Antithrombotic therapy in TAVI
Manolis Vavuranakis, Konstantinos Kalogeras, Angelos Michail Kolokathis, Dimitrios Vrachatis,Nikolaos Magkoutis, Gerasimos Siasos, Euaggelos Oikonomou, Maria Kariori, Theodoros Papaioannou,Maria Lavda, Carmen Moldovan, Ourania Katsarou, Dimitrios Tousoulis
The First Department of Cardiology, Hippokration Hospital, Medical School, National & Kapodistrian University of Athens, Ilioupoli, Greece
1 Introduction
Transcatheter aortic valve implantation (TAVI) has been established as the main treatment option for high-risk or inoperable patients with symptomatic severe aortic stenosis(AS), for whom conventional surgical replacement has been previously denied.[1-4]Despite the early encouraging results regarding the patients’ outcome after TAVI, the risk of major periprocedural complications such as ischemic events and bleedings remains high in this frail population.[5]At the same time, many issues concerning optimal antithrombotic therapy are still under debate.[1,6-10]
A widely used, authority based practice is to prescribe lifelong aspirin in addition to clopidogrel for a 3-to 6-month period.[11,12]However, a variety of recent studies have questioned this practice regarding antiplatelet treatment.[13]Antithrombotic therapeutic approach becomes further more complex when there is a need for concomitant anticoagulant treatment. The usual clinical scenario of such a requirement is the presence of concomitant atrial fibrillation (AF).[14]Thus, considering that bleeding complications have a major effect on long-term outcomes in that population, it is of paramount importance to obtain data on optimal antithrombotic therapy in patients undergoing TAVI.[15-18]
There is a paucity of data derived from randomized control trials (RCTs) or registries concerning the appropriate antithrombotic regimen in patients undergoing TAVI, with no specific guidelines established up to now regarding these emerging issues.[19,20]In the present review, we aimed to identify all relevant studies evaluating antithrombotic therapeutic strategies in relation to clinical outcomes after TAVI.
2 Methods
A standardized protocol was used for study selection. We performed a systematic search of EMBASE, MEDLINE for the following search terms: (TAVI or TAVR) and (antithrombotic or antiplatelet). Studies were included in the study selection if they fulfilled the criteria of a Valve Academic Research Consortium (VARC)-reporting study and the outcomes or endpoints were defined according to the updated VARC.[21,22]
2.1 Thromboembolic events and pathophysiology in TAVI
Similar to most of other vascular or surgical interventions,TAVI carries a significant thromboembolic and concomitant bleeding risk not only during the procedure but also during the periprocedural period. Despite the fact that stroke rates are relative low after TAVI (about 3%), ischemic events documented with cerebral imaging methods remain of high frequency (66%-86%).[6,23]Indeed, the risk of stroke remains high during the periprocedural period due to the mechanics of valve implantation. Stenotic aortic valve is characterized by increased calcification and simultaneously large burden of tissue factor and thrombin, increasing inflammation, all of them predisposing to peripheral embolization.[24]Insertion and crossing of a bulky device through this high thrombogenetic environment, in addition to frequently required corrective maneuvers makes TAVI a high embolic risk procedure.
Accordingly, in the PARTNER (Placement of Aortic Transcatheter Valve) trial, TAVI was shown initially to have a higher risk for cerebrovascular events (CVEs) compared to surgical therapy.[3,25]These events have been post-procedurally correlated with adverse outcomes at 1, 12,and 24 months.[26,27]Almost half of all CVEs occur > 24 h after TAVI. Apart from artificial surface exposure, flow turbulence through the valve orifice and hemostatic activation due to vessel wall disruption are considered the main factors contributing to this late ongoing thrombogenicity,while AF, chronic or paroxysmal, add further to the embolic risk.[27-29]
2.2 Bleeding in TAVI
Similarly to thromboembolic risk, bleeding risk is a major and common threat for patients undergoing TAVI. According to VARC criteria, the incidence of periprocedural major and life-threatening bleeding is estimated around 15%-32% and 5%-16%, respectively.[25,30]Either with transfemoral access where large sheath sizes are used and bulky vessel calcification exists, or with transapical access due to inadequate apical repair, bleeding risk remains a major factor affecting patient outcome.
Nevertheless, about 50% of severe aortic stenosis patients present with anemia at baseline, either due to gastrointestinal loss (Heyde’s syndrome), or no obvious source of bleeding.[31-33]As a consequence, these patients often receive blood transfusions after TAVI.
