TAVR in 2017―What we know? What to expect?
Panagiota Kourkoveli, Konstantinos Spargias, George Hahalis
1Department of Transcatheter Heart Valves, Hygeia Hospital, Athens, Greece
2Department of Cardiology, University of Patras, Patras. Greece
1 Introduction
Transcatheter aortic valve implantation (TAVI) or replacement (TAVR) represents nowadays a viable and established therapeutic option in patients with severe aortic stenosis (AS) who are considered high or prohibitive risk for conservative surgical treatment. It has been a long journey requiring almost ten years of preclinical research since the first in man TAVR in 2002. Five more years of clinical trials followed, before “Conformité Européene” (CE) marking and clinical use initiation in Europe.
In the last decade, TAVR has been performed in about 400,000 patients worldwide and indications keep growing at a rate of 40% annually. Real-world data published confirm the rapid adoption of TAVR, especially in the developed countries, shifting the treatment of AS from conventional surgery to a percutaneous transcatheter approach.[1]
The positive results of the first randomised clinical trial published in 2010,[2]which were the precursor of the inclusion of the method in the European Society of Cardiology(ESC) and the European Association for Cardio-Thoracic Surgery guidelines and by the American College of Cardiology/American Heart Association (ACC/AHA) Task Force on Practice Guidelines in 2012 and 2014, respectively.Based on these recommendations, TAVR can be performed in patients not suitable for conventional surgical treatment I(B) and as an alternative to surgery in high risk patients after approval by a multidisciplinary heart team, based on the individual risk profile and anatomy IIa (B).[3-5]
The field of TAVR has been rapidly evolving over the last years. Ongoing developments in procedural techniques and biomedical engineering have made TAVR a simple and safe method for high risk patients. The question of expanding the indication for TAVR to intermediate or even lower risk patients has been raised by two recent large randomized trials. Based on the results of these trials, the AHA/ACC have recently published new guidelines on the management of patients with valvular heart disease in which TAVR has an I (A) indication in high surgical risk patients and a brand new indication IIa (B) in patients with severe AS and of intermediate surgical risk depending on patient-specific procedural risks, values and preferences.[6]
The choice of proceeding with surgical aortic valve replacement (SAVR) versus TAVR or even medical treatment is based on multiple factors, including surgical risk, patient frailty, comorbid conditions, and patient preferences and values. Non-patient depended factors of extreme importance is the operators’ experience in performing transcatheter procedures, as well as the financial aspect, referring to the ability of each country’s national insurance system to cover the expenses.
TAVR has been studied in randomised controlled trials(RCTs), as well as in numerous observational studies and multicentre registries that include large numbers of high-risk patients with severe symptomatic AS. The aim of this review is to analyse and compare the results of the published RCTs referring to transfemoral TAVR vs. SAVR and try to draw conclusions regarding the optimal choice of treatment for patients with severe symptomatic AS.
2 Overview
Current indications and recommendations for TAVR in high surgical risk patients are essentially based on the following RCTs; the PARTNER cohort B[1](2010), PARTNER cohort A[3](2011), US Corevalve High Risk[7](2014),PARTNER 2A[8](2016) and SURTAVR[9](2017). The extension of the indication for TAVR in the intermediate surgical risk patients as published in the latest ACC/AHA update for valvular heart disease was based on the results of the PARTNER 2A trial and the single arms (High and Intermediate Risk) study SAPIEN 3.[10,11]
Most of our knowledge on TAVR is based on an extensive experience acquired with two devices, Edwards prosthesis (Edwards Lifesciences, Irvine, CA, USA) and the Medtronic CoreValve (Medtronic, Minneapolis, MN, USA).The “Placement of Aortic Transcatheter Valve (PARTNER)” trials used the balloon-expandable Edwards prosthesis (SAPIEN in PARTNER cohort A and B, SAPIEN XT in PARTNER 2A and SAPIEN 3 in SAPIEN 3 trial), while the Corevalve high risk trial used the self-expanding Medtronic Corevalve. The majority (84%) of patients in the SURTAVI trial received the Medtronic Corevalve while the remaining(16%) patients were treated with the new generation Evolut R transcatheter aortic valve (Figure 1).
