supervision from the ethical review committee,and effectively protect the legitimate rights and interests of the human participants.
· Medical researchers should objectively and accurately collect human samples, data, and information, and protect the life, health, privacy, and dignity of human participants.
· When performing studies involving humans or experimental animals,medical researchers should provide accurate medical records, including adverse reactions and events, and report information regarding severe adverse reactions and events according to related regulations.
· Medical researchers should be aware of public health and laboratory biology safety, consciously abide by the requirements of related laws and regulations, and accept inspection and supervision by related organizations when performing studies regarding emerging infectious diseases and diseases with unknown etiologies or known pathogen transformation.
· Medical researchers should store,share, and destroy human or animal samples, data, or materials after study completion according to regulations of scientific research.
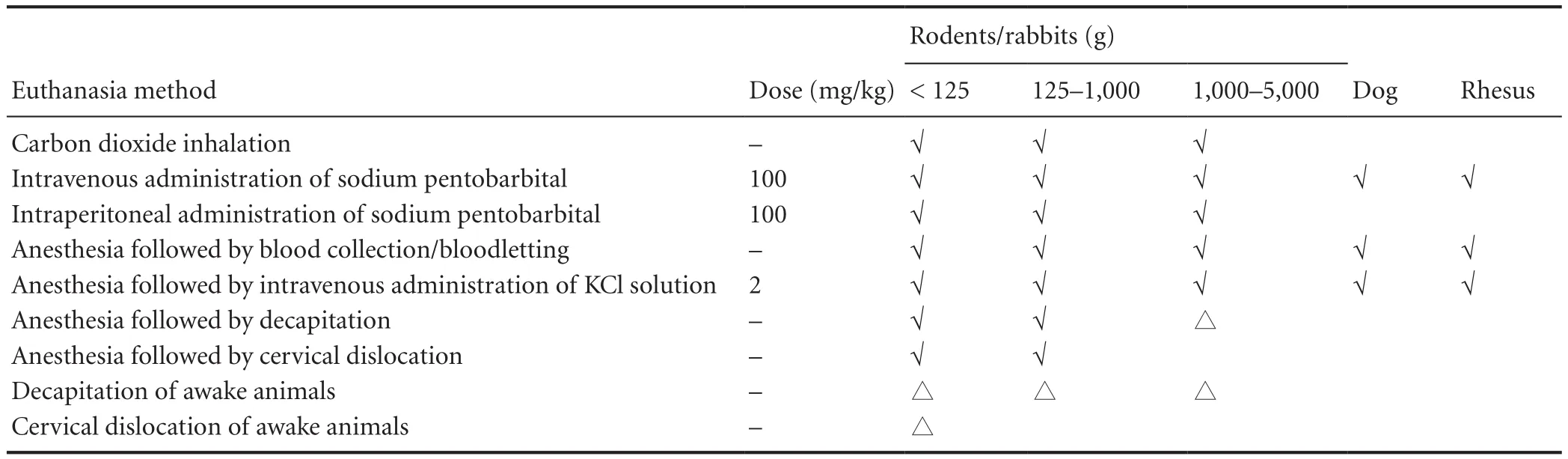
Table 1 Euthanasia methods in animal experiments
· Medical researchers should respect and protect the intellectual property rights of others and abide by the confidentiality rules of science and technology when performing academic communications and being invited to review another person’s submitted research paper or topic declaration.
· When citing published view-points,data, images, results, or other data,the source should be indicated and cited. When using other’s design ideas,experimental data, figures and tables,results and conclusions that have not been published, a written informed consent should be obtained from the authors, and acknowledgments and declarations should be openly stated.
· When publishing research papers,medical researchers should abide by related regulations. Medical researchers cannot be authors on a published research paper when they do not participate in conducting the research or contribute to the writing of the paper.
· Supervisors of the research project should take full responsibility to perform scientific research and assume responsibility for dishonorable events occurring during the writing of scientific papers.
· The original images, data (including computer databases), records, and samples involved in producing medical research papers should be kept in a secure storage area for potential recheck. Any errors or faults appearing in medical research achievements should be admitted and corrected publicly by the medical researchers.
· When performing collaborative scientific research, medical researchers should comply with good faith duty or contract, and authorship credit for publishing research papers or books,applying for patents, and acquiring awards should be based on substantial contribution.