2.3 Antithrombotic current recommendations in TAVI
Regarding anticoagulation during TAVI, unfractionated heparin (UFH) is widely administered, while the American College of Cardiology Foundation/American Association for Thoracic Surgery/Society for Cardiovascular Angiography and Interventions/Society of Thoracic Surgeons (ACCF/AATS/SCAI/STS) expert consensus document on TAVI advises maintenance of an activated clotting time (ACT) > 300 s with subsequent reversal using protamine sulfate (Table 1).[34]
Concerning antiplatelet therapy, the American guidelines recommend low-dose aspirin (81 mg qD) indefinitely and clopidogrel (75 mg qD) at short-term (3-6 months) after TAVI, while the European guidelines confirm that a combination of low dose aspirin and thienopyridine is required followed by a regimen consisting of aspirin or clopidogrel alone, despite the lack of firm data.[11,34,35]Finally, the Canadian statement on TAVI suggests the use of the same dose of aspirin indefinitely and clopidogrel (75 mg qD) for 30-90 days (Table 1).[36]
With regards to more complex scenario of patients requiring simultaneous anticoagulation therapy (concomitant AF), the American guidelines recommend low-dose aspirin in addition to oral anticoagulant (OAC) but avoidance of any other antiplatelet therapy whenever possible, while the European consensus document suggests that this population should be treated as if they have been stented without TAVI.[34,37]However, the Canadian recommendations discourage prescription of triple antithrombotic regimens (Table 1).[36]
3 Results
3.1 Randomized control trials
After study selection, four published RCTs mentioning the post-TAVI antithrombotic strategies have been finally identified (Table 2).[38]The Placement of Aortic Transcatheter Valves (PARTNER) trials A and B compared TAVI to SAVR and optimal medical therapy.[3,25]In both trials aspirin was given indefinitely (75-100 mg) and clopidogrel(75 mg) for six months. In the Medtronic CoreValve U.S.Pivotal Trial (CUSPT), in which TAVI vs. SAVR (surgical aortic valve replacement) were compared, aspirin (81-325 mg) indefinitely plus clopidogrel (75 mg) for three months was used.[39]In the comparison of transcatheter heart valves in high-risk patients with severe aortic stenosis: Medtronic CoreValve vs. Edwards SAPIEN XT (CHOICE) trial,
which compared TAVI with a balloon vs. a self-expandable prosthesis, suggested lifelong low-dose aspirin (100 mg)plus clopidogrel for three months.[40]Furthermore, in patients requiring concomitant oral OAC therapy, clopidogrel was prescribed for three months without aspirin (Table 2).
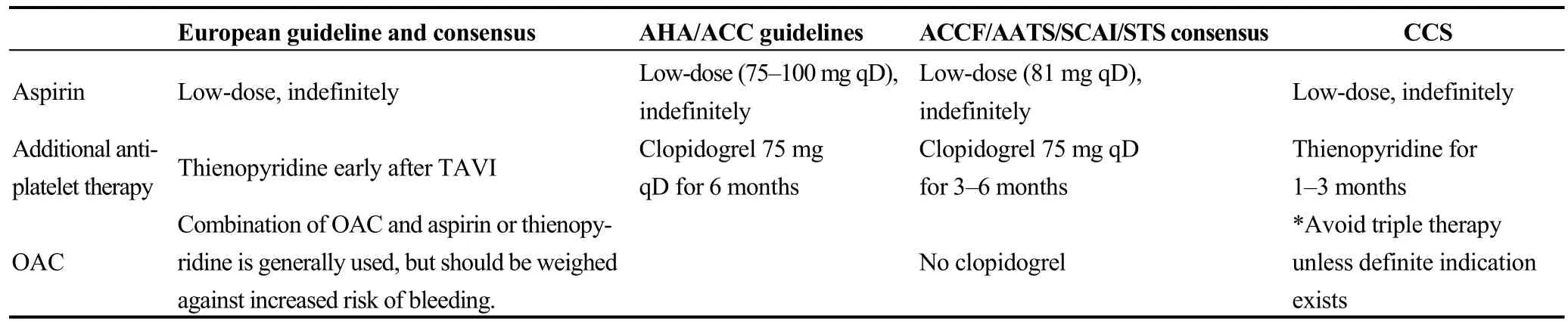
Table 1. Recommendations for antithrombotic therapy in TAVI.
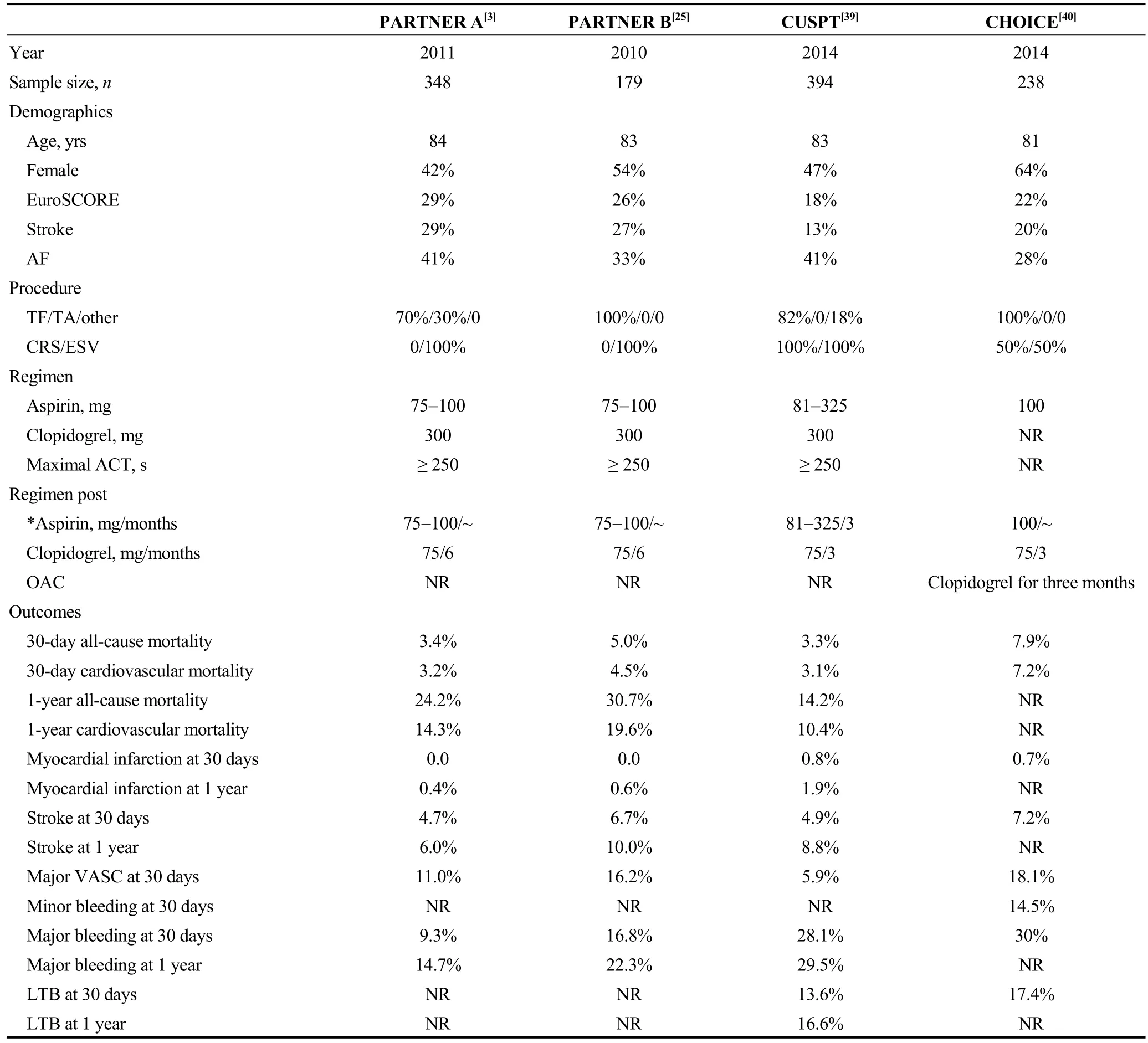
Table 2. Current RCTs mentioned in the antiplatelet regimen.
