Antifungal and cytotoxic activities of extracts obtained from underutilised edible tropical fruits
Cheong Wei Ong, Yik Sin Chan, Kong Soo Khoo, Hean Chooi Ong, Nam Weng Sit?
1Department of Biomedical Science, Faculty of Science, Universiti Tunku Abdul Rahman, Bandar Barat, 31900 Kampar, Perak, Malaysia
2Department of Chemical Science, Faculty of Science, Universiti Tunku Abdul Rahman, Bandar Barat, 31900 Kampar, Perak, Malaysia
3Institute of Biological Sciences, Faculty of Science, University of Malaya, 50603 Kuala Lumpur, Malaysia
1. Introduction
Countries in the tropical region such as Malaysia have a rich botanical diversity. According to Milow et al.[1], 355 species of trees and 165 species of non-trees bearing edible fruits or seeds are found in Malaysia. Fruits are frequently used as a food source and as ingredients in traditional medicine. However, some of these fruits have been less utilised commercially. Underutilised tropical fruits are those fruits with less popularity, highly seasonal and have underexploited potential for contributing to food security, nutritional or medicinal health, income generation, and environmental services[2].
Artocarpus altilis (A. altilis) (Parkinson ex F.A.Zorn) Fosberg (family Moraceae) is a tropical fruit tree native to South/Southeast Asia and Australasia[3]. It is known as ‘Sukun’ locally or breadfruit in English. In Malaysia, ripe fruits are peeled, sliced and fried in syrup or palm sugar until they are crisp and brown before consumption. Several reviews on A. altilis have highlighted that extracts or phytochemicals isolated from the fruit possess antibacterial, anticancer, antihypergylcaemic, anti-inflammatory and antioxidant properties[3,4].
Cynometra cauliflora (C. cauliflora) L., commonly known as “Namnam”, is a member of the bean family Leguminosae. This fruit tree is indigenous to eastern Peninsular Malaysia and is widely distributed in Southeast Asia, India and Sri Langka[5]. The fruit has a savoury taste and may be eaten raw or cooked with sugar to make a sweet compote,or fried in batter. The fruit has been used in folk medicine to treat loss of appetite[6]. Pharmacological studies on the fruit extracts reveal the presence of antioxidative[7], anti-lipase[8] and antiproliferative effects against human promyelocytic leukaemia cells[9].
Mangifera pajang (M. pajang) Kosterm. of the family Anacardiaceae is a mango species popularly known as “Bambangan” or “Pajang”.It originated from Borneo Island, which comprises Sarawak, Sabah(Malaysia), Brunei and Kalimantan (Indonesia)[10]. The flesh is aromatic, juicy, sweet-sour in taste, fibrous in texture and can be eaten fresh or make into juice. The grated kernel, together with the cut flesh is used by the Kadazan-Dusun community in Sabah to make a pickle called ‘nonsom bambangan’[10]. Besides nutritional values, the fruit extracts or compounds isolated from the fruit possess antioxidative[11-13], anticancer[12,14], antibacterial activities[12], and cytoprotective effects against liver cells[15].
Physalis minima (P. minima) L. is a herbaceous annual plant belonging to the family Solanaceae with many common names such as wild gooseberry, wild cape gooseberry, sunberry, ground cherry,etc[16]. It is believed to have originated from tropical America and is now distributed pantropically. The fruit is juicy and has been used in traditional medicine as a diuretic, a purgative, to relieve stomach pain,and to treat spleen disorders and constipation[17]. However, most of the studies on pharmacological activities and phytochemicals for P.minima have focused either on the leaves or the entire plant[16,18]. The fruit has thus far been only studied for antibacterial activity[19,20].
