Isotherm and kinetic studies on the adsorption of humic acid molecular size fractions onto clay minerals
Mohamed E.A.El-Sayed·Moustafa M.R.Khalaf·James A.Rice
Abstract Humic acid(HA)can adsorb onto mineral surfaces,modifying the physicochemical properties of the mineral.Therefore,understanding the sorption behavior of HA onto mineral surfaces is of particular interest,since the fate and transport of many organic and inorganic contaminants are highly correlated to HA adsorbed onto clay surfaces.Due to the extreme heterogeneity of HA,the extracted IHSS Leonardite humic acid(LHA)used in this work was fractionated using an ultrafiltration technique(UF)into different molecular size fractions(Fr1,>0.2 μm; Fr2, 0.2 μm–300,000 daltons; Fr3,300,000–50,000 daltons;Fr4,50,000–10,000 daltons;Fr5,10,000–1000 daltons). Equilibrium and the kinetics of LHA and fraction adsorption onto kaolinite and montmorillonite were investigated.The results demonstrated that the maximum adsorption capacity of LHA,Fr1,Fr2,Fr3,Fr4,and Fr5 was 5.99,13.69,10.29,7.02,5.98,and 5.09 on kaolinite while it was 8.29,22.62,13.17,8.91,8.62,and 5.69 on montmorillonite, respectively. The adsorption equilibrium data showed that the adsorption behavior of LHA and its fractions could be described more practically by the Langmuir model than the Freundlich model.The rate of humic acid fraction adsorption onto clays increased with decreasing molecular size fraction and increasing carboxylic group content.Pseudo-first-and second-order models were used to assess the kinetic data and the rate constants.The results explained that LHA and its fractions adsorption on clay minerals conformed more to pseudosecond-order.
Keywords Kaolinite·Montmorillonite·Leonardite humic acid·Humic acid fractions·Kinetics·Equilibrium
1 Introduction
Sorption of humic substances(HSs)to clays is a fundamental process in the environment.This process modifies surface properties and the reactivity of clay minerals(Al-Essa and Khalili 2018).Understanding the interactions of HSs and clay minerals is important for modeling the geochemical fate and transport of nutrients and contaminants in soil and water(Zaouri 2013).
Humic acid is a major fraction of HSs and generally contains both hydrophobic and hydrophilic moieties,as well as many reactive functional groups(e.g.–COOH,–C=O and–OH)in the component molecules.The existence of carboxylic and phenolic groups results in HA carrying a predominantly negative charge in aqueous solutions under normal environmental conditions (Maghoodloo et al.2011).
The clay minerals kaolinite and montmorillonite are highly abundant and composed of two basic building blocks containing silicon (Si2O5-2) and aluminum(Al(OH)6-3).Kaolinite and montmorillonite are layers of tetrahedral Si and octahedral Al sheets.Furthermore,the Si:Al ratio of kaolinite as nonexpanding clay is 1:1 while for montmorillonite,the ratio as expanding clay is 2:1.Clay minerals play the role of a natural scavenger of metals and organic matter (OM). Clay minerals have a high specific surface area,chemical and mechanical stability,layered structure,and high cation exchange capacity.Thus,the clay minerals are excellent materials for adsorption(Bhattacharyya and Gupta 2008). Moreover, nano-clay minerals have some important features such as having significantly wider surface areas than other particles,and they can be combined with different chemical groups to increase their efficacy (Derakhshani and Naghizadeh 2018).
Several authors reported different binding mechanisms between clay minerals and HA,such as cation bridging,ligand exchange,and van der Waals forces(Murphy and Zachara 1995).Polydispersity and polyelectrolytic properties of HA also play a major role in the adsorption process onto clay minerals.Thence,to better understand the interaction mechanism and due to the chemical heterogeneity and polydispersity of HA,UF has been used to reduce the chemical heterogeneity of HA by using suitable membrane filters.UF is a reasonably simple method for fractionation of HA into different molecular size fractions(Khalaf 2003).
The kinetics of LHA and its fractions adsorption on clay minerals are a significant factor in determining HA behavior in soil and aquatic systems and gives information about the adsorption capacity and its mechanisms.Accordingly,the objective of this study was to explore and understand the sorption mechanisms of HAs on clay minerals through kinetics and fractions sorption experiments.
2 Materials and methods
2.1 Clay minerals
Kaolinite(KGa-2)and montmorillonite(SWy-2),two clay minerals commonly found in soils,were purchased from the Source Clay Minerals Repository,University of Missouri-Columbia,Missouri.Clay minerals were pretreated as described elsewhere(Shang et al.2001).25 g of clay sample was placed in a 500 mL Erlenmeyer flask,and then 250 mL of pH 5 acetate buffer were added to remove carbonates.The mixture was heated in a water bath(90°C,2 h)before centrifugation.The supernatant was discarded.Hydrogen peroxide(30%)was added to the clay samples and the sample was digested(12 h,90°C,water bath)to oxidize organic matter.After organic matter oxidation,the sample was suspended in citric bicarbonate buffer(pH 8.3)at 80°C and an appropriate amount of dithionite powder was added to remove free iron oxides.Finally,the sample was transferred into a 1 L plastic bottle and the clay was shaken with 1 mol/L NaCl(6 h)before centrifugation.Then the supernatant was discarded.The salt washing step was repeated once.The Na-saturated clay was washed once with distilled and deionized water and dialyzed against water until it was free of chloride(silver nitrate test).Clay minerals were dried in an oven at 100°C.
BET surface area of clay minerals was obtained from nitrogen gas adsorption/desorption isotherms at 77 K,using a Micromeritics ASAP 2000 analyzer. Prior to measurements,all samples were degassed to 0.1 Pa and 150°C for 2 h.Furthermore,particle size distribution was done by x-ray diffraction technique,and elemental composition was done by scanning electron microscopy/energy-dispersive X-ray analysis(SEM/EDAX)techniques.