Between the aforementioned trials, baseline characteristics are comparable. Despite the fact that thromboembolic events seem comparable, vascular complications are lower in the CUSPT compared to the PARTNER trials. However,major bleedings rate at 30 days are higher in the CUSPT and CHOICE trial compared to the PARTNER trials [28.1%and 30% vs. 9.3% and 16.8%, respectively] (Table 2).
3.2 Registries
Regarding published registries about TAVI, overall seventeen have been identified and finally, out of them, only six were VARC-reporting studies (Table 3).[30,41-45]Among them, there is a wide variance in baseline characteristics.While concerning antiplatelet therapy, the loading dose of aspirin is not stated in most of them. Clopidogrel loading dose ranges from 300 mg to 600 mg while its’ duration ranges from 3-6 to 6-12 months. Concerning procedural anticoagulation,the maximal reported ACT was > 200 s or 250-300 s.
Among these VARC-reporting studies, major bleeding rates range from 1.6% to 20.9%, while life-threatening bleeding rates range from 1.2% to 13.9%. However, thromboembolic events seem comparable [myocardial infarction(MI) 0 to 2% and stroke 2% to 6.1%]. Finally, mortality rates at 1-month vary from 2.4% to 9.7% (Table 3).
3.3 Non-randomized studies for antiplatelet therapy
Four studies concerning antiplatelet regimens after TAVIhave been identified mainly comparing aspirin single antiplatelet therapy (SAPT) to dual antiplatelet therapy (DAPT)(Table 4). In the study from Ussia, et al.,[13]aspirin was prescribed indefinitely (100 mg) in all patients, while clopidogrel was added for three months (75 mg qD) in the clopidogrel-arm group. No further information concerning oral anticoagulant therapy is provided by the authors. At 30 days post procedure, bleeding was comparable between the two groups (18% vs. 18%). Besides, no difference was observed in composite endpoint of one month mortality, MI,and stroke.
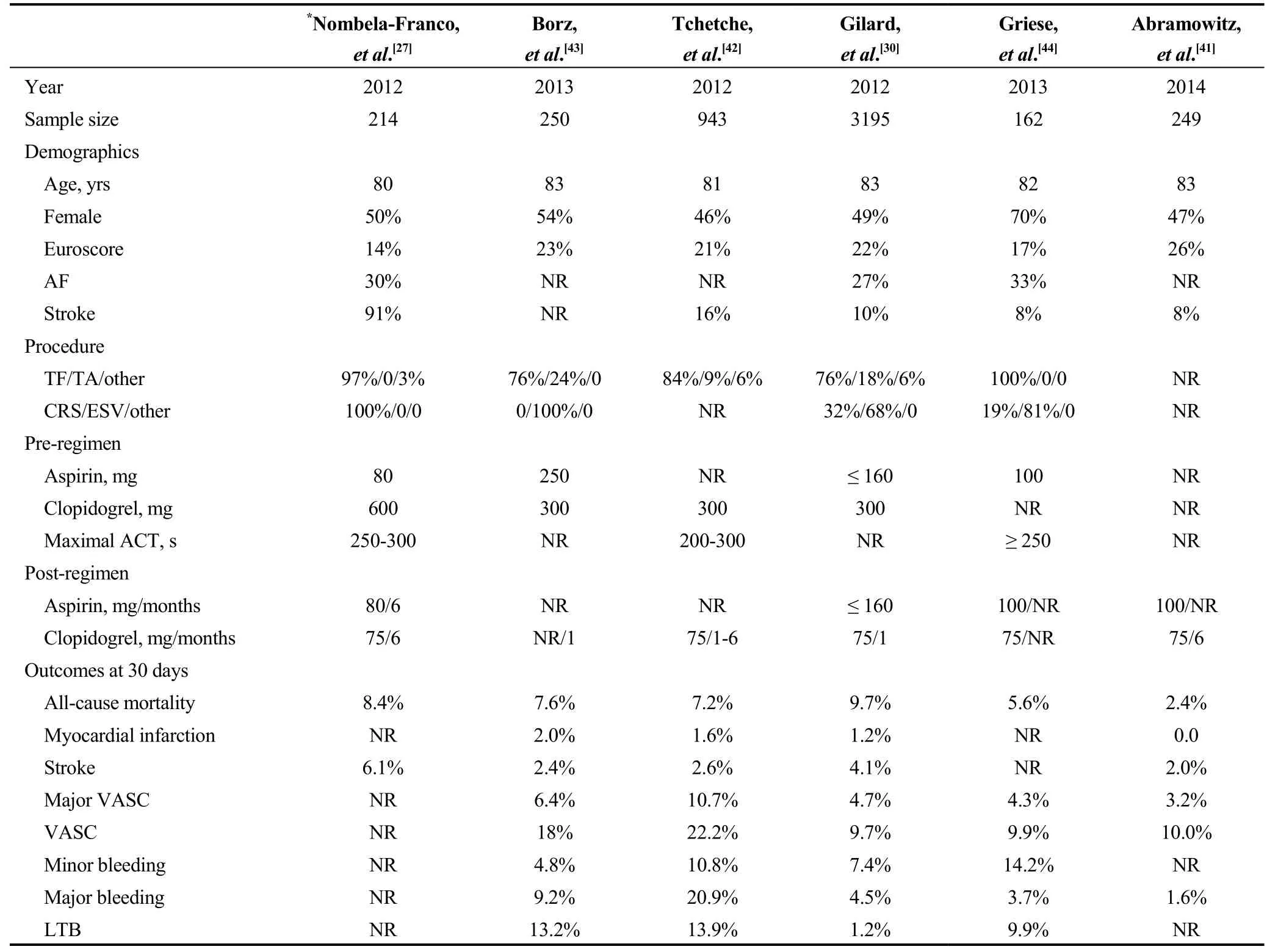
Table 3. Registries mentioning the antiplatelet regimen after TAVI.
