Surveilling Russell body Helicobacter pylori-negative gastritis: A case report and review of literature
Milena Peruhova, Monika Peshevska-Sekulovska, Viktoriya Georgieva, Gabriela Panayotova, Dorian Dikov
Abstract
Key words: Russell body gastritis; Helicobacter pylori-negative; Treatment; Mature plasma cells; Case report
INTRODUCTION
The first case ofHelicobacter pylori(H. pylori)-related Russell body gastritis (RBG) was announced in 1998 by Tazawaet al[1]. They described thatH. pylori-positive gastritis is characterized by localized accumulation of plasma cells containing Russell bodies (RB) in gastric mucosa[1]. RB represent nondegradable, condensed immunoglobulin disposed in endoplasmic dilated rough reticulum cistern of plasma cells[2].
Most of the cases reported in the English literature so far are aboutH. pylori-positive RBG[3,4]. The main theory regarding the pathogenesis of RBG includes chronic infection withH. pylorileading to abnormal secretion of immunoglobulin or their derivates by plasma cells and subsequent formation of intracellular RB[5].
The fewH. pylori-negative RBG cases that have been published were associated with HIV infection, alcohol and drug abuse, concomitant carcinoma and plasma cell neoplasms.
The clinical and endoscopic manifestation of RBG are variable and non-specific[6,7]. This rare type of chronic gastritis should be distinguished from other malignancies of the stomach such as carcinoma, lymphoma and plasmacytoma.
We present a case from our practice withH. pylori-negative RBG, who underwent endoscopic and histological surveillance three times over a period of one year.
We also made a review of twenty-one cases ofH. pylori-negative RBG published in the literature up to now with their specific and unique clinical, endoscopic and histopathological features.
CASE PRESENTATION
Chief complaints
We present a case of a 51-year-old male who underwent endoscopic screening for mild iron deficiency anemia. The patient had no upper and lower gastrointestinal (GI) complaints.
History of present illness
The iron-deficiency anemia was diagnosed 2 mo before the admission to the hospital.
History of past illness
He was without other co-morbidities and past history for illness.
Physical examination
From the physical examination, he had pale skin and visible mucous membranes.
Laboratory examinations
The laboratory work-up showed hemoglobin = 107 g/L, serum iron was 10.2 μmol/L (11.6-31.3 μmol/L), ferritin 18.43 ng/mL (30-400 ng/mL), total iron binding capacity 83.2 μmol/L (45-72 μmol/L). Inflammatory serological markers were within the normal limits–CRP was 0.30 ml/L (0-5 mg/L). Other biochemistry test results as well as carcinoembryonic antigen and carbohydrate antigen 19-9, were within the normal limits.
Imaging examinations
The colonoscopy was unremarkable. However, upper GI endoscopy revealed diffuse hyperemia, edema and nodularity of the gastric mucosa in the fundus and body, with a clear demarcation line between the body and the antrum. (Figure 1) The duodenal mucosa was intact. Due to non-specific endoscopic findings and iron-deficiency anemia our preliminary diagnoses were diffuse gastric carcinoma or gastric lymphoma. Therefore, multiple biopsies were taken from the stomach. We did not obtain duodenal, ileal and colonic biopsies, as there were no endoscopic abnormalities of the mucosa. We performed whole-body computed tomography with contrast enhancement. It showed neither pulmonary and abdominal space-occupying lesions, nor bone lytic lesions and enlarged lymph nodes.
Histologically, in the biopsy of fundic mucosa, we observed inflammatory infiltrate rich in plasma cells with numerous eosinophilic hyaline bodies (RB) and so-called mature plasma cells (Mott cells), consisting entirely of cytoplasmic RB (Figure 2A). Several hyperplastic lymphoid follicles were also observed. The plasma cells expressed CD79a (Figure 3A), CD138 and they showed polyclonal pattern, both expressed kappa (Figure 3B) and lambda IgM light immunoglobulin chains (Figure 3C). А large number of eosinophils were seen and the neutrophilic leucocytes were rare.
