Structural Investigation of Alkaline-Earth Phosphosilicate Glasses Containing Six-Coordinated Silicon by Solid-State NMR
Feng Shi , Lili Hu , Jinjun Ren ,*, Qiuhong Yang School of Materials Science and Engineering, Shanghai University, Shanghai 00444, P. R. China.
2 Key Laboratory of Materials for High Power Laser, Shanghai Institute of Optics and Fine Mechanics, Chinese Academy of Sciences, Shanghai 201800, P. R. China.
Abstract:Phosphate glass is widely used in optical applications; however,its generally low chemical stability and poor thermal mechanical properties hinder the application of phosphate glass to the rapidly evolving laser industry. The addition of a small amount of silicon can form a six-coordinate Si (Si(6)) network and improve the above-mentioned poor properties of phosphate glass. Therefore, it is important to characterize and understand the structural details of phosphosilicate glasses. It is difficult to investigate the glass structure because of its complicated and disordered characteristics.However, solid-state nuclear magnetic resonance (NMR) spectroscopy can provide detailed local structural information, regardless of the presence of its long-range order. To study the effect of alkaline earth metals on Si(6) species formation, we prepared phosphosilicate glasses (2MO-3P2O5)(1?x)·(SiO2)x(M = Ca, Sr, Ba) by conventional melt-quenching, and the glass structure was investigated by solid-state NMR and Raman spectroscopy. The 31P and 29Si NMR spectra indicated that the glass networks consisted of P(2) and P(3) tetrahedrons linked via four- and six-fold coordinated silicon units (Si(4) and Si(6)). The fraction of six-coordinated silicon Si(6) decreased with increasing SiO2 content. Similarly, the Raman spectra showed that the vibration band of the P=O stretching mode in P(3)linked with Si(6) neighbors reduced as the silica content increased. The connectivities between various phosphorus species were probed by 31P one- and two-dimensional refocused INADEQUATE experiments. This experimental technique is based on homonuclear J-coupling and yields correlation peaks between nuclei engaged in P―O―P linkages (P(2) and P(3) units).The signals from isolated 31P nuclei are suppressed because of the absence of J-coupling, which precludes the formation of double quantum coherences. The results indicated the segregation of P(2) and P(3) units in the prepared glass, which were also compared with those in the previously reported Na2O-P2O5-SiO2 glasses. They differed from alkali phosphosilicate glasses, where each P(3) unit exhibited a maximum average of one Si(6)―O―P(3) linkage, and in the alkaline earth phosphosilicate glasses, the average was approximately 0.4–0.7. When the content of Si(6) units reached its maximum, further increase in the SiO2 content did not increase the Si(6) content, and the surplus Si were present as Si(4).Alkaline earth metal ions exhibit weaker stabilizing effects for Si(6) species. Based on the results presented herein, we constructed sketches to illustrate the local structural organization of the glass. The relationships between the compositions and structures are important for glass composition and property design. It is important to improve the performance of phosphate glass by changing its composition, particularly for large laser device applications.