In all the aforementioned trials, surgical risk was estimated using a risk model developed by the Society of Thoracic Surgeons (STS score), which uses an algorithm based on the presence of coexisting illnesses in order to estimate the 30-day operative mortality, and in contrast to previous non randomised trials in which the Logistic EuroSCORE was used.
In PARTNER cohort A, patients were deemed to be at high risk for operative complications or death on the basis of coexisting conditions that were associated with an estimated risk of postoperative 30-day mortality of at least 15%.PARTNER cohort B included inoperable patients with an estimated risk of 30-day morbidity and mortality > 50%. The final determination of high operative risk in both arms was made by the heart team at each study centre, using as a guideline a score of at least 10% on the STS risk model.
The US Corevalve High Risk trial included patients that were considered to be at increased surgical risk by the heart team with an estimated risk of 30-day mortality of 15% or more and risk of death or serious complications within 30 days after surgery of less than 50%. Surgical risk assessment included consideration of the STS score and other factors not included in the STS assessment. This score provides an estimate of the rate of death at 30 days among patients undergoing surgical aortic-valve replacement on the basis of a number of demographic and procedural variables.
PARTNER 2A trial included patients with intermediate surgical risk. In this trial, the guideline was a STS risk score of at least 4.0%; the upper limit applied by the case review committee was 8.0%. The same risk model was applied in the SAPIEN 3 trial. Finally, SURTAVR trial included patients with symptomatic, severe AS determined by the local multidisciplinary heart team to be at intermediate surgical risk, which was defined as an estimated risk of 30-day mortality of 3% to 15%, according to the STS, as well as non-traditional factors (coexisting illnesses, frailty, and disability).
3 Results
Table 1 summarizes published data from the RCTs conducted so far regarding surgical risk estimation and mortality rates. Table 2 shows the reduction of the length of hospital stay and the improvement in the survival rates in patient after TAVR and SAVR over the years. As mentioned before, surgical risk was estimated using the STS score.There is a significant correlation between observed and estimated surgical mortality, as calculated with STS score,which underlines the significance of this statistical tool to account for the impact of patients’ risk factors on operative mortality and morbidity. On the other side, logistic Euro-Score, which was used in addition to STS score in patients included in the US Corevalve High Risk and SAPIEN 3 intermediate risk showed significant deviation from the observed mortality. In particular, the logistic EuroSCORE in US Corevalve High Risk trial was 17.5% ± 13.1% in the TAVR arm and 18.6% ± 13% in the SAVR arm. In the single arm SAPIEN 3 trial, the logistic EuroSCORE was 13.2% ± 5.1%.
In the landmark PARTNER cohort B trial, the survival benefit from TAVR in patients unsuitable for surgical treatment was remarkable with a mortality reduction up to 50% during the follow up period. Despite the initial procedure-related mortality, TAVR significantly improved survival with just six patients needed to treat (NNT) to have one more alive at one year, five patients to treat to have one more alive at two years and only four to treat to have one more alive at three years.
PARTNER cohort A trial randomized high-risk patientswith severe aortic stenosis to SAVR or TAVR. As seen in Table 1, STS scores in cohort A and B were comparable(mean STS score 11%-12%), while no significant difference was seen in terms of hospital stay between the two arms of cohort A trial (mean about 10 days). The significantly lower 30-day mortality in the TAVR arm can be associated with the less invasive nature of the method. At five years, however, rates of death from any cause were similar between the two groups. The comparable mortality rates across all time points could be related with the poor baseline status of the patients enrolled.

Table 1. Published data from the RCTs conducted so far regarding surgical risk estimation and mortality rates.