2.1.1 Preparation of clay mineral suspensions
Clay mineral suspension(kaolinite and montmorillonite)5 g/L stock solutions were prepared in order to obtain consistent solid concentrations for the equilibrium adsorption experiments.These suspensions were prepared in 0.01 mol/L of NaCl for LHA and fraction adsorption isotherms at pH 6(Khalaf et al.2009).
2.2 Humic acid and fractions
The LHA was isolated from the IHSS Leonardite by alkali extraction(Swift 1991)and fractionated into five humic acid molecular size fractions using the UF technique as described below.
2.2.1 Fractionation of LHA by ultrafiltration
The LHA sample was fractionated into five size-fractions using a cross-flow ultrafiltration technique(MinitanTM,Millipore).Different cellulose membranes(Millipore)with nominal molecular weight cutoffs of 1,10,50,300 kDa and 0.2 μm were used.An aqueous LHA solution at a concentration of 1.5 g/L was prepared by dissolving an appropriate amount of the LHA in NaOH.In brief,the alkaline LHA solution was microfiltered using a 0.2 μm Nylon filter and an Amicon ultrafiltration stirred cell(model 8050)under a pressure of 2.5 bar using argon gas.The retentate was washed with small portions of Millipore water.The washed retentate(Fr1)was carefully pipetted out of the UF cell.The filtrate obtained was then fractionated using an Amicon UF cell(model 8400)and a series of Amicon membranes of successively smaller pore size to obtain HA fractions with different nominal molecular sizes which were classified as Fr1 >0.2 μm,Fr2,0.2 μm–300 kDa;Fr3,300–50 kDa;Fr4,50–10 kDa,and Fr5,10–1 kDa.All separations were done under the same conditions.After each run,the membrane was removed,rinsed with distilled water, and stored overnight in a refrigerator at 4°C.The complete separation process was repeated at least three times to assess the reproducibility of the fractionation technique.The recovery percentage of the whole LHA fraction process was calculated at 92%.The separated LHA fractions were freeze-dried,weighed,and stored in the dark at 3°C.LHA and five fractions were characterized by UV–visible and13C DP solid state NMR spectroscopy.
A carbon-type distribution of LHA and the fractions were determined by solid state13C DP-MAS NMR using a Bruker ASX300 spectrometer(rotation speed=13 kHz;recycle delays were determined for each sample and were between 3 and 15)(Li et al.2004).
LHA and the five fractions were characterized using a Milton Roy UV–visible spectrometer.The degree of aromaticity was calculated by determining the ratio of absorbance of LHA and its fractions at 465 and 665 nm(E4/E6 ratio)(Purmalis and Klavins 2013;Helal et al.2011).Aqueous solutions of LHA and each fraction were prepared in the same manner as those used in the adsorption experiments.
2.2.2 Preparation of LHA and fraction solutions
Stock solutions of LHA and fractions were prepared by dissolving LHA in an aqueous solution of NaOH(0.5 mol/L)with shaking for 1 h.The adjustment pH to 6 was done by adding HCl or NaOH.Amounts of NaCl were added to LHA and fraction solutions to adjust to the desired ionic strength of 0.01 mol/L.Final LHA or fraction concentrations were 1 g/L.
2.3 Adsorption of LHA and fractions onto clays
A fraction of well-mixed clay suspension at the desired pH and ionic strength was pipetted into a series of LHA or fraction solutions at varying concentrations(0.083–0.75 g/L)to give the final volume of 30 mL in 50 mL polyethylene centrifugation tubes.The pH value checked up through the experimental lifetime.The suspensions were shaken for 24 h on a horizontal shaker(Lab line Instruments, Melrose Park, IL, USA) to reach equilibrium(Shaker et al.2012).The final(kaolinite or montmorillonite)concentrations for all adsorption experiments were 2.5 g/L.Preliminary experiments verified that,after 4 h,no measurable change occurred in the adsorbed amounts.Each sample was centrifuged for 5 min at 20,000 rpm(Damon/IEC Division Model High-Speed Centrifuge).The amount of the adsorbed LHA or fractions was calculated from the difference between the total added LHA or fraction concentration and the LHA or fraction concentration in the supernatant by the respective total organic carbon contents.Total organic carbon contents were determined with a Shimadzu TOC-VSCN with a solid-sampling module SSM 5000.
The amounts of LHA or fractions adsorbed were calculated from the mass balance equation for each isotherm bottle using Eq.(1):

where V is the volume of solution used in the adsorption experiment(L),Ci and Ce are the initial and the equilibrium concentrations of LHA and fraction solutions(mg/L),respectively,and m is the dry weight of the adsorbent(g)(Komy et al.2014).
Two models of Langmuir(1916)and Freundlich(1906)in their related linearized expressions have been used as Eqs.(2)and(3)respectively.

where Ceis the concentration of adsorbate (mg/L) at equilibrium,qethe amount of adsorbate at equilibrium(mg/g),and aL(L/mg)and KL(L/g)are constants.

where Kf((mg/g)(mg/L)-1/n)and n are constants incorporating all factors affecting the adsorption process,including the capacity and intensity of adsorption.If n is close to 1,the surface heterogeneity could be assumed to be less significant,and as n approaches 10,the impact of surface heterogeneity becomes more significant(Noroozi et al.2007).