Key Words:Solid state NMR;Glass structure;Six-coordination silica;Akaline-earth;Phosphosilicate glasses
1 Introduction
The properties of glass materials are strongly dependent on their compositions and concentrations involved. Usually, the performances of glasses are not simple superposition of corresponding constituents1–4, which has gathered much more attention of researchers to the interpretation of glasses structures.Mechanisms of property change can be explored effectively by clarifying glass structures. However, due to lack of any welldefined and repeating inter-connectivity patterns of the glass networks in the long range5, an exhaustive description of glasses structures is absent even for many simple glass systems.Researches on this issue are heavily focused on the local structures describing the short- and medium-range order of glass materials5–10. Recent years, various advanced solid-state nuclear magnetic resonance (SSNMR) spectroscopy technologies have been applied to characterize glass structures involving shortrange and medium-range features even for polybasic glasses11–16.Phosphate glasses with the addition of silicon is a major interest to the researchers for its six-coordinated Si (Si(6)) network former17,18. This is because a knowledge of the local structure of silicon in such glasses is profound to understand property changes in application environments. Such glasses are broadly used for the host material of optical devices, fiber communications and high-power/high-energy laser applications.Especially in the latter, the new generation of high-averagepower (HAP) laser glasses suffer a challenge for improving thermal shock resistance and assuring acceptable laser properties. The addition of silicon can well satisfy this requirement19–22. This is because Si(6)exist in these glasses. Si(6)can increase the network strength of glass structures much more significantly than Si(4). A small amount of Si(6)can greatly improve the thermo-mechanical properties and chemical stability of glasses25–31. Si(6)units have been observed in some phosphosilicate glasses and SiP2O7crystal23,24,27,30. Past studies suggest that Si(6)is dependent on the ratio of P/Si and modifying cations27–29. It was reported that the network modifiers, such as Na+ions, can stabilize Si(6)units via charge compensation in Na2O-P2O5-SiO2 glasses, and only low amounts of Si(6)units are being formed in binary SiO2-P2O5glasses18,30. Fleet et al.31,32reported that Si(6)is found to be largely independent on the details of the large cation sites in the glasses and the fraction of these sites occupied, based on the results from Si K-edge XANES. Recently, we have reported the detailed structural organization of29Si-rich sodium phosphosilicate glasses in the medium-range by comprehensive29Si and31P NMR techniques,and proposed a calculation model for quantitatively predicting the structural speciation based on glass compositions33. It makes sense for such a theoretical prediction route to be further extended to broader glass composition ranges.
In this work, we attempted to explore the effect of alkaline earth on the formation of Si(6)and P(3)units in MO-P2O5-SiO2(M = Ca, Sr, Ba) glass systems. The structural information of these samples was obtained by31P and29Si MAS NMR and Raman spectroscopy. Distributions of the P(n)and the Si(n)units and formation of the Si(6)unit were discussed in terms of the modifying cations and concentration of SiO2, combined with previous research on sodium phosphosilicate glasses.
2 Experimental
2.1 Sample preparation and characterization
The glass samples (2MO-3P2O5)(1?x)·(SiO2)x(M = Ca, Sr, Ba),with x = 0, 0.1, 0.15, 0.2, were prepared from mixtures of Ca(PO3)2(Taiyangkj, 99%), Ba(PO3)2(Taiyangkj, 99%),Sr(PO3)2(Taiyangkj, 99%), NH4H2PO4(Aladdin, 99%) and SiO2(Aladdin, 99.9%), and 0.1% (mole fraction) MnCO3(Aladdin,99%) were added in all batches to reduce the long T1relaxation time for both31P and29Si nuclei. The mixtures were pre-calcined at 500 °C in a platinum crucible for 1 h to remove NH3and H2O,and then melted for 20 min at 1100–1200 °C depending on the composition. The melts were subsequently cooled on a stainless steel plate to room temperature and stored in a drying cabinet.As-prepared samples are labeled as MPX, where M is C, S, and B for the CaO, SrO and BaO phosphosilicate glasses. X is corresponding to the mole fraction (%) of SiO2. The compositions were analyzed by inductively coupled plasma atomic emission spectroscopy (ICP-AES), and results were summarized in Section 3.3. Raman spectra were collected from 400 to 1500 cm?1by a Renishaw inVia Raman microscope using a 488 nm argon-ion laser as an excitation source.
