Research and Safety Evaluation of Thallium in Cosmetic Products
Zhou Jue
Shenzhen Consumers Council, China
Mo Yanmei, Feng Mei, Chen Xiaolei, Xu Baihe
Shenzhen Institute of Consumption Quality, China
Abstract
Key words
cosmetic product; thallium; safety evaluation; Maximum Theoretical Safety Limit
Thallium (Tl) is not a kind of essential trace metal element, it is highly toxic to human body.Thallium has been reported to have more toxicity than Lead (Pb) and Mercury (Hg), and its effects are cumulative.Generally, the lethal dose of Tl for adults is 8~12 mg/kg, its poisoning effects mainly show gastrointestinal irritation and neurological symptoms.[1]
The source of thallium in cosmetics
Thallium is an element which occurs naturally in the earth’s crust, mainly exists in various metallic ores, such as SeTICuAg2, TIAsS2 and so on.The average content of Thallium is 0.1~1.0 μg/g in the earth’s crust and 0.01~0.02 μg/L in the sea.[2]The report (environmental health criteria 182 thallium) released by World Health Organization International Programme On Chemical Safety (WHO IPCS) shows that[3]:cement plants using thallium-containing pyrite (up to 21 mg/kg dry weight) and base metal smelters (up to 2.1 mg/kg dry weight) released large quantities of thallium to the atmosphere.Plants growing in uncontaminated soil normally contain 0.01 to 0.3 mg thallium/kg dry weight, while those growing near cement plants using thallium-enriched pyrite have been reported to contain much larger amounts (100 to 1000 mg thallium/kg dry weight).Therefore thallium may be brought into cosmetics from the ingredients derived from natural sources, such as water, mineral, plant extracts and so on, especially in the thalliumcontaminated areas.
Hazard classification
International agency for research on cancer (IARC):Not classified thallium as a carcinogen.
Guidance on Labelling and Packaging in accordance with Regulation (EC) No 1272/2008:Acute Tox.2-H300 Fatal if swallowed; Acute Tox.2-H330 Fatal if inhaled; STOT RE 2:H373 y cause damage to organs through prolonged or repeated exposure; Aquatic Chronic 4-May cause long lasting harmful effects to aquatic life.[4]
Office of Environmental Health Hazard evaluation (OEHHA) on thallium:Not listed in California Proposition 65 list.
Detection of thallium content in cosmetics sold in Shenzhen