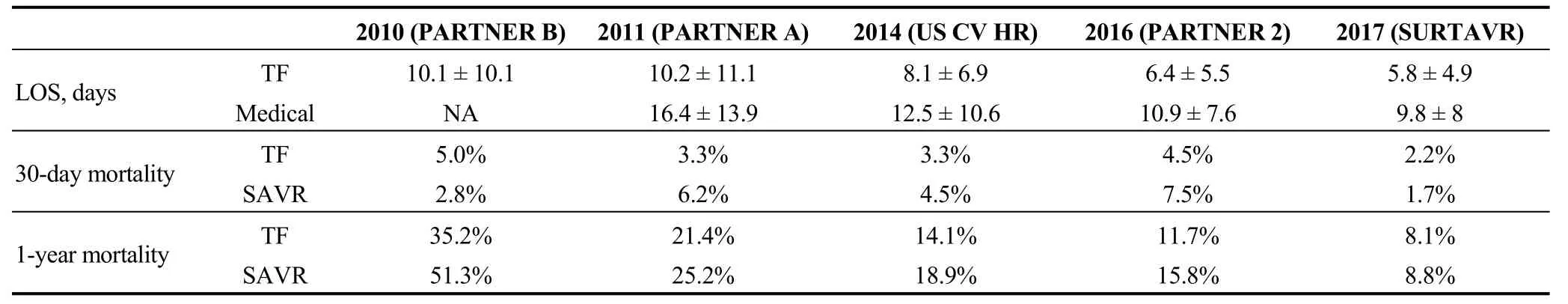
Table 2. Length of hospital stay and mortality rates of TAVR and SAVR over the years based on published data from trials.
On the contrary, US Corevalve High Risk trial included high risk patients but with a significantly lower STS score(mean: 7%-8%) and shorter in hospital stay for both the TAVR arm (about eight days) and the SAVR arm (about 12 days compared to about 16 days in PARTNER cohort A trial). The superior survival rate seen at 30 days for TAVR vs. SAVR was maintained at all time points until today(three years of follow up). In this trial, the number of patients need to be treated with TAVR vs. SAVR, in order to have one more survivor (NNT) in years two and three was 14.
A subgroup analysis of US Corevalve High Risk trial which included 383 patients with calculated STS score <7% (intermediate risk patients) showed better two years’survival in patients treated with TAVR compared to those treated with SAVR and with a lower NNT of 9 (Figure 2).
PARTNER 2A trial included intermediate risk patients with a mean STS score of 5%-6%, 2% lower compared to that of patients in the US Corevalve High Risk trial. The interquartile range showed that 25% of these patients had STS score < 4.4%, meaning that a great amount of these patients were between 3% and 4%. This was a non-inferiority trial which showed significant improvement in survival in the TAVR arm. In this trial, the number of patients requiring treatment in order to have one more survivor free of cerebrovascular events (NNT) was 25 in the second and third year. The total length of stay in PARTNER 2A was lower compared to all other trials (mean of 6 days for TAVR vs. mean of 9 days for SAVR) confirming that the patients included were of lower surgical risk.
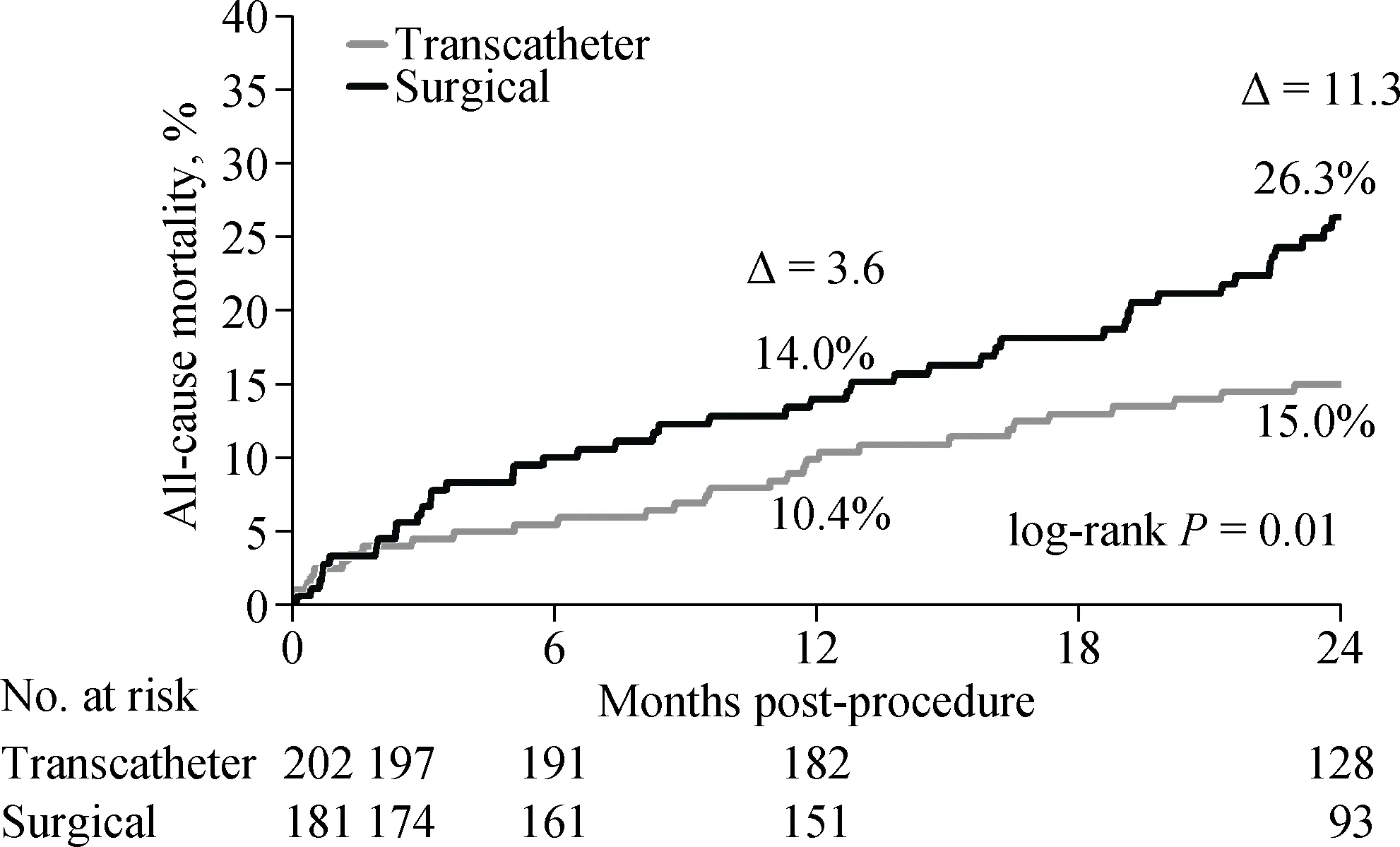
