could microRNA expression tell us more about colorectal serrated pathway carcinogenesis?
Milena Peruhova, Monika Peshevska-Sekulovska, Boris Krastev, Gabriela Panayotova, Viktoriya Georgieva,Rossitza Konakchieva, Georgi Nikolaev, Tsvetelina Veselinova Velikova
Abstract In the last two decades, the vision of a unique carcinogenesis model for colorectal carcinoma (CRC) has completely changed. In addition to the adenoma to carcinoma transition, colorectal carcinogenesis can also occur via the serrated pathway. Small non-coding RNA, known as microRNAs (miRNAs), were also shown to be involved in progression towards malignancy. Furthermore, increased expression of certain miRNAs in premalignant sessile serrated lesions (SSLs) was found, emphasizing their role in the serrated pathway progression towards colon cancer. Since miRNAs function as post-transcriptional gene regulators, they have enormous potential to be used as useful biomarkers for CRC and screening in patients with SSLs particularly. In this review, we have summarized the most relevant information about the specific role of miRNAs and their relevant signaling pathways among different serrated lesions and polyps as well as in serrated adenocarcinoma. Additional focus is put on the correlation between gut immunity and miRNA expression in the serrated pathway, which remains unstudied.
Key Words: MicroRNA; Serrated pathway; Carcinogenesis; Colorectal carcinoma; Sessile serrated lesions; Adenocarcinoma
INTRODUCTION
Colorectal cancer (CRC) is the most prevalent cancer in Western countries and the second cause of cancer-related death[1]. Obesity, sedentary lifestyle, tobacco and alcohol consumption are considered the driving factor behind the growth of CRC[2]. In the last two decades the vision of a unique carcinogenesis model for CRC has completely changed. The most prevalent genetic events accompanying CRC development are mutations that de-regulate the Wnt signaling cascade. In particular, inactivating mutations in the tumor suppressor adenomatous polyposis coli (APC) are considered the earliest genetic lesions sufficient to initiate tumorigenesis[3].
In addition to the adenoma to carcinoma sequence, colorectal carcinogenesis can also occurviathe serrated pathway. After the identification of serrated carcinomas by Jasset al[4]in 1992, the underlying genetic and epigenetic alterations have been described. In most serrated polyps, the pathway is believed to be the acquisition of a mutation in a gene that regulates mitogen-activated protein kinase (MAPK) pathway, disruptions to the Wnt signaling pathway and widespread methylation of CpG islands[5,6].
A class of small non-coding RNAs, designated as microRNAs (miRNAs), are involved in progression towards malignancy. miRNAs act as tumor suppressors or oncogenes depending on the characteristics of their downstream targets[7].They function as post-transcriptional gene regulators and have been increasingly recognized as useful biomarkers for CRC[8].
A plethora of studies have documented aberrant miRNA levels in CRC, but only a few of them relate to serrated pathway carcinogenesis[9]. There is even less data about different miRNA expression profiling in serrated adenomas with different grades of dysplasia[10]. In contrast to the conventional colorectal carcinogenesis, the pivotal role of miRNAs in the serrated pathway is still to be elucidated because of the insufficient number of studies conducted to clarify separate steps in serrated carcinogenesis[11].
Many of the published reviews in the English literature about the serrated pathway have been focused on histological, endoscopic, and molecular features[12,13]. However, there are a few data about post-transcriptional gene regulation, in particular, the expression of miRNAs in the serrated pathway in CRC. We aimed to interrogate the role of miRNAs in relevant signaling pathways in serrated carcinogenesis.
Emerging new approaches revealed increased expression of certain miRNAs in premalignant sessile serrated lesions (SSLs), emphasizing their role in the serrated pathway progression towards colon cancer[14]. This could make miRNAs potential biomarkers for screening in patients with SSLs[15,16].
In this review, we summarized the most relevant information about the specific role of miRNAs among different serrated lesions and polyps as well as in serrated adenocarcinoma (SAC). Additionally, the review is the first that looks at the correlation between gut immunity and miRNA expression in the serrated pathway.