There was no evidence of nuclear atypia and mitosis of the plasma cells, which ruled out lymphoma. Giemsa staining forH. pyloriinfection was negative, as well as immunohistochemical detection. Negative staining for cytokeratin AE1/AE3 excluded the signet-ring cell carcinoma. This microscopic finding corresponds to the so-called RBG.
We ruled out HIV, HCV and HBV infections, as well as autoimmune diseases, such as autoimmune thyroiditis and rheumatoid arthritis. However we did not check for M protein in the serum, we did not perform Bence-Jones protein urine test, TB-spot, EBER in situ hybridization or trephine biopsy of the bone marrow.
FINAL DIAGNOSIS
Histopathological findings confirmedH. pylori-negative RBG.
TREATMENT
The patient was treated with proton-pump inhibitor (PPI) (Esomeprazole)-20 mg bid for 8 wk and intravenous iron medication.
OUTCOME AND FOLLOW-UP
Long-term clinical and endoscopic surveillance was scheduled. Three months later, he came for follow-up. His blood tests showed slight increase of his hemoglobin level (117 g/L). He underwent second gastroscopy with endoscopic findings identical to the previous one. Diathermic snare was used which allowed obtaining of larger and deeper tissue specimen of gastric mucosa. Histology report revealed dense accumulation of plasma cells in lamina propria, with decreased distribution of RB (Figure 2B). Intestinal metaplasia was observed in the areas with plasma cell infiltration but without dysplasia. Histopathological findings from third gastroscopy, performed twelve months after the initial diagnosis, demonstrated that the chronic inflammatory infiltrate in the fundic mucosa is less pronounced, rich in plasma cells, with almost absent RB and Mott cells (Figure 2C).
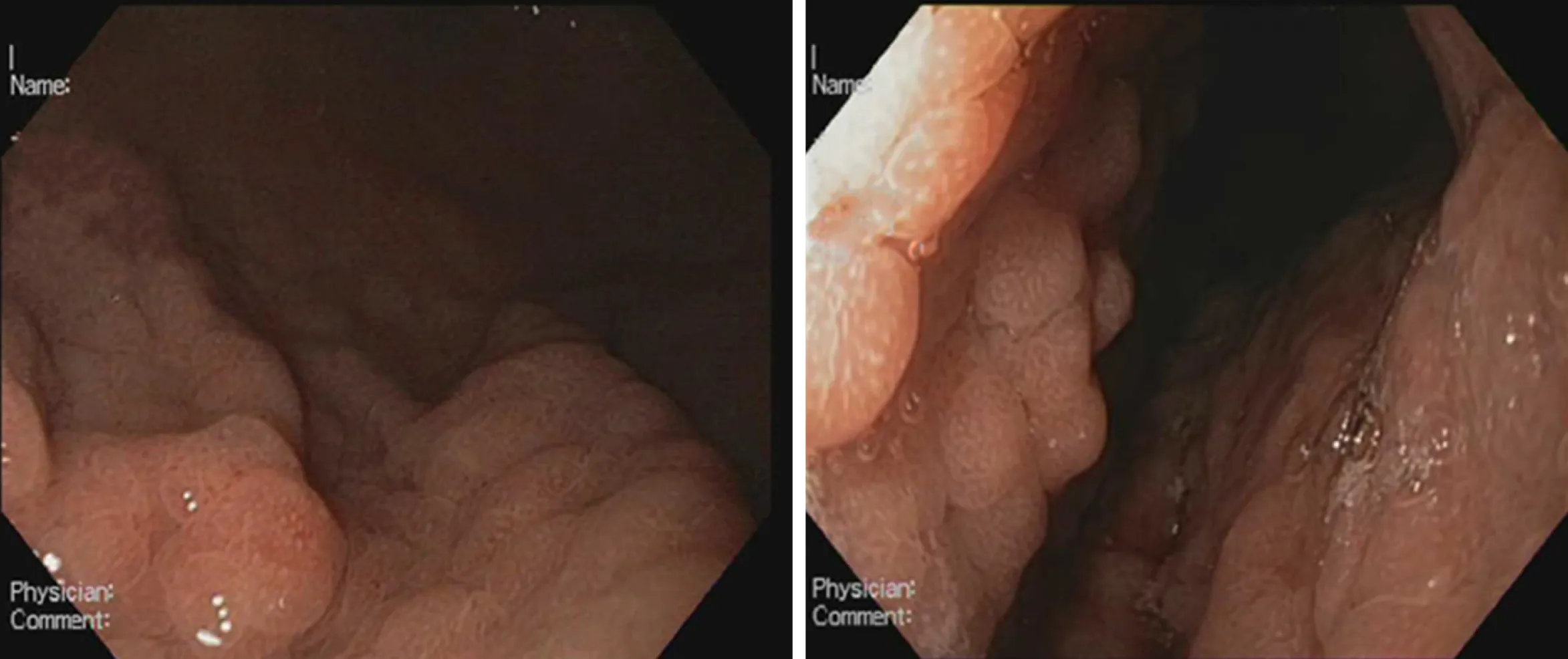
Figure 1 Endoscopic appearance of Helicobacter pylori-negative Russell body gastritis.
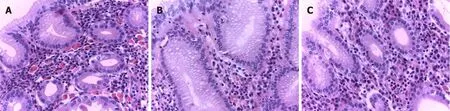