2.2 Solid state NMR spectroscopy
All of the NMR experiments were recorded at room temperature on a Bruker Avance III HD 500M spectrometer(11.7 T). Samples were ground to fine power in an agate mortar and packed into either 4 or 2.5 mm rotors. Spectrum deconvolutions were done with DMFIT software package34.31P MAS NMR were carried out at 202.5 MHz using a 4 mm MAS NMR probe operated at spinning rate of 12.0 kHz, with 90° pulse length of 4.5 μs and a recycle delay of 30 s. Chemical shifts are reported relative to 85% H3PO4.29Si MAS NMR experiments were acquired at a resonance frequency of 99.3 MHz, using a 4 mm MAS NMR probe operated at a spinning rate of 8 kHz. The 90° pulse length of 6 μs and a recycle delay of 300 s were used for up to 800–1600 scans depending on the compositions. The chemical shifts of the29Si were referenced to Tetrakis(tetramethyl) silicate silane. Different phosphorus units were further probed by31P MAS one-dimensional (1D) and twodimensional (2D) refocused INADEQUATE experiments.Those31P nucleus involved in the P―O―P connectivity can be selected by 1D-refocused INADEQUATE experiments35,36,whereas isolated P species were filtered out because of the absence of J-coupling in them precluding the formation of double quantum coherences. 1D-refocused INADEQUATE spectra were recorded at a spinning speed of 12.0 kHz with a 4 mm MAS probe, using 90° pulses of 4.3 μs length and a double quantum coherence buildup time of 2τ = 7.5 ms. The recycle delay is 30 s. All single-quantum coherences were suppressed by appropriate phase cycling. In 2D-refocused INADEQUATE experiments, the correlation peaks appear at the sum of the offset frequencies in F1 dimension, whereas the F2 dimension represents the regular double-quantum filtered one-pulse spectrum. Double quantum coherences involving two chemically equivalent P-atoms show autocorrelation signals on the diagonal. The pulse sequences and coherence transfer pathway are shown in Fig. 1. 2D INADEQUATE spectra were recorded on a 2.5 mm MAS NMR probe at a spinning speed of 25.0 kHz, using 90° pulses length of 2.2 μs and a recycle delay of 30 s. A double quantum coherence buildup time of 2τ = 9.14 ms was used.
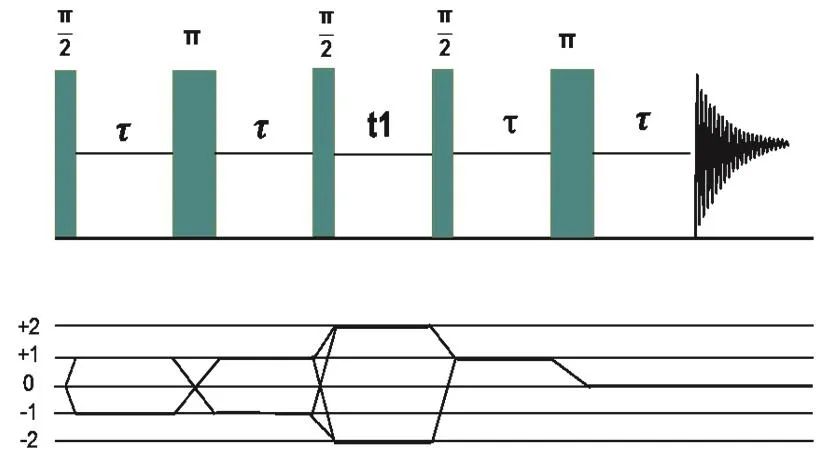
Fig. 1 Pulse sequence (top) and corresponding coherence transfer pathway (bottom) diagram of refocused INADEQUATE experiment.
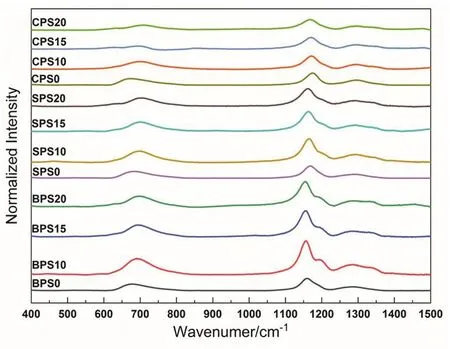
Fig. 2 Raman spectra of glass samples in(2MO-3P2O5)(1?x)·(SiO2)x system.