2.4 Kinetic experiments
Kinetics experiments were carried out by shaking certain amounts of adsorbents(kaolinite or montmorillonite)with a 50 mL solution containing LHA or fraction(to achieve 435 mg/L concentration)at 25°C.At pre-determined time intervals for 24 h,portions of the mixture were drawn by a syringe and then centrifuged,and LHA or fraction concentration was determined as described before.In adsorption kinetics,the amount of adsorption at time t,qt(mg/g)was calculated by the following formula:

where Ciis the initial concentration of LHA or fraction solutions(mg/L),Ctis the concentration at any time t(mg/L),V is the volume of solution used in the adsorption experiment(L),and w is the dry weight of adsorbent(g).
These models include the irreversible pseudo-first-order kinetics(Chang and Juang 2005)and the pseudo-second-order(Wu et al.2009;Asfaram et al.2015).The linear form of the irreversible pseudo-first-order model can be formulated as:

where Ci(mg/L)is the initial concentration of LHA or fraction and Ct(mg/L)is the equilibrium concentration of LHA or fraction at time t respectively,and k(min-1)is the rate constant.The values of k and correlation coefficients were determined.
The linear pseudo-second-order kinetics can be formulated as:
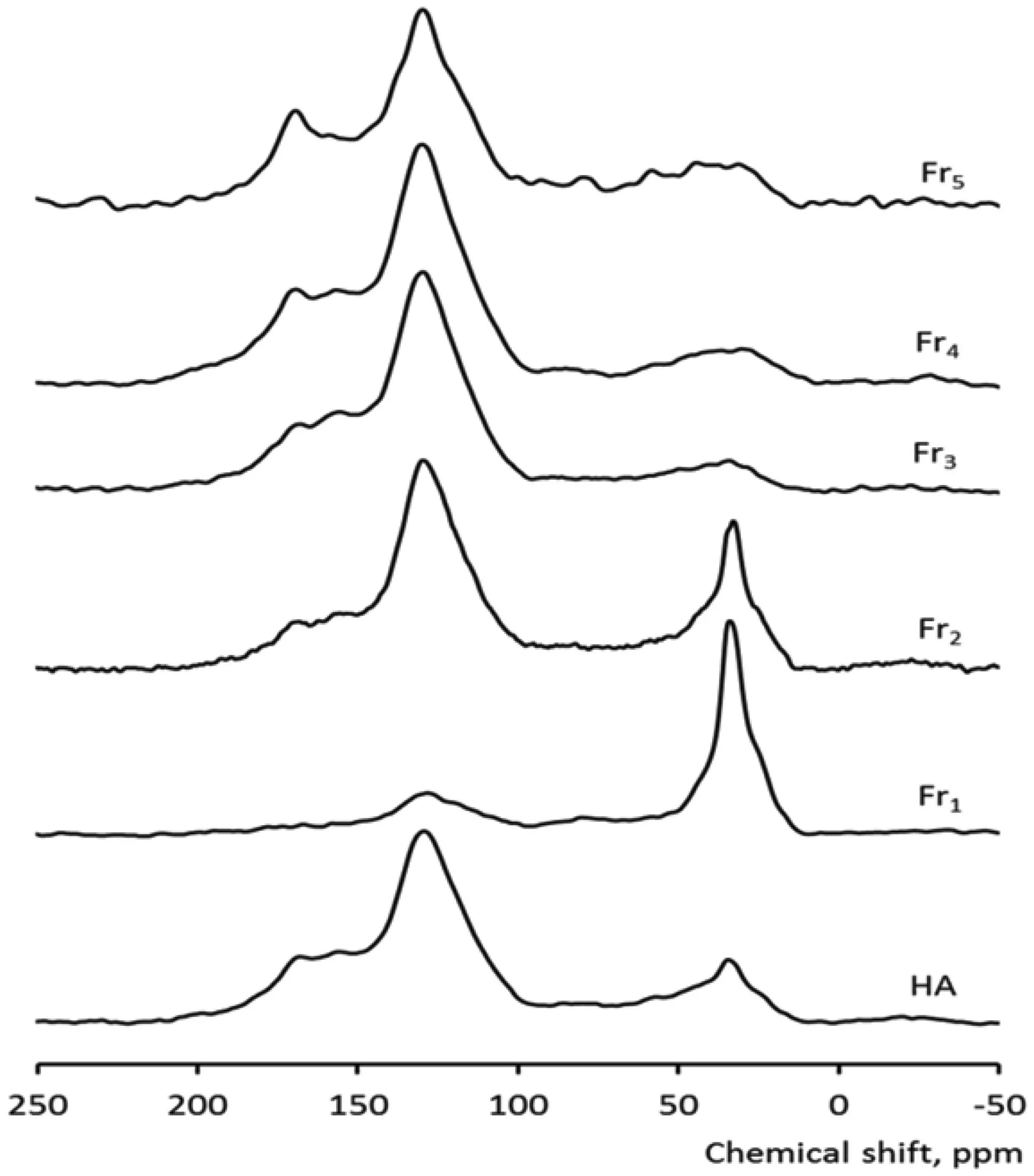
Fig.1 13C DP MAS NMR spectra of LHA and fractions

where qeand qtare surface loading of LHA at equilibrium and time t respectively,and K2(g/mg min)is the rate constant.The linear plots of t/qtas a function of t provided not only the rate constant K2but also an independent evaluation of qe.
3 Results and discussion
3.1 Characteristics of clay minerals
The elemental analysis of kaolinite and montmorillonite were determined by SEM/EDAX.The SEM/EDAX results showed that the chemical composition analysis of kaolinite was (SiO248.66%, Al2O340.80%, Na2O 3.9%, MgO 2.71%,K2O 1.55%,CaO 0.45%,Ti 0.91%)while that of montmorillonite was(SiO260.41%,Al2O322.66%,Na2O 5.9%,MgO 4.49%,K2O 1.1%,CaO 0.35%,Ti 1.33%).