Despite the reported pharmacological properties of these four underutilised fruits, very little information is available regarding their antifungal potential against human pathogens. The escalation of invasive fungal infections in immunocompromised or immunosuppressed patients,the development of drug-resistant fungal strains, the significant side effects associated with synthetic antifungal drugs during chemotherapy and the relative high cost of treatment[21,22] have justified the search for newer, safer and cheaper antifungal agents among natural resources.Since plants produce a diverse variety of secondary metabolites such as alkaloids, anthraquinones, flavonoids, tannins, triterpenes, etc. as part of their defence mechanisms against microbial infections[23], they are promising sources of new antifungal compounds. This study was therefore conducted to evaluate the antifungal activity of the extracts obtained from the fruits of A. altilis, C. cauliflora, M. pajang and P.minima against a panel of human fungal pathogens. The fruit extracts were also assessed for cytotoxicity using African monkey kidney epithelial (Vero) cell line.
2. Materials and methods
2.1. Fruit species
The mature fruit (before fully ripened) of A. altilis was obtained from an orchard in Sitiawan, Perak, Malaysia while the ripe fruit (brownish yellow colour) of C. cauliflora was plucked from a garden in Tanah Merah, Kelantan, Malaysia. The fully ripened fruit of M. pajang with yellow flesh was bought from a wet market in Miri, Sarawak, Malaysia while the ripe fruit (yellow colour) of P. minima was purchased from a farm in Cameron Highlands, Pahang, Malaysia. The species identity of the fruits was confirmed by Professor Hean Chooi Ong, an ethnobotanist affiliated with Institute of Biological Sciences, Faculty of Science, University of Malaya, Malaysia.
2.2. Preparation of fruit extracts
Only the edible parts of the fruits were used for extraction. The fresh flesh of A. altilis (1 564.32 g) and C. cauliflora (657.90 g), the whole fresh fruit of P. minima (360.73 g), and the fresh flesh (1 933.18 g) and kernel (275.93 g) of M. pajang were subjected to sequential solvent extraction using hexane, chloroform, ethyl acetate, ethanol, methanol and distilled water. All the organic solvents used were of analytical grade. The maceration process was carried out at room temperature and with agitation using an orbital shaker (Yihder Technology, New Taipei City, Taiwan) at 120 rpm for three cycles (one day/cycle). The collected extract solvents were removed using a rotary evaporator(Buchi Labortechnik AG, Flawil, Switzerland) to obtain the dry extracts while the water extract was lyophilised using a freeze-dryer (Martin Christ Gefriertrocknungsanlagen GmbH, Osterode, Germany). All the dry extracts were kept at -20 ℃ prior to bioassay.
2.3. Antifungal assay
Four species of yeasts [Candida albicans (C. albicans) ATCC?90028TM, Candida parapsilosis (C. parapsilosis) ATCC?22019TM,Candida krusei (C. krusei) ATCC?6258TM, Cryptococcus neoformans(C. neoformans) ATCC?90112TM] and two species of filamentous fungi[Aspergillus fumigatus (A. fumigatus) ATCC?204305TM, Trichophyton interdigitale (T. interdigitale) ATCC?9533TM] were tested in the study.All the species were obtained from American Type Culture Collection.The yeasts were grown on Sabouraud dextrose agar (SDA) (Merck,Darmstadt, Germany) while the filamentous fungi were maintained on potato dextrose agar (PDA) (Merck, Darmstadt, Germany).
A colourimetric broth microdilution method deploying p-iodonitrotetrazolium chloride as the growth indicator was used for the antifungal assay with modifications[24]. The stock solution for each fruit extract was prepared at 10 mg/mL in a methanol-water mixture (2:1, v/v),and filter-sterilised using 0.45 μm syringe filters. The fruit stock solution was then diluted two-fold serially with RPMI-1640 medium (Biowest,Nuaille, France) in 96-well, U-shaped microplates (Sigma-Aldrich, St.Louis, Missouri, USA) to obtain eight final concentration levels, i.e. 0.02,0.04, 0.08, 0.16, 0.31, 0.63, 1.25 and 2.50 mg/mL. Dilutions beyond 0.02 mg/mL were performed whenever necessary. Medium, growth (fungus only), negative (extract only) and positive (antibiotic griseofulvin/amphotericin B) controls were included in each microplate during the assay. The final volume for each well was 100 μL. The inoculum preparations and the incubation conditions for yeasts and filamentous fungi were carried out according to the Clinical and Laboratory Standards Institute guidelines M27-A3 and M38-A2, respectively[25,26]. After the designated incubation periods, 20 μL of p-iodonitrotetrazolium chloride(Sigma-Aldrich, St. Louis, Missouri, USA) at 0.4 mg/mL was added to each well to determine the minimum inhibitory concentration (MIC)value. Subsequently, 20 μL of the content from those wells which did not show any growth was inoculated onto SDA or PDA plates using a spread plate method. The lowest concentration of an extract corresponds to no fungal colonies observed on the plates is taken as the minimum fungicidal concentration (MFC) of the extract. The assay was conducted in triplicate.