3 Results and discussion
3.1 Raman
The Raman results of all glasses were shown in Fig. 2. The glasses without SiO2have four peaks which can be ascribed to symmetrical stretching modes of P―O―P at 640 cm?1, PO?2(P(2)) at 1160 cm?1, P=O (P(3)) at 1290 cm?1, and a weak peak at about 1330 cm?1, respectively. With the addition of SiO2,several changes were observed in the Raman spectra. A new shoulder peak at 1200 cm?1appears, which was attributed to the P=O symmetrical stretching modes of P(3)units with Si(6)in their next nearest coordinate shells37–40. The vibrational frequency of P=O stretching mode in P(3)linking with a Si(6)neighbors is lower than that in P(3)without Si(6)neighbors. This is because silicon is less electronegative than phosphorus39. The bands at about 1330 cm?1increase with the addition of SiO2.Similar peaks were observed in modifier-free phosphosilicate glasses39. They could be ascribed to the P=O stretching of Si(6)-related double single phosphorus center (O=P―O―P=O).
3.2. 31P and 29Si MAS NMR
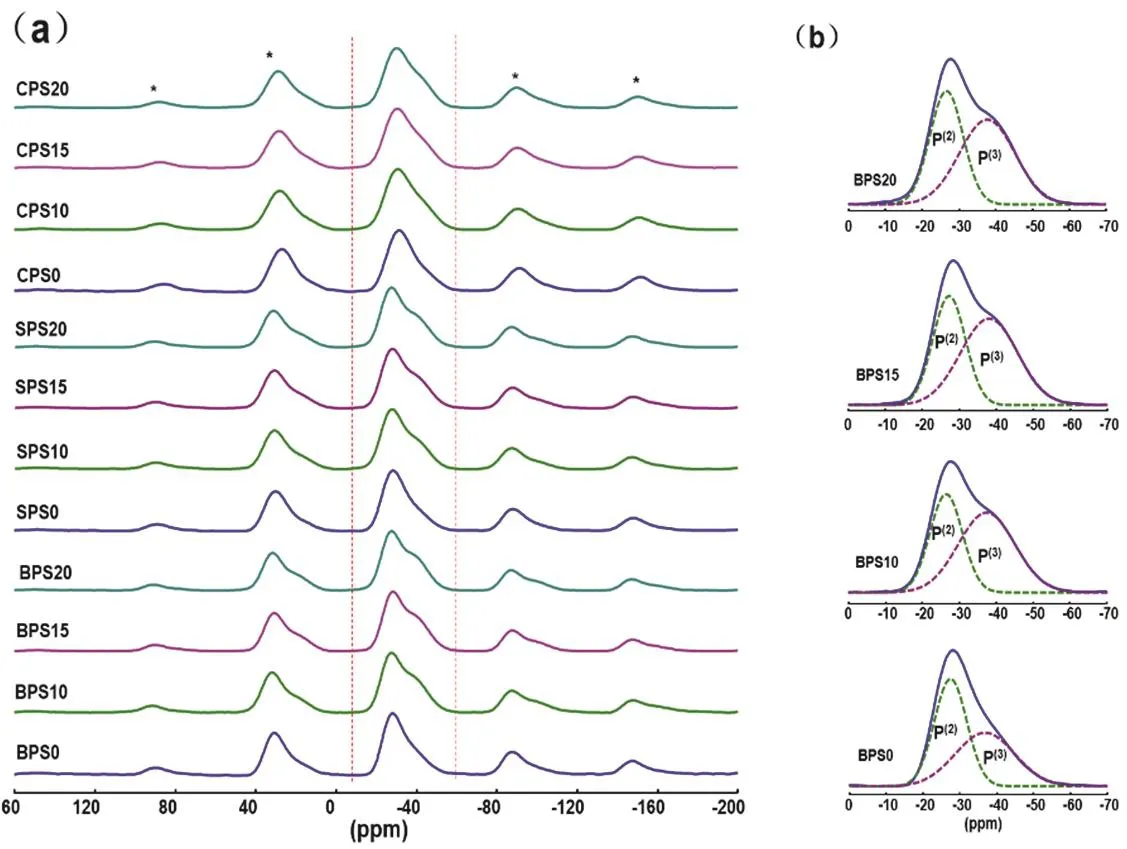
Fig. 3 31P MAS NMR spectra.