BET surface area and particle size of kaolinite and montmorillonite properties were determined.Montmorillonite and kaolinite particles were 80±5 nm and 850±20 nm on the nanoscale,respectively,and the BET surface area of montmorillonite (107 m2/g) was much greater than that of kaolinite(11.2 m2/g).BET surface area is the measure of the accessible surface area per unit mass of soil minerals and is equal to the external surface area.
3.2 Characteristics of LHA and fractions
LHA was fractionated by UF into five fractions.Each fraction had relatively less heterogeneous properties than the whole material,as described later.The fractionation process was repeated three times.The(%TOC)distribution of LHA fractions was 9±0.01%, 4±0.004%,31±0.017%,33±0.04%,and 23±0.19%for Fr1,Fr2,Fr3,Fr4,and Fr5 respectively.The fraction with 50–10 kDmolecular weight was most obvious while fraction with 0.2 μm–300 kD molecular weight was lowest.
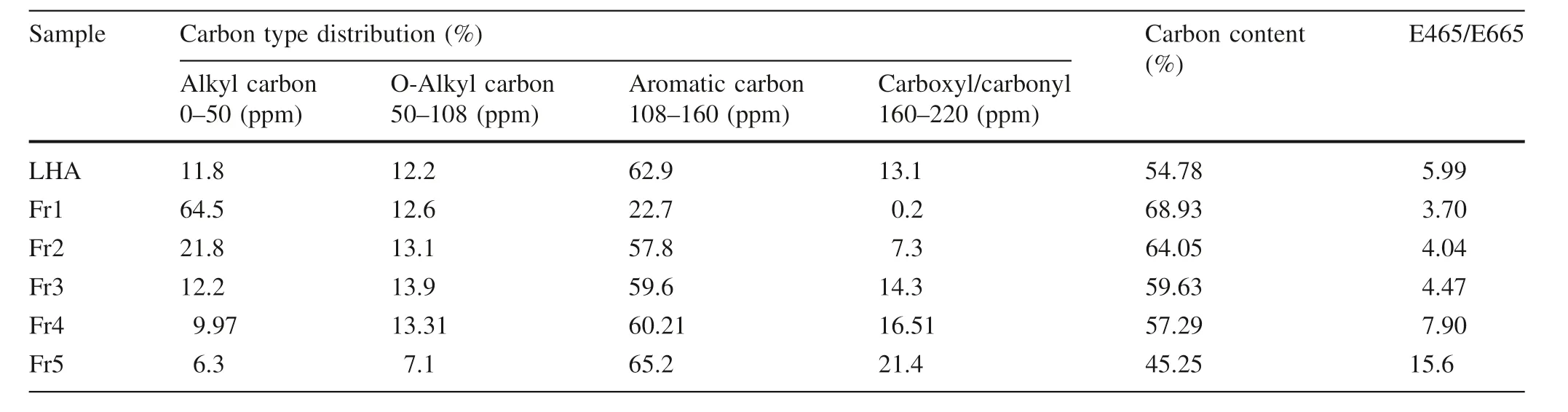
Table 1 A carbon type distribution based on the integration of the 13C-NMR spectra,degree of aromaticity,and carbon content percentage for LHA and its size fractions
The solid-state13C NMR spectra are shown in Fig.1 for the bulk and supernatant whole LHA samples. The assigned peaks and the estimated peak areas obtained by integration of the spectra are listed in Table 1.13C-NMR analysis of the LHA fractions revealed that the chemical forms of carbon varied between the different size fractions.The Fr1 fraction contained predominantly aliphatic carbon(0–110 ppm)with lower contents of aromatic and carboxyl carbon.By contrast,the Fr3,Fr4,and Fr5 fractions of much smaller molecular weight had a much higher content of aromatic(110–160 ppm)and phenolic and carboxyl carbons(140–160 ppm and 160–185 ppm,respectively)and lower levels of aliphatic carbons (Nagao et al. 2009;Tanaka 2012;Mukasa-Tebandeke et al.2015).Furthermore,fraction Fr2 had approximately the same proportion of aliphatic and aromatic carbon.These findings support the degree of aromaticity determined by UV spectroscopy as related in the E4/E6 ratio and the carbon content of each fraction(Table 1).The degree of aromaticity increased with decreasing LHA molecular size fractions while the carbon content decreased.
