Pediatric recurrent Clostridium difficile infections in immunocompetent children: Lessons learned from case reports of the first twelve consecutive patients
Angela Chu,Sonia Michail
Angela Chu,Sonia Michail,Department of Pediatrics,Miller Children's and Women's Hospital,Long Beach,CA 90826,United States
Angela Chu,Sonia Michail,Department of Pediatrics,UC Irvine School of Medicine,Irvine,CA 92612,United States
Sonia Michail,Pediatric Gastroenterology,Hepatology and Nutrition Center,Miller Children's and Women's Hospital,Long Beach,CA 90826,United States
Sonia Michail,University of Southern California,Los Angeles,CA 92708,United States
Abstract
Key words: Recurrent Clostridium difficile infection; Eosinophilic colitis; Inflammatory bowel disease; Fecal microbiome transplant
INTRODUCTION
The incidence ofClostridium difficileinfections (CDI) in both adults and pediatrics is increasing[1-5].CDI can result in a spectrum of disorders that ranges from carrier and asymptomatic state to causing significant morbidity and even mortality[6].Infants frequently test positive but are asymptomatic[7].Part of the rise in CDI could be from increasing testing among infants,which needs to be done with caution given the high prevalence of asymptomatic colonization in young infants[7].There is also a higher incidence of colonization and colitis withC.difficilein pediatric inflammatory bowel disease (IBD) compared to adult IBD as well as patients with celiac disease[8].
There have been multiple studies showing correlation between certain risk factors predisposing to the development of CDI.Risk factors such as acid suppressing agents,especially H2 receptor antagonists,exposure to antibiotics and immunosuppressants,comorbidities such as cancer,cystic fibrosis and IBD,and hospitalization have been known to increase the incidence of CDI for some time[1,9].These studies are charged with the task of understanding the risk for developing the infection in general,however,there is a paucity of studies that describe a select population of children that haverecurrenceof this infection.While community acquired CDI is more common in pediatrics than adults,recurrent CDI is not common in children[10].A study by Kociolek in 2015[11]showed an association between recurrent CDI and malignancy and IBD.The study identified thirty children with recurrent infection and demonstrated that the majority of these subjects (19 subjects or 63%) have malignancy,underwent solid organ transplant or have IBD.
In this study,we aimed to understand CDI in a very unique population of children who are not immunocompromised and do not have any identified IBD.This study describes important discoveries of unidentified underlying gastrointestinal conditions which may not be recognized unless the child is adequately evaluated by a specialist in the field.The study also describes the success,and the durable gut microbial changes after fecal microbial transplant in this population.These discoveries contribute to the successful outcome in management of these subjects by identifying and addressing the underlying disease.
MATERIALS AND METHODS
Institutional Review Board (IRB) approval was obtained to study pediatric patients with recurrent CDI,defined as two or more distinct episodes of CDI associated with diarrhea or bloody diarrhea who were referred for evaluation to pediatric gastroenterology service.Subjects younger than one year and older than twenty-one years of age were excluded.All subjects with known immunosuppression or IBD prior to referral were excluded.Subjects had been followed up for at least one year.
Stool microbiome methods
The 16S bacterial DNA region from stool DNA and negative controls were amplified by PCR using a shared forward primer 806rB (CAAGCAGAAGACGGCATACGAGATAGTCAGCCAGCCGGACTACNVGGGTWTCTAAT) for all samples,while each sample had its own unique identifying reverse primer,which were modified from the original 515F-806R primer pairs.All samples were pooled and sequenced using custom sequencing primers; R1 (TATGGTAATTGTGTGYCAGCMGCCGCGGTAA),R2 (AGTCAGCCAGCCGGACTACNVGGGTWTCTAAT) and Index(AATGATACGGCGACCACCGAGATCTACACGCT).Paired-end sequencing (2 ×150bp) using Illumina MiSeq Reagent Kit v2 flowcell was performed on an Illumina MiSeq System.
Reads were de-multiplexed using QIIME v1.9.1.Statistical analyses were performed using the “phyloseq” (v1.20.0) package in the R statistical environment.
RESULTS
Twelve consecutive children were identified that fit the criteria described above.Children averaged 7.5 years of age (range 1-17 years).All children were treated with at least one course of metronidazole and one course of enteral vancomycin prior to referral.Nine children were exposed to antibiotic therapy prior to their first CDI.Three children had multiple antibiotic courses including amoxicillin.The most common single antibiotic course prior to CDI was amoxicillin as well.Three children did not receive antimicrobials prior to their first CDI.Two of the three children who did not receive antibiotics prior to their first CDI,were found to have an underlying gastrointestinal disease.The identification of the underlying disease changed the management of these patients.Five of the 12 children were previously healthy.The remaining children had different co-morbidities as described in Table1 without a known history of colitis or immunodeficiency prior to referral.There were 9 patients that failed antibiotic treatment of CDI and required fecal microbiome transplant(FMT),which ultimately relieved CDI symptoms.Of these nine patients,4 had a gastrostomy or gastrojejunostomy tube (Table1),seven had history of antibiotic use,and 3 had history of acid suppressants.