Figure 2. Kaplan-Meyer mortality curves in US Corevalve High Risk with STS score < 7%. STS: Society of Thoracic Surgeons.
The most reassuring results were those of SAPIEN 3 study which included intermediate surgical risk patients as in PARTNER 2A. Patients in SAPIEN 3 study had the lowest length of stay (average of four days) and the lowest 30 days mortality seen so far (1.1%). One year mortality was also very low (7.4%), almost 50% lower than in the surgical cohort of PARTNER 2A trial.
In March 2017, Medtronic unveiled the first-ever clinical data from the Surgical Replacement and Transcatheter Aortic Valve Implantation (SURTAVR) trial, which were presented at the ACC 66thAnnual Scientific Session.[9]The SURTAVR trial enrolled intermediate risk patients with mean STS score of only 4.4% and compared TAVR patients to SAVR patients. Total length of stay however was not lower compared to that of high surgical risk patients included in PARTNER 2A trial and SAPIEN 3IR study. This could be related with the increased need of permanent pacemaker implantation following the implantation selfexpanded transcatheter aortic valve. Rates of all-cause mortality for TAVR at two years, even though in absolute numbers were, the best ever seen, no statistical significance was however documented.
To summarize, TAVR seems to be a viable method for high risk patients (STS > 8%-10%). Thirty days and short term mortality rates, as well as length of hospital stay in patients treated with TAVR are significantly lower compared to the SAVR cohort. Long term survival benefit however is rather low and difficult to estimate mainly due to the poor performance status of these patients. On the other hand, intermediate surgical risk patients seem to benefit more for the transcatheter method with data showing clinical superiority of TAVR in terms of long term survival and in hospital stay.