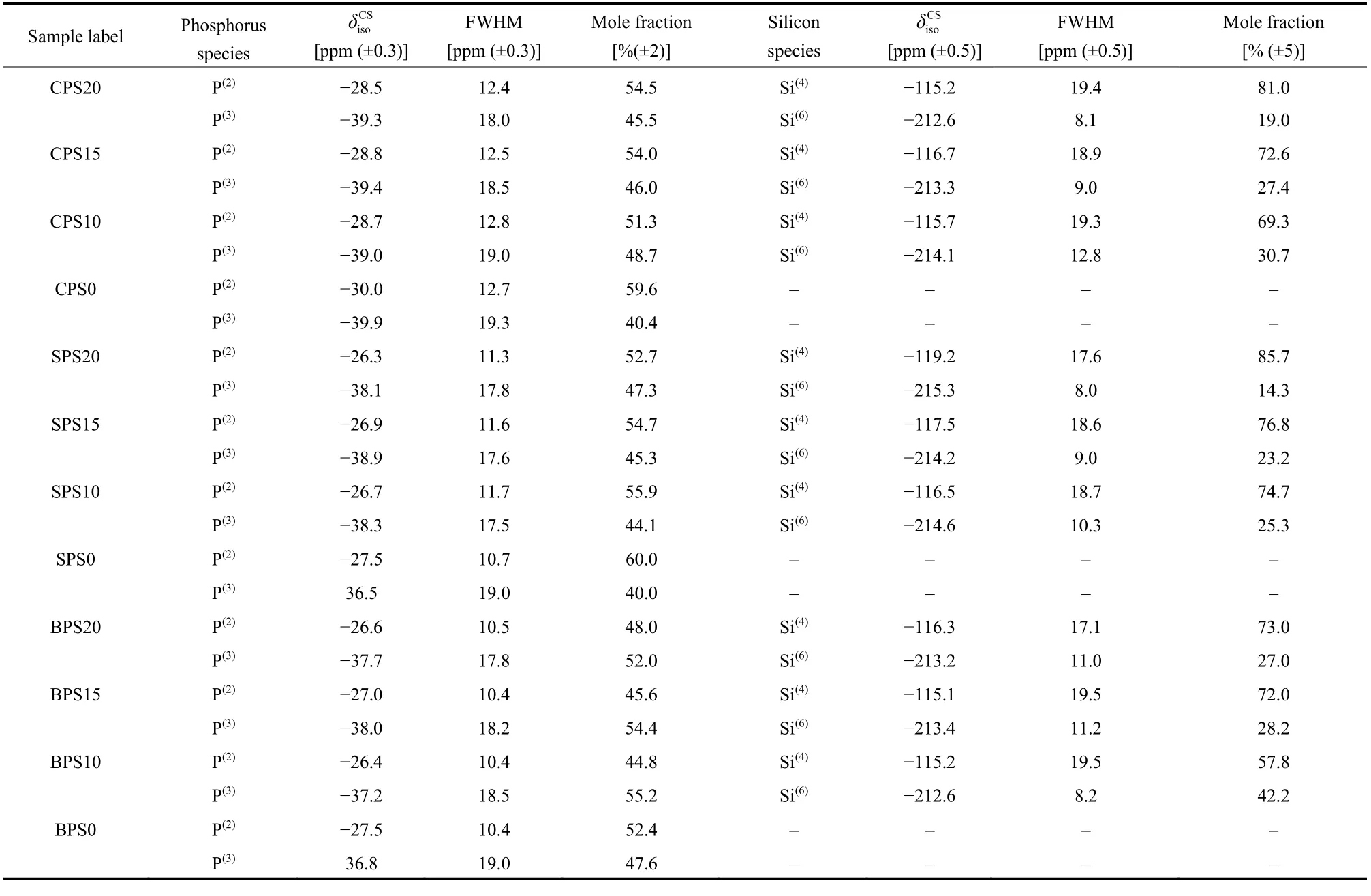
Table 1 31P and 29Si NMR spectral fitting parameters of glasses in the system (2MO-3P2O5)(1?x)·(SiO2)x shown in Fig. 3 and Fig. 5.