2.4. Cytotoxicity assay
The African monkey kidney epithelial (Vero) cell line (ATCC?CCL-81TM) was used for cytotoxicity study. The cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) (Sigma-Aldrich, St. Louis, Missouri, USA) supplemented with 5% of foetal bovine serum (FBS) (Biowest USA, Riverside, Missouri, USA), 1% of penicillin solution (10 000 U/mL), 1% of streptomycin solution (10 mg/mL) (EMD Millipore, Darmstadt, Germany) and 3.7 g/L of sodium bicarbonate (Merck, Darmstadt, Germany). The cells were maintained at 37 ℃ in a humidified incubator (Memmert GmbH, Schwabach,Germany) with 5% carbon dioxide.
For the cytotoxicity assay, the fruit stock solution was prepared at 256 mg/mL in a dimethyl sulfoxide-ethanol mixture (60:40, v/v) and filtersterilised using 0.45 μm syringe filters[27]. The stock solution was then diluted two-fold serially in DMEM (supplemented with 1% FBS) to obtain eight different final concentrations, i.e., 5, 10, 20, 40, 80, 160,320 and 640 μg/mL. The dimethyl sulfoxide-ethanol mixture in the microplate was maintained at a final concentration of less than 0.25%(v/v) to avoid any toxicity to the Vero cells[28]. The cells (4 × 104cells/well) were seeded in 96-well, flat-bottom microplates (TPP Techno Plastic Products AG, Trasadingen, Switzerland) and incubated at 37 ℃and 5% CO2for 24 h. One hundred microliters of the prepared extract solution was then added to the cells. The treated cells were incubated at 37 ℃ and with 5% CO2for another 72 h. Medium and cell controls were included in each microplate. After incubation, the viability of Vero cells was measured by the Neutral Red uptake assay[29]. The absorbance for each well was read at 540 nm using a microplate reader (BMG Labtech GmbH, Ortenberg, Germany). The assay was conducted in three independent experiments with duplicate in each experiment.
2.5. Data analysis
The MIC and MFC values are expressed as the mean of three consistent replicates. Fungal susceptibility index (FSI), expressed in% value is calculated as 100 × number of extracts effective against each fungal strain ÷ number of total extracts. Fifty percent cytotoxic concentration (CC50) of an extract was determined from the plot of percentage of cell viability versus extract concentration. The data were analysed with one-way analysis of variance (ANOVA) using IBM SPSS Statistics for Windows software, Version 20.0 (IBM, Armonk, New York, USA). The significance level was set at P<0.001. A post hoc test,either using the Tukey’s (equal variance assumed) or Dunnett’s(equal variance not assumed) test was conducted to determine which concentration of extract produced a significant result.
3. Results
3.1. Antifungal assay
By considering one extract against one fungus as one bioassay, as shown in Table 1, 45.6% of the bioassays (82/180) showed fungistatic activity whereas 26.1% (47/180) of the bioassays displayed fungicidal activity.The dimorphic yeast C. neoformans was the most susceptible fungus as its growth was inhibited by all 30 extracts evaluated in this study with a MIC range of 0.02 to 2.50 mg/mL. Hence, FSI for C. neoformans was 100%. Half of these extracts were also able to kill the yeast but at a higher MFC range, which was 0.08 to 2.50 mg/mL. In contrast, the filamentous fungus A. fumigatus was the most resistant fungus (FSI = 0%) as none of the extracts showed activity against it. The calculated FSI values for C. albicans, C. parapsilosis, C. krusei and T. interdigitale were 56.7%,50.0%, 36.7% and 30.0%, respectively.