Figure 2 Russell body gastritis of the fundus mucosa in the standard histological stain Hematoxylin-eosin, × 400. A: The initial biopsy specimen shows abundant plasma cell inflammatory infiltrate, rich in Russell body and mature plasma cell; B: The follow-up biopsy revealed no change in plasma cell inflammatory infiltrate, but with decreased distribution Russell body and mature plasma cells; C: Third biopsy, twelve months after the initial diagnosis, demonstrated that chronic inflammatory infiltrate in the fundus mucosa is less pronounced, rich in plasma cells, with almost absent Russell body and mature plasma cells.
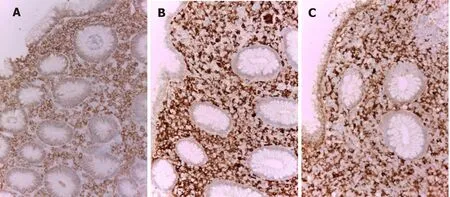
Figure 3 Immunohistochemical stains in Russell body gastritis (initial biopsy) × 200. A: The inflammatory infiltrate in the gastric fundus mucosa is CD79a positive, which is in support of its homogeneous plasmocytic nature; B: Kappa; C: Lambda. The plasma cells are polyclonal both kappa (B) and lambda (C) light chains are positive.
DISCUSSION
RBG is a rare form of chronic gastritis which mostly affects the antrum and has a male predominance[8,9]. The diagnosis is histologic, and it is characterized by accumulation of plasma cells containing RB and Mott cells in gastric mucosa. According to the literature, mucosal infiltration with RB and Mott cells may be associated with infectious or autoimmune processes[8,10-13]. The diagnostic and differential-diagnostic histological algorithm includes immunohistochemical detection of plasma cell nature of the inflammatory infiltrate (CD138 and CD79a positivity), as well as its polyclonality (both kappa and lambda light immunoglobulin M chains expression). These immunohistochemical profiles, as well as the absence of nuclear atypia, mitotic activity, lymphoepithelial lesions and monoclonal infiltrates are most in keeping with a benign process and not with a lymphoproliferative disease (lymphoma or myeloma).
Signet-ring stomach adenocarcinoma, where the cells resemble RB, but show nuclear atypia, mitotic activity and cytokeratin expression must also be excluded[14,15].