MORPHOLOGICAL ASPECTS OF SERRATED POLYPS AND SAC
Based on the literature, the percentage prevalence of serrated pathway is highly variable, ranging from 15% up to 30% of all CRCs[17-20].
According to the 5th edition of WHO classification of colorectal serrated lesions and polyps, they are classified into three histopathological subtypes: Hyperplastic polyps (HPs), SSLs, and traditional serrated adenomas (TSAs)[21](Figure 1). TSAs are extremely rare < 1% of all colorectal polyps, while HPs are the most common, comprising approximately 75% of all serrated polyps. SSLs (previously known as sessile serrated adenomas or sessile serrated polyps) cause nearly 25% of serrated polyps[22].
HPs are usually small, rarely cause symptoms, and have minimal malignant potential. However, it was established that HPs could progress to SSLs or TSAs for a period of 7.5 years[23]. In this context, HPs may predispose to cancer because of their ability to transform into serrated lesions[24]. These lesions could be found anywhere in the colon, but they are mostly placed in the distal colon (70%-80%)[25]. It was established that HPs, with right-side localization, are more likely to have malignant potential[26-28].
Clinical characteristics, such as size, location, and endoscopic appearance, can support the identification of SSLs but are not sufficient for their identification. Approximately 10% of SSLs could lead to sporadic CRCsviathe serrated polypcarcinoma sequence[29].
In most series, TSAs account for < 1% of all colorectal polyps, represent about 1%-2% of the serrated lesions and are located predominantly in the left colon[30-32].
SAC is characterized by mainly right-sided location of the colon, specific molecular features and female predominance. Percentage prevalence of SAC is about 7.5%-8.7% of all CRCs and according to the literature it has worse prognosis than conventional CRC[6,33].
EPIGENETIC AND GENETIC ASPECTS IN SERRATED PATHWAY
CpG methylator phenotype
Toyotaet al[34]introduced the CpG island methylator phenotype (CIMP) in 1999.Methylation is an epigenetic process where a methyl group (CH3) is added to the cytosine nucleotide at a CpG dinucleotide group. The process of methylation of gene promoters is a physiological mechanism by which gene expression is regulated without altering the DNA sequence[35,36].
Transcriptional silencing of essential tumor suppressor genes, caused by aberrant DNA methylation, could promote neoplastic growth. This aberrant methylator has been called the CIMP and is thought to be important in the serrated pathway in CRC[37].
Using eight markers, Oginoet al[38]classified CIMP in CRC into three subgroups, CIMP-low (CIMP-L), CIMP-high (CIMP-H), and CIMP-negative, according to the numbers of methylated promoters.
With the growing impact of translational research and molecular pathology, the CRC pathogenesis became more elucidated based on the association of CIMP and key mutations inKRAS,BRAF,PIK3CA,TP53, andAPC. Furthermore, microsatellite instability (MSI), caused by dysfunction of DNA mismatch repair (MMR) genes, is considered another critical pathway in carcinogenesis[39].
MSI mechanism in CRC
The MSI mechanism in CRC was first described in relation to Lynch syndrome, where germline mutations take place in specific MMR genes such asMLH1,MSH2,MSH6, andPMS2[40]. Germline deletions at 3’ end of theEPCAMgene which lead to decreased MSH2 expression were also demonstrated as a recurrent cause of Lynch syndrome[41]. Furthermore, functional relevance ofMSH3mutations for the development and inheritance of CRC were reported, but their role in the serrated pathway needs further analysis and more cohort studies[42,43]. Evidence has shown that mutations in MSI are vital points in the developing malignancy in 3%-15% of all CRC[42,43]. About 80% of MSI CRCs are characterized by the hypermethylation ofMLH1, while 20% of MSI CRCs by mutations in MMR genes[44]. MSI status could be subclassified into MSI-high (MSI-H), MSI-low (MSI-L) and microsatellite stable (MSS) according to the number of mutations in microsatellite sequences[45].