Comparing the flesh and kernel of M. pajang fruit, all extracts fromthe kernel part were active against all the fungi, except A. fumigatus,with lower MIC and MFC ranges of 0.001-0.630 and 0.001-2.500 mg/mL, respectively. Extracts from the flesh part were only active against the four species of yeasts, with MIC and MFC ranges of 0.16-2.50 and 0.63-2.50 mg/mL, respectively. Besides A. fumigatus, all extracts of P.minima did not exhibit any antifungal activity against C. parapsilosis,C. krusei and T. interdigitale. Using the classification of antimicrobial potency proposed by Saraiva et al.[30], only the six extracts from the kernel of M. pajang were considered to be highly active against the fungi (depending on the species) tested, in which the achievable MIC value was less than 0.1 mg/mL. The ethanol and methanol extracts from the kernel exhibited the lowest MIC or MFC in this study, with a value of 0.001 mg/mL against C. krusei (Table 1).

Table 1 MIC and MFC of underutilised tropical fruit extracts against human fungal pathogens.
3.2. Cytotoxicity assay
The cytotoxicity of the fruit extracts was assessed on the Vero cells. This well-established cell line has been widely used for evaluating the effects of chemicals, toxins and other substances, including plant extracts on mammalian cells at the molecular level. The fruits of C. cauliflora and P. minima were considered non-toxic as their extracts did not exhibit any statistically significant toxicity (P>0.001) on the Vero cells, except at the highest concentration 640 μg/mL for the chloroform extract of C.cauliflora and the ethyl acetate extract of P. minima (Figure 1). For the A.altilis fruit, only the hexane and chloroform extracts at the concentration higher than 160 and 320 μg/mL, respectively showed statistically significant (P<0.001) toxicity to the cells. The corresponding CC50values(mean±SD) for these two extracts were (118.6 ± 2.6) and (253.4 ± 4.5)μg/mL, respectively.
The flesh and kernel parts of M. pajang fruit showed distinct differences in the toxicity towards the Vero cells. For the flesh part,extracts obtained using less polar solvents (hexane, chloroform and ethyl acetate) were toxic to the cells when their concentrations exceeded 10 μg/mL. The CC50values (mean±SD) for these three extracts were(30.6 ± 1.1), (13.5 ± 0.4) and (22.2 ± 2.5) μg/mL, respectively. On the other hands, the toxicity for the kernel part was mainly derived from the three more polar extracts, including hexane, ethanol, methanol and water. The strength of toxicity for these extracts were much lower, as evident by their higher CC50values (mean±SD) which were (481.6± 14.2), (358.7 ± 18.4), (158.4 ± 7.6) and (261.3 ± 8.9) μg/mL,respectively.