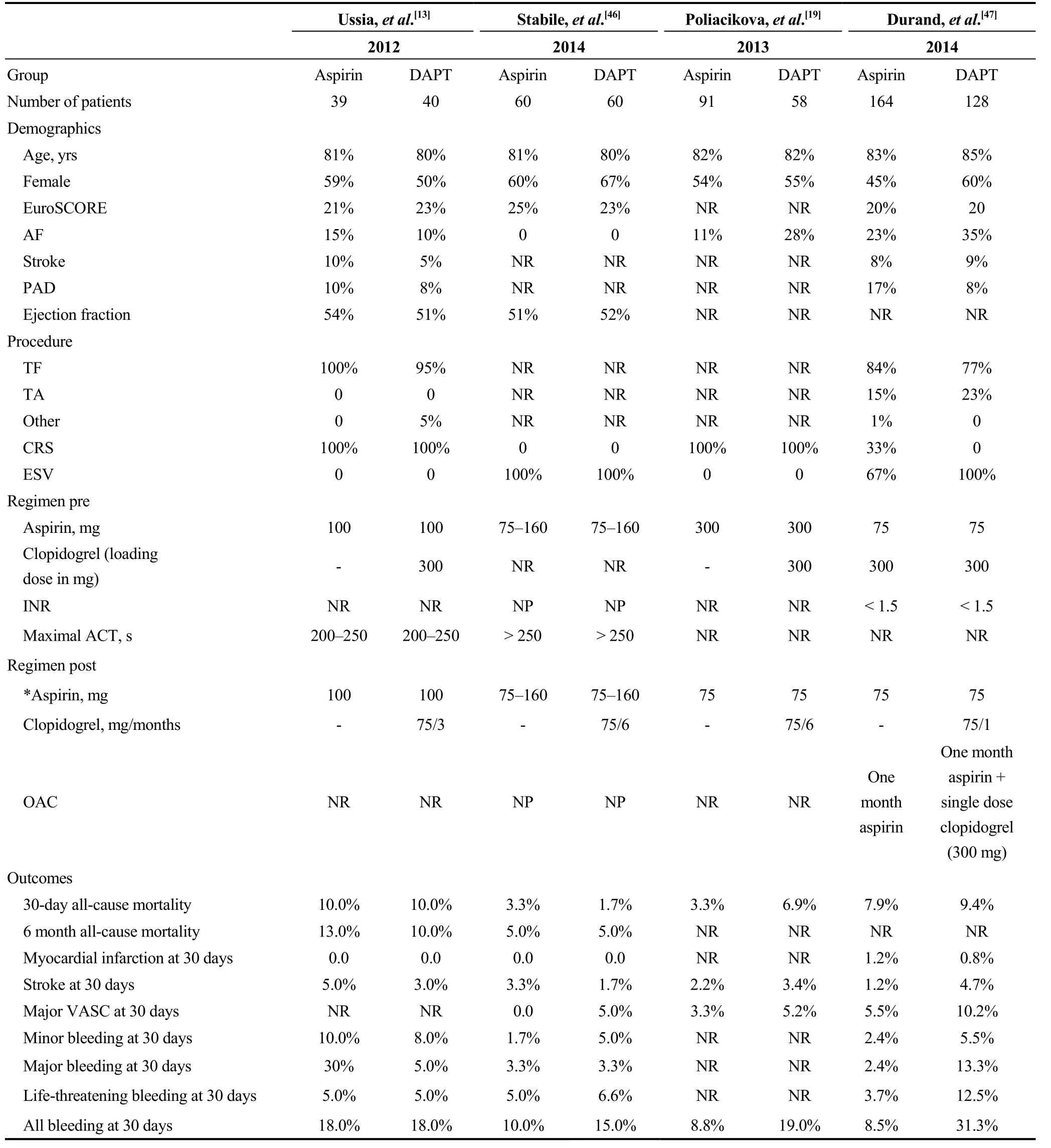
Table 4. Studies comparing aspirin vs. DAPT after TAVI.
