Exogenous application of glycine betaine improved water use efficiency in winter wheat(Triticum aestivum L.) via modulating photosynthetic efficiency and antioxidative capacity under conventional and limited irrigation conditions
Nazir Ahmed, Yushi Zhang, Ke Li, Yuyi Zhou, Mingcai Zhang*, Zhaohu Li
Engineering Research Center of Plant Growth Regulator, Ministry of Education, College of Agronomy and Biotechnology, China Agricultural University, Beijing 100193,China
Keywords:Winter wheat Glycine betaine Water use efficiency Limited irrigation Photosynthetic performance Antioxidant systems
ABSTRACT Improving water use efficiency (WUE) is an important subject in agricultural irrigation for alleviating the scarcity of water resources in semiarid regions of the North China Plain.Moreover,glycine betaine (GB) is one of the most effective compatible solutes synthesized naturally in plants for enhancing stress tolerance under abiotic stress,but little information is available on the involvement of GB in regulating crop WUE under field conditions.This study was conducted to explore the role of exogenously applied GB in improving WUE and plant physiological and biochemical responses in winter wheat subjected to conventional or limited irrigation during the 2015-2016 and 2016-2017 growing seasons.Exogenous application of GB significantly enhanced antioxidant enzyme activities and reduced the accumulation of malondialdehyde and hydrogen peroxide under limited irrigation conditions.Furthermore,GB-treated plants maintained higher leaf relative water content and membrane stability,which led to higher chlorophyll content and gas exchange attributes for better intrinsic and instantaneous water use efficiencies compared to control plants under limited irrigation conditions. GB-treated plants had higher indole-acetic acid and zeatin riboside levels but lower ABA levels compared to control plants under conventional and limited irrigation conditions.Additionally,GB enhanced the grain filling rate and duration,grain number per spike,and final grain weight,which resulted in higher grain yield compared to the control. Interestingly, GB significantly improved the integrative and photosynthetic WUE under conventional and limited irrigation conditions, although GB treatment did not markedly affect total water consumption. These results suggest the involvement of GB in improving WUEs in winter wheat by modulating hormonal balance,membrane stability, photosynthetic performance and antioxidant systems to maintain higher grain yield under conventional and limited irrigation conditions.
1. Introduction
Water shortage is one of the significant environmental stresses limiting crop growth and productivity worldwide,while global warming and climate change make the situation more serious, which poses a significant threat to global food security [1]. The North China Plain (NCP) is an important center of agricultural production in China,covering 21%of the total national farmland and producing nearly 26%of the total grain yield[2,3].Moreover,the NCP is one of the areas in China most severely impacted by water shortage, having fewer per capita water sources than countries severely impacted by water shortage such as Israel [4]. Otherwise, the NCP experiences a typical temperate monsoon climate and annual precipitation of approximately 400-800 mm with variable spatial and temporal distributions having 70%-80% of the annual precipitation in the summer(July to September).Here,the dominant cropping system is the annual double-cropping system of winter wheat (October to mid-June) and summer maize(mid-June to September),and winter wheat is grown in the dry season [5]. Nearly 70% of irrigation water depends on groundwater to meet winter wheat water requirements [6,7].Pumping groundwater to fulfill crop water requirements not only depletes groundwater reservoirs but also deteriorates soil and ecosystem health,drying up rivers and wetlands and increasing seawater intrusion and land subsidence [8]. Consequently, improving water use efficiency (WUE) is very important for alleviating water shortages and maintaining stable and sustainable wheat production under limited irrigation conditions in the NCP.
The increase in WUE can be achieved through droughttolerant and water-saving varieties, appropriate agronomic practices and management [9-11]. WUE is generally referred to as either integrative WUE(at the whole plant or crop level)or instantaneous and intrinsic WUE(at the leaf level involving photosynthesis). Crops can engender various morphological,physiological, biochemical and molecular responses to adapt to water deficit stress. Moreover, chemical signaling, involving phytohormones or compatible solutes, is considered the most critical regulator for modulating water use in plants subjected to water deficits [12]. Abscisic acid (ABA), as a key regulator in plant adaptation to abiotic stress,can manipulate stomatal conductance to regulate water use in wheat exposed to terminal drought.Exogenous ABA or a small molecule ABA mimic, a potent activator of multiple members of ABA receptors, decreases water loss and increases drought tolerance in plants [13,14]. Furthermore, compatible solutes such as betaines, proline, and soluble sugars are rapidly accumulated to retain water and maintain regular functioning in plants exposed to abiotic stresses [15]. Exogenous proline induces soluble sugar accumulation and effects photosystem II functioning to alleviate drought stress in Arabidopsis [16],whereas in maize, it increases the contents of antioxidant compounds and enhances their activities under water deficit conditions [17]. In general, compatible solutes protect plants from water stress through cellular osmotic adjustment,detoxification of reactive oxygen species, and protection and stabilization of membrane integrity [18]. These results demonstrate that the application of compatible solutes might be an effective strategy for improving WUE in winter wheat under limited water conditions.
Glycine betaine(GB)is one of the most effective compatible solutes and it protects cellular structures from stresses by maintaining an osmotic balance and stabilizing the quaternary structures of complex proteins [19]. In higher plants, GB is synthesized in chloroplasts from choline in response to abiotic stresses and accumulates at elevated levels in many crops grown under stress conditions [15,20,21]. Meanwhile,tolerant genotypes accumulate a higher concentration of GB than the sensitive genotypes in wheat exposed to stress [22].Moreover,GB applied exogenously or generated by transgenes can regulate the expression of stress-response genes and trigger stress tolerance to abiotic stresses [23,24]. Wheat, one of the major cereal crops in the NCP, is grown in the dry season [9,11]. It is essential to establish a possible watersaving strategy to increase the WUE of winter wheat under water-limited conditions in the NCP region.
The objective of this study was to evaluate the role of exogenously applied GB in improving WUE and drought tolerance in winter wheat under different irrigation conditions. The water consumption, plant water status and WUE were analyzed to assess the effects of GB application in wheat subjected to different irrigation schemes. Furthermore, the effect of GB on grain yield, photosynthesis,antioxidant systems and membrane stability, and hormone concentrations in wheat under different irrigation conditions was also investigated.The results of this study provide not only a new biological water-saving technique but also a scientific basis for the application of water-saving irrigation schemes.
2. Materials and methods
2.1. Site description
The field experiments were conducted during the 2015-2016 and 2016-2017 winter wheat growing seasons at the Wuqiao Experiment Station of China Agricultural University in Cangzhou, Hebei province, China (37°41′N, 116°37′E). This site is located in a warm temperate zone with a semiarid to semihumid monsoonal climate,and the mean annual rainfall is 562 mm (1998-2017), while most of the rainfall occurs during the rainy season from June to September. Moreover,the monthly rainfall during the wheat growing seasons is presented in Fig. 1. The mean annual temperature at the station is 12.9 °C with 201 frost-free days. Winter wheat and summer maize are predominantly grown in this region; from mid-June to early-October is the summer maize growing season and from early-October to mid-June is the winter wheat growing season. Generally, 3-5 irrigations with 75 mm each are applied during the winter wheat growing season,and 0-2 irrigations are carried out in the summer maize growing season[9,25].Moreover,the tillage systems were rotary-tillage for winter wheat and no-tillage for summer maize.Moreover,the soil of the experimental field is a light loam with 17.4 g kg-1organic matter, 1.16 g kg-1total N, 123.4 mg kg-1available K and 41.2 mg kg-1available P in the 0-20 cm tillage layer.
