Abnormal brain activity in adolescents with Internet addiction who attempt suicide: an assessment using functional magnetic resonance imaging
Yan Huang, Lu Xu , Li Kuang , Wo Wang, Jun Cao Mu-Ni Xiao
1 GCP Office, Chongqing Traditional Chinese Medicine Hospital, Chongqing, China
2 Chongqing Medical and Pharmaceutical College, Chongqing, China
3 Department of Psychiatry, the First Affiliated Hospital of Chongqing Medical University, Chongqing, China
4 Mental Health Center, University-Town Hospital of Chongqing Medical University, Chongqing, China
Abstract Internet addiction is associated with an increased risk of suicidal behavior and can lead to brain dysfunction among adolescents. However, whether brain dysfunction occurs in adolescents with Internet addiction who attempt suicide remains unknown. This observational cross-sectional study enrolled 41 young Internet addicts, aged from 15 to 20 years, from the Department of Psychiatry, the First Affiliated Hospital of Chongqing Medical University, China from January to May 2018. The participants included 21 individuals who attempted suicide and 20 individuals with Internet addiction without a suicidal attempt history. Brain images in the resting state were obtained by a 3.0 T magnetic resonance imaging scanner. The results showed that activity in the gyrus frontalis inferior of the right pars triangularis and the right pars opercularis was significantly increased in the suicidal attempt group compared with the non-suicidal attempt group. In the resting state, the prefrontal lobe of adolescents who had attempted suicide because of Internet addiction exhibited functional abnormalities, which may provide a new basis for studying suicide pathogenesis in Internet addicts. The study was authorized by the Ethics Committee of Chongqing Medical University, China (approval No. 2017 Scientific Research Ethics (2017-157)) on December 11, 2017.
Key Words: adolescents; amplitude of low-frequency fluctuation; brain activity; functional magnetic resonance imaging; Internet addiction; prefrontal lobe; resting state; suicidal attempt
Introduction
Suicide is an important factor related to mortality among people in the 15-34-year-old group (Phillips et al., 2002). Adolescent suicide not only has severe consequences for individuals and their families, but also negatively impacts society. Previous suicide attempt(s) is the most critical risk factor for suicidal death. The risk of death in individuals who have attempted suicide is 100 times higher than that in the general population (Phillips et al., 2002). According to the World Health Organization statistics, each victim performs 10 to 40 non-fatal suicide attempts (V?rnik, 2012).
Internet addiction, also known as Internet addiction disorder, is considered to have a great impact on public health, especially among adolescents (Bian et al., 2016; Fu, 2019; Zhong et al., 2019). Internet addiction is associated with physical and mental symptoms such as deterioration of health, emotional disorder, impulsive behavior, and maladjustment caused by repeated and uncontrolled use of the Internet (Huang et al., 2019). The deprivation of Internet use can lead to withdrawal reactions such as anxiety and depression (Guo et al., 2018; Lin et al., 2018; Huang et al., 2019). Moreover, with the rapid development of Internet technology, an increasing number of studies of adolescent Internet users have shown that Internet addiction and suicide-related behaviors are strongly related (Kim et al., 2006; van den Eijnden et al., 2008; Schilling et al., 2009; Yang et al., 2010; Wang and Meng, 2014; Zhang et al., 2014; Wu et al., 2015; Ji et al., 2016; Pan et al., 2018). Therefore, paying close attention to Internet addiction in teenagers can help to prevent their suicidal behavior.
Internet addiction has been reported to lead to abnormal changes in the brain function of adolescents (Zhou et al., 2011; He et al., 2012). Because the amplitude of low-frequency fluctuations (ALFF) can reflect the intensity of local spontaneous brain activity, it has been used as a surrogate biomarker for the evaluation of spontaneous brain activity (Wang, 2008; Yu, 2015). In subjects with Internet addiction, the ALFF method based on the resting blood oxygenation level-dependent (BOLD) signal is useful for evaluating disease-related abnormalities in local brain activity and is gradually being adopted to identify variations in local synchronization of automatic nerve activity in other illnesses (Zou et al., 2008; Xu, 2016). Previous studies have used the local ALFF of resting-state functional magnetic resonance imaging (rs-fMRI) to analyze the z-score value of the ALFF (zALFF) (Cao, 2014). The results showed that the abnormal intracranial zALFF changes in youth with suicide attempts and depression were mainly distributed in the frontal, temporal, and parietal lobes. This information provided a basis for using this imaging biomarker to evaluate and monitor the suicide risk of young depressive patients.