By Method 1.6 Chapter 4 in ChinaSafety and Technical Standards for Cosmetics(2015 Edition) to determine thallium content in 34 batches of cosmetic products be sold in Shenzhen, including 14 batches of cushions and 20 batches of sunscreens for children, all results of samples are within the range of not detected to 0.62 mg/kg, the detection rate is about 62%, about 35% samples contain thallium content in more than 0.03 mg/kg.All the test results are shown in Table 1.
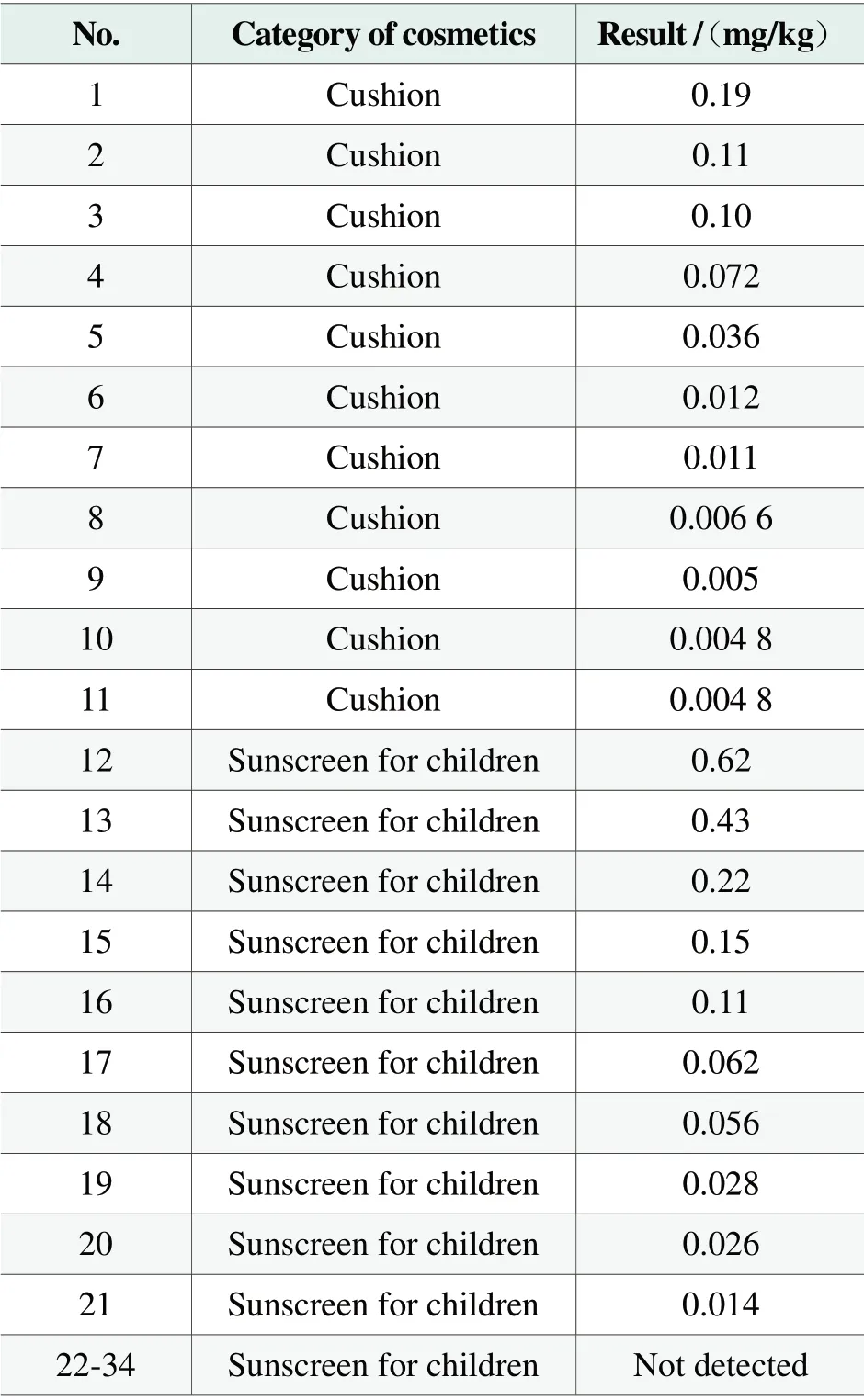
Table1.The content of thallium in 34 batches of cosmetic products sold in Shenzhen
Current regulatory requirements and limits
Thallium and compounds are listed as cosmetic banned ingredient according to ChinaSafety and Technical Standards for Cosmetics(2015 Edition).They are prohibited as materials for use in cosmetic products, but no specific limits in the regulation.The non-intended presence of a small quantity of Thallium which is technically unavoidable in good manufacturing practice, shall be safe for human health when used under normal or reasonably foreseeable conditions of use.
Thallium and compounds are also listed as cosmetic prohibited substances according to REGULATION (EC) No 1223/2009, Cosmetic Ingredient Hotlist-Canada and ASEAN Cosmetic Directive, which all are lack of regulatory limits for cosmetics and ingredients.However, there is no regulation for Thallium and compounds in U.S.Federal Food Drugs and Cosmetic.
Safety evaluation of thallium in cosmetic products
This risk evaluation procedure is subdivided in 3 parts[5]:Hazard Evaluation (Hazard Identification and Dose-Effect Relationship):based on the results of related studies, the intrinsic physical, chemical and toxicological properties are studied to identify whether the substance has the potential to damage human health and its level of hazard.In the case of an effect with a threshold, the dosage at which no adverse effects are observed (NOAEL), is determined.Exposure evaluation:in which the amount and the frequency of human exposure to the compound are determined (including oral, inhalation and dermal route).Risk characterization:base on the hazard identification, exposure evaluation and many other uncertainties, the probability that the substance under investigation causes damage to human health, the level of risk and whether the risk is controllable are determined.