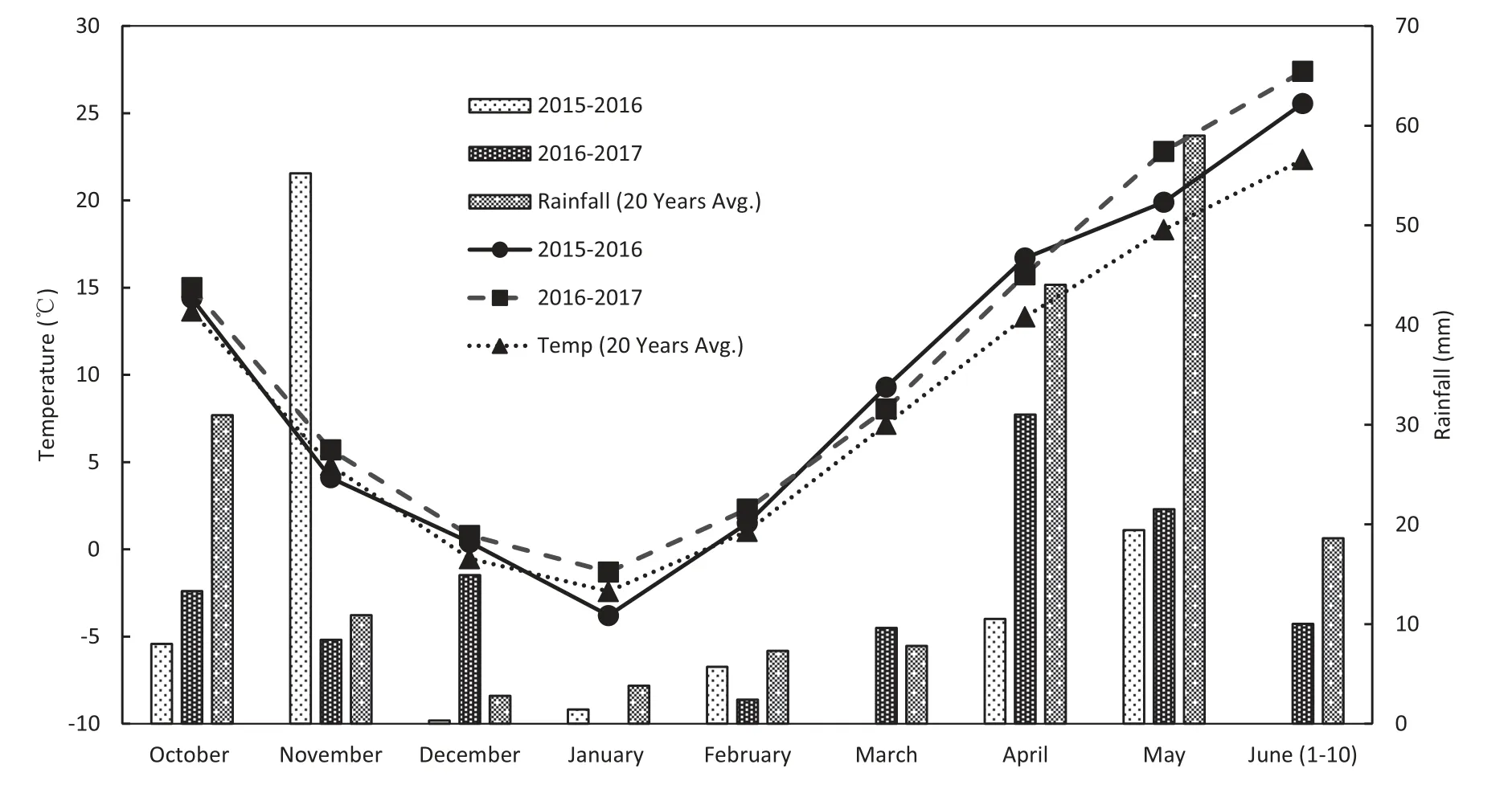
Fig.1-Monthly rainfall distribution(bar)and mean temperature(line)during the winter wheat growing season in 2015-2016 and 2016-2017 and the 20-year average.
2.2. Experimental design and crop management
Two water treatments were applied during the wheat growing season, including limited-irrigation (irrigation with 75 mm of water before sowing, Lim.-Irri.) and conventional irrigation(irrigation with 75 mm of water before sowing, at the elongation stage, and at the anthesis stage each, Con.-Irri),and the irrigation water was appropriately applied in each plot by 4-inch plastic pipe. Furthermore, GB 50 mmol L-1(based on the preliminary field experiment) was applied and deionized water was used as a control. The foliar application of GB was applied at 50 mL m-2during the booting and flowering stages, while the control plots were treated with the same amount of deionized water. The application of GB and deionized water (control) treatments was repeated for two days under different irrigation conditions. The experiment utilized a completely randomized block design with three replicates. Each plot size was 8 m × 10 m, and a 1-meter-wide nonirrigated strip was maintained to avoid the effects of adjacent plots. Jimai-22, a local dominant winter wheat variety in Hebei province,was used as the test material and was sown at a density of 6 × 106plants ha-1(with 15 cm × 15 cm row spacing) on October 8, 2015, and October 10, 2016. In two winter wheat growing seasons, each plot received 185 kg ha-1N, 207 kg ha-1P2O5, and 75 kg ha-1K2O before sowing.Additionally,10% fenoxaprop-P-ethyl at a rate of 500 mL ha-1was used to control weeds during the growing season.
Winter wheat was harvested on June 10,2016,and June 14,2017. All plants from the 1 m2site (seven 1-meter-long rows with 15 cm distances)were harvested at maturity by avoiding border rows from each replicate for the determination of grain yield and biomass. From these samples, plant height, spike length, spikelet spike-1and grain spike-1were determined,and above ground biomass plant-1, grain yield plant-1, 1000-grain weight, and grain yield per m2were determined after drying at 80 °C in a hot air oven (SANYO, Model, MOV-202,Japan) to constant weight, while grain yield m-2was calculated to determine yield at 14% moisture content. Moreover,two hundred uniform spikes within the same flowering time were tagged, and 20 tagged spikes and flag leaves from each treatment were sampled at 6-day intervals from anthesis to maturity. Furthermore, these samples were used for direct assays or frozen in liquid nitrogen and stored at -80 °C for physiological and biochemical analyses.
2.3. Soil water storage and evapotranspiration
A 5 cm diameter soil auger was used to take soil samples from 0 to 200 cm soil depth at increments of 20 cm, and then soil samples were dried at 105 °C to constant weight for determination of soil water content(SWC,g g-1)at pre-sowing and at harvesting stages. After that, soil water storage (SWS, mm)was calculated as described in Xu et al. [26] and the equation was the following:
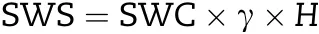
where γ(g cm-3)is the soil bulk density,and H(mm)is the soil thickness.Meanwhile,the difference between SWS at sowing and maturity was used to calculate the reduction of soil water storage (△SWS). Total water consumption (ET) including soil evaporation and plant transpiration during the whole wheat growing season was calculated according to Saleh et al. [27]and the water balance equation was described as following:
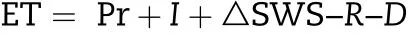
where Pr (mm) is the precipitation and I (mm) is irrigation amount,respectively;R(mm)is the surface runoff,which was assumed as not significant; D (mm) is the water drainage below the crop root zone, which was negligible since soil moisture measurements indicated that drainage at the site was insignificant. Water use efficiency (WUE, kg m-3) was calculated by the equation:WUE = grain yield/ET according to Wang et al. [28].
2.4. Grain weight and grain filling rate
Twenty tagged spikes from each treatment sampled at six days’intervals from flowering to maturity.All the grains from each spike were removed and divided into superior grains and inferior grains. The most basal grains in the middle spikelets(4 to 12 spikelets)from the bottom of a spike were considered as superior grains and the most distal grains in the midst spikelets(4 to 12 spikelets)from the lower part of a spike were considered as inferior grains [29]. The selected grains were used for fresh grain weight and then dried at 80 °C and weighed until a constant weight observed.Moreover,the grain filling process was simulated using Richards’growth equation[30],according to Yang et al.[31]:
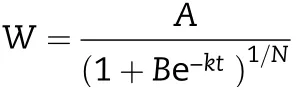
The grain-filling rate(G)was calculated as the derivative of Richards’growth equation.