· Medical researchers should have a scientific attitude and social responsibility and avoid false statements and news hyperbole in academic communication, achievement generalization,and popular science propaganda.Medical researchers should treat academic criticism and questions from peer reviewers with respect.
Requirements for ethical issues related to animal experiments
· Medical researchers should abide by international guidelines for the care and use of laboratory animals, theWeatherall report on the use of non-human primates in research (2006), and The National Centre for the Replacement, Refinement and Reduction of Animals in Re-search (NC3Rs).
· The authors’ institutional animal care and use committee that approved the animal experiments and the associated permit number(s) should be stated in research papers.
· The methods section ofNRRpapers must include ethics statements, e.g.“The protocol was approved by the Animal Ethics Committee of XXX University (approval number: XXX).All surgery was performed under sodium pentobarbital anesthesia, and all efforts were made to minimize animal pain, distress, and death.” Guidelines for euthanasia methods approved byNRRfor use in animal experiments is shown in Table 1.
· Data and methods published inNRRshould be shared for replication of the results by others. Original experimental data or DOI numbers registered in data repository should be provided upon submission to facilitate understanding of peer reviewers and transparency to other audiences.
· Original data from studies involving genes, proteins, mutants and diseases should be registered on recognized public databases e.g.figshare or re3data, and the registered identifier should be provided upon submission.
· A small amount of data or certain special data may be submitted as supplementary data online.
· For data mining from other open-access databases, the source must be indicated.
· Studies involving animal experiments should be reported according to ARRIVE guidelines (https://www.nc3rs.org.uk/arrive-guidelines).
Requirements for ethical issues related to clinical trials
· All studies performed involving human should be registered in clinical trials registry platform, such as clinicaltrials.gov, prior to participant recruitment. The registry platform and register identifier should be provided upon submission and included in the abstract of the manuscript.
· The ethics committee and the approval number(s) should be stated in papers. Prospective clinical studies with no registration will not be accepted byNRR. In addition, informed consent of study and protocol verion should be indicated.
· Clinical manuscripts should be written according to the reporting guidelines at www.equator-network.org. Additionally, checklists and a flow chart should be provided upon submission.
Patient Consent Privacy
Identifying information should not be published in written descriptions,photographs, sonograms, CT scans,etc., and pedigrees unless the information is essential for scientific purposes and the patient (or parent or guardian,wherever applicable) gives informed consent for publication. Authors should remove patients’ names from figures unless they have obtained informed consent from the patients. The journal abides by ICMJE guidelines: 1.Authors, not the journals nor the publisher, need to obtain the patient consent form before the publication and have the form properly archived. The consent forms are not to be uploaded with the cover letter or sent through email to editorial or publisher offices.2. If the manuscript contains patient images that preclude anonymity, or a description that has obvious indication to the identity of the patient, a statement about obtaining informed patient consent should be indicated in the manuscript.
Plagiarism
· EachNRRpaper will be checked twice, using Crosscheck to verify originality after submission and prior to publication. The check report will be sent to the authors.
· The similarity of anyNRRpaper should not be over 5% against one single published paper, nor over 20%against all published papers.
· Similarity between new submitted manuscript and the published by the same research team or author should be not over 50%
· No retracted articles should be cited.
· For dishonorable events including redundant (duplicate) publication,suspected plagiarism, and undisclosed conflicts of interest,NRRwill abide by COPE guidelines (http://publicationethics.org/resources).
Corrections and Retractions
NRRpublishes corrections, retractions, and expressions of concern as appropriate, and as quickly as possible. We follow the ICMJE and COPE guidelines where applicable.
· Correction: A notice of correction will be issued by NRR to correct substantial errors that appear in published articles when these errors significantly affect the content or understanding of the work (e.g., error in data presentation or analysis) or when the error affects the publication’s metadata (e.g., misspelling of an author’s name). In these cases,NRRwill publish a correction that will be linked to the original article. In very rare cases, we may choose to correct the article itself and repost it online. If that course is taken, a correction notice will also be created to document the changes to the original article.
·Author-Initiated Retractions:NRRwill retract an article at the authors’request at any time unless it is under review for a possible violation of Responsible Conduct Regarding Scientific Communications.At the authors’ option, the retraction notice may simply state that the article has been retracted at the authors’ request. Alternatively,the authors may provide a brief explanation of the error(s) prompting the retraction. However, statements of retraction may not assign blame to specific authors or laboratories.