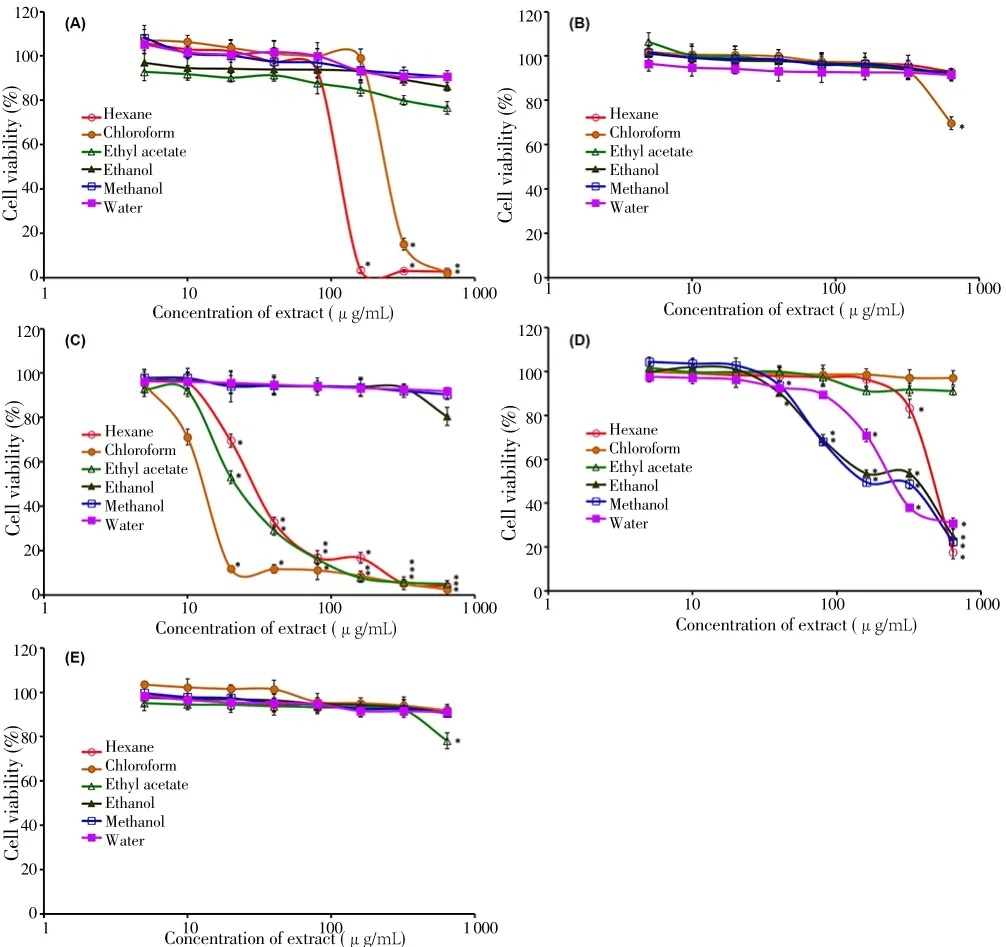
Figure 1. Viability of African monkey kidney epithelial (Vero) cells treated with different concentrations of underutilised tropical fruit extracts derived from (A)A. altilis, (B) C. cauliflora, (C) flesh of M. pajang, (D) kernel of M. pajang and(E) P. minima.
4. Discussion
To the best of our knowledge, this is the first report of C. cauliflora fruit extracts possessing antifungal activity against human pathogens.More studies need to be carried out to evaluate the phytochemicals and other antimicrobial activities such as antibacterial activity of this fruit.In a study using a normal mouse fibroblast (NIH/3T3) cell line as a model for cytotoxicity study, the cell viability assessed using MTT assay was reduced to 80.0% when the cells were treated with the methanol extract of the fruit at 30 μg/mL[9]. However, our study indicates that the methanol extract was not toxic (P>0.001) to the Vero cells (assessed using the Neutral Red uptake assay) even at the highest concentration of 640 μg/mL. The differences could be due to the types of cell line or the assay used to measure the cell viability[27]. Although the fruit samples for both studies were obtained from the east coast of Peninsular Malaysia,the fresh flesh was used in the current study while Tajudin et al.[9]studied the dried whole fruit. The difference in the material used could also be a variable that contributes to the differences in cytotoxicity.
This study reveals that the fruit of P. minima possesses limited antifungal property whereby the extracts were only active against two species of fungi, i.e. C. albicans and C. neoformans. In contrast, the fruit is known to have antibacterial property. Extracts obtained from the fresh or dried fruits using solvents of different polarity (hexane, chloroform,diethyl ether, ethyl acetate, acetone, ethanol, methanol, aqueous, or water) have shown different degrees of inhibitory activity against Grampositive and Gram-negative bacteria[19,20]. The results of this study have provided us with a broader understanding on the antifungal potential of A. altilis fruit on different species of fungal pathogens. Our findings are in agreement with the study by Jalal et al.[31] who evaluated the antimicrobial activities of the hexane, dichloromethane and methanol extracts derived from the dry pulp (flesh) of A. altilis fruit. C. albicans was the only fungus studied and the MIC range reported was 0.5-4 mg/mL.