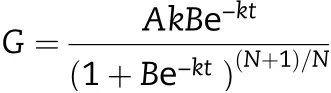
where W is the grain weight (mg); A is the final grain weight(mg); t is the time after anthesis (day); B, k, and N are the coefficients determined by regression. The grain filling properties, including the maximum grain filling rate (Gmax)and the time when Gmaxappeared(Tmax),were calculated with the above equation according to Yang et al. [31]. The active grain-filling period(P)was defined as the duration from 5%of A to 95%of A.And the average grain-filling rate(Gmean)during this period was therefore calculated as 0.9A/P.
2.5. Analysis of CO2 gas exchange, chlorophyll content and relative water content
A portable photosynthesis system with an infrared gas analyzer(LI-6400,LI-COR, Lincoln, USA)was used to measure the photosynthetic gas exchange parameters, including net photosynthetic rate (Pn), stomatal conductance (gs) and transpiration rate (E), of flag leaves from each treatment in the field from 9:00 to 11:00 a.m. at 6, 12, 18, and 24 days after anthesis (DAA). The flag leaf was placed in the chamber, and the instrument was calibrated with a photon flux density of 11,000 μmol m-2s-1. The flow rate through the chamber was set at 500 μmol s-1, and the leaf temperature was approximately 25 °C. The ambient CO2concentration was approximately 400 μmol CO2mol-1air. Flag leaves of randomly selected plants (1 plant from each replicate) from each treatment were used for gas exchange measurements. However, the photosynthetic (instantaneous and intrinsic) WUE was measured by gas exchange analysis.The ratio between Pnand E was presented as an instantaneous WUE, while the intrinsic WUE was recorded as the ratio between Pnand stomatal conductance(i.e.,Pn/gs)according to Wang et al.[32].
The chlorophyll(Chl)content was determined according to the methods of Arnon [33] and Davies [34]. Fresh flag leaves(0.5 g) were chopped into segments of 0.5 cm and extracted with 5 mL acetone (80%) at room temperature overnight. The samples were centrifuged at 10,000 ×g for 20 min, and the absorbance of the supernatant was recorded at 645, 652, 663,and 480 nm on a spectrophotometer (Hitachi, U-2800). The final concentrations of total chlorophyll content were calculated according to Arnon[33]and Davies[34].
Relative water content (RWC) was calculated using flag leaves from randomly selected plants;the leaves were cut into approximately 1 cm discs and weighed to obtain fresh weight(FW).Later,these leaf discs were soaked in distilled water for 4 h, and the turgid weight (TW) was recorded. The leaf discs were then dried in an oven until constant weight at 80 °C to obtain the dry weight (DW). RWC was calculated as RWC% =(FW - DW)/(TW - DW) × 100.
Thirty fully expended flag leaves (10 for each replicate)were detached from GB-treated and control plants under different irrigation conditions, and flag leaf areas were recorded by a leaf area meter (Yaxi-1241, Beijing Yaxin Science Instrument Technology Company Limited, Beijing,China)and represented as cm2.
2.6. Antioxidant enzyme extraction and assays
Antioxidant enzyme extraction was performed as described by Seckin et al.[35]with slight modifications.Flag leaves were collected at 12 days and 18 days after flowering,immediately placed into liquid nitrogen, and then stored at -80 °C until use. Approximately 500 mg of flag leaf sample was rapidly extracted in a prechilled mortar on an ice bath with 5 mL of ice-cold 50 mmol L-1sodium phosphate buffer (pH 7.8) containing 1 mmol L-1EDTA-Na2,1%(w/v)PVPP and 10 mmol L-1magnesium chloride. Samples were centrifuged at 12,000 ×g for 25 min, and the supernatant was used for the determination of antioxidant enzyme activities.Likewise,catalase(CAT,EC 1.11.1.6) activity was estimated by monitoring the initial rate of disappearance of H2O2at 240 nm according to Cakmak and Horst[36].Meanwhile,the activity of superoxide dismutase (SOD, EC 1.15.1.1) was determined using the method of Xue et al.[37].Thus,the peroxidase activity(POD,EC 1.11.1.7)was determined by the guaiacol oxidation method[38].
2.7. Electrolyte leakage and membrane stability index
Leaf membrane damage was estimated by the recording of electrolyte leakage (EL) as reported by Bajji et al. [39] with slight modifications.Plant material(500 mg)was washed with distilled water and then placed in 15 mL test tubes with 10 mL of deionized water and incubated for 4 h at 25 °C. Subsequently, the electrical conductivity (EC) of the samples (R1)was recorded.The samples were then autoclaved at 120 °C for 20 min, the samples were cooled at room temperature, and the final conductivity (R2) was recorded on an EC meter. The EL was defined as follows: EL% = (R1/R2) × 100. However, the membrane stability index (MSI) was calculated using the following equation:MSI% = (1 - R1/R2) × 100.
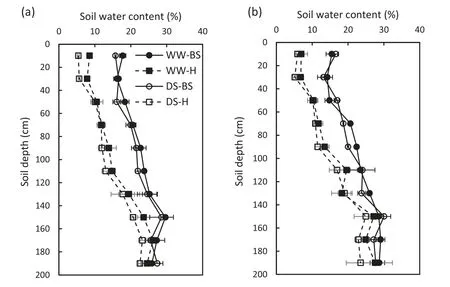
Fig.2- Changes in soil water content in the 0-200 cm soil profile under conventional(Con.-Irri.)and limited(Lim.-Irri.)irrigation conditions before sowing(BS)and at harvest stage(H)during the 2015-2016(a) and 2016-2017(b)growing seasons.Each value represents the mean ± SE (n = 3).
2.8.Determination of hydrogen peroxide and malondialdehyde content
Hydrogen peroxide (H2O2) content was estimated according to Alexieva et al.[40]with slight modifications.Fresh leaf material(500 mg)was homogenized in an ice bath with 5 mL of 0.1%(w/v)trichloroacetic acid (TCA). The homogenate was centrifuged at 4 °C and 10,000 ×g for 10 min,and 0.5 mL of the supernatant was added to 0.5 mL of 10 mmol L-1potassium phosphate buffer(pH 7.0) and 1 mL of 1 mmol L-1potassium iodide (KI) solution.The absorbance of the aliquot was measured at 390 nm. The content of H2O2was calculated by comparison with a standard calibration curve previously made using different concentrations of H2O2and presented as H2O2μmol g-1FW.
Lipid peroxidation was recorded in term of malondialdehyde (MDA) content according to Heath and Packer [41] with slight modifications. Leaf material (500 mg)was homogenized in 5 mL of 0.1% (w/v) TCA solution. The homogenate was centrifuged at 10,000 ×g for 20 min and 1 mL of the supernatant was added to 2 mL of 0.5%(w/v)TBA in 20%TCA. The mixture was incubated in boiling water for 30 min,and the reaction stopped by placing the reaction tubes in an ice bath. Then the samples were centrifuged at 10,000 ×g for 5 min,and the absorbance of the supernatant was recorded at 532 nm and 600 nm. The following formula was used to calculate Malondialdehyde content using its absorption coefficient and expressed as μmol MDA g-1fresh weight.
2.9. Hormone extraction, purification, and quantification
Flag leaf samples were collected from each treatment at 12 and 18 DAA and placed immediately in liquid nitrogen.Furthermore,the protocol for the extraction and purification of indole acetic acid(IAA),zeatin riboside(ZR),and abscisic acid(ABA)were similar to those described by Liu et al.[42]and Yang et al.[31].Moreover,the quantification of IAA,ZR,and ABA was measured by an enzyme-linked immunosorbent assay(ELISA).The recovery rates of IAA,ZR,and ABA were(95.1 ± 1.3)%,(86.1 ± 0.05)%,and(92.2 ± 0.5)%,respectively.Hormone contents were presented on a fresh weight basis(ng g-1FW).