A previous study showed that structural abnormalities indeed exist in the gray matter of adolescents with Internet addiction (Zhou et al., 2011). However, these brain abnormalities in Internet addicts are mainly functional abnormalities (He et al., 2012). The core symptoms of Internet addiction are the same as those of substance dependence and pathological gambling. The potential neurobiological mechanisms and models need to be established to guide the treatment of Internet addiction (Du et al., 2011).
According to the previous results, we speculated that compared with young Internet addicts with no suicidal attempts, addicts with a history of suicidal attempts exhibit differential templates of neural activity in the resting state. This study attempted to assess the changes in local brain activity in Internet addicts with suicidal attempts using the ALFF method.
Participants and Methods
Design
The present study was an observational cross-sectional study. We focused on adolescent students with Internet addiction (Internet Addiction Test score ≥ 40) (Fu et al., 2010). Young’s Internet Addiction Test, a valid and authentic measure of addictive Internet use (Fu et al., 2010), was applied to evaluate Internet addiction in adolescents. Suicidal attempt was evaluated using the Scale of Suicide developed by the Beijing Center for Psychological Crisis Research and Intervention (Kayi? et al., 2016). In this investigation, 41 young Internet addicts, aged from 15 to 20 years, were enrolled from the Department of Psychiatry, the First Affiliated Hospital of Chongqing Medical University, China from January to May 2018. The participants included 21 individuals who had attempted suicide 1-6 months before an fMRI scan and 20 Internet-addicted individuals without a suicidal attempt history. This study was authorized by the Ethics Committee of Chongqing Medical University (approval No. 2017 Scientific Research Ethics (2017-157)) on December 11, 2017 (Additional file 1). The subjects were informed of the purpose, methods, possible risks, and discomforts of participating in the study. All participants signed a written informed consent form (Additional file 2). The study was conducted in accordance with the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) statement. Figure 1 shows a flow chart outlining the selection of young Internet addicts with and without suicide attempts.
The subjects were included if they (1) were aged between 15 and 20 years; (2) had an Internet Addiction Test score ≥ 40; (3) had no history of drug treatment or electroconvulsive therapy within the 2 weeks before enrollment; (4) were right-handed; and (5) voluntarily participated in the study. The subjects were excluded if they had (1) other mental disorders, such as schizophrenia, schizophrenic affective disorder, bipolar disorder, or post-schizophrenic depression; (2) complicated or organic brain disease or severe brain trauma; (3) kidney-, heart-, or liver-related diseases, diabetes mellitus, or other serious somatic diseases; (4) a history of substance abuse or dependence (drugs, cocaine, or alcohol) or a family history of mental disorders; or (5) a contraindication for undergoing MRI.
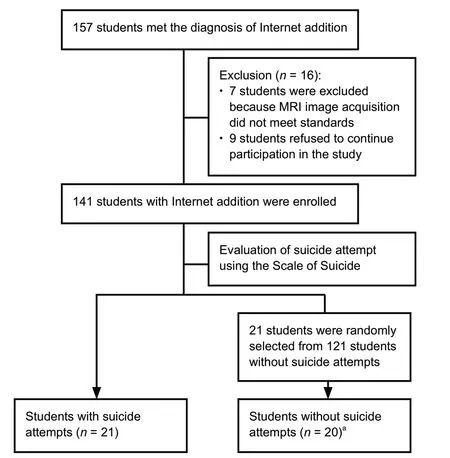
Figure 1 Flow chart of the selection procedure for young Internet addicts with and without suicide attempts.
Imaging data acquisition
Brain imaging was performed at the Department of Radiology, the First Affiliated Hospital of Chongqing Medical University. All MR images were obtained with a 3.0 T GE Signa Hdxt MR scanner (SignaHDxt; GE Healthcare, WI, USA) with a standard eight-channel head coil (Cao et al., 2015, 2016; Benzagmont et al., 2019; Chung et al., 2019). The participants were asked to stay awake, close their eyes, breathe at a constant rate, not move their heads, and try not to indulge in any thinking activity. T1-weighted and BOLD images were obtained for structural and functional brain assessment, respectively. A total of 240 time points were obtained over 8 minutes (Cao et al., 2015). The scanning parameters were as follows (Cao et al., 2016): (1) Three-dimensional T1-weighted images: repetition time = 8.3 ms, thickness/gap = 1.0/0 mm, echo time = 3.3 ms, flip angle = 15°, field of view = 240 × 240 mm2, size of matrix = 256 × 192. (2) Functional images: 33 axial slices, repetition time = 2000 seconds, echo time = 40 ms, flip angle = 90°, thickness/gap = 4.0/0 mm, field of view = 240 × 240 mm2, size of matrix = 64 × 64.