In a similar design non-randomized study [single antiplatelet for TAVI (SAT-TAVI) trial], DAPT with clopidogrel was administered for six months in one arm, while SAPT was administered in the second arm.[46]Accordingly to Ussia, et al.,[13]bleeding at 30 days was not statistical different between two groups (10% for SAPT vs. 15% for DAPT). Similarly, 30-day rates for mortality, MI, and stroke results were comparable. However, in the SAT-TAVI trial,vascular access related complications (VASC) were reported significant lower for aspirin monotherapy vs. DAPT(5% vs. 13%, P < 0.05). In this study, patients requiring OACs were excluded.
In a third non-randomized study, Poliacikova et al.[19]compared retrospectively aspirin monotherapy vs. DAPT.Specifically, aspirin was administered indefinitely in all patients while clopidogrel was prescribed for six months in DAPT arm. Concerning thrombotic events and 30-day mortality, no difference between groups was recorded. Contrary to that, bleeding at 30 days was higher in DAPT vs. SAPT group (19% vs. 8.8%, P = 0.069). As well, the composite end point of all-cause mortality, acute coronary syndrome, stroke and major bleeding was higher in DAPT vs. aspirin group(27.6% vs. 12.1%, respectively).
Finally, in a multi-center study (FRANCE 2 registry), aspirin monotherapy vs. DAPT were prospectively compared.[47]The antithrombotic therapeutic regimens were as follows. In both groups, aspirin administration began the day before TAVI (75 mg without loading) and was given indeterminately. In the DAPT group, additionally to aspirin,clopidogrel was started (300 mg) the day prior to TAVI and continued for one month (75 mg qD). For patients requiring OACs, if in SAPT group, aspirin was administered for 30 days and if in DAPT group, except for 30 days aspirin (as in the SAPT group) a loading dose of 300 mg clopidogrel was given without continuation. Compared to the SAPT group,major and minor vascular access site related complications(VASC) were more frequent in the DAPT group (10% for DAPT vs. 6% for SAPT and 9% for DAPT vs. 2% for SAPT, respectively). Similarly, major and life-threatening bleedings were also recorded more frequent in the DAPT vs.SAPT group (13% vs. 2% and 13% vs. 4%, respectively).Accordingly, the number of patients needed blood transfusions was higher among the DAPT group (25% vs. 7%).However, mortality rates and thromboembolic events were not statistical different between the two groups.
3.4 Anticoagulant treatment
Concerning anticoagulation during implantation, only one published RCT has been finally identified.[48]The BRAVO-3 trial investigated whether a direct thrombin inhibitor, bivalirudin, offers an alternative to heparin as the procedural anticoagulant agent in patients undergoing TAVI.A total of 802 patients were randomized to undergo transfemoral TAVI with bivalirudin versus unfractionated heparin administered during the procedure. Regarding the two primary endpoints of major bleeding within 48 h or before hospital discharge (whichever occurred first) and 30-day net adverse clinical events (combination of all-cause mortality,myocardial infarction, or stroke and major bleeding), no significant difference was recorded between the two groups(6.9% for bivalirudin vs. 9.0% for UFH, P = 0.27 for major bleeding and 14.4% for bivalirudin vs. 16.1% for UFH, P =0.50 for combined adverse clinical events). However, at 48 h, the bivalirudin group had significantly fewer MI but more acute kidney injury events than the UFH group; at 30 days, these differences were no longer significant.
Finally, regarding long term antithrombotic therapy in TAVI patients with concurrent AF, Vavuranakis, et al.[14]have published a single center experience with the following therapeutic combination regimen; the AF-group patients were prescribed clopidogrel (75 mg/day) plus OAC for the first three months. Consequently, aspirin (100 mg/day) plus OAC were recommended until follow-up contact. Non-AF group patients were treated with DAPT for the first three months (clopidogrel 75 mg/day plus aspirin 100 mg/day),followed by SAPT with aspirin until follow-up. In a mean follow-up of 23.4 ± 14 months no statistical difference was found between groups concerning the primary endpoint of major adverse cardiac events (death, MI, coronary revascularization and stroke) (P = 0.705, phi coefficient = 0.06).Similarly, no difference between groups was found concerning the secondary end-point of major bleeding, as defined by Bleeding Academic Research Consortium (BARC)definition (P = 0.658, phi-coefficient = 0.14).
3.5 Forthcoming studies
Further evidence is needed in order to establish the appropriate antithrombotic treatment in patients undergoing TAVI, whether anticoagulation is required or not. Ongoing trials attempt to clarify this debated issue.
The dual antiplatelet therapy versus oral anticoagulation for a short time to prevent cerebral embolism after TAVI(AUREA) (NCT01642134) trial assesses the efficacy of aspirin 80 mg/day plus clopidogrel 75 mg/day for three months, compared with acenocumarol in preventing cerebral thromboembolism identified by magnetic resonance(primary endpoint) at three months in patients without indication for anticoagulation. The Antiplatelet Therapy for Patients Undergoing Transcatheter Aortic Valve Implantation (POPular-TAVI) (NCT02247128) trial is a prospective randomized, controlled, open-label multicenter clinical trial to test the hypothesis that monotherapy with aspirin or OAC after TAVI is safer than the addition of clopidogrel for three months, without compromising clinical benefit.
Future studies will also need to investigate the potential role of newer antiplatelet (ticagrelor, prasugrel) or OAC regimens (dabigadran, rivaroxaban, apixaban and edoxaban)in antithrombotic strategy during and after TAVI.
4 Discussion
Undeniably, the concern for antithrombotic therapy after TAVI is of increasing importance especially after the knowledge that transcatheter heart valve thrombosis is more frequent than initially believed, with a frequency of 1%-5%for asymptomatic cases and much higher (40%) for symptomatic patients. The importance for optimal antithrombotic therapy is further underlined by the close correlation between asymptomatic transcatheter heart valve thrombosis and asymptomatic embolic events.