·Retractions:The editors reserve the right to retract an article at any time after publication without the consent of the authors if an investigation by an appropriate authority reveals a violation ofNRR’s Ethics Policy.
· To request a correction/retraction,please contact the editorial office directly at szb@nrren.org .
Data Avialibility
NRRencourages authors to upload original experimental data on journal website or Figshare prior to or after publication, including original data,images, or tables. Open access of data will increase study transparency, accelerate the scientific research pace,and establish crediblity of scientific research.
Figshare is an internationally respected data repository that can upload, store, and share original data of the study. With the permission of open-access copyright, the authors can display and share their data, which facilitates retrieval, reading, download,and sharing.
Data-Sharing Statement in Clinical Trials
As of 1 June 2018, manuscripts submitted to NRR that report the results of clinical trials must contain a data-sharing statement as described inTable 2.http://www.icmje.org/news-and-editorials/data_sharing_june_2017.pdf
Open-Access Publication
NRRis co-published by Wolters Kluwer-MedKnow, a global open access medical publisher. Therefore,NRRapplies the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License to works we publish. This license allows others to remix, tweak,and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Anyone interested in reading your research can get free access to your paper at our official website (www.nrronline.org) or PubMed Central.
Open Access Fees
Authors of invited papers will be granted complete waiver for the open-access fee.
When a paper is accepted for publication, the authors will be issued an invoice of the open-access fee. The open-access fee is used for online open access, development and maintenance of editorial system, and data processing for better exposure on Google Scholar,iPhone, iPad and Android smartphones,as well as for PubMed indexing and CrossRef registration. The open-access fee will, at the discretion of the editorial committee based on the quality of manuscript, be reduced or waived for manuscripts from low-income countries.Articles decision making is not associated with author’s ability to pay. The cost for language polishing is not covered in open-access fee.
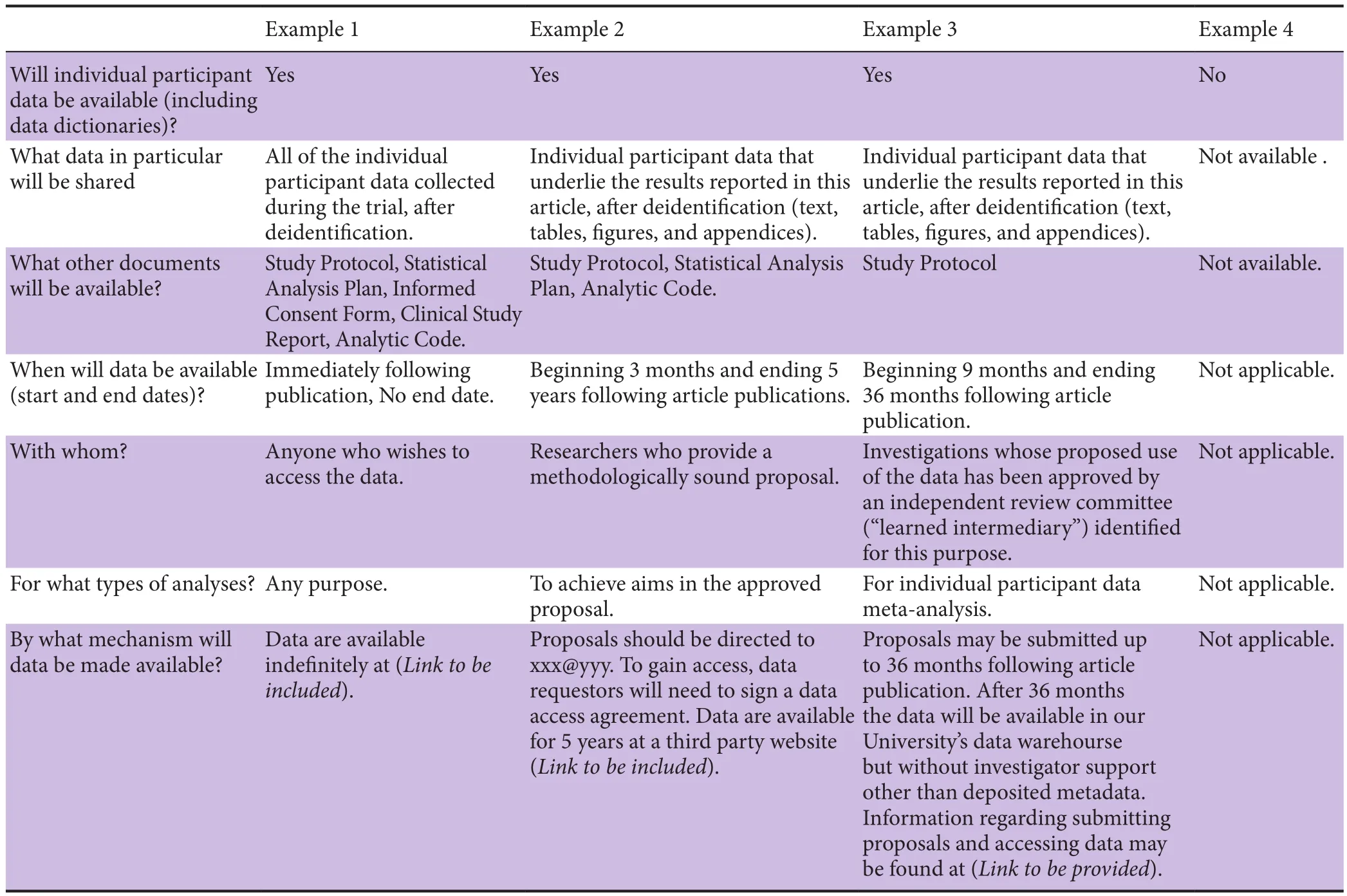
Table 2 Examples of Data Sharing Statements That Fulfill These ICMJE Requirements*
Copyright/Permissions after Publication
· The publisher retains all rights concerning assembling, printing, reproducing, translating, disseminating,exhibiting, publishing, retrieving and indexing part or of all the contents of the article.