Data processing
Each structural MR image of the subject was evaluated by two experienced radiologists. Data pre-processing was carried out using Data Analysis and Processing for Brain Imaging software (http://rfmri.org/dpabi) based on the Matrix-Laboratory (MATLAB 7.9), developed by MathWorks (Natick, MA, USA). After converting the original MRI data into the Digital Imaging and Communications in Medicine (DICOM) format, the first 10 volumes of each series of functional time were deleted for calibration of the scanner and adjustment of participants to the environment of scanning. Subsequently, various image processing steps were performed, including time-level and head-motion correction, segmentation, registration, removal of covariates (head-motion, white matter signal, cerebrospinal fluid signal, and polynomial trend), and spatial standardization. After correcting for slice timing and head movement, subjects with head movement greater than 2 mm in any orientation or head rotation greater than 2° were excluded. The pre-processing procedure included movement correction, time alignment across slices, within subject enrollment between the echo-planar and T1 images, T1 segmentation, and normalization to transform the BOLD fMRI datasets to Montreal Neurologic Institute space with voxels re-sampled to 3 × 3 × 3 mm3. The estimated head movement was less than 1.0 mm and 1.0° in the x, y, and z directions for all the subjects. Therefore, all subjects met the motion criterion, and no subject was eliminated from the final analysis (Cao et al., 2015, 2016; Zhong et al., 2019).
zALFF analysis
The ALFF was meas ured using WFU PickAtlas 3.0 (AAL atlas of Wake Forrest University, NC, USA). The ALFF value of each voxel was normalized using z-score transformation to improve the reliability and normality across the subjects. All individual ALFF maps were standardized and transformed into zALFF values. The z values were computed by subtracting the total mean voxel-wise ALFF values (mALFF) of the whole brain from the raw ALFF value for each voxel and then dividing by the standard deviation (SD) of the ALFF values of all voxels based on the following formula: z score = (ALFF - mALFF) / SD. The zALFF values were used for further statistical analysis (Cao et al., 2016).
Statistical analysis
Continuous data are expressed as mean and SD or as medians with interquartile ranges. Categorical data are presented as frequencies and percentages. Before statistical analysis, data were tested for normality. Two-sample t-tests and chisquared tests were used to compare differences between groups regarding demographic and clinical characteristics using SPSS 23.0 (IBM Corp., Armonk, NY, USA). The zALFF values of the bilateral orbitofrontal lobes of the two groups were compared by a two-sample t-test. The obtained minimum voxel-wise value was 26 after AlphaSim correction, and the significance before and after correction was the same, P < 0.001. The significance threshold was P = 0.05.
Results
General population data of Internet addicted individuals with and without a suicidal attempt
No significant difference was observed in gender composition and age between the two groups (both P > 0.05), but the Internet Addiction Test score was higher in the suicidal attempt group than in the non-suicidal attempt group (P = 0.033; Table 1).
zALFF differences in different brain areas between Internet addicts with and without a suicidal attempt
Compared with the suicidal attempt group, the activity in the gyrus frontalis inferior of the right pars opercularis (Frontal_Inf_Oper_R) and the right pars triangularis (Frontal_Inf_Tri_R) was significantly enhanced in the non-suicidal attempt group (P < 0.001; Figure 2 and Table 2).