4 Cost effectiveness
In general terms, cost effectiveness of an applied therapy compared to another depends on two factors: (1) the total cost difference between the two therapies, referring not only to the cost directly related to therapy application but also to the lifetime cost and; (2) the effectiveness of each therapy on patients. Based on the observed data form the PARTNER cohort B trial,[12]we project that TAVR will increase life expectancy by about 1.6 years compared to standard medical care with an estimated cost of $50,200 per life-year gained. The main element in this analysis is the significantly increased cost of TAVR compared to standard medical treatment, which is somehow supplanted by the clinical efficacy of TAVR.
In PARTNER cohort A trial,[13]the authors made an attempt to directly evaluate the incremental cost-effectiveness ratio of TAVR relative to aortic valve replacement (AVR)among patients who are acceptable candidates for high-risk SAVR. In the mathematic model, the 12-month cost in TAVR was calculated $1,250 lower compared to SAVR,while the cost for quality adjusted life year (QALY) was less than $50,000 in 70.9% of cases and became negative in 60% of the simulations (net benefit for TAVR). This result reflects the unbearable cost of classic surgical treatment of the very high risk patients included in the study.
In US Corevalve High Risk trial,[14]the cost of SAVR in patients at intermediate surgical risk was as expected lower and life time cost projection was $17,849 in favor of SAVR.Therefore, despite the clinical superiority of TAVR, the cost for TAVR vs. SAVR was $55,090 per QALY gained, which is similar to the one calculated for TAVR vs. medical treatment in PARTNER cohort B trial.
To date, there is no available data regarding cost effectiveness for PARTNER 2A and SAPIEN 3 Intermediate Risk trials. Based on published data from previous trials investigators estimate that the results for PARTNER 2A will be similar to US Corevalve High Risk. The outstanding clinical results of SAPIEN 3 Intermediate Risk are expected to have a positive impact on cost effectiveness of TAVR.
In conclusion, and in accordance with the documented clinical efficacy of TAVR, the aforementioned cost effectiveness analysis is in favor of the transcatheter method mainly in high surgical risk patients, who remain however eligible for surgery.
5 Future challenges
The long-term durability of TAVR valves remains a question, especially now that the application of the method has expanded to lower risk and younger patients. Multiple studies with prosthetic surgical valves have shown that structural valve deterioration (SVD) in these valves fluctuates between 10% and 20% in a 10 years’ period.[15]SVD is defined as the deterioration of patient’s functional status due to valve’s malfunction, which could cause stenosis and/or regurgitation.
A study of Toggweiler, et al.[16]demonstrated favourable outcomes five years after successful TAVI with excellent hemodynamics and signs of moderate prosthetic valve failure observed in only 3.4% of patients. No patient developed severe valvular regurgitation or stenosis.
A more recent study of Dvir, et al.,[17]which was presented in the EuroPCR meeting in May 2016, showed that in a total of 378 patients, who had undergone a TAVR with a previous generation SAPIEN valve, the observed valve’s degeneration was 10% in six years (a further increase up to 30% was noticed in patients with chronic renal failure). In this case, study valve’s degeneration had a different definition,that of at least moderate regurgitation and/or mean transaortic gradient > 20 mmHg, which was not evident one-month post procedure and was not due to infective endocarditis.
As a conclusion and based on the published data, it is safe to say that eight years is an acceptable time frame for good valve performance. Therefore, it is justified to use these valves in patients with an estimated life expectancy of at least eight years or in patients that have exceeded it (estimated life expectancy in Greek population for 2015; 81 years for women and 78.3 years for men). When it comes to younger patients, due to remaining high incidence of paravalvurar regurgitation after TAVR (despite the reassuring results with the new generation valves), TAVR shouldn’t be recommended.
6 Conclusions
Whether TAVI will become the standard treatment of care over the next years is uncertain but possible. Despite the outstanding results and the increasing frequency of TAVR the number of SAVR performed remains grossly unchanged. The reassuring results of PARTNER 2A and SURTAVI studies that were recently published, confirm the clinical superiority of transfemoral TAVR in intermediate surgical risk patients, is expected to change clinical practice and expand the method in this group of patients, in which SAVR is the gold standard of therapy. Until then, long term durability of TAVR valves as well as lower rates of paravalvular leak, stroke and permanent pacemaker implantation needs to be established.