Hazard evaluation
The acute toxicity of thallium and compounds is extremely strong, and it begins with gastrointestinal diseases, which are manifested as gastrointestinal bleeding, gastroenteritis, nausea, vomiting, etc.[6]Repeated exposure to thallium may be fatal or affect the nervous system, eyes, liver and kidneys.The toxicological target organs of thallium also include skin hair follicles, which may cause hair loss and alopecia.
Considering the hair loss and alopecia caused by thallium poisoning, in order to evaluate the toxic effects of thallium fully, we believe that it is appropriate to use the threshold related to hair loss for safety evaluation in cosmetic products.Based on the subchronic (90-day) toxicity study of thallium (I) sulfate in Sprague-Dawley rats by MRI (1988), considering the adverse effect:alopecia, U.S.Environmental Protection Agency(EPA) corrected the study data, then used the NOEAL of 0.04 mg/kg/day TI related to hair follicle atrophy and the BMDL10 of 0.01 mg/kg/day TI related to hair loss as the starting value to evaluate the dose effect.[7]
EPA considers that the subchronic (90-day) toxicity study of thallium (I) sulfate in Sprague-Dawley rats by MRI (1988) represents a data deficiency, a lack of adequate developmental toxicity studies and a two-generation reproductive toxicity study, and additional uncertainty associated with the limited data available on neurotoxicity in light of the potential for neurotoxicity to represent a sensitive endpoint for thallium exposure.So, a database UF of 10 was applied to correct the result.
Therefore, we should use a database UF of 10 to correct the BMDL10 of 0.01 mg/kg/day Tl, and then choose the BMDL10 of 0.001 mg/kg/day Tl after corrected as threshold for safety evaluation of thallium in cosmetic products.Calculation process as follows:
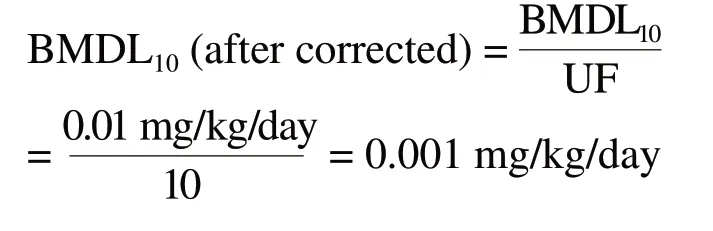
Exposure evaluation
Exposure value
Thallium and compounds are cosmetic banned ingredients and they are prohibited as materials for use in cosmetic products, but thallium is an element which occurs naturally in the earth’s crust, especially exist in various metallic ores, even exist in uncontaminated water, sea water and soil.In addition, a article[3]reported that plants also contained thallium, especially those that grew in contaminated areas.Therefore, when this kind of material is used for cosmetics, thallium will be brought into the cosmetics as an impurity or a trace substance unavoidably.

These material are commonly used in various types of cosmetics, such as minerals (including colorants), water, and plant extracts.According to the requirement of The SCCS’s notes of guidance for the testing of cosmetic ingredients and their safety evaluation 9th revision,[8]the cosmetic aggregate exposure value 269 mg/kg/day is reasonable to be used.