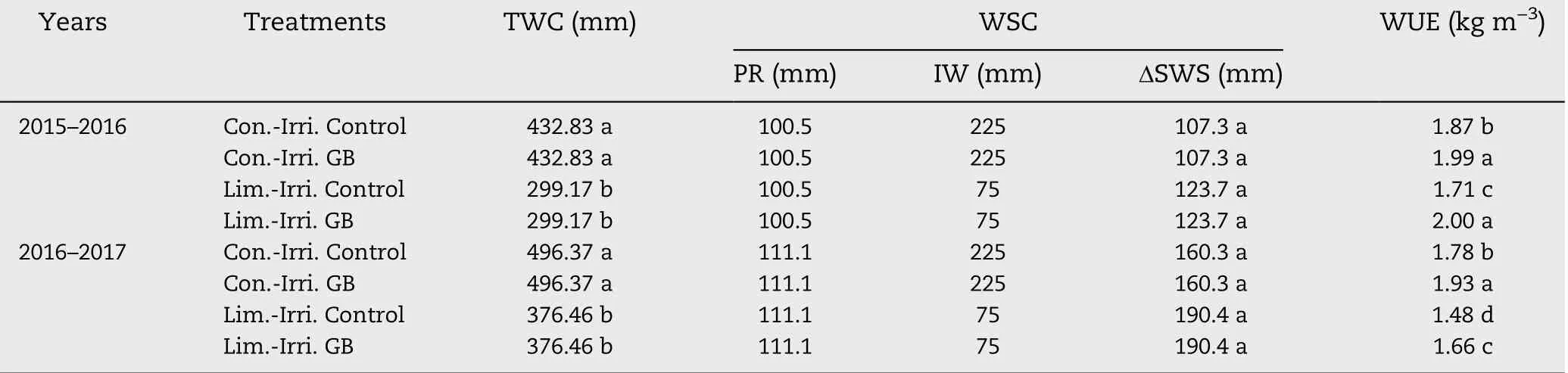
Table 1-Effect of GB on total water consumption (TWC), water source composition (WSC), reduction of soil water storage(ΔSWS), and water use efficiency (WUE) of winter wheat under conventional (Con.-Irri.) and limited (Lim.-Irri.) irrigation conditions during 2015-2016 and 2016-2017 growing seasons.
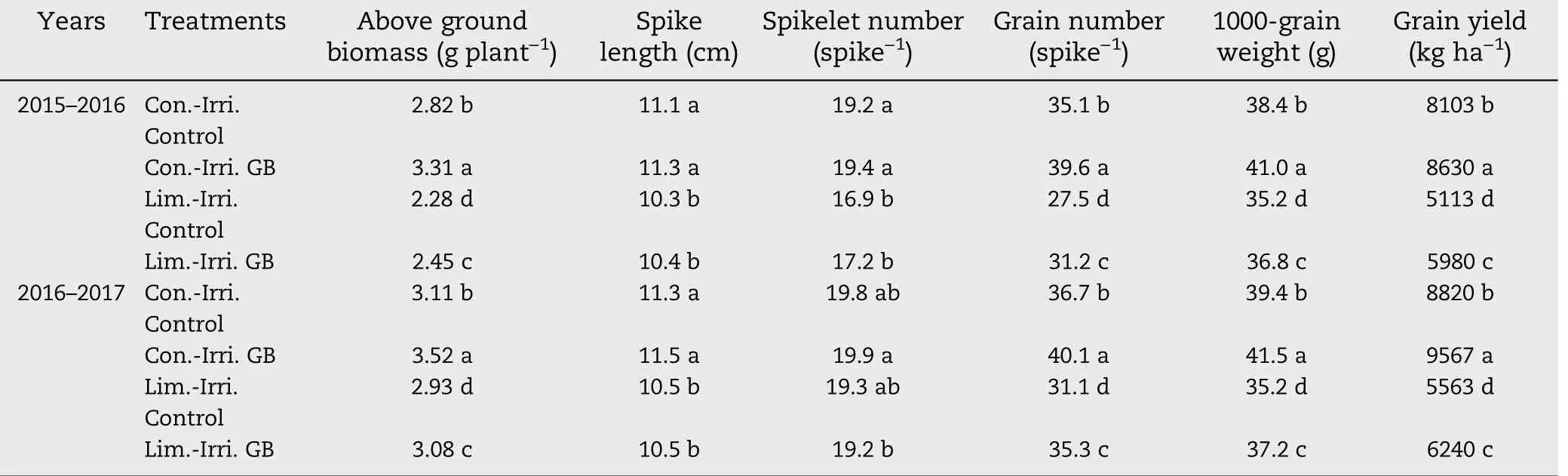
Table 2-Effect of glycine betaine (GB) on above ground biomass, yield components and grain yield under conventional(Con.-Irri.)and limited(Lim.-Irri.)irrigation conditions during 2015-2016 and 2016-2017 growing seasons.
2.10. Statistical analysis
Statistical analysis and data computations were made using Microsoft Excel 2016 (Microsoft Corporation, Redmond, WA,USA)and Statistix 8.1(Analytical Software,Tallahassee,USA).Significantly different treatment means were separated using the Fisher’s least significant difference(LSD at P <0.05)test.
3. Results
3.1.Climatic conditions during the winter wheat growing seasons
The total rainfall in the winter wheat growing season(October-June) was 100.5 mm in 2015-2016 and 111 mm in 2016-2017, which was less than that of the 21-year average rainfall by 45.7% and 40.0%, respectively (Fig. 1). Moreover,rainfall distribution was highly variable within the winter wheat growing seasons.Thus,the rainfall was higher by 11.2%in 2015-2016 and lower by 23.5%in 2016-2017 than that of the 21-year average from the sowing to jointing stage (October-March).The rainfall was reduced by 75.4%in 2015-2016 and by 48.6% in 2016-2017 compared to the 20-year average rainfall from the booting stage until ripening(April-June).Meanwhile,the rainfall during the summer maize growing season could affect soil water storage for winter wheat water consumption.The rainfall during the summer maize growing season(June-October) was 370.1 mm in 2015 and 566.5 mm in 2016, while the 20-year average rainfall was 437.6 mm in this region.
The mean temperature during the winter wheat growing season was 9.8 °C in 2015-2016 and 10.7 °C in 2016-2017,while it was 8.6 °C across 20 years.Moreover,the mean minimum winter temperature was observed in January and was -3.8 °C in 2015-2016 and -1.5 °C in 2016-2017 and -2.4 °C across 20 years.During the grain filling period(May-June),the mean temperature was 22.7 °C in 2016 and 25.1 °C in 2017 and was 20.3 °C across 20 years.
3.2. Water consumption characteristics and water use efficiency
The SWC in the 0-200 cm soil profile was affected by irrigation practices during both growing seasons (Fig. 2). In the2015-2016 growing season,there was no evident difference in SWC between conventional and limited irrigation treatments in the 0-200 cm soil profile before sowing, while the SWC under conventional irrigation maintained higher levels compared to that under limited irrigation at the harvest stage.Similar changes were observed in the 2016-2017 growing season.
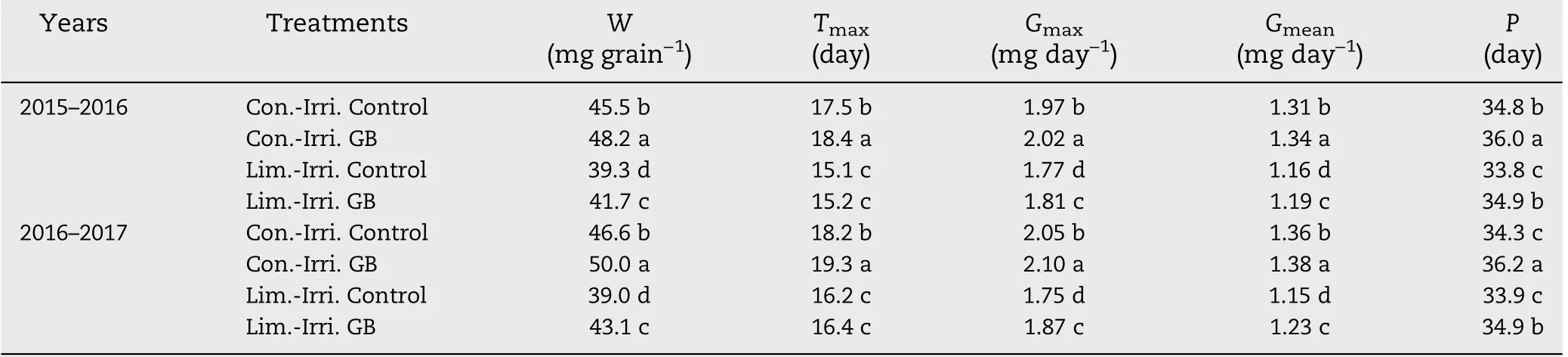
Table 3-Effect of GB on grain filling characteristics of winter wheat under conventional (Con.-Irri.) and limited (Lim.-Irri.)irrigation conditions during 2015-2016 and 2016-2017 growing seasons.