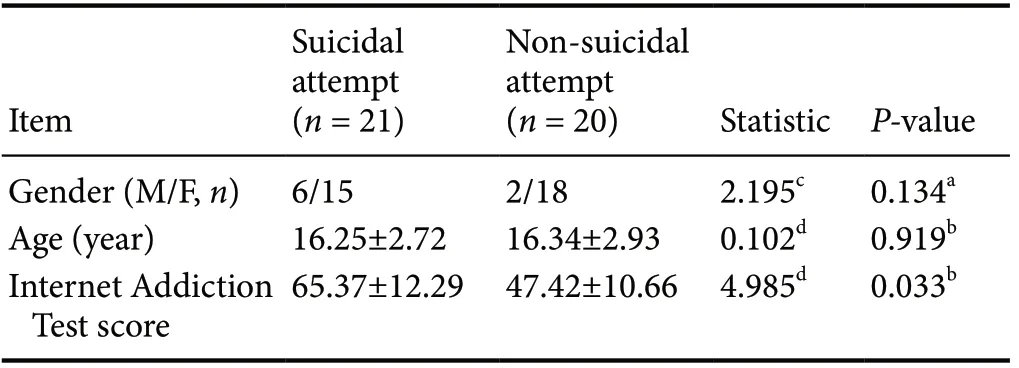
Table 1 General demographic data and Internet Addiction Test scores of individuals with Internet addiction with and without a suicidal attempt
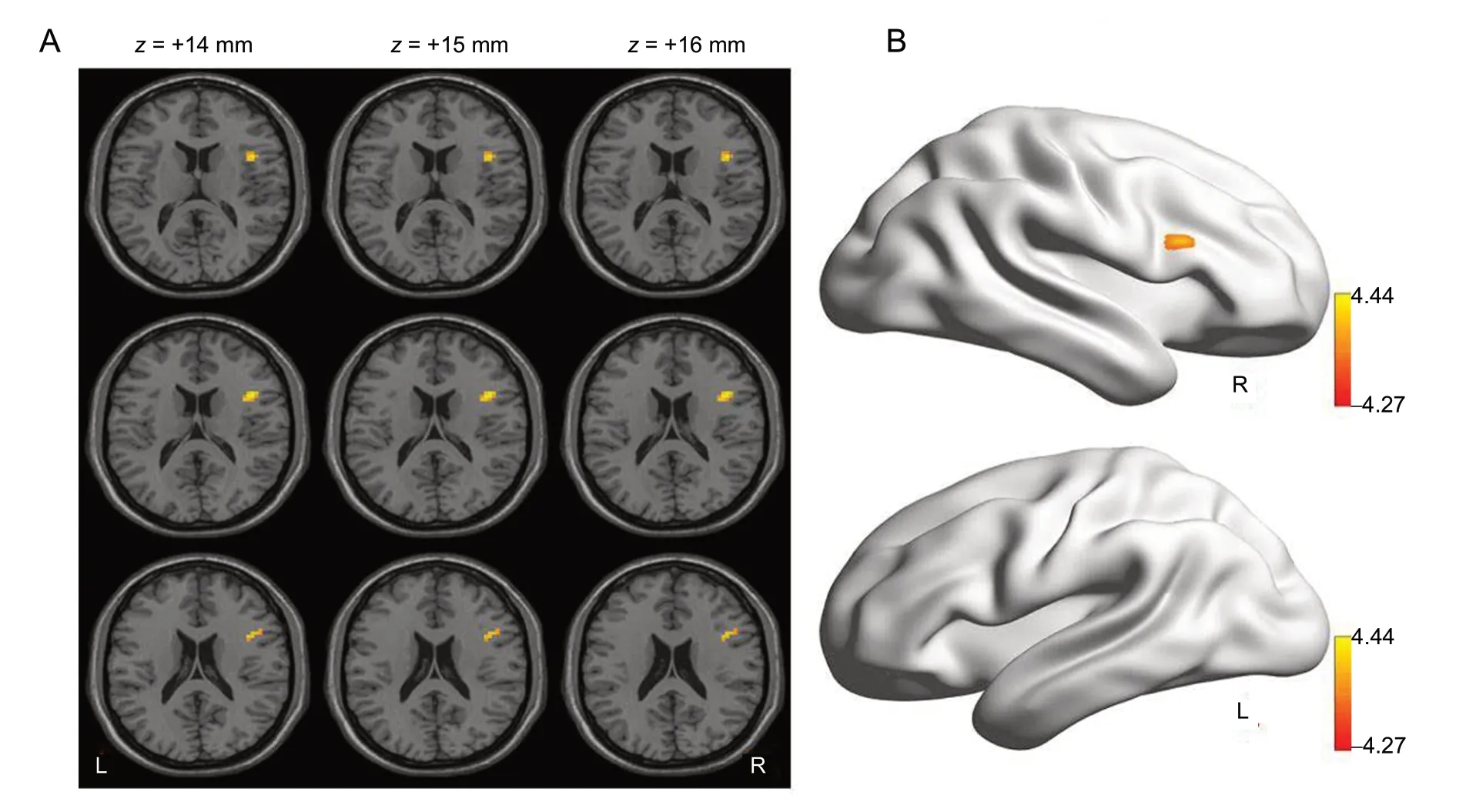
Figure 2 Statistical maps indicating t-test results of the z-score of the amplitude of low-frequency fluctuation differences between the suicidal attempt and non-suicidal attempt groups.