Absorption rate
Thallium can enter body through inhalation, oral, and dermal route.[1]In the past, thallium was used as a depilatory agent, and it is also externally used to cure scalp spasms and tuberculosis-related night sweats, which also means that thallium can be absorbed well through the skin.Furthermore, the dermal LD50 value of thallium(I) sulfate in rats was 405 mg/kg Tl,[3]indicating that it is dermal toxic, which indirectly prove that thallium can be absorbed well through the skin.It can be seen that thallium can be absorbed well through on the skin, but there is no or inadequate absorption data available.According to the requirement of The SCCS’s notes of guidance for the testing of cosmetic ingredients and their safety evaluation 9threvision,[8]the conservative absorption rate 100% is reasonable to be used for safety evaluation of thallium in cosmetic products
Risk characterisation
According to the requirement of The SCCS’s notes of guidance for the testing of cosmetic substances and their safety evaluation 9threvision[8]and China’s guidance for safety evaluation in cosmetic products (Consultation Draft),[9]it is generally accepted that the MoS value should at least be 100 to declare a substance with a threshold safe for use, which is also applicable to children.The value of 100 consists of a factor 10 for the extrapolation from animal to man and another factor 10 taking into account the interindividual variations within the human population.Formula as follows:
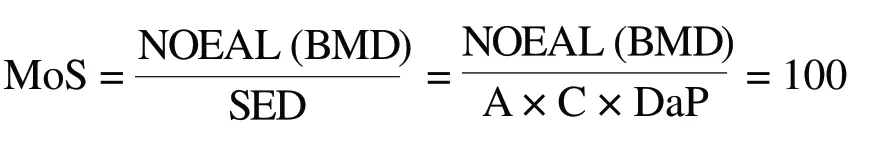
BMDL10:used as dose descriptors.According to the requirement of The SCCS’s notes of guidance for the testing of cosmetic substances and their safety evaluation 9th revision,[8]the BMDL10 0.001 mg/kg/day Tl can replace the value of NOAEL of to calculate MoS value.
A:estimated daily exposure to a cosmetic product per unit of weight.The cosmetic aggregate exposure value is 269 mg/kg/day.
DaP:dermal absorption expressed as a percentage of the test dose assumed to be applied in reallife conditions.The absorption rate is 100%.C:concentration of the ingredient under study in the finished cosmetic product.
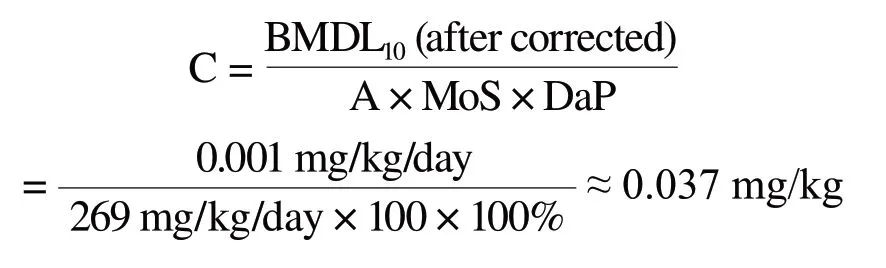
After calculation, the maximum theoretical safety limit of thallium in cosmetics is calculated to be 0.037 mg/kg.The calculation formula as follows:
Background exposure evaluation
Theoretically the maximum theoretical safety limit of thallium in cosmetics 0.037 mg/kg only considers the exposure to thallium from cosmetics, and it does not take into account drinking water, food or environmental intake.
According to the report released by International Programme on Chemical Safety World Health Organization(WHO IPCS), 1996,[3]the amount of thallium ingested by drinking water is expected to be less than 1 μg/day for most people.The amount of thallium ingested by dietary is estimated to be less than 5 μg/day in thallium-contaminated areas, the vast majority of which is derived from vegetables.Therefore, considering all sources of thallium ingested, the dietary intake can be used as the background exposure value.According to the adult weight of 60 kg defaulted in safety evaluation in cosmetic products,[8]then the background exposure value is calculated to be about 8.3×10-5mg/kg/day.The calculation formula as follows:
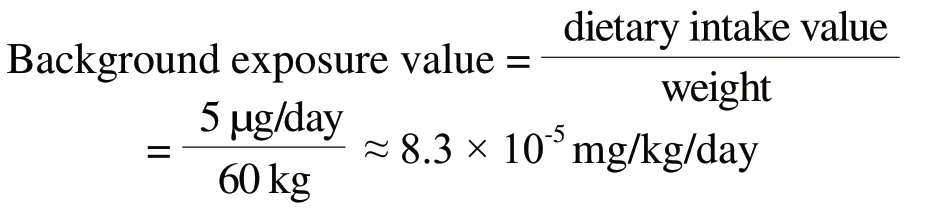