Use of dual antiplatelet therapy with aspirin plus clopidogrel after TAVI has been loosely based on coronary and peripheral vascular therapies. Furthermore, there is no evidence on duration of therapy or what agents should be used.Simultaneously with severe aortic stenosis, a high proportion of these patients suffer from AF, stroke and coronary artery disease with recent MI or coronary intervention. Thus,all these issues make difficult the development of a systematic antithrombotic approach.
Current clinical practice on antithrombotic therapy after TAVI remains empirical and/or authority based. Despite the lack of evidence, the American guidelines suggest aspirin(81 mg qD) indefinitely and clopidogrel for 3-6 months,while the Canadian statement on TAVI recommends the use of clopidogrel for 1-3 months.[34,36]Similarly, the European guidelines confirm the above treatment, despite the lack of evidence.[11]
Direct comparison of the different antithrombotic regimens is challenging, because of the significant variety in the baseline characteristics of the available study populations.Besides, especially in RCTs mentioned above, antithrombotic therapy remains quite vague. Finally, antithrombotic strategy in cases with need for OAC is not definitely reported, while major outcomes or bleeding events are often not reported according to VARC.
Antithrombotic treatment is initiated during implantation procedure for protection from embolic events, possibly occurring at this period. However, results from BRAVO-3 trial do not preconceive the use of bivalirudin instead of UFH for procedural anticoagulation, but have proven the safety of bivalirudin in this high frailty group of patients.[48]
In case of concomitant AF, long term antithrombotic therapy is a challenging and complex field.[49]Evaluation of the thrombotic (CHA2DS2-VASc score) and bleeding (HASBLED score) risk remains the optimal patient approach with simultaneous assessment of patient frailty. Combination of OAC plus usually aspirin in the first 3 months is generally used in high thrombotic risk patients (CHA2DS2-VASc score ≥ 2). Contrary to that, in cases of low risk (CHA2DS2-VASc score < 2) exclusive antiplatelet therapy is preferred,while in high bleeding risk patients (HAS-BLED ≥ 3) left atrial appendage closure remains an option.
Data regarding the exact mechanism of thromboembolic events after TAVI are lacking. It remains unclear whether clot formation is platelet or thrombin based. It has been shown that the endothelialization of the valve stent probably occurs within the first three months after implantation.[50]This is in line with the observation that thromboembolic events are greater within this early post intervention period and supports the strategy of more intense early antithrombotic therapy.[51,52]However, the use of DAPT during that period after TAVI must be further tested with well organized RCTs. Existing data from aforementioned small non-RCTs do not demonstrate any superiority of DAPT in comparison to aspirin monotherapy. Cerebrovascular events rates are similar whereas bleeding events may happen even more frequently.
It has been recently supported that rationale for anticoagulation therapy apart from concomitant AF, is further based on high stroke rates of TAVI patients and ‘subclinical leaflet thrombosis’.[3]The implanted valve is a complex device with metal frame, biologic prosthesis and remaining native valve with complex flow patterns and possible embolic complications. Thus, “subclinical leaflet thickening or thrombosis” has been observed in quite remarkable rates(20%). Accordingly, anticoagulation directed at reducing stroke risk and possible leaflet thrombosis may be a novel investigation field in the near future.
Finally, as transcatheter based treatment for aortic steno-sis is targeting at lower age and lower risk patients, optimal antithrombotic therapy becomes of paramount importance.It seems that in these populations, long term valve durability and elimination of possible leaflet thrombosis are highlighted as the main pursued issues, in parallel with diminishing bleeding complications. Individualized antithrombotic regimens according to patient characteristics will be probably the optimal approach in the near future.
In conclusion, diminishing ischaemic and bleeding complications after TAVI remains the main challenge in patients undergoing TAVI. Due to the high risk and frailty of the treated population antithrombotic therapy after TAVI should be carefully evaluated. The inconsistency of clinical and demographic characteristics of these patients makes a headto-head comparison of alternative antithrombotic regimens quite challenging. Current practice supports DAPT for up to 6 months and is mainly based on experience from coronary and peripheral vascular therapies without existing evidence of additional protection from dual antiplatelet therapy. Use of anticoagulants directed at reducing stroke risk and valve thrombosis is still debatable and well-organized RCTs are needed in this field.
References
1 Vavuranakis M, Vrachatis DA, Tousoulis D. Bioengineering,clinical and therapeutical trends in transcatheter aortic valve implantation. Curr Pharm Des 2016; 22: 1851-1852.
2 Vahanian A, Alfieri O, Al-Attar N, et al. Transcatheter valve implantation for patients with aortic stenosis: a position statement from the European Association of Cardio-Thoracic Surgery (EACTS) and the European Society of Cardiology(ESC), in collaboration with the European Association of Percutaneous Cardiovascular Interventions (EAPCI). EuroIntervention 2008; 4: 193-199.
3 Leon MB, Smith CR, Mack M, et al. Transcatheter aorticvalve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010; 363: 1597-1607.
4 Vavuranakis M, Voudris V, Vrachatis DA, et al. Transcatheter aortic valve implantation, patient selection process and procedure: two centres’ experience of the intervention without general anaesthesia. Hellenic J Cardiol 2010; 51: 492-500.
5 Thomopoulou S, Vavuranakis M, Karyofyllis P, et al. Fouryear clinical results of transcatheter self-expanding Medtronic CoreValve implantation in high-risk patients with severe aortic stenosis. Age Ageing 2016; 45: 427-430.
