Antidiabetic and antioxidant activity of ethyl acetate extract fraction of Moringa oleifera leaves in streptozotocin-induced diabetes rats via inhibition of inflammatory mediators
Ghazi A. Bamagous, Saeed S. Al Ghamdi, Ibrahim Abdel Aziz Ibrahim, Amal M. Mahfoz, Mohamed A. Afify,Mahdi HM. Alsugoor, Ahmed Ali Shammah, Palanisamy Arulselvan, Thamaraiselvan Rengarajan
1Department of Pharmacology and Toxicology, Faculty of Medicine, Umm Al-Qura University, Makkah, Saudi Arabia
2College of Health Sciences, AL-Qunfudah branch, Umm Al-Qura University, Saudi Arabia
3Family and Emergency Medicine, FAMS, Umm Al-Qura University, Makkah, Saudi Arabia
4Muthayammal Centre for Advanced Research, Muthayammal College of Arts and Science, Rasipuram, Namakkal, Tamilnadu, 637408, India
5Scigen Research and Innovation Pvt. Ltd., Periyar Technology Business Incubator, Periyar Nagar, Thanjavur, 613403, India
1. Introduction
Diabetes mellitus is a severe ailment that has been listed as prevalent chronic ailment in many countries[1]. Inadequate physical exercise,imbalanced diet, obesity, excessive alcohol consumption, environmental influence and viral infection are the common risk factors known to increase the possibility of diabetes. These unhealthy behaviors result in improper metabolism of carbohydrates, proteins and lipids, leading to metabolic disorders such as hyperlipidemia and hyperglycemia[2].
The pathological disorders related to diabetes such as nephropathy,foot ulcers, retinopathy and sexual dysfunction are common[3-5]. The common treatment of diabetes in elderly people is oral hypoglycemic drugs and diet control[6]. However, the hypoglycemic drugs are only able to control blood glucose level instead of curing the disease.Discovering the potential therapeutic treatment for diabetes remains a challenge for many decades. The detailed etiological factor of diabetes is still unclear yet, compromised antioxidant defense system, elevated oxidative stress and increased lipid peroxidation are notably having vital roles in the progression and manifestation of the disease[7,8]. Therefore,a large-scale research is conducted on medicinal plants with antioxidant potential, as an alternative to the current treatment for diabetes[9].
Plants are natural source of antioxidants which are known to have therapeutic potentials for various diseases[10]. In that case, Moringa oleifera (M. oleifera) Lam (Moringaceae), or horsera dish plant,is learned to possess high nutrient contents such as iron, calcium,vitamins A, C, D & E, phosphorus, zinc, potassium, manganese and protein along with high contents of antioxidants such as flavonoids,phenolic acids, tannins, saponins, glucosinolates & isothiocyanates,and many more bioactive compounds[11-13]. The whole plant,including bark, root, stem, leaves, flowers, pods and seed, is used for medicinal purpose and as nutrient consumption traditionally by the Indian people[14]. This is a reason for M. oleifera to be called as the God’s gift to man or as the tree of life, since the whole tree,which is rich in nutrients and antioxidants, can be consumed for betterment of human health[15]. The antioxidant potential of M. oleifera with numerous pharmacological values is well-described in recent reports[16,17]. Pharmacological evidences of M. oleifera have shown anti-inflammatory, anti-fungal, anti-diabetic, hepatoprotective, antimicrobial, diuretic, anti-hypertensive, wound healing, anti-ulcerative and anti-cancer properties[18,19]. Antioxidants identified in M. oleifera that include vanillin, epicathechin, p-coumaric acid, ferulic acid,quercetin, kaempferol, cinnamic acid, chlorogenic acid, caffeic acid and glucosinolates (glucomoringin) are assumed to be responsible for the therapeutic potential of this plant[8,19]. Many medicinal plants are proven to possess remedy properties for curing diabetes mellitus. M.oleifera is notable for its medicinal and nutritional contents and has been reported to possess strong antioxidative and antidiabetic effects.
Anti-diabetic properties of M. oleifera have been proven in few previous studies[9,20,21]. Aqueous and methanolic leaf extracts of M.oleifera are described to have anti-hyperglycemic effect on alloxaninduced diabetic mice and streptozotocin (STZ) induced rats, due to high contents of antioxidants[2,8,14,17,19,22]. Through this research, we evaluated the anti-diabetic mechanism(s) of M. oleifera leaves in ethyl acetate extract fraction on STZ-induced diabetic adult male rats. The ethyl acetate extract fraction of M. oleifera was chosen for this study since this fraction is documented to possess anti-inflammatory effects in lipopolysaccharide-induced macrophages, along with high antioxidant content and significant wound healing properties on normal and diabetic human dermal fibroblasts[23-25]. To our knowledge, we are the first to report the anti-diabetic potential of ethyl acetate extract fraction of M. oleifera on STZ-induced diabetic rats.
2. Materials and methods
2.1. Plant collection and extract preparation
Mature leaves of M. oleifera were harvested from the University’s garden in February, 2017. Then the leaves were washed and air dried.The plant material was verified by a botanist according to its taxonomy(voucher number SK 1561/08). Dried leaves were ground to powder and extracted at room temperature with 90% ethanol. Extracts were concentrated under vacuum pressure using rotary evaporator (Buchi Labortech AG, R215) and lyophilized to obtain crude powder. The crude extract fraction was subjected to liquid-liquid extraction with ethyl acetate in a separating funnel and the ethyl acetate fraction was collected. Residual extract fraction was re-extracted with ethyl acetate following the same method. The filtrates were combined, concentrated,lyophilized and placed in -20 ℃ for further use.
2.2. Animals
Sprague-Dawley rats of 8-12 weeks old, weighing 200-250 g were procured from the University’s animal facility. Animals were segregated into four groups (6 rats each group) and acclimatized for one week prior to experiment. The rats were exposed to normal light cycle, (55 ± 5)% humidity at room temperature with water and chow ad libitum. All animals were cared in accordance to the National Institute of Health’s ethical guidelines and our institute’s Animal Ethics Committee (IAEC No. 04/011/09).