References
1 Eggebrecht H, Mehta RH. Transcatheter aortic valve implantation (TAVR) in Germany 2008-2014: on its way to standard therapy for aortic valve stenosis in the elderly? EuroIntervention 2016; 11: 1029-1033.
2 Leon MB, Smith CR, Mack M, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010; 363: 1597-1607.
3 Vahanian A, Alfieri O, Andreotti F, et al. Guidelines on the management of valvular heart disease (version 2012). Eur Heart J 2012; 33: 2451-2496.
4 Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011; 364: 2187-2198.
5 Nishimura RA, Otto CM, Bonow RO, et a l. AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task force on practice guidelines. Circulation 2014; 129: e521-e643.
6 Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease. J Am Coll Cardiol 2017; 70: 252-289.
7 Adams DH, Popma JJ, Reardon MJ, et al. Transcatheter aorticvalve replacement with a self-expanding prosthesis. N Engl J Med 2014; 370: 1790-1798.
8 Leon MB, Smith CR, Mack MJ, et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med 2016; 374: 1609-1620.
9 Reardon MJ, Van Mieghem NM, Popma JJ, et al. Surgical or transcatheter aortic-valve replacement in intermediate-risk patients. N Engl J Med 2017; 376: 1321-1331.
10 Thourani VH, Kodali S, Makkar RR, et al. Transcatheter aortic valve replacement versus surgical valve replacement in intermediate-risk patients: a propensity score analysis. Lancet 2016; 387: 2218-2225.
11 Kodali S, Thourani VH, White J, et al. Early clinical and echocardiographic outcomes after SAPIEN 3 transcatheter aortic valve replacement in inoperable, high-risk and intermediate-risk patients with aortic stenosis. Eur Heart J 2016; 37:2252-2262.
12 Reynolds MR, Magnuson EA, Wang K, et al. Cost-effectiveness of transcatheter aortic valve replacement compared with standard care among inoperable patients with severe aortic stenosis results from the placement of aortic transcatheter valves (PARTNER) Trial (Cohort B). Circulation 2012; 125:1102-1109.
13 Reynolds MR, Magnuson EA, Lei Y, et al. Cost-effectiveness of transcatheter aortic valve replacement compared with surgical aortic valve replacement in high-risk patients with severe aortic stenosis. Results of the PARTNER (Placement of Aortic Transcatheter Valves) Trial (Cohort A). J Am Coll Cardiol 2012; 60: 2683-2692.
14 Reynolds MR, Lei Y, Wang K, et al. Cost-effectiveness of transcatheter aortic valve replacement with a self-expanding prosthesis versus surgical aortic valve replacement. J Am Coll Cardiol 2016; 67: 29-38.
15 Rahimtoola SH. Choice of prosthetic heart valve in adults: An update. J Am Coll Cardiol 2010; 55: 2413-2426.
16 Toggweiler S, Humphries KH, Lee M, et al. 5-year outcome after transcatheter aortic valve implantation. J Am Coll Cardiol 2013; 61: 413-419.
17 Dvir D. First look at long-term durability of transcatheter heart valves. Assessment of valve function up to 10-years after implantation. Presented at EuroPCR, Paris, France, May 17-20, 2016.
 Journal of Geriatric Cardiology2018年1期
Journal of Geriatric Cardiology2018年1期
- Journal of Geriatric Cardiology的其它文章
- Restrictive perimembranous ventricular septal defect with left to right Shunt post urgent aortic balloon valvuloplasty and transcatheter aortic valve replacement
- Transcatheter aortic valve replacement and stroke: a comprehensive review
- The role of echocardiography and CT angiography in transcatheter aortic valve implantation patients
- Transcatheter versus surgical aortic valve replacement in severe, symptomatic aortic stenosis
- Antithrombotic therapy in TAVI
- Endocarditis after transcatheter aortic valve implantation: a current assessment