6 Kahlert P, Knipp SC, Schlamann M, et al. Silent and apparent cerebral ischemia after percutaneous transfemoral aortic valve implantation: a diffusion-weighted magnetic resonance imaging study. Circulation 2010; 121: 870-878.
7 Moretti C, D'Amico M, D'Ascenzo F, et al. Impact on prognosis of periprocedural bleeding after TAVI: mid-term follow-up of a multicenter prospective study. J Interv Cardiol 2014; 27: 293-299.
8 Stepinska J, Czerwinska K, Witkowski A, et al. Risk factors for bleeding complications in patients undergoing transcatheter aortic valve implantation (TAVI). Cardiol J 2013; 20:125-133.
9 Vavuranakis M, Siasos G, Zografos T, et al. Dual or single antiplatelet therapy after transcatheter aortic valve implantation? A systematic review and meta-analysis. Curr Pharm Des 2016; 22: 4596-4603.
10 Magkoutis NA, Fradi S, Azmoun A, et al. Antiplatelet therapy in TAVI: current clinical practice and recommendations. Curr Pharm Des 2016; 22: 1888-1895.
11 Vahanian A, Alfieri O, Andreotti F, et al. Guidelines on the management of valvular heart disease (version 2012). Eur Heart J 2012; 33: 2451-2196.
12 Vahanian A, Alfieri O, Al-Attar N, et al. Transcatheter valve implantation for patients with aortic stenosis: a position statement from the European Association of Cardio-Thoracic Surgery (EACTS) and the European Society of Cardiology(ESC), in collaboration with the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J 2008; 29: 1463-1470.
13 Ussia GP, Scarabelli M, Mule M, et al. Dual antiplatelet therapy versus aspirin alone in patients undergoing transcatheter aortic valve implantation. Am J Cardio l 2011; 108:1772-1776.
14 Vavuranakis M, Kolokathis AM, Vrachatis DA, et al. Atrial fibrillation during or After TAVI: incidence, implications and therapeutical considerations. Curr Pharm De s 2016; 22:1896-1903.
15 Vavuranakis M, Vrachatis DA, Siasos G, et al. Managing complications in transcatheter aortic valve implantation. Hellenic J Cardiol 2015; 56 Suppl A: S20-S30.
16 Vavuranakis M, Vrachatis DA, Kalogeras KI, Stefanadis CI.Vascular sealing implications in transfemoral transcatheter aortic valve implantation. Rev Esp Cardiol (Engl Ed) 2014;67: 869.
17 Vavuranakis M, Kariori M, Voudris V, et al. Predictive factors of vascular complications after transcatheter aortic valve implantation in patients treated with a default percutaneous strategy. Cardiovasc Ther 2013; 31: e46-e54.
18 Vavuranakis M, Kalogeras K, Vrachatis D, et al. Inferior epigastric artery as a landmark for transfemoral TAVI. Optimizing vascular access? Catheter Cardiovasc Interv 2013; 81:1061-1066.
19 Poliacikova P, Cockburn J, de Belder A, et al. Antiplatelet and antithrombotic treatment after transcatheter aortic valve implantation―comparison of regimes. J Invasive Cardiol 2013;25: 544-548.
20 Lynch DR Jr., Dantzler D, Robbins M, Zhao D. Considerations in antithrombotic therapy among patients undergoing transcatheter aortic valve implantation. J Thromb Thrombolysis 2013; 35: 476-482.
21 Leon MB, Piazza N, Nikolsky E, et al. Standardized endpoint definitions for transcatheter aortic valve implantation clinical trials: a consensus report from the valve academic research consortium. J Am Coll Cardiol 2011; 57: 253-269.
22 Kappetein AP, Head SJ, Genereux P, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. J Am Coll Cardiol 2012; 60: 1438-1454.
23 Ghanem A, Muller A, Nahle CP, et al. Risk and fate of cerebral embolism after transfemoral aortic valve implantation:a prospective pilot study with diffusion-weighted magnetic resonance imaging. J Am Coll Cardiol 2010; 55: 1427-1432.
24 Marechaux S, Corseaux D, Vincentelli A, et al. Identification of tissue factor in experimental aortic valve sclerosis.Cardiovasc Pathol 2009; 18: 67-76.
25 Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011; 364: 2187-2198.
26 Stortecky S, Windecker S, Pilgrim T, et al. Cerebrovascular accidents complicating transcatheter aortic valve implantation:frequency, timing and impact on outcomes. EuroIntervention 2012; 8: 62-70.
27 Nombela-Franco L, Webb JG, de Jaegere PP, et al. Timing,predictive factors, and prognostic value of cerebrovascular events in a large cohort of patients undergoing transcatheter aortic valve implantation. Circulation 2012; 126: 3041-3053.
28 Sun JC, Davidson MJ, Lamy A, Eikelboom JW. Antithrombotic management of patients with prosthetic heart valves: current evidence and future trends. Lancet 2009; 374: 565-576.
29 Amat-Santos IJ, Rodes-Cabau J, Urena M, et al. Incidence,predictive factors, and prognostic value of new-onset atrial fibrillation following transcatheter aortic valve implantation. J Am Coll Cardiol 2012; 59: 178-188.
30 Gilard M, Eltchaninoff H, Iung B, et al. Registry of transcatheter aortic- valve implantation in high-risk patients. N Engl J Med 2012; 366: 1705-1715.
31 Gurvitch R, Toggweiler S, Willson AB, et al. Outcomes and complications of transcatheter aortic valve replacement using a balloon expandable valve according to the Valve Academic Research Consortium (VARC) guidelines. EuroIntervention 2011; 7: 41-48.