2.3. Experimental protocol
All the animals were fasted overnight before the start of experiment.Diabetes was induced with STZ for all the animals except for the normal control animals (Group 1). The blood glucose levels of diabetic induced rats were checked after 3 days of STZ induction through tail vein puncture to confirm hyperglycemia. Animals of Group 1 were treated with distilled water alone (control). Group 2 animals served as diabetic control, induced with freshly prepared STZ (i.p.) at 55 mg/kg b.w. in citrate buffer (0.1 M, pH 4.5)[26]. Animals of Group 3 served as experimental group, which was given STZ and M. oleifera extract fraction (oral gavage) at a dose of 200 mg/kg b.w. for 30 consecutive days. Group 4 animals served as positive control, which was given STZ and 5 mg/kg b.w.[26] of glibenclamide (oral gavage) for 30 consecutive days. After 24 hours of final dose of drug, blood was extracted and rats were sacrificed to excise the pancreas which was washed with icecold phosphate buffered saline after excision. The blood samples were centrifuged at 4 000 rpm for 10 min at 4 ℃ to obtain the serum for biochemical analysis. A portion of the pancreas tissue was stored in 10% formalin for histopathological analysis and the rest of the tissue was homogenized and centrifuged at 13 000 rpm at 4 ℃ for 20 min.Resulting supernatant was subjected to biochemical analysis. Moreover,the animal body weight, feed efficiency ratio, food and water intake,relative kidney and liver weight were recorded.
2.4. Serum biochemical analysis
Blood glucose monitoring system (AP-Plus?, Mednet, Germany)was used to determine blood glucose levels. Glycosylated hemoglobin(HbA1c) levels were determined by anion exchange method using commercial kit (LifeChem?GHb, Hyderabad, India) in accordance with standard protocols described by manufacturer. Serum insulin levels were measured with Insulin Mouse ELISA kit (American Diagnostica Inc., CA, USA). Serum creatinine, total protein, globulin and albumin concentrations were determined using a Randox kit with an automated Randox Daytona analyzer (Randox Laboratories Ltd., USA) following protocols from the manufacturer. Serum alanine aminotransferase(ALT), alkaline phosphatase (ALP), aspartate aminotransferase (AST),total cholesterol (TC), lactate dehydrogenase (LDH), triglycerides(TG), low-density lipoprotein-cholesterol (LDL-C) and highdensity lipoprotein-cholesterol (HDL-C) levels were estimated using the appropriate kit (Stanbio Reagent kit, TX, USA) following the manufacturer’s instructions. Serum IL-1β levels were measured with RayBio?Rat ELISA kit (Ray Biotech Inc., Georgia, USA) according to the standard protocols of the manufacturer.
2.5. Biochemical analysis of pancreatic tissue
Lipid peroxidation levels in pancreatic tissue were determined spectrophotometrically from the reaction of malondialdehyde(MDA) with thiobarbituric acid conjugate formation at 535 nm.The results were stated as nanomole of MDA formed per milligram protein. Superoxide dismutase (SOD) activity was measured by rate of inhibition of pyrogallol auto-oxidation on a spectrophotometer at 470 nm. Glutathione peroxidase (GPx) activity was estimated spectrophotometrically at 340 nm by the rate of nicotinamide adenine dinucleotide phosphate oxidization. Activity of glutathione S-transferase (GST) was measured by the rate of 1-chloro-2,4-dinitrobenzene -conjugate formation spectrophotometrically at 340 nm. Catalase (CAT) activity was estimated by monitoring the decomposition of H2O2spectrophotometrically at 240 nm.Levels of reduced glutathione (GSH) were measured through spectrophotometrical measurement of 5-thiol-2-nitrobenzoic acid formation at 412 nm. Vitamin C (Vit-C) concentrations were estimated spectrophotometrically at 520 nm by the rearranged formation of bis-2,4-dinitrophenyl hydrazine. Vitamin E (Vit-E) concentrations were estimated by ferric ion reduction measured spectrophotometrically at 536 nm. Bradford method was employed for protein estimation in each sample using crystalline bovine serum albumin as standard[27].
The pro-inflammatory cytokines TNF-αand IL-6 levels in pancreatic tissues were determined with Millipore’s MILLIPLEX?MAP rat cytokine magnetic bead panel kit (Millipore Cooperation, MA, USA)following the manufacturer’s guidelines. The assay was conducted on Bio-Plex?platform (Bio-Rad, USA) and analyzed using Bio-Plex Manager TM software version 6.0 (Hercules, USA).
2.6. Histopathological analysis
The pancreatic tissues fixed in 10% formalin were routinely processed and embedded in paraffin. The tissue sections (5-6 μm) underwent H&E staining following standard protocol. The stained sections were mounted with DPx to be analyzed and photographed under highresolution light microscope.
2.7. Statistical analysis
All data were analyzed with one-way ANOVA (SPSS 19.0, USA)followed by Tukey’s multiple comparisons test. P values less than 0.05(P< 0.05) were considered as significant.
3. Results
3.1. Effect of M. oleifera on the body weight, feed efficiency ratio, food and water intake, relative kidney and liver weight
The effect of M. oleifera on animal body weight, feed efficiency ratio,food and water intake, relative kidney and liver weight were shown in Table 1. The body weight of Group 2 animals showed significantly reduced weight gain as compared to normal rats of Group 1 (P<0.05).Diabetic rats treated with M. oleifera (Group 3) showed significantly better weight gain (P<0.05) in contrast to diabetic control rats. Rats of Group 4 showed significantly high weight gain similar to that of normal rats in Group 1.