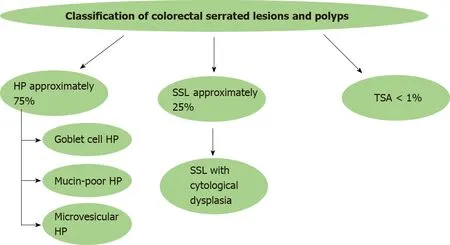
Figure 1 Schematic presentation of classification of colorectal serrated lesions and polyps. HP: Hyperplastic polyp; SSL: Sessile serrated lesion; TSA: Traditional serrated adenoma.
Alteration of MMR genes due to epigenetic silencing by sporadic, acquired hypermethylation of theMLH1gene promoter leads to the serrated pathway in CRC[44].
Serrated colorectal malignancies are characterized by CIMP-H,MLH1promoter hypermethylation, and MSI andBRAFmutations[46].
BRAF / KRAS gene mutations in serrated CRC
Serrated colorectal lesions rarely bеаr truncatingAPCmutations, but the most frequent genetic alterations involveBRAFmutations, whereasKRASmutations are less common[47]. BothKRASandBRAFbelong to the MAPK signaling pathway, mediating cell proliferation, apoptosis and differentiation[48].
BRAFgene encodes a protein called B-Raf, which plays a pivotal role in regulating the MAPK/ERKs signaling pathway[49]. Recent findings in molecular biology demonstrated that mutations inBRAFare found in about 10% of CRC patients[50].BRAF-mutated CRCs are associated with the female gender, often right-sided, mucinous histology, and advanced stage[51].BRAFmutations are considered as early events in CIMP cancers by inhibition of normal apoptosis in colonic mucosa[52]. Many recent studies classified two different molecular phenotypes of CRC based onBRAFmutation status:BRAFV600E- and non-V600-mutated CRC[53]. А correlation between serrated carcinogenesis andBRAFV600E mutation was established, which induce CIMP-H status and methylation ofMLH1promoter[54]. In contrast to the conventional adenomas, the earliest event in serrated precursor lesions areBRAFmutations and hypermethylation, which leads to transformation of aberrant crypt foci (ACF) to microvesicular HP and then to SSLs. Methylation and loss of key tumor suppressor genes such asp16andMLH1are the key points in SSLs’ progression to SAC[55]. Interesting information about theBRAFmutated/MSS SACs was reported by Bondet al[55]. They found out that hypermethylation events occurred inBRAFmutated SACs more often than in conventional pathway (respectively 60% and 3%)[55].BRAFV600Emutated CRCs are with dismal prognosis and resistance to standard systemic chemotherapy[56,57].
Another significant driver in the serrated pathway isKRASmutations[58]. Opposite to the traditional model of Vogelstein, where aberrant activation of Wnt pathway has been observed, high frequency ofKRASmutations was established in TSAs. In contrast to SSLs, TSA lesions showedMGMThypermethylation, but notMLH1promoter hypermethylation. Based on this evidence, a non-MLH1mutating SSL could progress to a TSA and ultimately develop into aBRAF-mutated MSS tumor (Figure 2)[59,60].