Total water consumption (TWC) was composed of precipitation, irrigation water and reduction of soil water storage(ΔSWS) during the winter wheat growing season, and limited irrigation plots had less TWC than that under conventional irrigation in both growing seasons (Table 1). Conversely, the ΔSWS in the 0-200 cm soil profile layer was higher under limited irrigation compared to conventional irrigation. Moreover,the overall integrative WUE under limited irrigation was less than that under conventional irrigation. Although GB application could not change the TWC, GB significantly improved integrative WUE compared to the control under limited or conventional irrigation during both growing seasons.
3.3. Shoot biomass, yield and yield-related components, and grain filling rate
Conventional irrigation promoted biomass accumulation in winter wheat, and a marked increase was recorded in aboveground biomass in both growing seasons (Table 2). GB application increased aboveground biomass accumulation by 13.2% to 17.4% compared to the control under conventional irrigation, while under limited irrigation, aboveground biomass accumulation increased by 7.5% to 11.9% during 2015-2016 and 2016-2017, respectively. Moreover, conventional irrigation significantly increased spike length and spikelet number per spike compared to limited irrigation, but there was no significant difference in spike length and spikelet number per spike between GB-treated and control plants under limited or conventional irrigation during both growing seasons.
Conventional irrigation significantly affected grain numbers per spike, 1000-grain weight and grain yield in both growing seasons, while plants under conventional irrigation had higher values than those under limited irrigation (Table 2).GB application resulted in higher grain numbers spike-1by 12.8% under conventional irrigation and by 13.5% under limited irrigation compared to the control in 2015-2016. Meanwhile, GB significantly increased the 1000-grain weight and grain yield under limited or conventional irrigation in 2015-2016. Similar results were observed in the 2016-2017 growing season.
The grain filling characteristics of winter wheat were markedly modified by irrigation and GB application treatments (Table 3, Fig. 3). Plants grown under conventional irrigation had a higher grain weight, maximum filling rate,mean filling rate and longer grain filling duration compared to limited irrigation in both growing seasons. GB significantly increased the maximum and mean filling rate, active grain filling duration, and grain weight compared with the control under conventional and limited irrigation during both growing seasons.
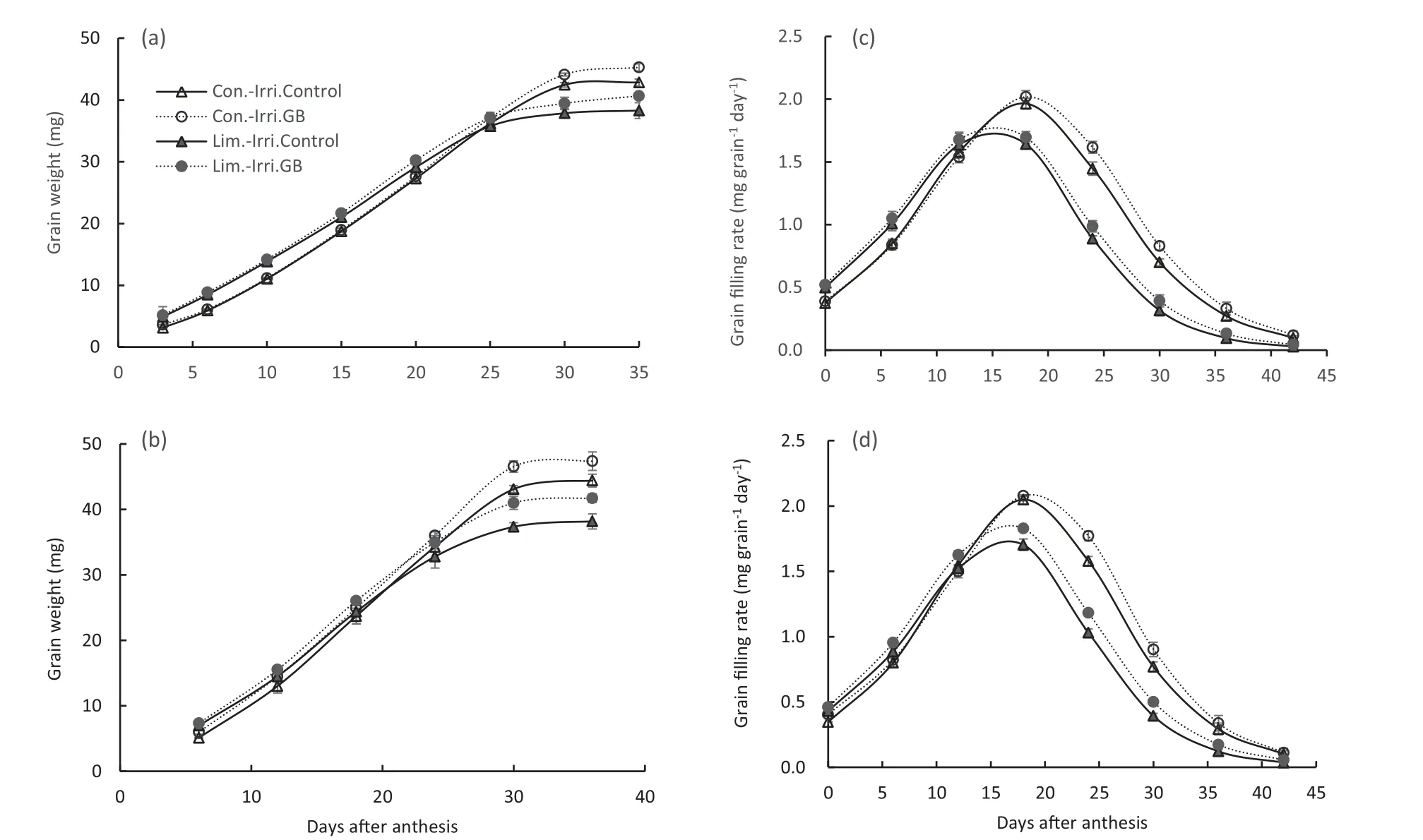
Fig.3-Effect of GB on grain weight(a,b)and grain filling rate(c,d)of winter wheat under conventional(Con.-Irri.)and limited(Lim.-Irri.) irrigation conditions during 2015-2016(a,c) and 2016-2017(b, d) growing seasons.Each value represents the mean ± SE (n = 3).
3.4. Flag leaf area, chlorophyll content and photosynthetic parameters
Conventional irrigation produced a larger flag leaf area compared to limited irrigation (Fig. 4). The flag leaf area of GB-treated plants was higher by 5.5% under conventional irrigation and by 7.8% under limited irrigation compared to the control plants. Plants under conventional irrigation showed a slower pigment degradation rate and had higher chlorophyll content than those under limited irrigation from 6 to 24 DAA (Fig. 4). There was no significant difference in chlorophyll content between GB and control treatments under conventional irrigation. However, GB-treated plants had higher chlorophyll contents than control plants from 6 to 24 DAA under limited irrigation.
Limited irrigation significantly decreased the net photosynthesis rate (Pn), stomatal conductance (gs), and transpiration rate (E) compared to conventional irrigation in both growing seasons (Fig. 5). Moreover, GB application increased Pnby 22.5% and 23.1% under conventional irrigation and by 32.0% and 38.4% under limited irrigation compared to the control at 12 and 18 DAA, respectively, in the 2015-2016 growing season. Thus, GB-treated plants also had higher Evalues by 6.4% and 10.7% and gsvalues by 8.8% and 15%compared to control plants under limited irrigation at 12 and 18 DAA, respectively. Correspondingly, similar patterns were observed in the 2016-2017 winter wheat growing season.Conventional irrigation resulted in higher leaf RWC than limited irrigation at 12 and 18 DAA in both growing seasons(Fig.6).Meanwhile,GB-treated plants maintained higher RWC levels than control plants under conventional and limited irrigation.