Water and food intake of Group 2 animals were higher (P<0.05) as compared to Group 1 animals. The food and water intake levels were reduced in Group 3 (P<0.05) and Group 4 (P<0.05) towards the level of normal control rats. Feed efficiency ratio that was a measuring rate of weight of animal divided by food intake was also calculated. An average food efficiency ratio of diabetes control rats (Group 2) were significantly lower than Group 1 rats (P<0.05) whereas rats in Group 3 and Group 4 showed higher food efficiency ratio as compared to Group 2 rats(P<0.05).
Relative kidney and liver weight of Group 2 rats were high in comparison with Group 1 rats. The results of Group 3 rats and Group 4 rats showed significantly reduced relative kidney and liver weight(P<0.05) in comparison with Group 2 rats.
3.2. Effect of M. oleifera on blood glucose, serum insulin and glycosylated hemoglobin levels
The effect of M. oleifera on serum insulin, glycosylated hemoglobin and blood glucose levels were demonstrated in Figure 1. Blood glucose and glycosylated hemoglobin levels were significantly elevated in Group 2 animals in comparison to Group 1 (P<0.05). In contrast, glycosylated hemoglobin and blood glucose levels were reduced significantly(P<0.05) towards the normal levels in Group 3 and Group 4 animals.The level of serum insulin in diabetes induced rats of Group 2 was significantly less (P<0.05) than that of normal animals of Group 1.Remarkably, the serum insulin levels were increased (P<0.05) in Group 3 and Group 4 rats as compared to Group 2 rats.
3.3. Effects of M. oleifera on serum albumin, globulin,creatinine and total protein concentrations
The effects of M. oleifera treatment on the concentrations of serum globulin, albumin, creatinine and total protein were shown in Table2. The concentrations of serum albumin, globulin and total protein were significantly lowered (P<0.05) in Group 2 rats as compared to normal rats of Group 1. Rats of Group 3 treated with M. oleifera significantly increased (P<0.05) the serum albumin, globulin and total protein concentrations as compared to Group 2 rats. Serum creatinine concentration was increased significantly in diabetic rats of Group 2 in comparison to Group 1 rats. Treatment with M. oleifera in Group 3 rats significantly reduced (P<0.05) the serum creatinine concentration in comparison with diabetic rats of Group 2. Group 4 also demonstrated similar results as Group 3.
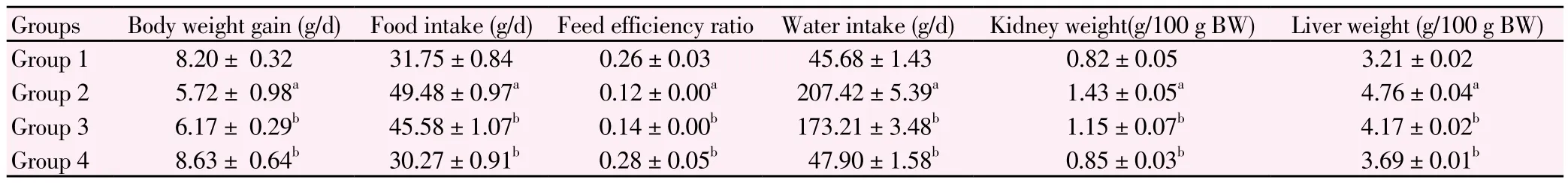
Table 1 Levels of fluid, food intake, body weight, relative weight of kidney and liver in four groups of rats.
3.4. Effect of M. oleifera on serum hepatic marker enzymes
The effect of M. oleifera on serum hepatic marker enzymes concentrations was demonstrated in Figure 2. Mean activities of serum AST, LDH, ALT and ALP were elevated (P<0.05) in diabetes-induced rats of Group 2 as compared to Group 1. High concentrations of these enzymes represented the level of liver damage caused by diabetes.Group 3 significantly reduced (P<0.05) the levels of serum ALT, AST,LDH and ALP in comparison with rats of Group 2. Group 4 showed almost similar results to Group 1 in the concentrations of serum AST,LDH, ALT and ALP.
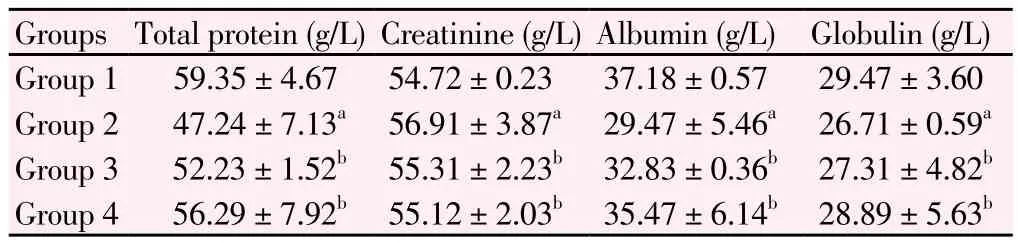
Table 2 Levels of serum total protein, creatinine, albumin and globulin concentrations in four groups of rats.