Fig. 3 summarizes31P MAS NMR spectra of samples in(2MO-3P2O5)(1?x)·(SiO2)xsystem and the deconvolutions of the31P spectra of representative samples. The spectra show one main peak at around ?28 ppm and a shoulder at about ?38 ppm, which are assigned to P(2)and P(3)units, respectively. The fitting parameters of all the spectra are summarized in Table 1. These two signals in all spectra are generally analogous because of the constant ratio of P/M in original composition. However, some differences still can be observed as the addition of SiO2and the change of alkaline-earth oxides (MO). The fractions of P(3)units obviously increase when SiO2was added into the glasses. But when the content of SiO2increases from 10% to 20%, the fractions of P(3)units have no significant change. These can be interpreted to the presence of P(3)―O―Si(6)linkages. Our previous research on29Si-enriched sodium phosphosilicate glasses has proved that Si(6)connects exclusively with P(3)forming P(3)―O―Si(6)linkages33. During the formation of P(3)―O―Si(6)linkages, the glass modifiers are transferred from P(2)to the P(3)to stabilize the Si(6)units, resulting in the increase of P(3)units. When the content of Si(6)reaches a certain value,the increase of SiO2will not increase the content of Si(6)any more and the surplus silica will exist as Si(4). Subsequently, the P(3)units will not increase either33. Here, the P(3)fraction has almost no significant change when SiO2content changes from 10% to 20%, which indicates the content of Si(6)has reached the maximum value.
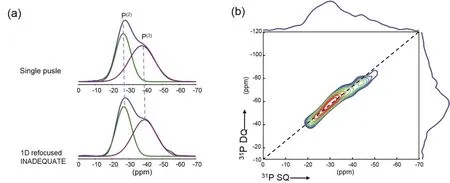
Fig. 4 31P refocused INADEQUATE spectra of sample BPS10.

Fig. 5 29Si MAS NMR spectra of samples in(2MO-3P2O5)(1?x)·(SiO2)x system.
The information of connectivity between various phosphorus species can be provided by 2D refocused INADEQUATE spectrum. Fig. 4b shows the 2D refocused INADEQUATE spectrum of sample BPS10 as a representative. Note that autocorrelations of P(2)and P(3)dominate in the 2D spectra. Both the single pulse and 1D refocused INADEQUATE spectra (see Fig.4a) indicate that there is comparable P(2)and P(3)content.However, no significant cross-correlation between P(2)and P(3)species was observed, which indicates the segregation of P(2)and P(3)in the glasses.
Fig. 5 shows29Si MAS NMR spectra of samples. Two signals are observed near ?115 and ?213 ppm, which should be ascribed to Si(4)and Si(6)units, respectively. There is a very weak signal at around ?166 ppm, which indicates there might be some Si(5)units in these glasses41–44. The fitting parameters of29Si NMR spectra are listed in Table 1. The Si(5)signals are too weak to fit.In all the three glass systems, the fraction of Si(6)decreases with increasing the content of SiO2,. Similar results were observed in the Na2O-SiO2-P2O5glass systems33,38. In the Na2O-SiO2-P2O5glass system, the Si(6)content increases up to the maximum value when SiO2reaches a certain value and, with furthermore increment of SiO2content, the surplus silica will exist as Si(4).Subsequently, the fraction of Si(6)decreases with the increase of SiO2content after Si(6)content reaches the maximum value.Please note that although the fraction of Si(6)decreases obviously, the content of Si(6), which equals to the fraction of Si(6)multiplying the content of SiO2, has not changed such much.This is because Si(6)content will be kept to the maximum value.The significant Si(4)signals here indicate that in these glasses Si(6)have reached their maximum values.