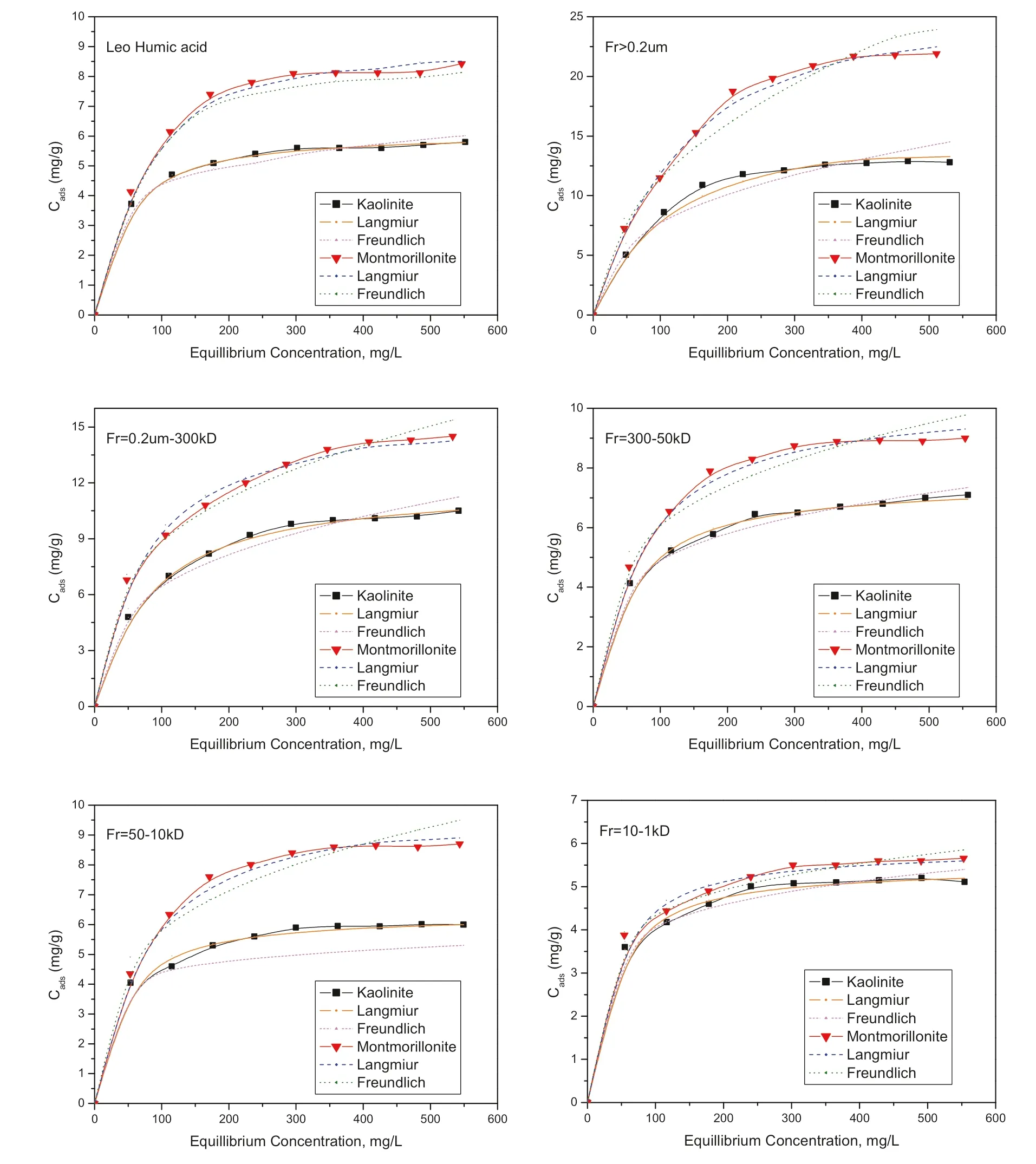
Fig.2 Adsorption of LHA and fractions onto kaolinite and montmorillonite with Langmuir and Freundlich model fitting
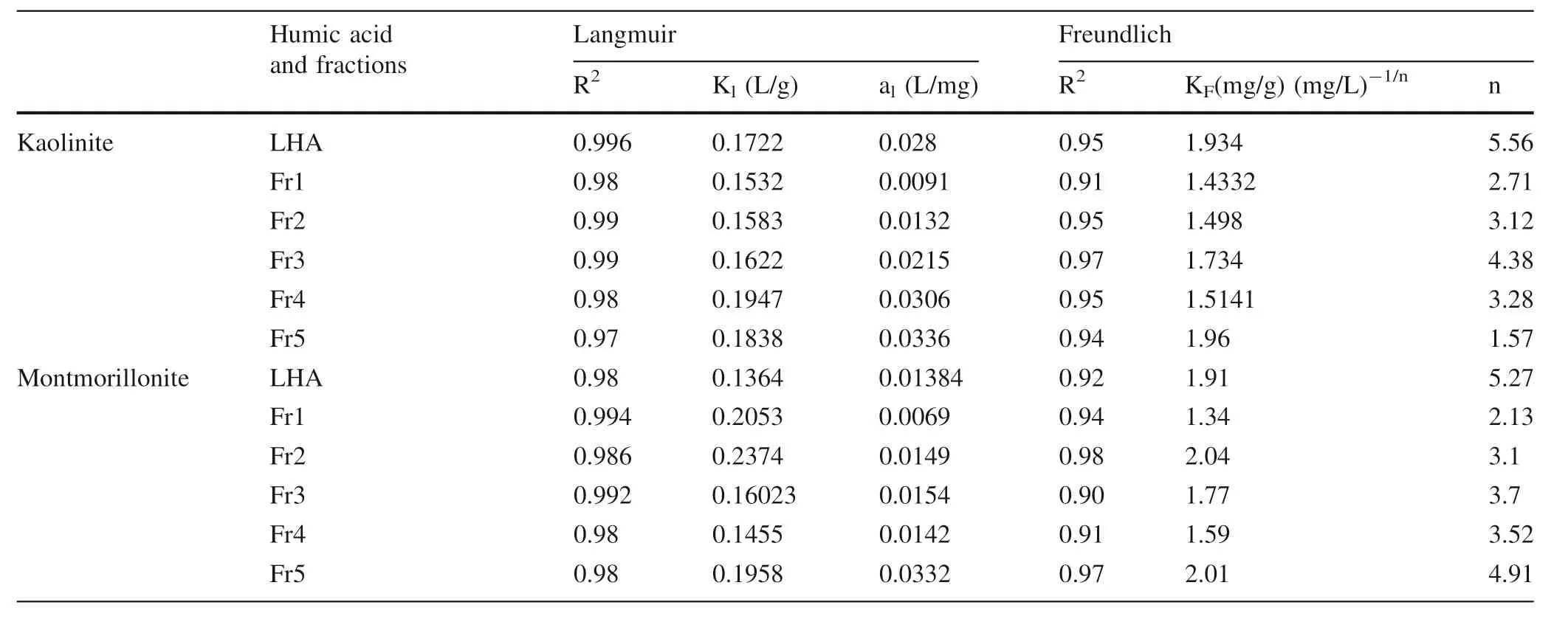
Table 2 LHA and fractions adsorption fitting by Langmuir and Freundlich equations
3.3 Humic acid and fraction adsorption
Adsorption data for HA and fractions onto clay minerals at pH 6 and 0.01 mol/L NaCl are shown in Fig.2.The results showed that the amount of LHA and fractions adsorbed onto montmorillonite are greater than that adsorbed onto kaolinite.This is due to the fact that the surface area and cation exchange capacity for montmorillonite was more than kaolinite(Tomba′cz et al.2004;Khalaf et al.2009).When referencing the amount of LHA fraction adsorption onto clay,it was obvious that the molecular size and the structure of the LHA fractions played an important role in the adsorption process.Moreover,the largest molecular size fraction was more adsorbed while the smallest was less adsorbed onto kaolinite and montmorillonite.These results mean that the adsorbed amount increased with increasing LHA molecular size.As shown from the13C-NMR results(Fig.1), this observation suggests that the larger HA molecular size fractions,which have more aliphatic carbon(less hydrophilic),were more strongly adsorbed in comparison to the smaller molecular size fractions (more hydrophilic),which have higher contents of aromatic carbon. Because adsorption is site-specific through the hydroxyl functional groups on clay surfaces and the hydrophobic moieties on siloxane sheets, only limited numbers of the ionizable groups of HA fractions may react with the clay surfaces.By suggesting limited surface site availability,a lower amount of Fr5 was required to cover the clay surfaces compared with Fr1.It is also observed that there is a decrease in the slope of the adsorption isotherm in the plateau regions with decreasing the LHA fraction size.This indicates a lower contribution