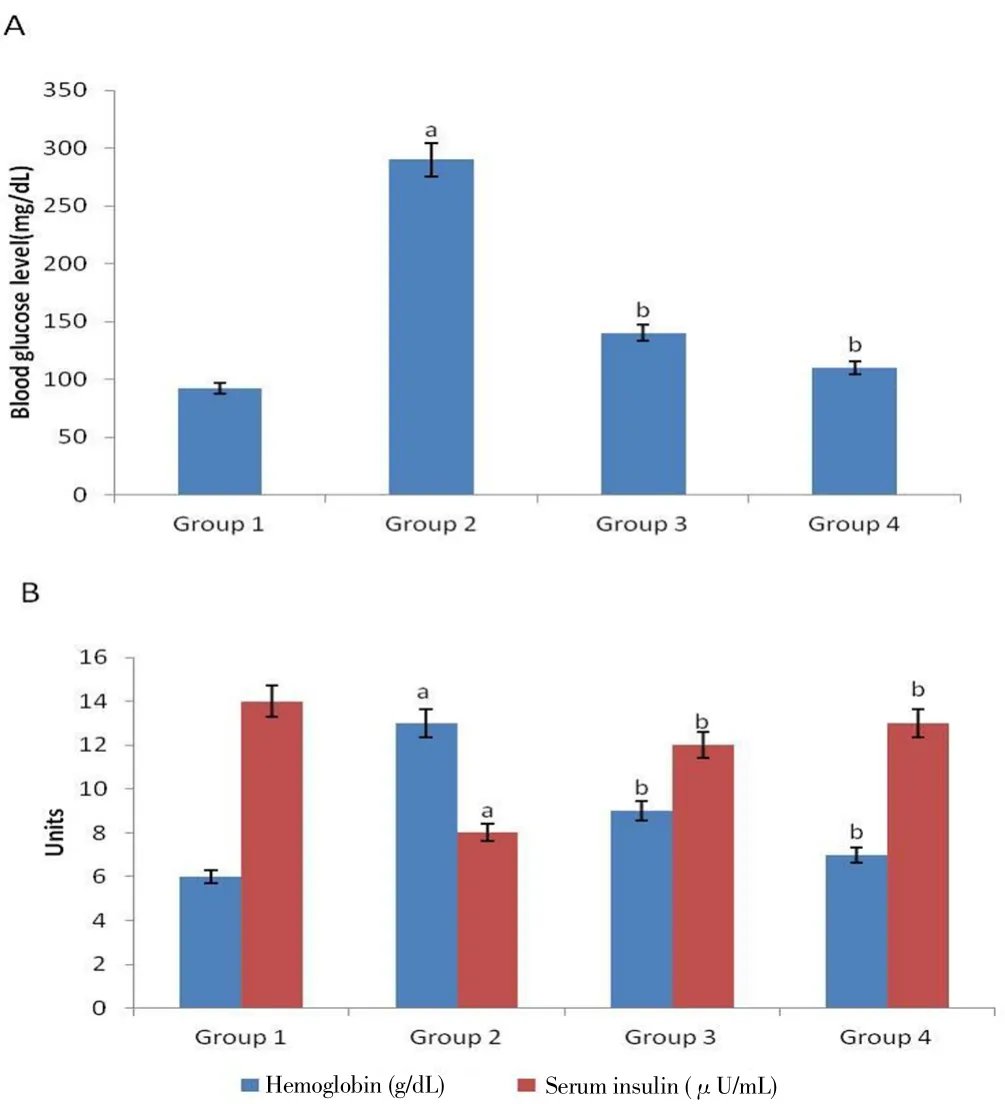
Figure 1. Mean blood glucose levels, glycosylated hemoglobin percentages and serum insulin levels in Sprague-Dawley rats.
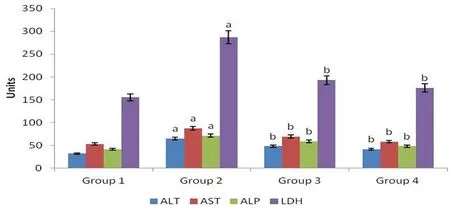
Figure 2. Mean activities of hepatic marker enzymes in serum of control and experimental groups of rats.
3.5. Effect of M. oleifera on the lipid profile levels
Lipid profile levels were determined in M. oleifera treated rats as presented in Figure 3. Diabetes-induced rats of Group 2 exhibited elevated TC, TG and LDL-C levels in comparison with Group 1 rats(P<0.05). The effect of M. oleifera was observed in M. oleifera treated rats of Group 3 as TG, TC and LDL-C levels were reduced (P<0.05) in contrast to Group 2 rats. The level of HDL-C was found significantly less in Group 2 rats in contrast to Group 1 rats while the level was increased (P<0.05) in M. oleifera treated rats of Group 3. Group 4 displayed similar results as Group 1 for lipid profile levels.
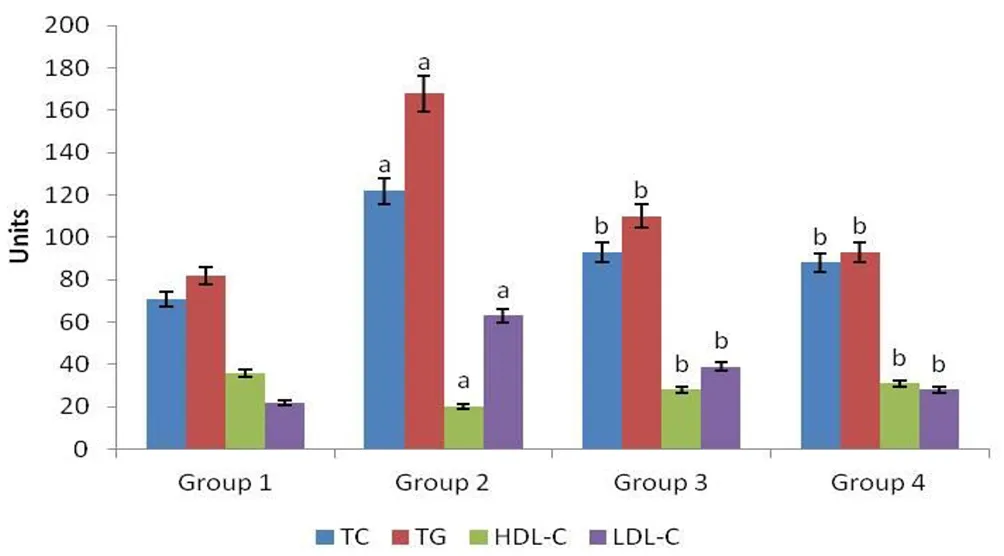
Figure 3. Effect of ethyl acetate fraction of M. oleifera extract on levels of lipid profile in four groups of rats.