3.3. Distributions of local structures
Table 2 lists the fractions of P(3)and Si(6)units and related ratios in glasses from this work and relevant literatures. Previous studies on sodium phosphosilicate glasses have proved that one P(3)unit can have an average of one P(3)―O―Si(6)linkages at most and per Si(6)connects with six P(3)units through six P(3)―O―Si(6)linkages. Na+ions are necessary for the formation of Si(6)33. In order to study the effect of glass modifiers on the formation of Si(6)units, the [6Si(6)/P(3)] values are calculated and listed in Table 2. It could be found that in Na2O-P2O5-SiO2glass systems, when Si(6)content has reached the maximum value,which is indicated by the exist of Si(4)units in the glasses, the maximum [6Si(6)/P(3)] values are close to 1. This indicates that per P(3)unit can have maximum one P(3)―O―Si(6)linkages.However, here in the alkaline earth phosphosilicate glasses, the maximum values are close to 0.4–0.7, which is significantly smaller than that in Na2O-P2O5-SiO2glass systems. This indicates alkaline earth ions have much weaker stabilizing effect on the formation of Si(6)than Na+ions.The sketches of Si(6)and Si(4)are shown in Fig. 6 to illustrate glass structural organization.
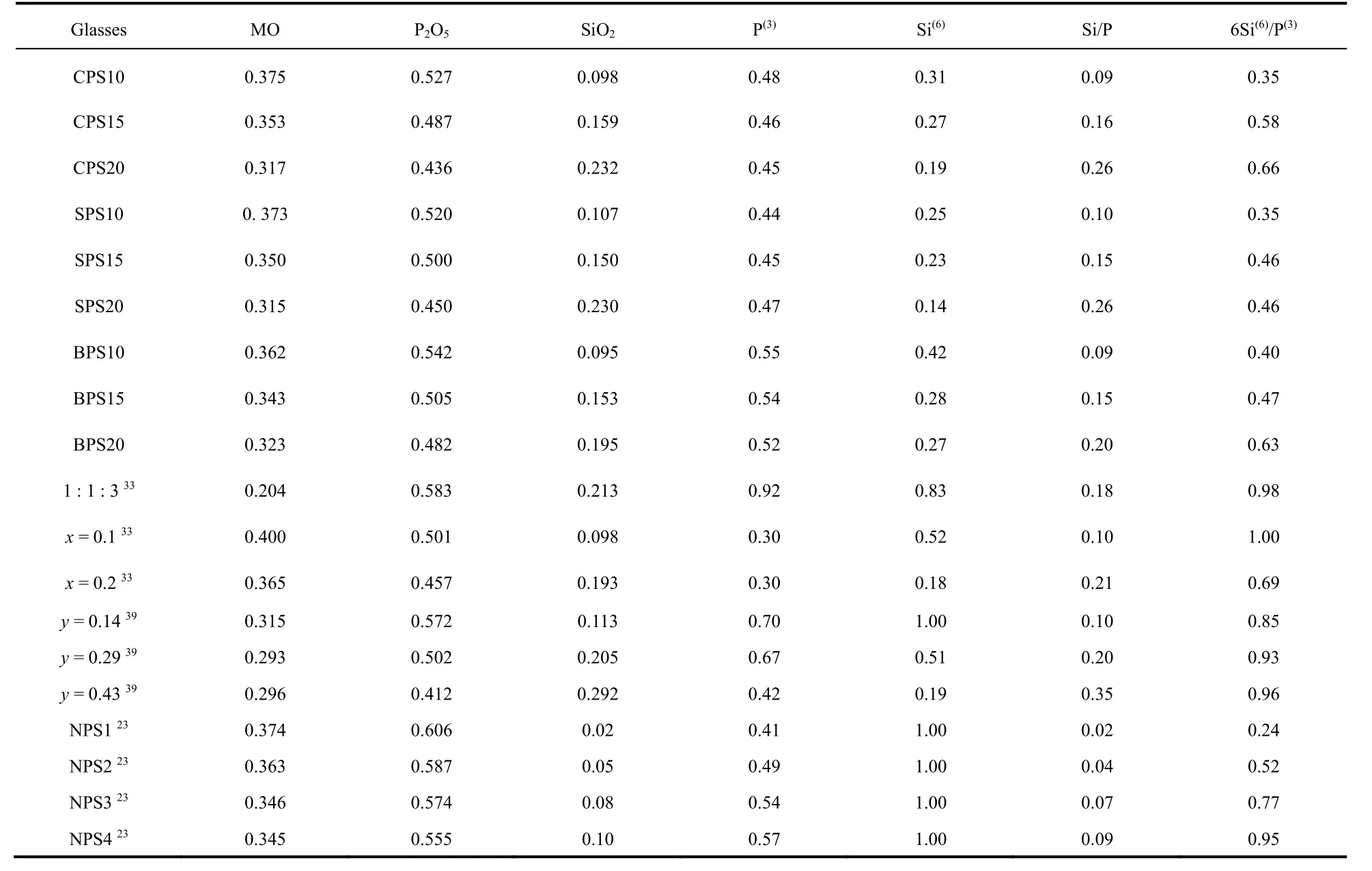
Table 2 Compositions, fractions of P(3) (±0.05) and Si(6) (±0.05) units, the content ratio of silicon and phosphorus, and the fraction ratio (±0.1) of Si(6) and P(3) in glass samples from this work and literature a.