of the hydrophobic interactions due to the decrease in the aliphatic carbons(Fig.1).Hence,the hydrophobicity of LHA molecular size fractions and clay minerals also had an important role in the adsorption process beside the functional groups of LHA and clay.Similarly,previous workers(Khalaf 2003;Dunnivant et al.1992)have directly or indirectly indicated that dissolved humic substances with higher molecular size exhibited higher adsorbed amounts on mineral surfaces compared to the smaller (more hydrophilic)ones(El-sayed et al.2019).
In addition,adsorption isotherm data are an essential way of predicting the mechanisms of adsorption. The results of the adsorption isotherm shape of LHA and fractions,the structure of LHA and fractions,and the highly variable adsorption amounts indicated that the mechanism of HA adsorption onto clay minerals will be a mix between two or more of following mechanisms:ligand exchange,van der Waals,water bridging,anion exchange,and hydrogen binding.The actual mix depends on the composition and ratio of each LHA fraction.Moreover,the ligand exchange mechanism could be the specific and predominant mechanism due to the LHA fractions percentage,structure,and slight increase in pH values after the adsorption process.
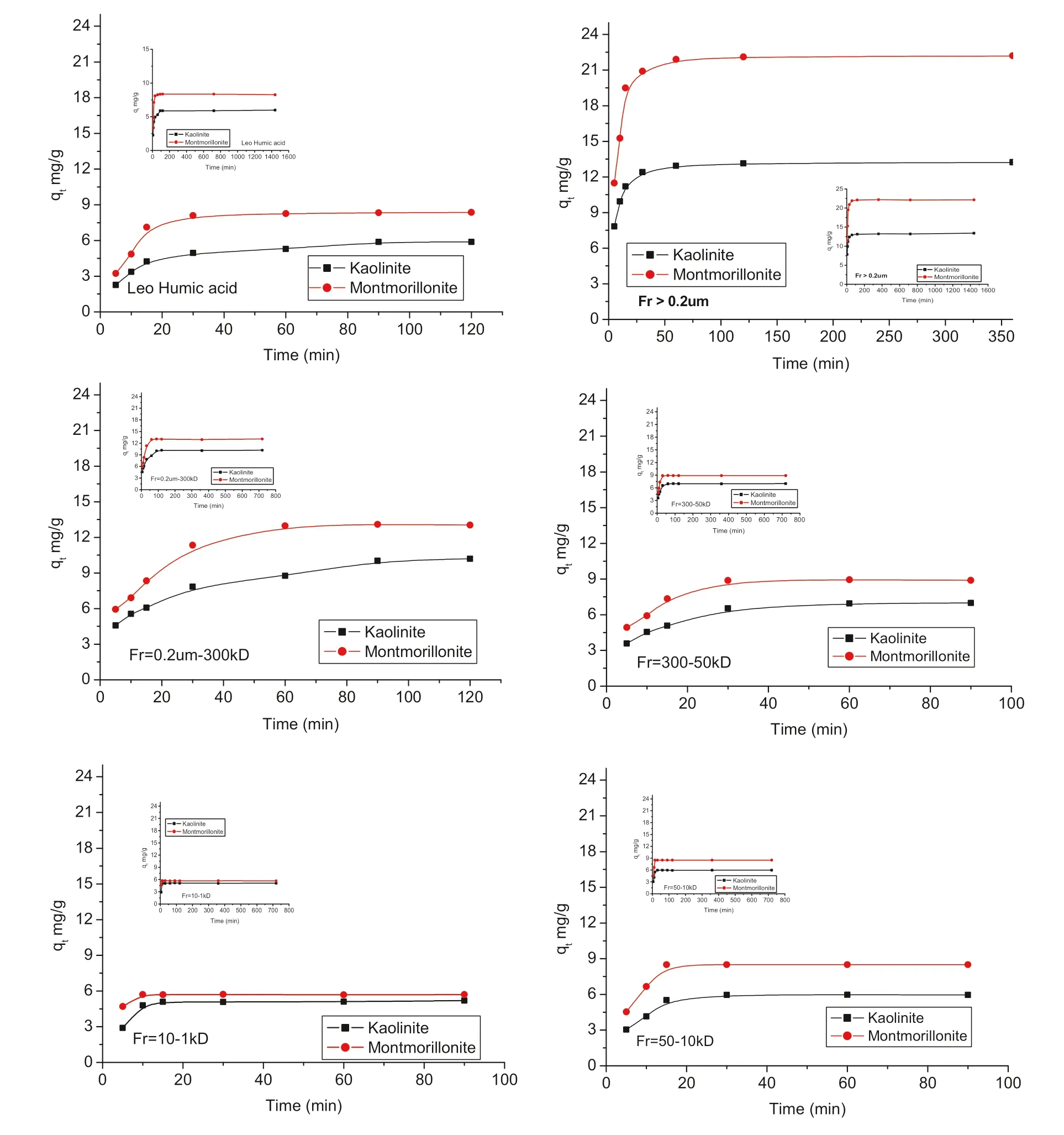
Fig.3 The changing of LHA and fractions adsorption onto kaolinite and montmorillonite with time
Furthermore,the results of LHA and fraction adsorption on clay minerals,cf.Fig.2 and Table 2,were compared with Langmuir and Freundlich adsorption isotherm models,where the Langmuir and Freundlich models have been frequently used to fit experimental data. Lists of the parameters obtained along with R2values are predicted in Table 2.The results clarified that the Langmuir model fitted to the experimental data was better than the Freundlich model. This means that LHA and fraction adsorption onto kaolinite and montmorillonite particles occurred strongly on homogeneous surfaces.