3.6. Alteration of oxidative stress by ethyl acetate fraction of M. oleifera
Lipid peroxidation level of animals in all groups was presented in Figure 4. Oxidation of lipids in pancreas due to diabetes was measured in terms of MDA formation. The diabetic Group 2 rats showed significantly elevated levels of MDA (P<0.05) compared to normal rats of Group 1. This situation was significantly reversed by M. oleifera in Group 3 which showed lower levels of MDA formation compared with Group 2. There was no significant difference between MDA formation of Group 4 and Group 1 rats.
The concentrations of non-enzymatic and enzymatic antioxidants were determined in the pancreas of experimental and control rats(Figure 5). Concentrations of non-enzymatic antioxidants (Vit-C,GSH, Vit-E) and enzymatic antioxidants (CAT, GST, SOD, GPx)in the pancreas of diabetic Group 2 rats were reduced significantly(P<0.05) in contrast with normal rats of Group 1. This showed that the pancreatic tissues were undergoing oxidative stress where the antioxidants were consumed largely. Interestingly, the ethyl acetate fraction of M. oleifera was able to conserve the concentrations of all the enzymatic and non-enzymatic antioxidants significantly (P<0.05),exhibiting high concentrations of GPx, GST, CAT, SOD, Vit-E, GSH and Vit-C in pancreas of Group 3 rats in contrast with Group 2 rats.The pancreas of glibenclamide treated rats of Group 4 exhibited almost similar contents of non-enzymatic and enzymatic antioxidants to those of normal rats of Group 1.
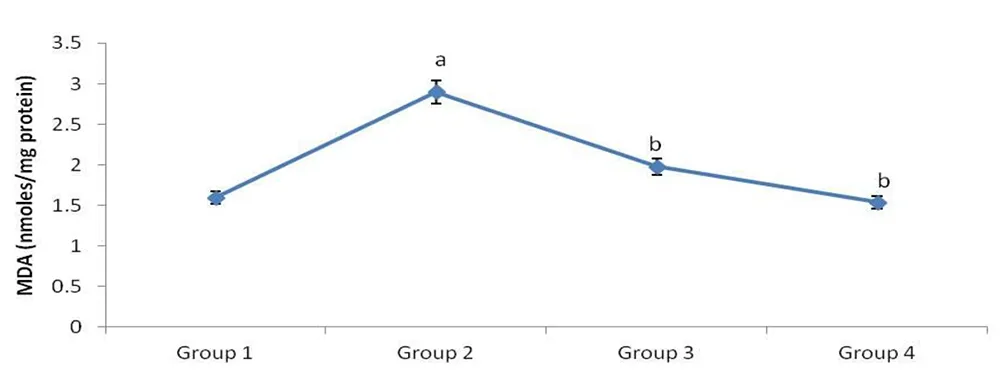
Figure 4. Mean level of MDA in pancreatic tissue samples of four groups of rats.
3.7. Effect of M. oleifera on pancreatic TNF-α, IL-6 and serum IL-1β levels
The effects of M. oleifera on inflammatory biomarkers IL-1β, TNF-αand IL-6 were displayed in Figure 6. Measurement of IL-6, TNF-α and IL-1β in diabetic rats of Group 2 showed significantly elevated levels as compared to normal rats of Group 1. In contrast, treatment of M. oleifera in Group 3 rats significantly reduced the expressions of TNF-α, IL-6 and IL-1β in comparison with Group 2. Rats of Group 4 also demonstrated reduced IL-6, IL-1β and TNF-αlevels.

Figure 5. Effect of ethyl acetate fraction of M. oleifera extract on levels of enzymatic and non-enzymatic antioxidants in pancreatic tissue samples of control and experimental groups of rats.
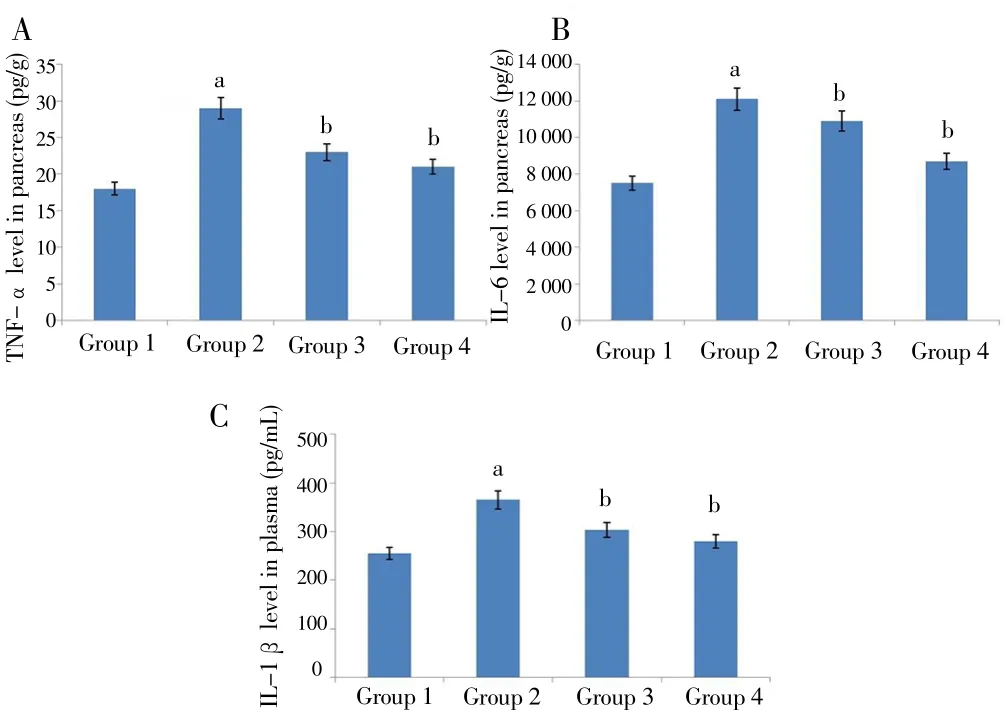
Figure 6. Effect of ethyl acetate fraction of M. oleifera extract on levels of inflammatory markers in four groups of rats.