The flesh and kernel extracts of M. pajang fruit showed different antifungal activity and cytotoxicity against Vero cells, suggesting that the phytochemicals distribution between the two parts is highly different. The antifungal activity of the kernel extracts was more active and stronger against the fungi tested compared to that of the flesh extracts. Similar observations have been documented by other studies. Abu Bakar et al.[11] found that the 80% methanol extract of the kernel possesses significantly higher antioxidant activity than the flesh, as measured by DPPH radical scavenging and ferric reducing antioxidant power assays. The ethanol extract of the kernel, but not the flesh displays anticancer activity against colon, liver and ovarian cancer cell lines[14] and cytoprotective effect on human hepatocellular HepG2 cell line against oxidative damage[15]. However, Ahmad et al.[12]reported that none of the extracts (petroleum ether, chloroform, ethyl acetate and methanol) from the dried kernel powder showed antifungal activity against C. albicans, Aspergillus ochraceaus and Saccharomyces cerevisiae. Our study indicates that all six extracts from the fresh kernel were active against the three Candida spp., C. neoformans and T.interdigitale with MIC values ranging from 0.001 to 0.630 mg/mL. It is unclear whether these differences are due to the fungal species or the type of material (fresh vs dry) used in the study. Several aromatic esters(benzaldehyde, benzyl alcohol, methyl gallate), flavonoids (diosmin,hesperidin, naringin, rutin), phenolic acids (caffeic, chlorogenic,p-coumaric, ferulic, gallic, synapic acids) and a sterol (β-sitosterol)have been isolated from the kernel[14,15]. Phenolic compounds such as flavonoids and phenolic acids from plants have been known to be active against human pathogens[32,33]. The phytochemicals present in the kernel that are responsible for the antifungal activity remain to be studied.
Despite the fact that the fresh flesh of M. pajang or the fruit juice has been consumed by the indigenous community regularly without any report of adverse effects, our study shows that the less polar extracts(hexane, chloroform and ethyl acetate) caused significant toxicity towards the Vero cells. Further studies using human cell lines are required to corroborate this observation. The toxicity caused by the flesh extracts of M. pajang fruit on the Vero cells was mainly due to phytochemicals with low polarity. On the other hands, the noncytotoxic property for the more polar extracts supports the safe consumption of juice and the development of functional drink using M. pajang fruit[13]. As for the kernel part, phytochemicals with higher polarity in nature are responsible in reducing the viability of Vero cells.The kernel of M. pajang fruit is usually grated together with the cut flesh to make a pickle, which is then left to ferment for weeks before being eaten[10]. Toxicity has not been reported following the consumption of the pickle by the indigenous community. It is possible that the process of making the pickle might alter the toxicity of the phytochemicals present in the kernel and flesh.
Among the four types of underutilised tropical fruits evaluated in this study, the kernel extracts of M. pajang fruit are promising sources of new lead compounds with potent fungistatic or fungicidal activity against human pathogens and low or non-toxic to eukaryotic cells.Further studies are warranted to identify the active compounds and their mechanisms of action. It is also of interest to identify the chemical profile of each fruit extract. Continuous exploration and exploitation of underutilised tropical fruits is necessary to increase the economic value of the fruits, and to ensure optimal use of local resources.
Conflict of interest statement
We declare that there is no conflict of interest.
Acknowledgements
The authors would like to thank Universiti Tunku Abdul Rahman Research Fund (IPSR/RMC/UTARRF/2012-C2/S03) for the financial support, and Ms. Chee Kei Kong for the technical assistance.
[1] Milow P, Malek SB, Edo J, Ong HC. Malaysian species of plants with edible fruits or seeds and their valuation. Int J Fruit Sci 2014; 14(1): 1-27.
[2] Jaenicke H, H?schle-Zeledon I. Strategic framework for underutilized plant species research and development, with special reference to Asia and the Pacific, and to Sub-Saharan Africa. International Centre for Underutilised Crops, Colombo, Sri Lanka and Global Facilitation Unit for Underutilized Species, Rome, Italy; 2006.