3.5. Photosynthetic water use efficiency
Photosynthetic WUEs were categorized as instantaneous and intrinsic WUEs. Different irrigation schemes could significantly affect WUEs at both integrative and instantaneous or intrinsic levels.The decrease in Pnalso reflected a reduction in the WUE of flag leaves under limited irrigation, and the WUE of flag leaves from limited irrigation dropped more sharply than those of flag leaves from conventional irrigation treatments (Fig. 7). However, GB improved flag leaf net photosynthesis under both conventional and limited irrigation, and higher integrative and instantaneous or intrinsic WUEs were obtained from GB-treated plants compared to control plants under both irrigation conditions.
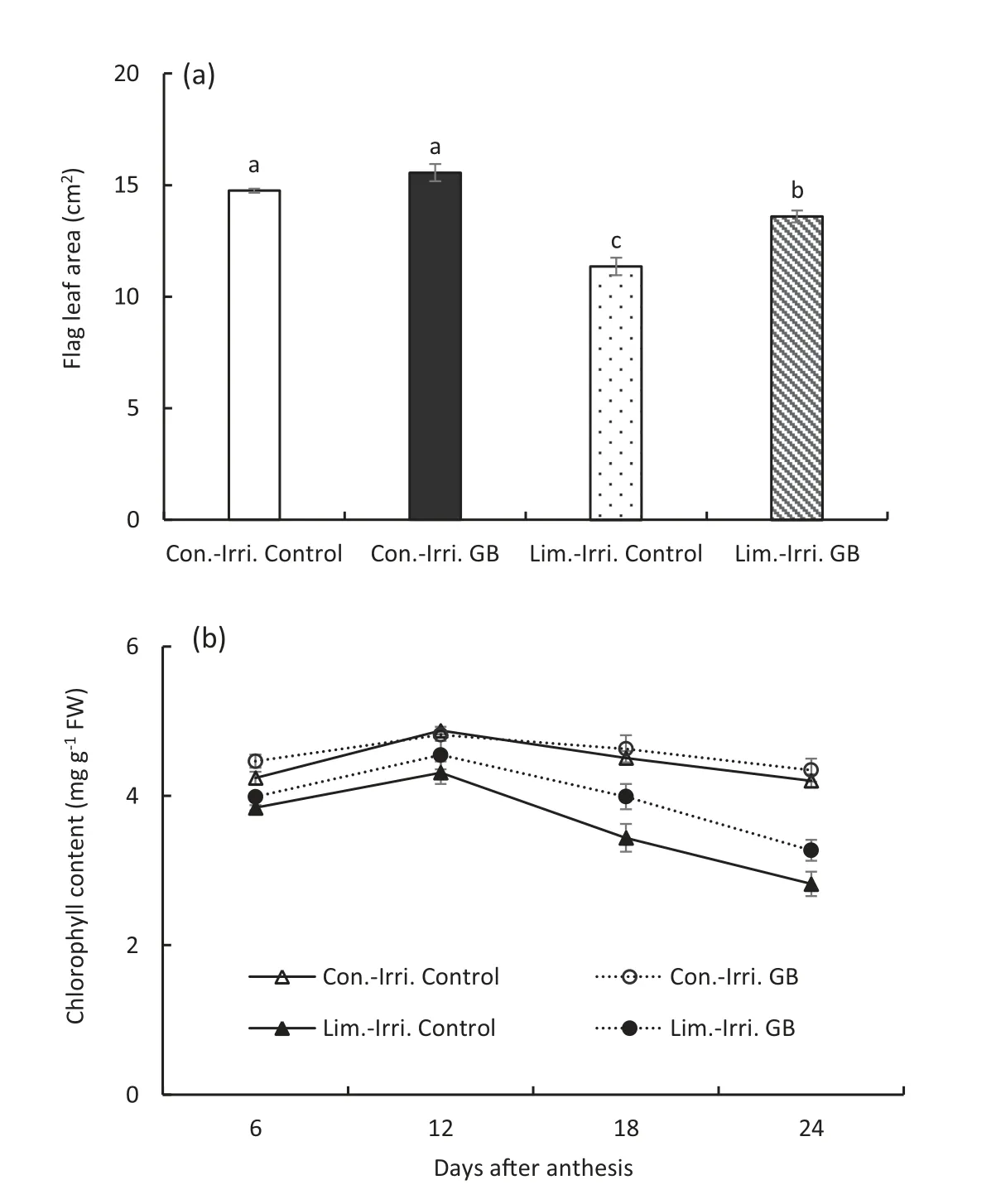
Fig.4-Changes of leaf area(a)and chlorophyll content(b)in the flag leaves of GB-treated and control plants under conventional(Con.-Irri.)and limited(Lim.-Irri.)irrigation conditions during the 2016-2017 growing season.Each value represents the mean ± SE(n = 3).Error bars in Fig.4-a showing the same letter are not significantly different at P ≤0.05 as determined by the LSD test.
3.6. Hormone content
Plant hormones are the key regulators of the grain development process under normal as well as under stress conditions.The IAA and ZR contents in flag leaves decreased,while the ABA content increased significantly from 12 to 18 DAA during the grain filling stage under different irrigation treatments (Fig. 8). Moreover, IAA and ZR contents in GBtreated plants were significantly higher than those of control plants at 12 and 18 DAA under both conventional and limited irrigation schemes. However, limited irrigation significantly increased ABA content compared to conventional irrigation.Thus, GB-treated plants exhibited lower ABA content at 12 and 18 DAA than the control plants under conventional and limited irrigation conditions.
3.7. Hydrogen peroxide, lipid peroxidation, electrolyte leakage and antioxidant enzymes
The MDA and H2O2contents in leaves were higher under limited irrigation than under conventional irrigation(Fig.9-a,b). Thus, there was no difference in MDA and H2O2values among treatments under conventional irrigation. Under limited irrigation, the MDA and H2O2values in GB-treated plants were lower by 14.7%and 15.9%at 12 DAA and by 16.6%and 11.2%at 18 DAA than those of control plants,respectively.

Fig.5- Effect of GB on net photosynthetic rate(Pn) (a,d), stomatal conductance(gs)(b,e), and transpiration rate(E) (c,f)of flag leaves under conventional(Con.-Irri.)and limited(Lim.-Irri.)irrigation conditions during the 2015-2016(a, b and c) and 2016-2017(d, e and f)growing seasons. Each value represents the mean ± SE(n = 3).
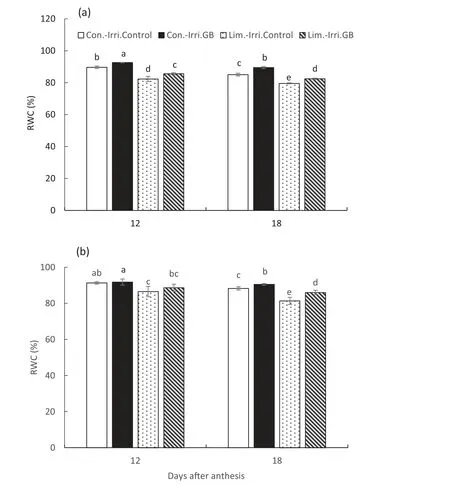
Fig.6- Effect of GB on flag leaf relative water content(RWC)under conventional(Con.-Irri.)and limited(Lim.-Irri.)irrigation conditions during the 2015-2016(a)and 2016-2017(b) growing seasons.Each value represents the mean ± SE(n = 3). Bars showing the same letter are not significantly different at P ≤0.05 as determined by the LSD test.
Limited irrigation significantly raised leaf EL values compared to conventional irrigation,while EL values in GB-treated plants were respectively lower by 11.2%and 17.9%than those of control plants at 12 and 18 DAA under limited irrigation(Fig. 9-c). Conversely, limited irrigation markedly decreased the membrane stability index (MSI) values (Fig. 9-d), but GB increased MSI values by 9.5% and 26.4% compared to the control at 12 and 18 DAA under limited irrigation,respectively.