3.8. Histopathological analysis of pancreas by M. oleifera treatment
Histopathological sections of four groups of rats were shown in Figure 7. The pancreas of normal rats of Group 1 exhibited healthy islets of Langerhans with granulated cytoplasm and acini tissues whereas the pancreas of diabetic rats of Group 2 showed irregular acini tissues with necrotic cells and degenerated cytoplasm along with disrupted outlining of islets of Langerhans. Treatment with ethyl acetate fraction of M. oleifera in rats of Group 3 significantly restored the arrangements of islets of Langerhans and pancreatic acinar tissues as compared to pancreas of Group 2 rats. Rats of Group 4 showed almost similar arrangements of islets of Langerhans and pancreatic acinar tissues with the normal rats of Group 1. The histopathological findings were in agreement with the biochemical results.

Figure 7. Histopathology sections of four groups of rats.
4. Discussion
Diabetes is usually induced in experimental animal models through an intraperitoneal injection of STZ, which is cytotoxic to pancreatic β-cells[27]. It is learned that STZ mediates diabetes through free radical formation, eventually causing damage to the pancreatic cells[9]. Studies have shown that diabetes induced rats exhibit similar symptoms of a non-insulin dependent diabetic human such as loss of weight, persistent hyperglycemia, altered insulin secretion, glucose intolerance, and so on[27,17]. We chose to evaluate the anti-diabetic effect in the ethyl acetate fraction of M. oleifera on STZ-induced diabetic rats, through antioxidant supplementation. It was clearly shown that STZ caused loss of body weight, increase in average water and food consumption as well as relative kidney and liver weight. The enlargement of organs is a sign of acute inflammation which might be due to overproduction of cells(hyperplasia) or hypertrophy[28]. These attributes are signs of diabetes in rats, which are similar to previous studies[2,8,19]. Ethyl acetate extract fraction of M. oleifera reversed these symptoms significantly which proves overall protection from diabetes. Safety in oral administration of M. oleifera has been described by Stohs and Hartman[29], which supports the dose regimen (200 mg/kg b.w.) of ethyl acetate extract fraction in our study.
Excessive oxidative stress causes endothelial cell dysfunction, which is an early feature and crucial metabolic significance of chronic hyperglycemia[30]. STZ-induced rats of Group 2 exhibited low mean serum insulin and significant high mean blood glucose levels (P<0.05),however ethyl acetate fraction of M. oleifera maintained the serum insulin and glucose levels around normal. M. oleifera leaf extract might have stimulated the insulin production by β-cells of pancreas, causing the anti-hyperglycemic effect in diabetic rats. Glycosylated hemoglobin,a marker of ambient glycemia, is formed through non-enzymatic reaction of excess blood glucose with hemoglobin[31,26]. STZ elevated the glycosylated hemoglobin level (P<0.05) in rats which was reduced significantly by ethyl acetate fraction of M. oleifera (P<0.05). This proves that M. oleifera has antihyperglycemic properties which cause reduction of blood glucose, eventually causing reduced glycosylated hemoglobin level in diabetic rats.
Diabetic condition causes heavy loss of blood protein, causing irregular filtering in the kidney and buildup of toxic wastes[19].Globulin, albumin, total protein and creatinine concentrations in serum were determined in experimental and control rats. The diabetic rats of Group 2 showed significantly decreased globulin, albumin and total protein concentrations (P<0.05) and increased creatinine concentration (P<0.05) as compared to normal control rats, which were significantly reversed by ethyl acetate fraction of M. oleifera(P<0.05). This shows the diabetic-induced rats had deranged kidney functions (nephropathy) due to heavy loss of blood protein (albumin and globulin), which are biomarkers in disease state and high creatinine concentration, which is a marker of kidney function[32]. M. oleifera was able to maintain the normal function of kidney in diabetic-induced rats through regenerating and enhancing the kidney function. The increase of TG, TC and LDL-C levels along with reduced HDL-C level are common symptoms of disease in STZ-induced rats, which was exhibited by the diabetic rats of Group 2. Ethyl acetate extract of M. oleifera increased the HDL-C level and reduced the TG, TC and LDL-C levels and these results are similar to other studies reported earlier on lipid profile of diabetic rats treated with plant extracts[33,34].Liver is an important organ in maintaining normal glucose concentration in body, which functions in insulin disposal and produces inflammatory cytokines. Increased levels of serum ALP, ALT, AST and LDH are symptoms of liver damage where the enzymes leak into the bloodstream from cytosol upon hepatic cellular damage[27]. STZ-induced rats of Group 2 also exhibited similar results, proving hepatic damage. Oral administration of M. oleifera was able to prevent the oxidative stress related hepatic damage through antioxidant supplementation. Lipid peroxidation is caused by oxidative stress during diabetic conditions.Oxidative stress due to high production of ROS in diabetic state causes damage to structural biomolecules, leading to dysfunction of organs[19].Similarly, enzymatic and non-enzymatic antioxidants play vital roles in scavenging ROS to prevent oxidative stress related organ damage.Hyperglycemia during diabetic condition could cause depletion of enzymatic and non-enzymatic antioxidants as a consequence of excessive ROS production[35]. Diabetic rats of Group 2 also showed similar results such as elevated MDA level and reduced pancreatic GSH, Vit-C, Vit-E, SOD, GPx, CAT and GST levels (P<0.05). The administration of M. oleifera leaves extract fraction was able to inhibit oxidative stress, proven by reduced MDA level and increased nonenzymatic and enzymatic antioxidants (P<0.05). Histopathological alterations by ethyl acetate extract fraction of M. oleifera were in agreement with these biochemical results in the structural protection of pancreatic tissues. The anti-hyperglycemic effect of ethyl acetate extract fraction of M. oleifera can be attributed to the phytochemical constituents of the leaves such as flavonoids, terpenoids, alkaloids,saponins, sterols and polyphenols[36,37].