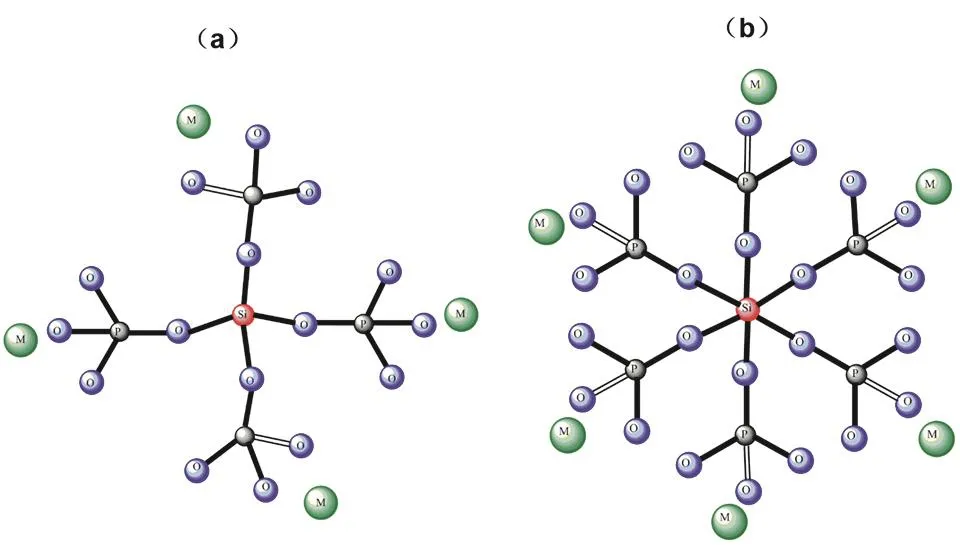
Fig. 6 The sketch of Si(4) (a) and Si(6) (b) in (2MO-3P2O5)(1?x)?(SiO2)x glass system.
4 Conclusions
In summary, we have studied the structure of (2MO-3P2O5)(1?x)·(SiO2)x(M = Ca, Sr, Ba) glasses by solid state NMR techniques. P(2), P(3), Si(6)and Si(4)structure units exist in these glasses. Segregations of P(2)and P(3)was observed. Si(6)units connect with P(3)units. In these alkaline earth phosphosilicate glass systems, average P(3)units can have at most 0.4–0.7 P(3)―O―Si(6)linkage. When the content of Si(6)units reaches the maximum value, the further increment of SiO2content will not continue to increase the content of Si(6). The surplus Si atoms will exist as Si(4). In Na2O-P2O5-SiO2glasses, per P(3)unit has a maximum of one Si(6)―O―P(3)linkage, which indicates that the alkaline earth metal ions have weaker effects on stabilizing Si(6).This work has developed the relationship between the glass compositions and structures of alkaline earth phosphosilicate glass systems. The results here could be used for the designs of glass compositions and properties.
Acknowledgment:We thank Yongchun Xu for the ICPAES measurement and Sasa Yan for assistance with Roman spectroscopy.