[3] Jagtap UB, Bapat VA. Artocarpus: A review of its traditional uses,phytochemistry and pharmacology. J Ethnopharmacol 2010; 129(2): 142-166.
[4] Sikarwar MS, Hui BJ, Subramaniam K, Valeisamy BD, Yean LK, Balaji K. A review on Artocarpus altilis (Parkinson) Fosberg (breadfruit). J Appl Pharm Sci 2014; 4(8): 91-97.
[5] Lim TK. Edible medicinal and non-medicinal plants: Volume 2, Fruits.Netherlands: Springer; 2012.
[6] Sedgley M, Gardner JA. International survey of underexploited tropical and subtropical perennials. Wageningen, Netherlands: International Society for Horticultural Science; 1989.
[7] Rabeta MS, Nur Faraniza R. Total phenolic content and ferric reducing antioxidant power of the leaves and fruits of Garcinia atrovirdis and Cynometra cauliflora. Int Food Res J 2013; 20(4): 1691-1696.
[8] Ado MA, Abas F, Mohammed AS, Ghazali HM. Anti- and pro-lipase activity of selected medicinal, herbal and aquatic plants, and structure elucidation of an anti-lipase compound. Molecules 2013; 18(12): 14651-14669.
[9] Tajudin TJ, Mat N, Siti-Aishah AB, Yusran AA, Alwi A, Ali AM.Cytotoxicity, antiproliferative effects, and apoptosis induction of methanolic extract of Cynometra cauliflora Linn. whole fruit on human promyelocytic leukemia HL-60 cells. Evid Based Complement Alternat Med 2012;2012(2012): 127373. Doi: http://dx.doi.org/10.1155/2012/127373.
[10] Lim TK. Edible medicinal and non-medicinal plants: Volume 1, Fruits.Netherlands: Springer; 2012.
[11] Abu Bakar MF, Mohamed M, Rahmat A, Fry J. Phytochemicals and antioxidant activity of different parts of bambangan (Mangifera pajang) and tarap (Artocarpus odoratissimus). Food Chem 2009; 113(2): 479-483.
[12] Ahmad S, Sukari MA, Ismail N, Ismail IS, Abdul AB, Abu Bakar MF, et al. Phytochemicals from Mangifera pajang Kosterm and their biological activities. BMC Complement Altern Med 2015; 15(1): 83. Doi: http://dx.doi.org/10.1186/s12906-015-0594-7.
[13] Ibrahim M, Ismail A, Al-Sheraji SH, Azlan A, Abdul Hamid A. Effects of Mangifera pajang Kostermans juice on plasma antioxidant status and liver and kidney function in normocholesterolemic subjects. J Funct Foods 2013;5(4): 1900-1908.
[14] Bakar MFA, Mohamed M, Rahmat A, Burr SA, Fry JR. Cytotoxicity and polyphenol diversity in selected parts of Mangifera pajang and Artocarpus odoratissimus fruits. Nutr Food Sci 2010; 40(1): 29-38.
[15] Abu Bakar MF, Mohamed M, Rahmat A, Burr SA, Fry JR. Cellular assessment of the extract of bambangan (Mangifera pajang) as a potential cytoprotective agent for the human hepatocellular HepG2 cell line. Food Chem 2013; 136(1): 18-25.
[16] Chothani DL, Vaghasiya HU. A phyto-pharmacological overview on Physalis minima Linn. Indian J Nat Prod Resour 2012; 3(4): 477-482.
[17] Parkash V, Aggarwal A. Traditional uses of ethnomedicinal plants of lower foot-hills of Himachal Pradesh-I. Indian J Tradit Know 2010; 9(3): 519-521.
[18] Zhang WN, Tong WY. Chemical constituents and biological activities of plants from the genus Physalis. Chem Biodivers 2016; 13(1): 48-65.
[19] Patel T, Shah K, Jiwan K, Shrivastava N. Study on the antibacterial potential of Physalis minima Linn. Indian J Pharm Sci 2011; 73(1): 111-115.