Inflammation is a common response in tissues which are damaged by oxidative stress. Pro-inflammatory cytokines such as IL-1β, TNF-αand IL-6 are usually triggered during hyperglycemia-induced organ injuries,leading to development of diabetes[19]. These cytokines are associated with the pathogenesis of diabetes and related organ damage as a consequence of oxidative stress. Excessive production of these cytokines causes harm to the pancreatic β-cells thus disrupting insulin production[38]. In this study, ethyl acetate extract of M. oleifera prevented the inflammatory cytokines production from the adverse effect caused by STZ in rats. These details support the anti-inflammatory and antioxidant potential in the ethyl acetate extract fraction of M. oleifera as described by other studies[9,19]. Oral administration of M. oleifera has the ability to counteract the ROS formation induced by STZ in rats, which is basically credited to the rich antioxidant content of M. oleifera leaves.
The oral administration of ethyl acetate extract fraction of M. oleifera prevented oxidative stress in STZ-induced diabetic rats through supplementation of antioxidants such as flavonoids and polyphenols.Biomarkers of organ damage indicated that ethyl acetate extract fraction of M. oleifera has the ability to inhibit inflammation induced by STZ in rats, thus protecting the kidney and liver from damage by hyperglycemia. Ethyl acetate extract fraction of M. oleifera also increased the antioxidatives activity and prevented the inflammatory reactions in the pancreas tissue in STZ-induced diabetic rats. Therefore,the anti-diabetic properties in the ethyl acetate fraction of M. oleifera can be credited to the rich antioxidant content and anti-inflammatory mechanism promoted by the extract. Further research is recommended to be performed on the individual phytochemical constituents in the ethyl acetate extract of M. oleifera for anti-diabetic and antiinflammatory potentials.
Conflict of interest statement
The authors declare that there is no conflict of interest.
[1] Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract 2010; 87(1): 4-14.
[2] Latif AAE, Bialy BESE, Mahboub HD, Eldaim MAA. Moringa oleifera leaf extract ameliorates alloxan-induced diabetes in rats by regeneration of β-cells and reduction of pyruvate carboxylase expression. Biochem Cell Biol 2014; 92(5): 413-419.
[3] Smolek MK, Notaroberto NF, Jaramillo AG, Pradillo LR. Intervention with vitamins in patients with nonproliferative diabetic retinopathy: A pilot study.Clin Ophthalmol 2013; 7: 1451-1458.
[4] Liu J, Wang S, Feng L, Ma D, Fu Q, Song Y, et al. Hypoglycemic and antioxidant activities of paeonol and its beneficial effect on diabetic encephalopathy in streptozotocin-induced diabetic rats. J Med Food 2013;16(7): 577-586.
[5] Sreekutty MS, Mini S. Ensete superbum ameliorates renal dysfunction in experimental diabetes mellitus. Iran J Basic Med Sci 2016; 19(1): 111-118.
[6] Warjeet SL. Traditional medicinal plants of Manipur as anti-diabetics. J Med Plants Res 2011; 5(5): 677-687.
[7] Karthikesan K, Pari L, Menon V. Combined treatment of tetrahydrocurcumin and chlorogenic acid exerts potential antihyperglycemic effect on streptozotocinnicotinamide induced diabetic rats. Gen Physiol Biophys 2010; 29(1): 23-30.
[8] Yassa HD, Tohamy AF. Extract of Moringa oleifera leaves ameliorates streptozotocin-induced Diabetes mellitus in adult rats. Acta Histochemica 2014; 116(5): 844-854.
[9] Al-Malki AL, El Rabey HA. The anti-diabetic effect of low doses of Moringa oleifera Lam. seeds on streptozotocin-induced diabetes and diabetic nephropathy in male rats. Biomed Res Int 2015; 2015(2015): 381040.
[10] Gnanaraj C, Shah MD, Song TT, Iqbal M. Hepatoprotective mechanism of Lygodium microphyllum (Cav.) R.Br. through ultrastructural signaling prevention against carbon tetrachloride (CCl4)-mediated oxidative stress.Biomed Pharmacother 2017; 92: 1010-1022.
[11] Jongrungruangchok S, Bunrathep S, Songsak T. Nutrients and minerals content of eleven different samples of Moringa oleifera cultivated in Thailand. J Health Res 2010; 24: 123-127.
[12] Gowrishankar R, Kuma M, Menon V, Divi SM, Saravanan M,Magudapathy P, et al. Trace element studies on Tinospora cordifolia(Menispermaceae), Ocimum sanctum (Lamiaceae), Moringa oleifera(Moringaceae) and Phyllanthus niruri (Euphorbiaceae) using PIXE. Biol Trace Element Res 2010; 133(3): 357-363.
[13] Farooq F, Meenu R, Avinash T, Abdul A, Sahila F. Medicinal properties of Moringa oleifera: An overview of promising healer. J Med Plant Res 2012;6(27): 4368-4374.
[14] Jaiswal D, Rai PK, Kumar A, Mehta S, Watal G. Effect of Moringa oleifera Lam. leaves aqueous extract therapy on hyperglycemic rats. J Ethnopharmacol 2009; 123(3): 392-396.
[15] Mbikay M. Therapeutic potential of Moringa oleifera leaves in chronic hyperglycemia and dyslipidemia: A review. Front Pharmacol 2012; 3: 1-12.
[16] Atsukwei D, Eze ED, Moses DM, Adinoyi SS, Upkabi CN. Hypolipidaemic effect of ethanol leaf extract of Moringa oleifera Lam. in experimentally induced hypercholesterolemic Wistar rats. Int J Nutri Food Sci 2014; 3:355-360.
[17] Tuorkey MJ. Effects of Moringa oleifera aqueous leaf extract in alloxan induced diabetic mice. Interv Med Appl Sci 2016; 8(3): 109-117.