Plants under conventional irrigation maintained lower levels of SOD, POD, and CAT activities than those under limited irrigation (Fig. 10). Moreover, there was no difference in SOD, POD, and CAT activities between GB and control treatments under conventional irrigation. However, GBtreated plants had higher SOD, POD, and CAT activities than control plants at 12 and 18 DAA under limited irrigation.
4. Discussion
Water productivity potential is improved by using droughttolerant varieties, efficient irrigation practices and appropriate agronomic techniques for achieving higher grain yield with low water consumption in crop production [11].Limited irrigation significantly reduced the spikelet number spike-1, grain spike-1, and 1000-grain weight compared to conventional irrigation (Table 2). Moreover, lower integrative and photosynthetic WUEs were observed under limited irrigation compared to conventional irrigation during both growing seasons(Table 1, Fig. 7). These results indicated that limited irrigation significantly reduced the photosynthetic capacity for grain yield reduction by 36.9% compared to conventional irrigation during 2015-2016, and a similar pattern was recorded in 2016-2017. It is generally accepted that WUE is positively linked to yield and drought tolerance in numerous water-limited cases [9]. Moreover, the WUE can be increased by increasing the net photosynthetic rate or by reducing the total irrigation water input and frequency of irrigation in crop production [3,9,43]. In addition, photosynthetic (instantaneous and intrinsic) WUE is generally measured at the leaf level to evaluate crop WUE. Conventional irrigation increased both the photosynthetic rate (Pn) and transpiration rate (E) and resulted in greater instantaneous WUE compared to limited irrigation.GB treatment resulted in higher integrative (grain yield/crop evapotranspiration) and photosynthetic (instantaneous and intrinsic) WUEs under both irrigation schemes. GB could improve integrative and photosynthetic WUE by reducing the destructive effects of water stress and the higher net photosynthetic rate, which manifested as greater biomass accumulation [24,43,44].
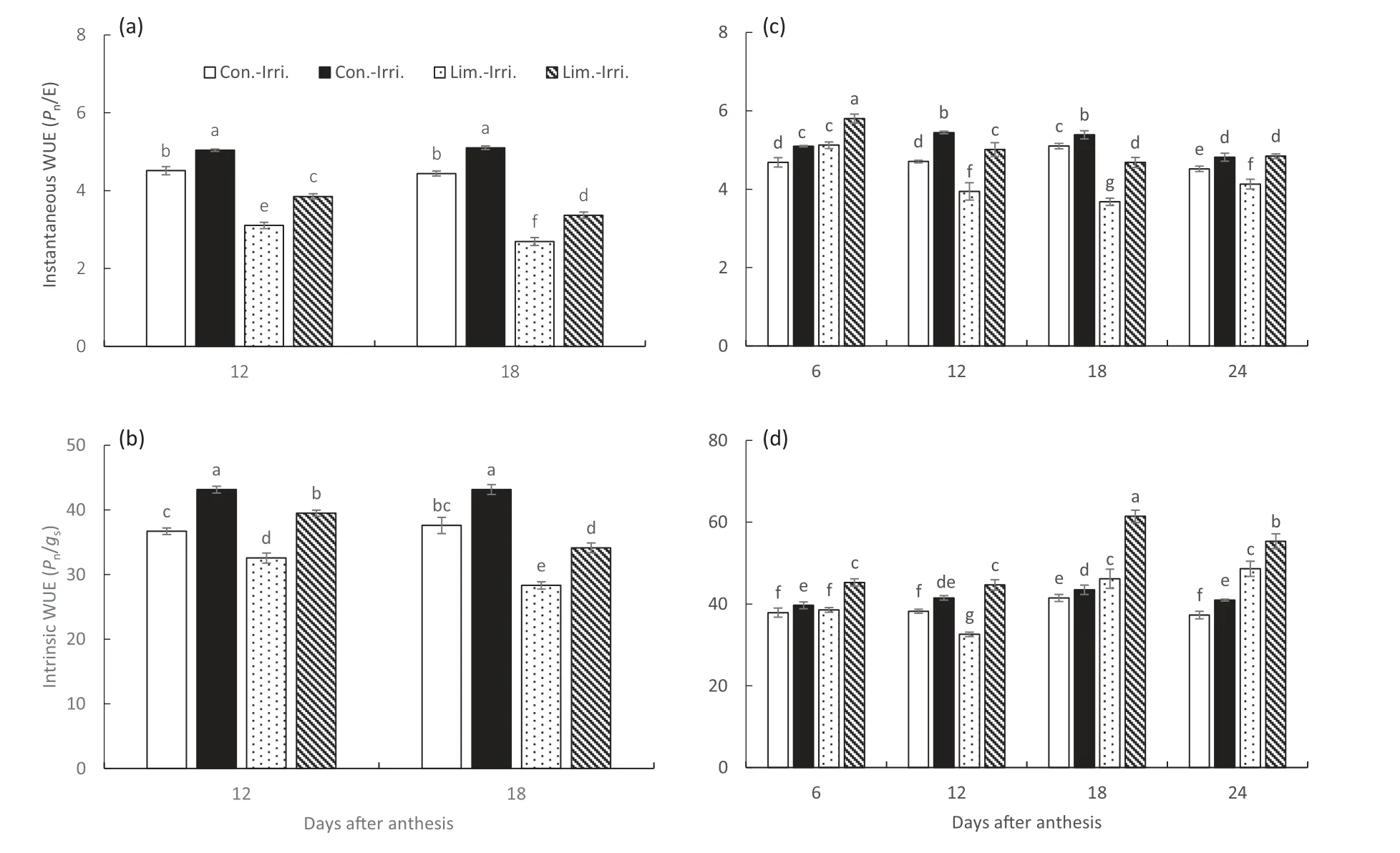
Fig.7-Effect of GB on instantaneous WUE(a,c)and intrinsic WUE(b,d)of winter wheat flag leaves under conventional(Con.-Irri.)and limited(Lim.-Irri.)irrigation conditions during the 2015-2016(a,b)and 2016-2017(c,d)growing seasons.Each value represents the mean ± SE(n = 3).Bars showing the same letter are not significantly different at P ≤0.05 as determined by the LSD test.
Limited irrigation significantly increased soil water depletion, which is beneficial for regulating the availability of soil water from the accessible rainwater in the soil during the summer season in the NCP.Although water deficit negatively affects wheat performance regardless of the growth stage,the crop is more sensitive during the flowering and grain filling phases, resulting in substantial yield losses [45]. Exogenous application of GB can improve plant biomass accumulation and yield traits in different crops subjected to water stress[15,22,24]. In the present study, GB application significantly increased the grain number and grain weight of spikes,which led to higher grain yield compared to the control under limited and conventional irrigation conditions (Table 2).Meanwhile, GB application improved the maximum and mean filling rate and delayed the active grain filling duration,thus increasing the grain weight (Fig. 3, Table 3). Grain yield culminates in the process of grain filling, which is closely related to flag leaf functionalities [46], and many studies declared that regulation of leaf senescence is most important factor for yield stability under suboptimal conditions[47].Stay green is the most vital strategy of crop plants to benefit seed set and seed development under drought stress [48].Generally, 30%-50% of the assimilates are contributed by flag leaf photosynthesis during grain filling in wheat, and maintaining flag leaf photosynthesis can produce better yields[49-53]. In the present study, limited irrigation significantly decreased the chlorophyll content, Pn, E, and gscompared to conventional irrigation in both growing seasons. Meanwhile,GB-treated plants had higher chlorophyll contents,Pn,gs,and E values than the counterpart plants under limited irrigation(Figs. 4 and 5). The higher net photosynthetic rate and stomatal conductance during grain filling are closely associated with better grain yield[54].Improved gsalong with higher RWC in GB-treated plants, might be the reason for the maintenance of the better net photosynthetic rate under limited irrigation conditions and the mitigation of adverse effects of water deficit stress.Exogenous application of GB can increase the proportion of bound water in the cell due to its hydrophilic features, which promotes turgor pressure in the stomatal guard cells and leads to increased stomatal conductance [54]. Here, GB-treated plants had higher chlorophyll contents and a lower pigment degradation rate under limited irrigation (Fig. 4), which suggests that GB application could delay leaf senescence and enhance the net photosynthetic rate under limited irrigation conditions.