Once the histologic diagnosis and differential-diagnosis have been made, the pathologist must prove or rule out the association withH. pyloriinfection. This is done with Giemsa staining or immunohistochemical detection.
Literature data for RBG histologic follow up are scarce.
We have the opportunity to follow up in three consecutive biopsies the histological evolution of gastric mucosa in RBG. Our results showed a tendency from decrease up to complete extinction of RB and Mott cells in this chronic gastritis over time and under the influence of treatment. Our histologic follow up results indicate that RBG is probably an inconstant and dynamic morphological finding developing within a rich of plasma cells chronic gastritis. Like other authors we observe decreased number of RB and Mott cells in the time[8]. In contrast to this study, our results show that the reason for decreased number of RB in the stomach is notH. pylorieradication. Probably, the factor causing RB formation is not onlyH. pyloriinfection, other factors may also play role such as local degenerative or vascular-circulatory phenomena.
In one study, a total of 15 cases of RBG with polyclonal plasma cells, containing RB (Mott cells) have been described[8]. Our case also showed polyclonal proliferation of plasma cells with RB with uneventful clinical follow up. The decreased number of Mott cells in the stomach afterH. pylorieradication shows thatH. pyloriis one of the factors causing RB formation. In practical terms, the latter can be used as an additional diagnostic sign in contrast to the increased follow up distribution of RB in the context of multiple myeloma-associated RBG[6].
Diffuse infiltration of plasma cells with RB in the gastric mucosa requires differential diagnosis with several diseases. Cytokeratin negativity and CD79a positivity exclude signet-ring cell carcinoma of the stomach. Kappa and lambda polyclonal immunoreactive pattern exclude lymphoplasmacytic lymphoma[1-3,5,7,16,17], plasmacytoma and monoclonal gammopathy of undetermined significance[4]. The lesion can be differentiated from MALT lymphoma by absence of nuclear atypia and lymphoepithelial lesions[8,18-20].
There are many unclear points about the etiology of RBG. According to Hasegawa[21], immunoglobulin accumulation could be in a result of an over or altered production as well as aberrant secretion and impaired excretion[21].
Most of the cases published in the literature have demonstrated a connection betweenH. pyloriinfection and antigenic stimulation of plasma cells[5,7]. On the other hand,H. pylori-negative RBG is rarely reported. To the best of our knowledge, only twenty-one cases are published in the literature so far. There are insufficient data about the etiology and progression of this entity. A possible relationship betweenH. pylori-negative RBG and the immune status has been proposed, with a number of cases reported in patients with HIV[10-12], alcohol[10,16,22]and drug abuse[10,13,16]and posttransplant patients[23].
Apart from chronic infections and immunosuppressive treatment, cancer could also be a trigger of immune dysregulation and RBC has been reported in patients with signet-ring cell carcinoma[14]. For this reason, it is of great importance to be able to discriminate between cancer-induced mucosal changes and RBG. During upper endoscopy this entity should be kept in mind because of the vast majority of differential diagnosis such as plasma cell neoplasms, signet-ring cell carcinoma and MALT lymphoma. It is also of great significance to obtain biopsies according to Sydney system.
Our case is about 51-year-old man with iron-deficiency anemia andH. pylorinegative RBG. We performed many diagnostic tests to rule out chronic infections, autoimmune diseases associated with B cell proliferation and different malignancies of gastric mucosa. We came to the conclusion that RBG in our patient is a benign process with uncertain prognosis and long-term clinical and endoscopic follow-up was scheduled. For a period of one year we observed histologic regression, most probably in a result of his 8-wk-treatment with PPI. Hence, PPI therapy could be considered as a feasible option of treatment for this rare type of gastritis.
In our article we present all published cases in English literature withH. pylorinegative RBG so far.
A summary of 22 reportedH. pylori-negative RBG is described in Table 1.