· After signing transfer of copyright with the journal, the authors still retain the rights.
· In not-for-profit circumstances, the use, dissemination and reproduction of part or of all contents of the article is permitted when cited properly.
Reprints/Subscriptions
We are committed to providing two hard copies and five high-quality reprints to authors for free. Reprints can be purchased as print or electronic reprints and as translated articles, and will be customized to your specific requirements and shrink-wrapped.
Dissemination after Publication
Once the paper is published, it will be scheduled for disseminationviae-Newslettter.
After publication,NRRprovides articles more visibilty on the social network, e.g. Mendeley, Facebook,Twitter, LinkedIn. We also recommend authors link your paper from as many websites as possible using citation and social book marking tools such as,
· ORCID, https://orcid.org/
· ResearchGate,https://www.researchgate.net/
· Kudos, https://www.growkudos.com/
· Mendeley,https://www.mendeley.com/
· Figshare, https://figshare.com/
· DataCite, https://www.datacite.org/
· Google+,https://plus.google.com/discover
· Facebook,https://www.facebook.com/
· Twitter, https://twitter.com/
· LinkedIn, https://www.linkedin.com/
· Wikipedia http://en.wikipedia.org/wiki/Acanthamoeba_keratitis#References etc.
Contact Us English website:http://www.nrronline.org/
Chinese website:http://www.sjzsyj.org/
Submission website:http://nrr.edmgr.com/
Telephone:+86(24)23380576/+86-13804998773
By Mail:Editorial Office of NRR,Shenyang PO Box 10001, Shenyang 110180, Liaoning Province, P. R. China
Author inquiries:bwb01@nrren.org
WeChat Official Account:sjzsyj_nrr
LinkedIn Account:NRR Liu
Mendeley Account:Neural Regeneration Research
Facebook Account:Liu NRR
Twitter Account:NRR Neural
- 中國神經(jīng)再生研究(英文版)的其它文章
- Injury of the Papez circuit in a patient with traumatic spinal cord injury and concomitant mild traumatic brain injury
- Critical role of SDF-1/CXCR4 signaling pathway in stem cell homing in the deafened rat cochlea after acoustic trauma
- Low-frequency pulsed electromagneticfield pretreated bone marrow-derived mesenchymal stem cells promote the regeneration of crush-injured rat mental nerve
- PTEN knockdown with the Y444F mutant AAV2 vector promotes axonal regeneration in the adult optic nerve
- Neuroprotective mechanisms of rutin for spinal cord injury through anti-oxidation and anti-inflammation and inhibition of p38 mitogen activated protein kinase pathway
- Does combined therapy of curcumin and epigallocatechin gallate have a synergistic neuroprotective effect against spinal cord injury?