32 Nuis RJ, Rodes-Cabau J, Sinning JM, et al. Blood transfusion and the risk of acute kidney injury after transcatheter aortic valve implantation. Circ Cardiovasc Interv 2012; 5: 680-688.
33 Dauerman HL, Lessard D, Yarzebski J, et al. Bleeding complications in patients with anemia and acute myocardial infarction. Am J Cardiol 2005; 96: 1379-1383.
34 Holmes DR Jr., Mack MJ, Kaul S, et al. 2012 ACCF/AATS/SCAI/STS expert consensus document on transcatheter aortic valve replacement. J Am Coll Cardiol 2012; 59: 1200-1254.
35 Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; 63: 2438-2488.
36 Webb J, Rodes-Cabau J, Fremes S, et al. Transcatheter aortic valve implantation: a Canadian Cardiovascular Society position statement. Can J Cardiol 2012; 28: 520-528.
37 Lip GY, Windecker S, Huber K, et al. Management of antithrombotic therapy in atrial fibrillation patients presenting with acute coronary syndrome and/or undergoing percutaneous coronary or valve interventions: a joint consensus document of the European Society of Cardiology Working Group on Thrombosis, European Heart Rhythm Association (EHRA),European Association of Percutaneous Cardiovascular Interventions (EAPCI) and European Association of Acute Cardiac Care (ACCA) endorsed by the Heart Rhythm Society (HRS)and Asia-Pacific Heart Rhythm Society (APHRS). Eur Heart J 2014; 35: 3155-3179.
38 Nijenhuis VJ, Bennaghmouch N, van Kuijk JP, et al. Antithrombotic treatment in patients undergoing transcatheter aortic valve implantation (TAVI). Thromb Haemost 2015; 113:674-685.
39 Adams DH, Popma JJ, Reardon MJ, et a l. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med 2014; 370: 1790-1798.
40 Abdel-Wahab M, Mehilli J, Frerker C, et al. Comparison of balloon-expandable vs. self-expandable valves in patients undergoing transcatheter aortic valve replacement: the CHOICE randomized clinical trial. JAMA 2014; 311: 1503-1514.
41 Abramowitz Y, Banai S, Katz G, et al. Comparison of early and late outcomes of TAVI alone compared to TAVI plus PCI in aortic stenosis patients with and without coronary artery disease. Catheter Cardiovasc Interv 2014; 83: 649-654.
42 Tchetche D, Van der Boon RM, Dumonteil N, et al. Adverse impact of bleeding and transfusion on the outcome post-transcatheter aortic valve implantation: insights from the Pooled-RotterdAm-Milano-Toulouse In Collaboration Plus (PRAGMATIC Plus) initiative. Am Heart J 2012; 164: 402-409.
43 Borz B, Durand E, Godin M, et al. Incidence, predictors and impact of bleeding after transcatheter aortic valve implantation using the balloon-expandable Edwards prosthesis. Heart 2013; 99: 860-865.
44 Griese DP, Reents W, Diegeler A, et al. Simple, effective and safe vascular access site closure with the double-ProGlide preclose technique in 162 patients receiving transfemoral transcatheter aortic valve implantation. Catheter Cardiovasc Interv 2013; 82: E734-E741.
45 Nuis RJ, Van Mieghem NM, Schultz CJ, Moelker A, van der Boon RM, van Geuns RJ, et a l. Frequency and causes of stroke during or after transcatheter aortic valve implantation.Am J Cardiol 2012; 109: 1637-1643.
46 Stabile E, Pucciarelli A, Cota L, et al. SAT-TAVI (single antiplatelet therapy for TAVI) study: a pilot randomized study comparing double to single antiplatelet therapy for transcatheter aortic valve implantation. Int J Cardiol 2014; 174:624-627.
47 Durand E, Blanchard D, Chassaing S, et al. Comparison of two antiplatelet therapy strategies in patients undergoing transcatheter aortic valve implantation. Am J Cardiol 2014;113: 355-360.
48 Dangas GD, Lefevre T, Kupatt C, et al. Bivalirudin versus heparin anticoagulation in transcatheter aortic valve replacement: the randomized BRAVO-3 trial. J Am C oll Cardio l 2015; 66: 2860-2868.
49 Mok M, Urena M, Nombela-Franco L, et al. Clinical and prognostic implications of existing and new-onset atrial fibrillation in patients undergoing transcatheter aortic valve implantation. J Thromb Thrombolysis 2013; 35: 450-455.
50 Merie C, Kober L, Skov Olsen P, et al. Association of warfarin therapy duration after bioprosthetic aortic valve replacement with risk of mortality, thromboembolic complications,and bleeding. JAMA 2012; 308: 2118-2125.
51 Heras M, Chesebro JH, Fuster V, et al. High risk of thromboemboli early after bioprosthetic cardiac valve replacement.J Am Coll Cardiol.1995; 25: 1111-1119.
52 Whitlock RP, Eikelboom JW. Prevention of thromboembolic events after bioprosthetic aortic valve replacement: what is the optimal antithrombotic strategy? J Am Coll Cardiol 2012; 60:978-980.
 Journal of Geriatric Cardiology2018年1期
Journal of Geriatric Cardiology2018年1期
- Journal of Geriatric Cardiology的其它文章
- Restrictive perimembranous ventricular septal defect with left to right Shunt post urgent aortic balloon valvuloplasty and transcatheter aortic valve replacement
- Transcatheter aortic valve replacement and stroke: a comprehensive review
- The role of echocardiography and CT angiography in transcatheter aortic valve implantation patients
- Transcatheter versus surgical aortic valve replacement in severe, symptomatic aortic stenosis
- Endocarditis after transcatheter aortic valve implantation: a current assessment
- TAVR in 2017―What we know? What to expect?