Aggregated data from all the reported cases show that patients are within the age range of 20 to 82 years with predominant age group between 70-80 years (40.9%). Male to female ratio was 2.14:1. Most of the patients had GI symptoms such as abdominal pain, dyspepsia and nausea. Endoscopic findings include vast spectrum of nonspecific features such as erythema, edema, erosions and ulcerations or well-formed nodular lesions. In eight of the patientsH. pylori-negative RBG was localized in the gastric antrum (36.36%), in three of them it was in body of the stomach (13.63%), in the cardia (13.63%), in more than one region of the stomach (13.63%) and without specific localization in three of the patients (13.63%). According to our data, RBs distribution is the rarest in the fundus (9.09%) (Figure 4). Of all the twenty-two cases, two patients had anemia as concomitant disease[24], three reported alcohol abuse[10,16,22]and three with HIV infection[10-12](Figure 5). Other associated conditions include multiple myeloma, chronic kidney failure, drug abuse, post kidney transplant, diabetes mellitus and colonic adenoma. Majority of the cases (ten) withH. pylori-negative RBG showed no evidence of concurrent medical conditions. In this literature review it is well visible that two of the cases of H. pylori-negative RBG are associated with lymphoproliferative disease (multiple myeloma)[6,24-28].
CONCLUSION
We would like to summarize that there are few cases ofH. pylori-negative RBG described in the literature. This condition should be kept in mind during endoscopic surveillance and differentiated from other benign and malignant entities. To the best of our knowledge, our report is the first that present a long-term follow up in a patientH. pylori-negative RBG, treated with PPI. We came to conclusion that PPI therapy leads to significant reduction of RB and plasma cells in the gastric mucosa.
So far little is known about its etiology and pathogenesis, thus larger studies must be conducted. The outcome of chronic stimulation of the Mott cells is unknown, therefore it is of paramount importance to actively follow up these patients.
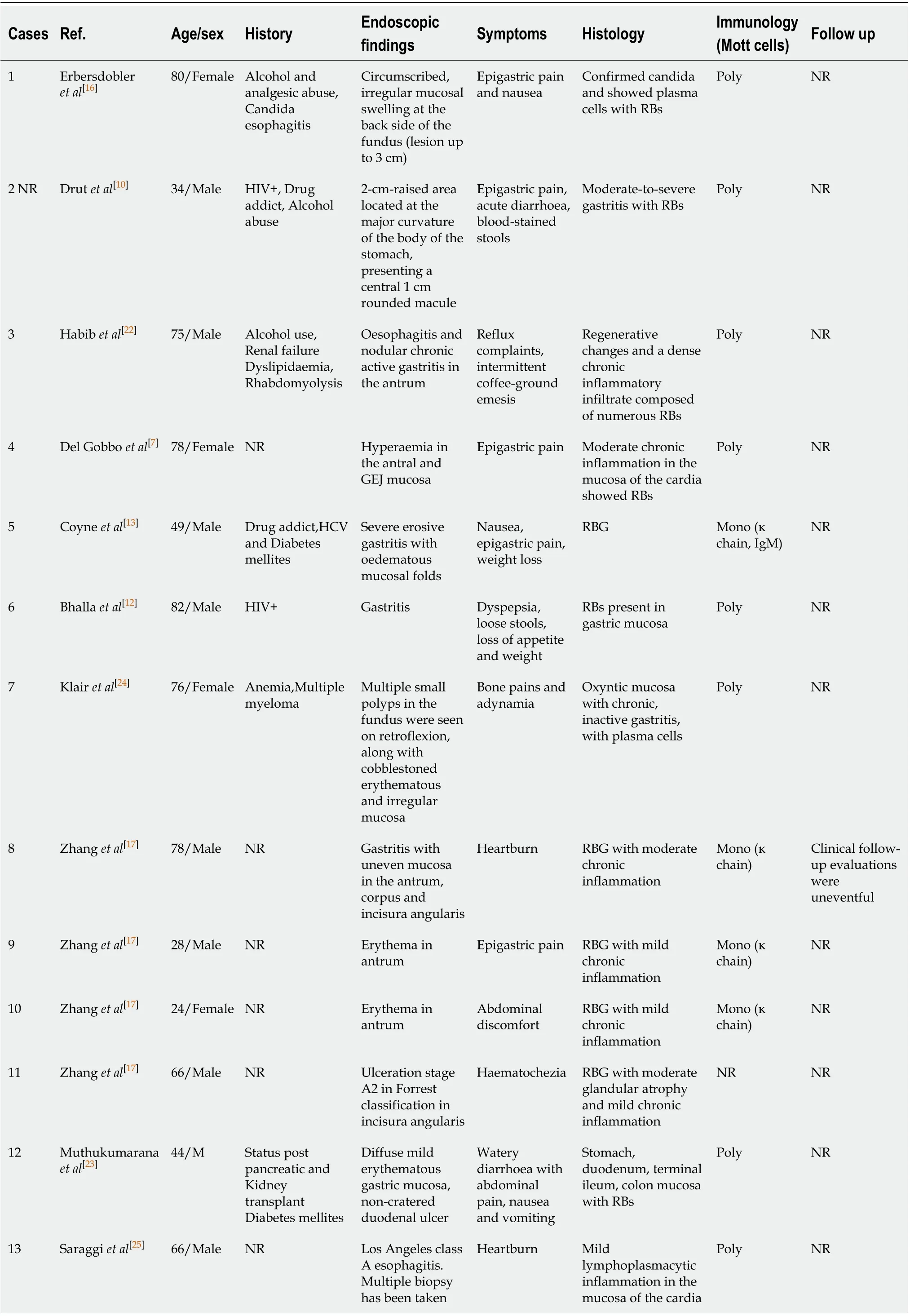
Table 1 A summary of 22 reported Helicobacter pylori-negative Russell body gastritis is described
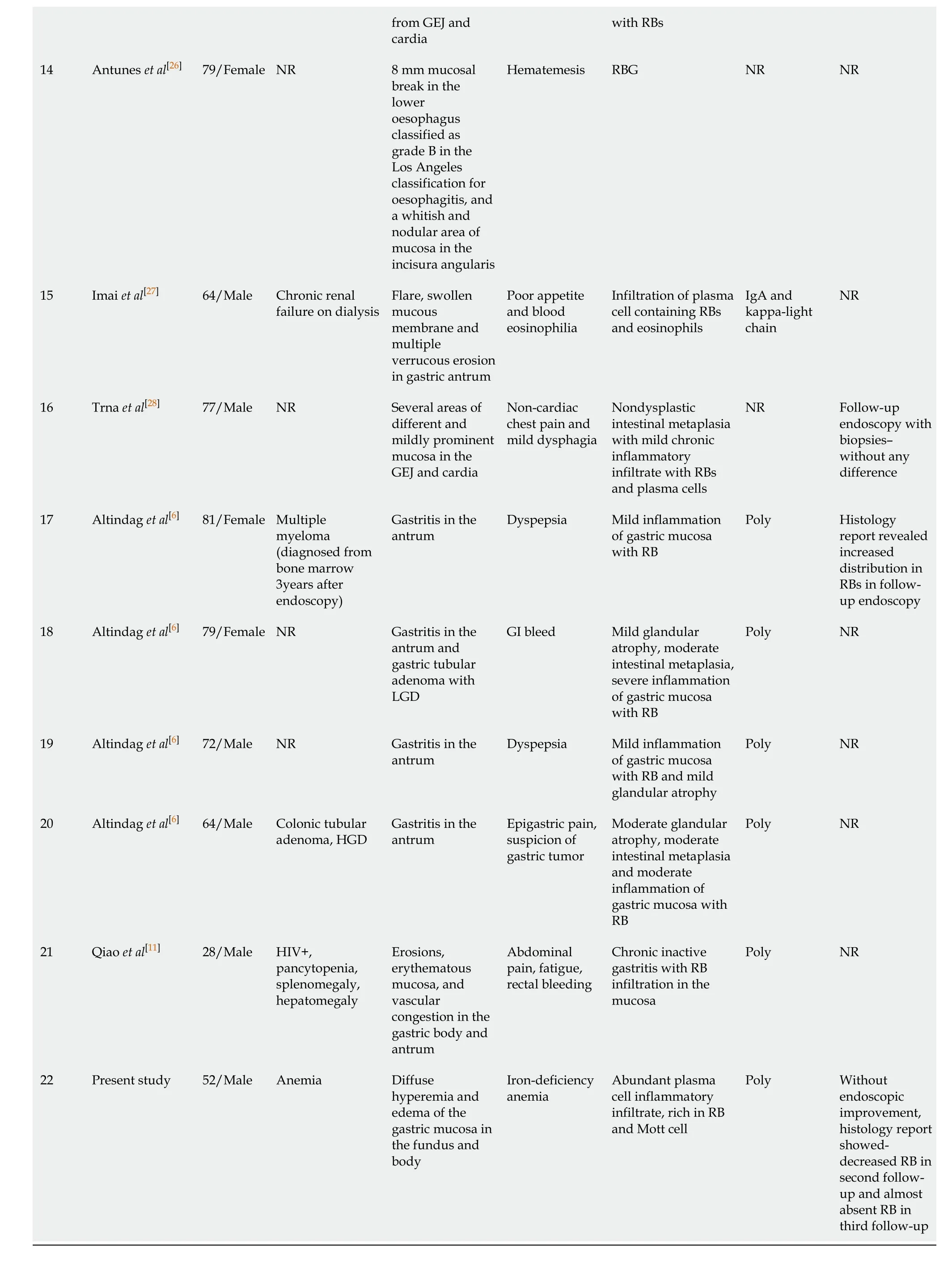