After a thorough gastrointestinal workup,two patients were found to have eosinophilic disease,one subject had eosinophilic colitis and another subject had eosinophilic esophagitis.The child with eosinophilic colitis was placed exclusively on crystalline amino acid formula which resulted in resolution and prevention of any further CDI even after future exposure to antimicrobial therapy.One patient was found to have IBD proctitis,and CDI resolved after treatment of IBD.There were three subjects diagnosed with lactase deficiency.
One of the children treated with FMT,experienced a change in disease phenotype fromC.difficilecolitis that required hospitalization for bloody diarrhea with endoscopic confirmation ofC.difficilecolitis,to an asymptomaticC.difficilecolonizer for 12 mo,followed by loss of colonization.No further CDI treatment was required despite the use of antimicrobial therapy for respiratory infection after FMT.From the FMT safety perspective,one subject developed transient fever for one day but was otherwise asymptomatic.Another subject developed bloating on the day of FMT.No serious adverse events were seen related to FMT.
Gut microbial profiles were examined before and after fecal transplant and compared to the donor profile.Children with recurrent CDI had very low abundance ofBacteroidaceae(Figure1) prior to fecal transplant as well as low diversity of microorganisms compared to healthy donor (1.3 = 0.2vs3.2 + 0.4,Shannon diversity index,P= 0.031).After fecal transplant,the fecal microbial profile diversity improved.This phenomenon seemed to be durable for the twelve months following fecal transplant (Figure2).Similarly,Bacteroidaceaebecame quite abundant after fecal transplant and this effect was seen over twelve months (Figure1).
DISCUSSION
In adults,a recent meta-analysis showed age > 65 years,additional antibiotic use during follow-up,use of proton-pump inhibitors (PPIs),and renal insufficiency were most frequently associated with recurrent CDI[12].There have been a few pediatric studies that describe risk factors for CDI in pediatric patients as well[1,9,11,13].Underlying chronic medical condition,recent antibiotic use (specifically cephalosporins as described by Crewset al[13]),acid-suppressing agents,gastro-intestinal feeding device,and past or prolonged hospitalization increase the risk of developing CDI in pediatrics[1,9,13].There is a paucity of data in the literature that focuses on recurrent infections.Kocioleket al[11]described a cohort of children who have recurrent CDI and found that the majority have malignancy or solid organ transplant,or IBD (n= 19,or 63%).Although there have been studies implicating IBD and immunosuppression increasing children's susceptibility to CDI[4,8],to our knowledge,no studies have directly linked recurrent CDI to undiagnosed underlying gastrointestinal disease.
With regards to antibiotic use in children who developed recurrent CDI,most of the children in our study received amoxicillin therapy,in contrast to the study by Crewset al[13]that showed more exposure to cephalosporins.Most children (two thirds),who did not receive antimicrobials prior to their first CDI were found to have an underlying gastrointestinal disease which was only identified when a work up was performed after referral to specialist.The authors recognize the limitation of the findings due to the small overall number of subjects in this sub-population.
Perhaps over one third of infants younger than 12 mo are colonized withC.difficile[14].The rate of colonization then drops to 15% between ages 1-8 years and then 5% after age 8 years,similar to the rate in adults[14].Due to the high rate of colonization in infants,patients under 12 mo of age were excluded from this study.
Five of the twelve children in our cohort had a gastrostomy or a jejunostomy feeding tube,which are known to be associated with an increased risk of acquiringC.difficile,in adults and children[14-16].This is likely due to spore contamination of equipment or formula,or use of formula that promotesC.difficilegrowth in the gut[14,15,17].Most of the children in our study have been hospitalized in the past,which again would expose them to an environment that could harborC.difficilespores and increasing their risk of acquiringC.difficile[14,18].
While there are many medical conditions known to predispose pediatric patients to CDI,such as hematopoietic stem cell transplant,IBD,cancer,fungal infections,and human immunodeficiency virus infection[14],those co-morbidities are diagnosed prior to the onset of CDI.In our study,5 out of 12 patients had underlying pathology that was not previously identified.There have been many single study reports of other medical conditions that are associated with CDI[14],such as cystic fibrosis[19],Hirschsprung's[20],and Henoch-Schonlein purpura[21].In our study,two patients had eosinophilic disease,which has not been described in prior studies as an association or risk factor for CDI.The discovery and treatment of an underlying colitis,namely eosinophilic colitis and IBD proctitis,resulted in prompt resolution of the recurrence of CDI.
Three of the twelve subjects were diagnosed with lactase deficiency by disaccharidase assay.Since there is overlap in symptoms with CDI and lactase deficiency,namely diarrhea and abdominal pain,the discovery and treatment of lactase deficiency allowed optimizing management and more appropriate assignment of symptoms to the correct underlying disease.However,as expected,management of lactase deficiency did not result in resolution of CDI recurrence.FMT in both subjects resulted in prompt resolution of symptoms.