3.4 Kinetic studies
The kinetic adsorption data were collected to understand the dynamics of the adsorption reaction and explore the rate constant.The kinetic parameters are generally helpful for predicting the adsorption rate and give important information to develop and model the adsorption processes(Ghaedi et al.2015).
The LHA and its fractions adsorption onto kaolinite and montmorillonite reached equilibrium within different times(cf.Fig.3),meaning different mechanisms occurred foreach fraction.LHA adsorption onto montmorillonite and kaolinite reached equilibrium within 60 and 90 min,respectively,while Fraction >0.2 μm reached equilibrium within 2 h in the case of kaolinite and montmorillonite.In addition,Fraction from 10 to 1 kD reached equilibrium within 10 and 15 min in case of montmorillonite and kaolinite respectively.Results explained that the adsorption process was highly dependent on carboxylic group content and became faster by increasing carboxylic group content.
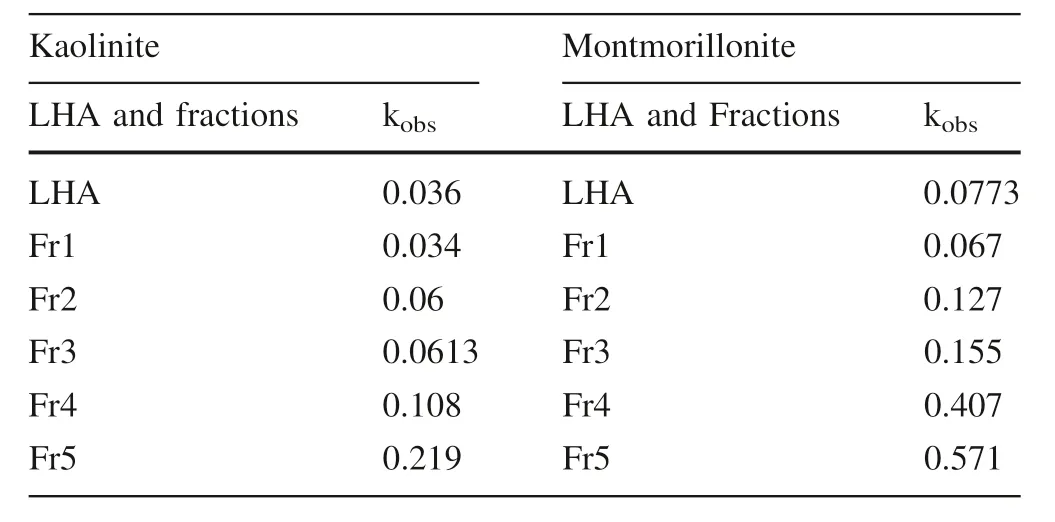
Table 3 The relationships between clay minerals and kobs of LHA and fractions
The values of kobsare affected by the change of the LHA and its fractions structure where kobsvalues increased with the increasing carboxylic groups’content of LHA fractions as shown in Table 3.The results indicated that the carboxylic groups’content played a very important role in the LHA and its fractions adsorption onto clay minerals.Moreover,kobsvalues for LHA and its fractions adsorption on montmorillonite were higher than kaolinite.
Pseudo-first-order and pseudo-second-order kinetic models were used to evaluate the kinetic data and the rate constants.The fitting of experimental data to the pseudofirst-order and pseudo-second-order equations,cf.Table 4,and the calculated correlation coefficient(R2)showed that LHA and its fraction adsorption on montmorillonite and kaolinite were more conforming to pseudo-second-order kinetics model.In the pseudo-second-order kinetics model,the rate-limiting step is the surface adsorption that involves chemisorption due to physicochemical interactions between the two phases.This means that the adsorption of LHA and its fractions on montmorillonite and kaolinite is mainly chemisorption.Additionally,qevalues agree with the experimental data.The initial adsorption rate,k2qe2,for montmorillonite is greater than that for kaolinite.This can be explained as due to the greater adsorption of LHA on montmorillonite than of that on kaolinite(as described in the adsorption section).