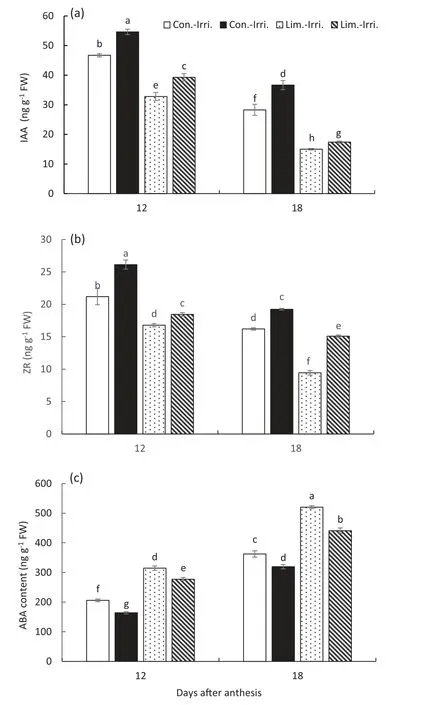
Fig.8- GB regulated the accumulation of zeatin ribosides(ZR,a),indole acetic acid(IAA,b), and abscisic acid(ABA,c)in flag leaves under conventional(Con.-Irri.)and limited(Lim.-Irri.)irrigation conditions during the 2016-2017 growing season.Each value represents the mean ± SE(n = 3).Bars showing the same letter are not significantly different at P ≤0.05 as determined by the LSD test.
Crop growth is mainly associated with hormonal balance under stress conditions [55,56], and growth decline can be minimized by external application of growth hormones or by gene manipulation [57-59]. GB-treated plants had higher IAA and ZR levels in flag leaves than the control plants under limited irrigation conditions (Fig. 8), and high IAA and ZR could maintain greater biomass and yield by modulating leaf functions. In general, ABA-induced stomatal closure is considered the main reason for the inhibition of photosynthesis in plants subjected to environmental stresses,including water stress [60,61]. In the present study, limited irrigation significantly promoted ABA accumulation in flag leaves compared to conventional irrigation (Fig. 8). However, GB application significantly decreased the ABA content in flag leaves compared to the control under limited irrigation, which might be due to an increase in the proportion of bound water in the cell for reducing the adverse effects of oxidative stress[54,62].

Fig.9-Effect of GB on malonyldialdehyde(MDA)content(a),hydrogen peroxide(H2O2)content(b),electrolyte leakage(c),and membrane stability index(d)in flag leaves under conventional(Con.-Irri.)and limited(Lim.-Irri.)irrigation conditions during the 2016-2017 growing season.Each value represents the mean ± SE(n = 3).Bars showing the same letter are not significantly different at P ≤0.05 as determined by the LSD test.
High electrolyte leakage and the accumulation of H2O2and MDA are generally considered symptoms of stress-induced damage [15,18]. The membrane integrity is altered by the stresses, which is always accompanied by an increase in electrolyte leakage from the cell [39]. In this study, limited irrigation markedly decreased membrane stability, as indicated by the increased electrolyte leakage and the content of H2O2and MDA in flag leaves(Fig.9). However,GB application alleviated adverse effects of water deficit by improving membrane stability, as evidenced by the reduced electrolyte leakage and lowered leaf H2O2and MDA contents. These results indicated that GB application could increase membrane stability by regulating ROS homeostasis and lower lipid peroxidation to protect cell organelles from the destructive effects of water stress. Antioxidant enzymes such as SOD,POD,and CAT are activated against oxidative damage[15,19].Moreover,GB application enhanced the activities of SOD,POD,and CAT compared to the control under limited irrigation(Fig.10). These results suggested that GB application could regulate the activities of antioxidant enzymes to modulate the accumulation of H2O2and MDA to maintain cell membrane stability. Similar results were also observed in wheat,maize,and rice[15,20,24].
In brief,GB application increased IAA and ZR contents and cell antioxidant enzyme activities but decreased ABA content in flag leaves. The GB-altered hormone levels and ROS removal activity reduced the damage to the cellular membrane system integrity by water stress and delayed leaf senescence, which benefited from the maintenance of the photosynthetic capability, grain filling rate, and finally increased WUE under limited irrigation conditions. Our results support the possibility of improving crop WUE and grain yield by exogenous application of GB, and GB enhanced the stress tolerance of crop plants by regulating hormone homeostasis and maintaining cellular membrane stability to improve gas exchange characteristics.
5. Conclusions
Limited irrigation induced oxidative stress and hampered plant growth and development resulting in substantial yield losses in winter wheat. Exogenous application of GB at booting and anthesis stages could enhance antioxidant(CAT,SOD,and POD)activities and reduce MDA and H2O2production to maintain higher RWC and membrane stability under limited irrigation.Meanwhile,GB-treated plants had higher IAA and ZR levels and lower ABA content in flag leaves,which led to higher chlorophyll content and photosynthetic capability for better integrative and instantaneous WUEs under conventional and limited irrigation conditions. Moreover, GB application enhanced the grain filling rate and delayed the active grain filling period for high grain weight under conventional and limited irrigation conditions.Taken together, GB altered hormone levels and antioxidant activity to maintain cellular membrane system integrity and photosynthetic capability to improve crop WUE and grain yield under conventional and limited irrigation conditions.
Acknowledgments
This research was supported by National Key Research and Development Program of China (2017YFD0300410), Special Fund for Agro-scientific Research in the Public Interest(201503121-11) and Introduction of International Advanced Agricultural Science and Technology Program of Ministry of Agriculture of the People’s Republic of China(2011-G19).
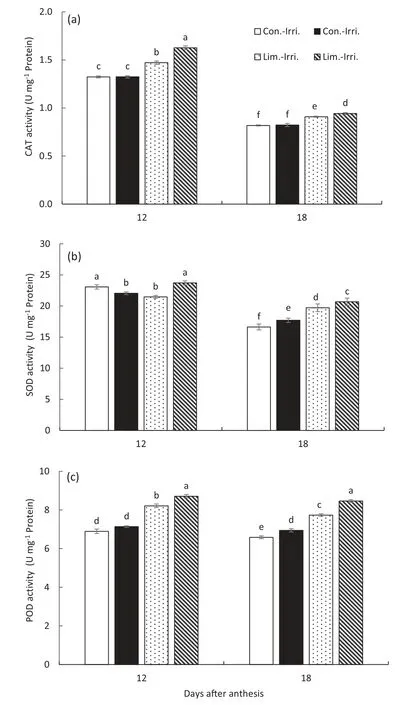
Fig.10-Effect of GB on activities of catalase(CAT,a),superoxide dismutase(SOD,b)and peroxidase(POD,and c)in flag leaves of GB-treated plants under conventional(Con.-Irri.)and limited(Lim.-Irri.)irrigation conditions during the 2016-2017 growing season.Each value represents the mean ± SE(n = 3).Bars showing the same letter are not significantly different at P ≤0.05 as determined by the LSD test.
- The Crop Journal的其它文章
- OstMAPKKK5,a truncated mitogen-activated protein kinase kinase kinase 5,positively regulates plant height and yield in rice
- Mapping QTL affecting the vertical distribution and seed set of soybean[Glycine max(L.) Merr.]pods
- Identifying key traits in high-yielding rice cultivars for adaptability to both temperate and tropical environments
- Deep genotyping of the gene GmSNAP facilitates pyramiding resistance to cyst nematode in soybean
- Molecular mapping and candidate gene analysis of the semi-dominant gene Vestigial glume1 in maize
- Draft genome sequence of a less-known wild Vigna: Beach pea (V. marina cv. ANBp-14-03)