GI: Gastrointestinal; NR: Not reported; RB: Russell body; IgA: Immunoglobulin A; GEJ: Gastro-oesophageal junction; RBG: Russell body gastritis.
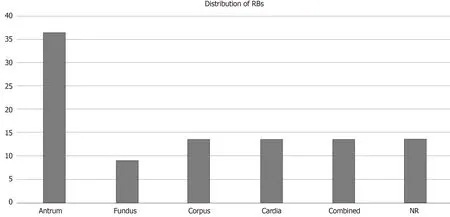
Figure 4 Distribution of Russell bodies in the stomach: in all cases from the literature. RB: Russell body.
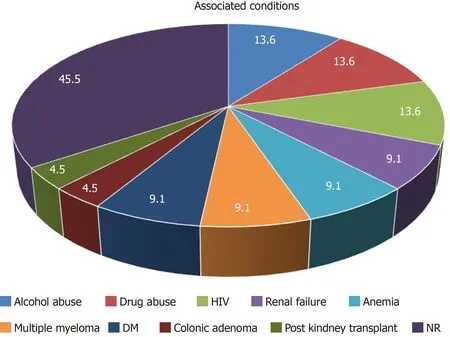
Figure 5 Associated conditions in patients with Helicobacter pylori-negative Russell body gastritis according to the available literature.
 World Journal of Gastroenterology2020年33期
World Journal of Gastroenterology2020年33期
- World Journal of Gastroenterology的其它文章
- Bowel function and quality of life after minimally invasive colectomy with D3 lymphadenectomy for rightsided colon adenocarcinoma
- Treatment repurposing for inflammatory bowel disease using literature-related discovery and innovation
- Effects of denosumab treatment in chronic liver disease patients with osteoporosis
- Neutrophil to lymphocyte ratio and albumin bilirubin grade in hepatocellular carcinoma: A systematic review
- Radiomics of rectal cancer for predicting distant metastasis and overall survival
- Liver fat accumulation measured by high-speed T2-corrected multi-echo magnetic resonance spectroscopy can predict risk of cholelithiasis