This result suggests varying binding mechanisms may be accountable for different clays(Feng et al.2005)and some LHA properties such as molecular weight,functional group compositions,and hydrophobicity lead the different adsorption of LHA and fractions on clay minerals as well as the reactive size of clay minerals(Zhang et al.2012).Moreover,the selectivity of mineral surfaces on LHA and fraction adsorption by different clay minerals surface deserve study in another paper.
4 Conclusion
In this study,LHA was fractionated into five molecular size fractions by UF to decrease LHA heterogeneity and better understand the LHA adsorption mechanism on clay minerals.The amount of LHA and fractions adsorbed onto montmorillonite was greater than that of those adsorbed onto kaolinite.The largest molecular size fraction wasmore readily adsorbed than the smallest fractions.Furthermore,the results showed that the Langmuir model fitted to the experimental data was better than the Freundlich model.This means that LHA and fraction adsorption onto clay particles occurred mostly on homogeneous surfaces.The kinetic adsorption data were collected and explained that the LHA and its fraction adsorption onto kaolinite and montmorillonite reached equilibrium within different times.Moreover,the results illustrated that LHA and its fraction adsorption on montmorillonite and kaolinite were more conforming to pseudo-second-order kinetics model.This result suggests varying binding mechanisms may be accountable for different clays and some LHA properties such as molecular weight,functional group compositions,and hydrophobicity lead the different adsorption of LHA and fractions on clay minerals as well as the reactive size of clay minerals.
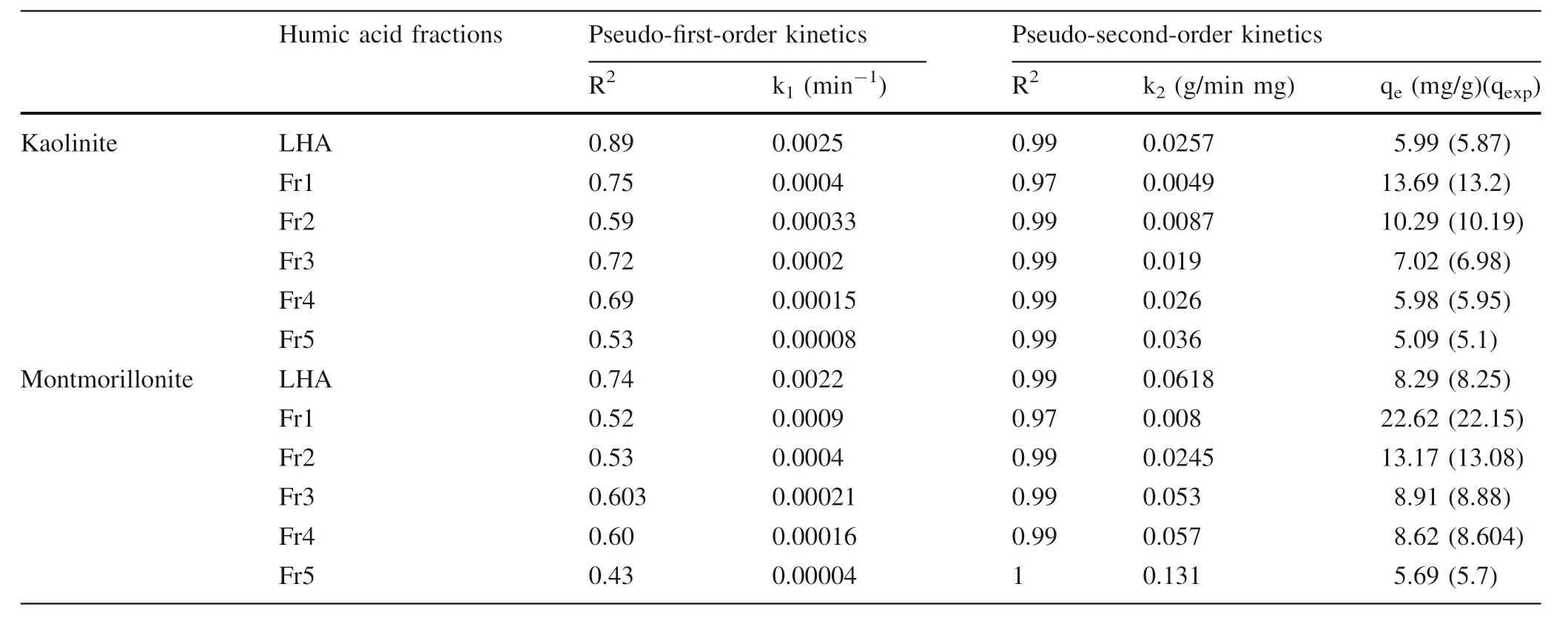
Table 4 Comparison of the pseudo-first-order and pseudo-second-order kinetic models for the adsorption of LHA and fractions onto clay minerals at 25°C
Acknowledgements This work was funded by a Fulbright Visiting Scholar fellowship to Mohamed El-sayed and performed at South Dakota State University.
Compliance with ethical standards
Conflict of interest The authors declare that they have no conflict of interest.
- Acta Geochimica的其它文章
- Whole-rock geochemistry of Tertiary sediments of Mizoram Foreland Basin,NE India:implications for source composition,tectonic setting and sedimentary processes
- Petrogenesis,geochemistry and geological significance of Paleocene Granite in South Gangdese,Tibet
- An experimental study on dynamic coupling process of alkaline feldspar dissolution and secondary mineral precipitation
- REE geochemistry of gangue minerals and their geological significance in the Muli antimony ore deposit in Yunnan,China
- Petrography and tectonic provenance of the Miocene Surma Group in parts of the Naga-Manipur hills,in and around Nungba,Northeast India
- Study on Late Cretaceous-Cenozoic exhumation of the Yanji area,NE China:insights from low-temperature thermochronology