[18] Igado OO, Olopade JO. A review on the possible neuroprotective effects of Moringa oleifera leaf extract. Niger J Physiol Sci 2017; 31(2): 183-187.
[19] Omodanisi EI, Aboua YG, Oguntibeju OO. Assessment of the antihyperglycaemic, anti-inflammatory and antioxidant activities of the methanol extract of Moringa oleifera in diabetes-induced nephrotoxic male wistar rats. Molecules 2017; 22(4): E439.
[20] Gupta R, Mathur M, Bajaj VK, Katariya P, Yadav S, Kamal R, et al.Evaluation of antidiabetic and antioxidant activity of Moringa oleifera in experimental diabetes. J Diabetes 2012; 4(2): 164-171.
[21] Edoga CO, Njoku OO, Amadi EN, Okeke JJ. Blood sugar lowering effect of Moringa oleifera Lam in albino rats. Int J Sci Tech 2013; 3(1): 88-90.
[22] Ampa L, Watchara K, Tanaree J. Anti-hyperglycemic properties of Moringa oleifera Lam. aqueous leaf extract in normal and mildly diabetic mice. Brit J Pharmacol Toxicol 2013; 4(3): 106-109.
[23] Arulselvan P, Tan WS, Gothai S, Muniandy K, Fakurazi S, Esa NM, et al.Anti-inflammatory potential of ethyl acetate fraction of Moringa oleifera in downregulating the NF-κB signaling pathway in lipopolysaccharidestimulated macrophages. Molecules 2016; 21(11): 1452.
[24] Gothai S, Arulselvan P, Tan WS Fakurazi S. Wound healing properties of ethyl acetate fraction of Moringa oleifera in normal human dermal fibroblasts. J Int Ethnopharmacol 2016; 5(1): 1-6.
[25] Gothai S, Muniandy K, Zarin MA, Tan WS, Kumar SS, Munusamy MA, et al. Chemical composition of Moringa oleifera ethyl acetate fraction and its biological activity in diabetic human dermal fibroblasts. Pharmacogn Mag 2017; 13(51): 462-469.
[26] Arulselvan P, Subramanian S. Effect of Murraya koenigii leaf extract on carbohydrate metabolism studied in streptozotocin induced diabetic rats.Int J Biol Chem 2007; 1(1): 21-28.
[27] Annadurai T, Muralidharan AR, Joseph T, Hsu MJ, Thomas PA, Geraldine P. Antihyperglycemic and antioxidant effects of a flavanone, naringenin, in streptozotocin–nicotinamide-induced experimental diabetic rats. J Physiol Biochem 2012; 68(3): 307-318.
[28] Rodriguez-Perez C, Quirantes-Pine R, Fernandez-Gutierrez A, Segura-Carretero A. Optimization of extraction method to obtain a phenolic compounds-rich extract from Moringa oleifera Lam leaves. Ind Crop Prod 2015; 66: 246-254.
[29] Stohs SJ, Hartman MJ. Review of the safety and efficacy of Moringa oleifera.Phytother Res 2015; 29(6): 796-804.
[30] Ayeleso A, Brooks N, Oguntibeju OO. Modulation of antioxidant status in streptozotocin-induced diabetic male Wistar rats following intake of red palm oil and/or rooibos. Asian Pac J Trop Med 2014; 7(7): 536-544.
[31] Punithavathi VR, Prince PSM, Kumar R, Selvakumari J. Antihyperglycaemic,antilipid peroxidative and antioxidant effects of gallic acid on streptozotocin induced diabetic Wistar rats. Eur J Pharmacol 2011; 650(1): 465-471.
[32] Santos AFS, Argolo ACC, Paiva PMG, Coelho LCBB. Antioxidant activity of Moringa oleifera tissue extracts. Phytother Res 2012; 26(9): 1366-1370.
[33] Wang W, He Y, Lin P, Li Y, Sun R, Gu W, et al. In vitro effects of active components of Polygonum multiflorum Radix on enzymes involved in the lipid metabolism. J Ethnopharmacol 2014; 153(3): 763-770.
[34] Krishnasamy G, Muthusamy K, Chellappan DR, Subbiah N. Antidiabetic,antihyperlipidaemic, and antioxidant activity of Syzygium densiflorum fruits in streptozotocin and nicotinamide-induced diabetic rats. Pharm Biol 2016;54(9): 1716-1726.
[35] Alhakmani F, Kumar S, Khan SA. Estimation of total phenolic content,in-vitro antioxidant andanti-inflammatory activity of flowers of Moringa oleifera. Asian Pac J Trop Biomed 2013; 3(8): 623-627.
[36] Tende JA, Ezekiel I, Dikko AAU, Goji ADT. Effect of ethanolicleaves extract of Moringa oleifera on blood glucose levels of streptozocin-induced diabetics and normoglycemic wistar rats. Bri J Pharmacol Toxicol 2011; 2(1): 1-4.
[37] Valdez-Solana MA, Mejia-Garcia VY, Tellez-Valencia A, García-Arenas G, Salas-Pacheco J, Alba-Romero JJ, et al. Nutritional content and elemental and phytochemical analyses of Moringa oleifera grown in Mexico. J Chem 2015; 2015: 1-9.
[38] Seca AML, Grigore A, Pinto DCG, Silva AMS. The genus Inula and their metabolites: From ethnopharmacological to medicinal uses. J Ethnopharmacol 2014; 154(2): 286-310.
 Asian Pacific Journal of Tropical Biomedicine2018年6期
Asian Pacific Journal of Tropical Biomedicine2018年6期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Probiotic based therapy for atopic dermatitis: Outcomes of clinical studies
- Antifungal and cytotoxic activities of extracts obtained from underutilised edible tropical fruits
- Evaluation of possible mechanisms of Cordia dichotoma fruits for hyperlipidemia controlling in Wistar albino rats
- Effects of black chokeberry extracts on metastasis and cell-cycle arrest in SK-Hep1 human liver cancer cell line