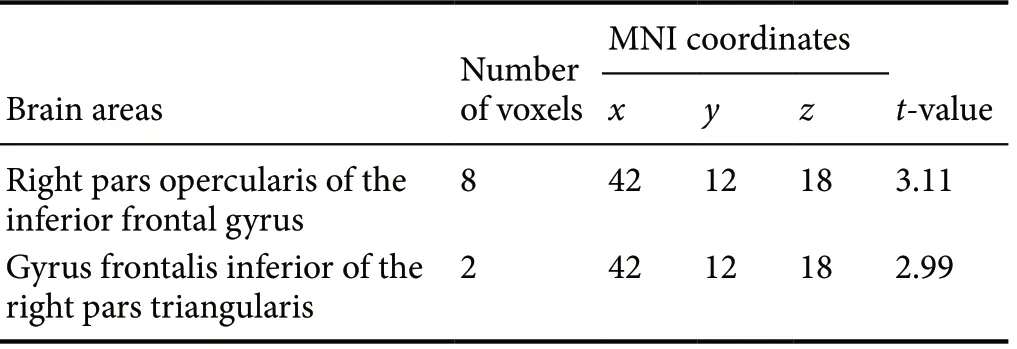
Table 2 Brain areas showing differences in the z-scores of the amplitude of low-frequency fluctuations between the suicidal attempt and non-suicidal attempt groups
Discussion
Internet addiction is characterized by compulsory Internet use and a high recurrence rate after withdrawal (Qi, 2011). A recent study showed that both Internet addiction and substance addiction originate from the same mechanism, leading to similar structural and functional changes in the brain (Ko et al., 2009). For example, hypofrontality changes the gray and white matter of the prefrontal lobe, reducing impulse control ability and predicting consequences in addicts (Mayberg, 2003; Helpern et al., 2011; Zhao et al., 2017). Qi (2011) found that abnormal changes in frontal lobe function might be involved in the generation and maintenance of Internet addiction.
Previous studies have shown that changes in brain function play a vital role in the occurrence of attempted suicide (Anand et al., 2007; Cao et al., 2016). Other studies have shown that changes in prefrontal lobe function play a critical role in suicidal behavior because impaired prefrontal lobe function could lead to decreased attention and memory, impaired executive function, increased negative emotions, increased sensitivity to frustration, or even cognitive and personality deficits (Jia et al., 2014). Prefrontal cortex dysfunction can lead to a decline in 5-hydroxytryptamine function (Fan et al., 2019), and many studies have confirmed that suicide attempts are associated with a decrease in brain 5-hydroxytryptamine function (Anand et al., 2007; Cao et al., 2016). Cao et al. (2015, 2016) found a decrease in brain activity associated with the left superior or middle frontal gyrus in patients with depression who attempted suicide.
The number of brain imaging studies comparing and analyzing Internet addicts with or without suicidal behaviors is relatively limited (Hamilton et al., 2012). In this study, rs-fMRI was used to explore the correlation between local changes in brain activity and suicidal behavior of adolescent Internet addicts and detect changes in automatic brain activity in the resting condition in the whole brain in the two groups. In the suicidal attempt group, activity was significantly increased in the gyrus frontalis inferior of the right pars opercularis and the right pars triangularis. The inferior frontal gyrus corresponds to Brodmann area 47 and is part of the prefrontal cortex. Roberts et al. (2017) confirmed that the inferior frontal gyrus is a critical brain area for emotional and cognitive control circuits, and any abnormal activity in this region is strongly associated with bipolar disorder. Our study confirmed that the prefrontal lobe of adolescents with attempted suicide because of Internet addiction had apparent functional abnormalities, which was consistent with previous studies (Kringelbach and Rolls, 2004; Gao et al., 2012). Internet addicts with an attempted suicide history have changes in the brain similar to those observed in patients with bipolar disorder. However, the causes related to such disorder remain unclear and will be examined in future studies.
Furthermore, this study confirms that the functional changes observed in the brain of Internet addicts who attempt suicide occur because of abnormal chemical changes in the prefrontal cortex. The subjects in the suicidal attempt and non-suicidal attempt groups were all Internet addicts, and their prefrontal lobes had abnormal changes. However, the results showed that the degree of change was more significant in the suicidal attempt group than in the non-suicidal attempt group, perhaps because the degree of Internet addiction in the suicidal attempt group was greater than that in the non-suicidal attempt group. Simultaneously, the prefrontal lobe of suicidal individuals exhibited abnormal functional changes. The inferior frontal gyrus activity in the suicidal attempt group was significantly increased because of the synergy of the two factors. It may be possible to explain this phenomenon in further studies by grouping subjects according to the degree of Internet addiction and observing the changes in brain function in suicidal subjects with increasing degrees of Internet addiction.