In order to consider the safety of consumers exposed to thallium fully, the background exposure value should be subtracted from BMDL10 in the safety evaluation of thallium in cosmetic products.Then, the maximum theoretical safety limit of thallium in cosmetics is calculated to be 0.034 mg/kg.The calculation formula as follows:

Calculated by WHO IPCS
According to the report released by International Programme on Chemical Safety World Health Organization (WHO IPCS), 1996,[3]it is unlikely to pose a risk to human health when the concentration of thallium in urine is less than 5 μg/L (equivalent to 10 μg thallium ingested by dietary).According to the adult weight of 60 kg defaulted in safety evaluation in cosmetic products,[8]the oral daily intake value is calculated to be about 8.3 × 10-5mg/kg/day, which does not pose a risk to human health .The calculation formula as follows:
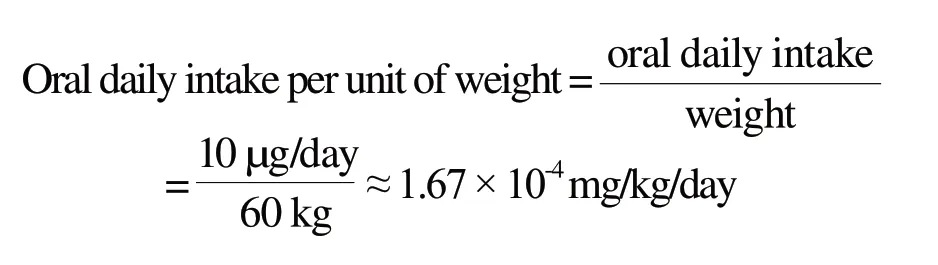
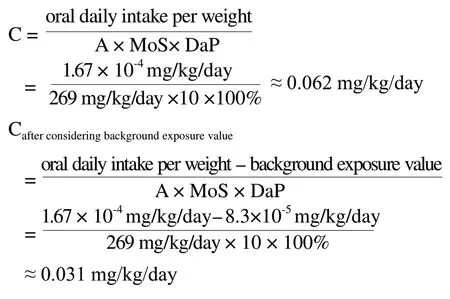
Since the above data is derived from the human body, there is no need to consider the extrapolation from animal to human (factor 10).It is only necessary to consider the interindividual variations (factor 10) when calculating the safety factor.Then the MoS value should at least be 10 to declare a substance safe.According to the cosmetic aggregate exposure value is 269 mg/kg/day, the absorption rate is 100% and the MoS value 100, the maximum theoretical safety limit of thallium in cosmetics is calculated to be 0.031 mg/kg.The calculation formula as follows:


Result and Discussion
Thallium and compounds are listed as cosmetic banned ingredients, but there is no specific limits in cosmetics internationally.Since thallium is an element which occurs naturally in the earth’s crust, it will be brought into the cosmetics as an impurity or a trace substance unavoidably when a variety of ingredients derived from natural sources is used for cosmetics.Therefore, the safety evaluation of thallium in cosmetic products is extremely necessary.
According to the report released by the United States Environmental Protection Agency (EPA), BMDL10 is calculated to be 0.001 mg/kg/day after corrected.Moreover, according to to the requirement of the SCCS’s notes and China’s guidance for safety evaluation in cosmetic products Public Draft, the maximum theoretical safety limit of thallium in cosmetics is calculated to be 0.034 mg/kg after considering the background exposure value.According to the suggestion by WHO IPCS, 1996, the safe concentration of thallium in urine is less than 5 μg/L, and the maximum theoretical safety limit of thallium in cosmetics is calculated to be 0.031 mg/kg after considering the background exposure value.On the basis of the data calculated, the the maximum theoretical safety limit of thallium in cosmetics calculated by two different evaluation data are not much different, 0.031~0.034 mg/kg can be used as a safety limit of thallium in cosmetics.
 China Detergent & Cosmetics2019年4期
China Detergent & Cosmetics2019年4期
- China Detergent & Cosmetics的其它文章
- Fluorinated Surfactants and Fluorinated Polymer Materials (IV):Strategy for Solving PFOS Problems
- Quantitative Evaluation Method of Amylase Performance in Detergents for Hand Dishwashing
- A Study on the Whitening Efficacy of Flavones in Lotus Leaf
- Refutation of Cosmetics Rumors
- Flight Inspection Analysis on National Cosmetics in 2018
- Standard Explanation of the Instruction for Use of Consumer Products—Labelling for Detergents