All the subjects receiving FMT had resolution of symptoms for at least one year.One subject became an asymptomatic colonizer ofC.difficileafter FMT.The colonization was seen for 12 mo followed by resolution of colonization.
FMT in this patient population appeared to be highly effective and safe.Fecal transplant resulted in improved gut microbial diversity and abundance ofBacteroides,which appeared to be durable and seen to persist for at least twelve months.The overall numbers are small and more research will be necessary to confirm these observations.
In this subset population,it is recommended that children with recurrent CDI who do not have immunodeficiency or identified IBD be evaluated by a provider who can investigate the presence of an underlying gastrointestinal disease.In about one third of these subjects,a gastrointestinal disorder may be discovered that can impact the management of recurrent infection.
In conclusion,there are likely risk factors that are still unknown that can predispose to CDI.Pediatric patients that have more than one episode of CDI recurrence have an increased likelihood of underlying gastrointestinal pathology especially if there has been no prior use of antimicrobials and should be investigated so that proper treatment can be offered.Fecal microbial transplant is a highly effective and safe therapy for these children and results in durable changes in the gut microbiome.
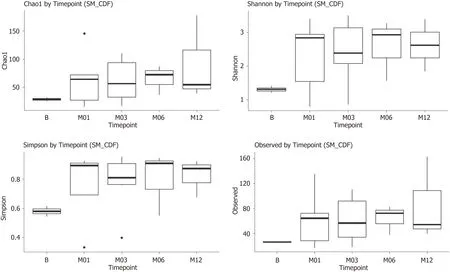
Figure2 Diversity index at baseline and 1,3,6 and 12 mo after fecal transplant,showing consistent increase in diversity compared to baseline.
ARTICLE HIGHLIGHTS
Research background
Childhood recurrentClostridium difficileinfections (CDI) may be difficult to control and may represent an unknown underlying pathology.Recurrence often occurs in immunodeficiency disorders and inflammatory bowel disease (IBD).
Research motivation
There have been multiple studies showing correlation between certain risk factors predisposing to the development of CDI.Risk factors such as acid suppressing agents,especially H2 receptor antagonists,exposure to antibiotics and immunosuppressants,comorbidities such as cancer,cystic fibrosis and IBD,and hospitalization have been known to increase the incidence of CDI for some time.These studies are charged with the task of understanding the risk for developing the infection in general,however,there is a paucity of studies that describe a select population of children that haverecurrenceof this infection.While community acquired CDI is more common in pediatrics than adults,recurrent CDI is not common in children.
Research objectives
The main objectives of this report are understanding CDI in a very unique population of children who are not immunocompromised and do not have any identified IBD.This study describes important discoveries of unidentified underlying gastrointestinal conditions which may not be recognized unless the child is adequately evaluated by a specialist in the field.The study also describes the success,and the durable gut microbial changes after fecal microbial transplant in this population.These discoveries contribute to the successful outcome in management of these subjects by identifying and addressing the underlying disease.
Research methods
Pediatric patients with recurrent CDI,defined as two or more distinct episodes of CDI associated with diarrhea or bloody diarrhea who were referred for evaluation to pediatric gastro-enterology service were identified.Subjects younger than one year and older than twenty-one years of age were excluded.All subjects with known immunosuppression or IBD prior to referral were excluded.Subjects had been followed up for at least one year.
Research results
We have observed 12 children in succession.All patients re ceived CDI antibiotics prior to referral.Five of the 12 patients had previously undiscovered potential pathologies,including eosinophilic colitis and IBD.After the treatment of basal colitis,the symptoms of CDI disappear and there is no need for CDI treatment.Nine patients required fecal microbial transplantation for antibiotic CDI failure,which is safe and effective (100% efficacy) for preventing recurrence.Intestinal microbial changes following fecal transplantation are characterized by a significant and sustained increase in diversity and the abundance ofBacteroides.
Research conclusions
Children with recurrent CDI deserve a through gastrointestinal workup as they may frequently have an underlying disease which can contribute to the management of the condition.When medical therapy fails in this population,fecal microbial transplant is a safe and durable therapy.Children with recurrent CDI may have unidentified gastrointestinal disease contributing to the recurrence of the infection.Children with recurrent clostridium difficile frequently have an unidentified gastrointestinal disorder,which when identified and addressed,can help with management of clostridium difficile recurrence.
Research perspectives
Children with recurrent CDI need a thorough gastrointestinal workup to optimize their care and management.Future research should focus on individualized medicine and targeting underlying disease on a case by case basis.
 World Journal of Meta-Analysis2019年7期
World Journal of Meta-Analysis2019年7期
- World Journal of Meta-Analysis的其它文章
- Phantom of the inflammasome in the gut: Cytomegalovirus
- Artificial intelligence for endoscopy
- Asymptomatic bacteriuria among hospitalized diabetic patients:Should they be treated?
- Effect of dl-3-n-butylphthalide on infarction volume in animal models of ischemic stroke: A meta-analysis