In this study, only the ALFF is used to compare and analyze the local brain function and to exclude the functional connectivity of brain areas. This is a preliminary exploratory study with a small sample size of 41 subjects, and it did not include stratified analysis according to the severity of Internet addiction.
In the resting state, the prefrontal lobe of the suicidal attempt group exhibited functional abnormalities compared with the non-suicidal attempt group, which may provide a new basis for studying the pathogenesis of suicide in Internet addicts.
Acknowledgments:The authors wish to show their appreciation to all investigators and all participants and express their thanks to all colleagues, for their valuable inputs to the study design and data collection.
Author contributions:Study concept and design, experimental implementation, data analysis, provision of reagents/materials/analysis tools, preparation of figures and tables, manuscript writing: YH; study concept, provision of reagents/materials/analysis tools: WW; study design, preparation of figures and tables: JC; experimental implementation, data analysis: MNX; study concept and design, experimental implementation, preparation of figures and tables, paper reviewer: LX; study concept and design, experimental implementation, manuscript writing: LK. All authors approved the final version of the paper.
Conflicts of interest:The authors declare that they have no conflicts of interest.
Financial support:This study was supported by a grant from Chongqing Science and Technology Commission of China, Nos. CSTC-2018jxj1130009, cstc2019 jscx-msxmX0279 (both to YH); the Traditional Chinese Medicine Scientific Research Fund from Chongqing Health Committee of China, No. 2019ZY023315 (to YH). The funders had no roles in the study design, conduction of experiment, data collection and analysis, decision to publish, or preparation of the manuscript.
Institutional review board statement:The study was authorized by the Ethics Committee of Chongqing Medical University, China (approval No. 2017 Scientific Research Ethics (2017-157)) on December 11, 2017.
Declaration of participant consent:The authors certify that they have obtained all appropriate participant consent forms from the participants or their legal guardians. In the forms, the participants or the legal guardians have given their consent for participants’ images and other clinical information to be reported in the journal. The participants or their legal guardians understand that the participants’ names and initials will not be published.
Reporting statement:The presentation of this study was in accordance with the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) statement.
Biostatistics statement:The statistical methods of this study were reviewed by the biostatistician of the Chongqing Traditional Chinese Medicine Hospital, China.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribu-tion-Non-Commercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:Juliana Dushanova, Bulgarian Academy of Sciencies, Bulgaria.
Additional files:
Additional file 1:Hospital Ethics Approval (Chinese).
Additional file 2:Informed Consent Form (Chinese).
CORRIGENDUM
Corrigendum: Genetic testing in neurology exploiting next generation sequencing: state of art
doi:10.4103/1673-5374.270164
In the article titled “Genetic testing in neurology exploiting next generation sequencing: state of art”, published on pages 265-266, Issue 2, Volume 15 of Neural Regeneration Research (Di Resta and Ferrari, 2020), the reference “Di Resta C, Spiga I, Presi S, Merella S, Pipitone GB, Manitto MP, Querques G, Parodi MB, Ferrari M, Carrera P (2018) Integration of multigene panels for the diagnosis of hereditary retinal disorders using Next Generation Sequencing and bioinformatics approaches. EJIFCC 29:15-25.” was cited inappropriately. The appropriate reference is “Di Resta C, Becchetti A (2010) Introduction to ion channels. Adv Exp Med Biol 674:9-21.” .
 中國(guó)神經(jīng)再生研究(英文版)2020年8期
中國(guó)神經(jīng)再生研究(英文版)2020年8期
- 中國(guó)神經(jīng)再生研究(英文版)的其它文章
- LIM kinases in synaptic plasticity and their potential as therapeutic targets
- Increased thalamocortical connectivity from the affected thalamus to the unaffected hemisphere in a stroke patient
- Expression of long non-coding RNAs in complete transection spinal cord injury: a transcriptomic analysis
- Inhibition of BACE1, the β-secretase implicated in Alzheimer’s disease, by a chondroitin sulfate extract from Sardina pilchardus
- Neuroprotective effect of deferoxamine on erastininduced ferroptosis in primary cortical neurons
- Human umbilical cord mesenchymal stem cells to treat spinal cord injury in the early chronic phase: study protocol for a prospective, multicenter, randomized, placebo-controlled, single-blinded clinical trial