[20] Patel PR, Ramana Rao TVR. Influence of growth and ripening of Physalis minima L. fruit on its antibacterial potential. Res J Med Plant 2012; 6(4):326-333.
[21] Brown GD, Denning DW, Gow NA, Levitz SM, Netea MG, White TC.Hidden killers: Human fungal infections. Sci Transl Med 2012; 4(165):165rv13. Doi: http://dx.doi.org/10.1126/scitranslmed.3004404.
[22] Roemer T, Krysan DJ. Antifungal drug development: Challenges, unmet clinical needs, and new approaches. Cold Spring Harb Perspect Med 2014;4(5): a019703. Doi: http://dx.doi.org/10.1101/cshperspect.a019703.
[23] Cos P, Vlietinck AJ, Berghe DV, Maes L. Anti-infective potential of natural products: How to develop a stronger in vitro ‘proof-of-concept’? J Ethnopharmacol 2006; 106(3): 290-302.
[24] Eloff JN. A sensitive and quick microplate method to determine the minimal inhibitory concentration of plant extracts for bacteria. Planta Med 1998;64(8): 711-713.
[25] Clinical and Laboratory Standards Institute. CLSI Document M27-A3.Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard-third edition. Wayne, PA: Clinical and Laboratory Standards Institute; 2008.
[26] Clinical and Laboratory Standards Institute. CLSI Document M38-A2.Reference method for broth dilution antifungal susceptibility of filamentous fungi; approved standard-second edition. Wayne, PA: Clinical and Laboratory Standards Institute; 2008.
[27] Chan SM, Khoo KS, Sit NW. Interactions between plant extracts and cell viability indicators during cytotoxicity testing: Implications for ethnopharmacological studies. Trop J Pharm Res 2015; 14(11): 1991-1998.
[28] Chan SM. In vitro quantitative assessment of antiviral activity of medicinal plants against Chikungunya virus. Master dissertation. Universiti Tunku Abdul Rahman, Malaysia; 2013.
[29] Repetto G, del Peso A, Zurita JL. Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat Protoc 2008; 3(7): 1125-1131.
[30] Saraiva AM, Castro RHA, Cordeiro RP, Peixoto Sobrinho TJS, Castro VTNA,Amorim ELC, et al. In vitro evaluation of antioxidant, antimicrobial and toxicity properties of extracts of Schinopsis brasiliensis Engl. (Anacardiaceae).Afr J Pharm Pharmacol 2011; 5(14): 1724-1731.
[31] Jalal TK, Ahmed IA, Mikail M, Momand L, Draman S, Isa ML, et al.Evaluation of antioxidant, total phenol and flavonoid content and antimicrobial activities of Artocarpus altilis (breadfruit) of underutilized tropical fruit extracts. Appl Biochem Biotechnol 2015; 175(7): 3231-3243.
[32] De Conti Louren?o RM, da Silva Melo P, de Almeida ABA. Flavonoids as antifungal agents. In: Razzaghi-Abyaneh M, Rai M. (eds.) Antifungal metabolites from plants. Berlin, Heidelberg: Springer; 2013, p. 283-300.
[33] Teodoro GR, Ellepola K, Seneviratne CJ, Koga-Ito CY. Potential use of phenolic acids as anti-Candida agents: A review. Front Microbiol 2015; 6:1420. Doi: http://dx.doi.org/ 10.3389/fmicb.2015.01420.
 Asian Pacific Journal of Tropical Biomedicine2018年6期
Asian Pacific Journal of Tropical Biomedicine2018年6期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Probiotic based therapy for atopic dermatitis: Outcomes of clinical studies
- Antidiabetic and antioxidant activity of ethyl acetate extract fraction of Moringa oleifera leaves in streptozotocin-induced diabetes rats via inhibition of inflammatory mediators
- Evaluation of possible mechanisms of Cordia dichotoma fruits for hyperlipidemia controlling in Wistar albino rats
- Effects of black chokeberry extracts on metastasis and cell-cycle arrest in SK-Hep1 human liver cancer cell line