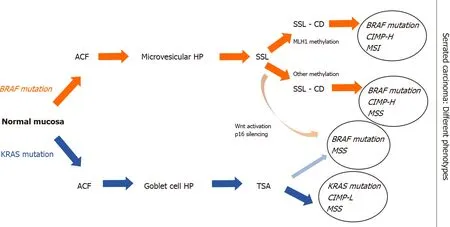
Figure 2 Outline of the schematic serrated pathway progression. In red color we indicate the steps of transformation of BRAF-mutated serrated lesions. BRAF mutations and hypermethylation lead to transformation of aberrant crypt foci to microvesicular hyperplastic polyp then to sessile serrated lesions (SSLs). Methylation and loss of key tumor suppressor genes such as p16 and MLH1 are the key points in SSLs’ progression to serrated adenocarcinoma. In blue color we indicate KRAS mutations in traditional serrated adenomas (TSAs), which showed MGMT hypermethylation, but not MLH1 promoter hypermethylation. In light red shading we indicate a non-MLH1 mutating SSL, which could progress to a TSA and ultimately develop into a BRAF-mutated microsatellite stability tumor. ACF: Aberrant crypt foci; HP: Hyperplastic polyp; SSL: Sessile serrated lesion; SSL-CD: Sessile serrated lesion with cytological dysplasia; TSA: Traditional serrated adenoma; CIMP: CpG island hypermethylator phenotype; CIMP-H: CIMP-high; CIMP-L: CIMP-low; MSI: Microsatellite instability; MSS: Microsatellite stability.
MIRNA PROFILE FROM PREMALIGNANT SERRATED LESIONS TO CRC
miRNAs were discovered in Caenorhabditis elegans by Leeet al[61]in 1993 while studying the genelin-14. However, the scientific community became aware of the importance of miRNAs seven years later when they were recognized as a specific class of biological regulators. miRNAs are small, single-stranded, non-coding RNAs (18-24 nucleotides) that can post-transcriptionally regulate the expression of various oncogenes and tumor suppressor genes[62]. Also, they play an essential role in cancer development, proliferation, regression, and metastasis. Even though their role in cancer progression is yet to be elucidated, several studies reported the influence of specific miRNA alterations in premalignant and malignant lesions[63-66]. miRNA expression profiling gives us the opportunity to understand and identify differences between benign and malignant lesions of the colon mucosa, as well as to stratify benign lesions according to their malignant potential[67].
The role of miRNA-125b, miRNA-222, miRNA-214, miRNA-335 in CRC carcinogenesis
In this scenario, several studies showed a unique miRNA signature in different types of colonic polyps, as well as in the progression of serrated lesions.
Tsikitiset al[68]profiled miRNA patterns in screen-detected polyps in relation to histologic features and cancer-related risk. miRNA expression analysis was carried out on biopsy specimens from 109 patients. The specimens were obtained from normal mucosa (NM), HPs, tubular adenomas (TAs), tubulovillous adenomas, or high-grade dysplasia (TVHGs), SSLs, and TSAs. They have not found a significant difference in the expression of miRNA between TSAs and SSLs. miRNAs expression pattern was similar in TSAs and HGTVs, whilst there were several differentially expressed miRNAs between HPNMs and TSAs. Additionally, they performed pairwise comparisons of non-serrated tissues and serrated lesions. miRNAs-222 and miRNA-214 were significantly downregulated by 2.35- and 1.51-fold respectively in serrated polyps, whereas miRNA-335 was significantly overexpressed by two-fold in nonserrated tissues. Tsikitiset al[68]drew the conclusion that the downregulation of miRNA-125b and miRNA-320a in the serrated pathway may be used as independent predictors of progression with a concordance index of 84.7%.
Opposite to the serrated pathway, in the conventional adenoma-carcinoma sequence, many studies showed a high expression of miRNA-125b in advanced tumor size. Another correlation was found between the overexpression of miRNA-125b, which leads to repression of the endogenous level of p53 protein in human CRC cells. Cancer progression and poor outcomes were associated with overexpression of miRNA-125b in the conventional colorectal pathway[69].
The role of miRNA-31 in carcinogenesis of serrated pathway of the colorectum
However, many studies showed that miRNA-31 plays a pivotal role in serrated carcinogenesis. In this scenario, miRNA-31 is located at 9p21.3 and is frequently overexpressed in sessile serrated adenomas. Aokiet al[70]analyzed in their case report miRNA-31 expression using quantitative reverse transcription-PCR in patients with early invasive CRC with HP component. Their results showed higher miRNA-31 expression in the carcinoma component compared to HP component. They revealed that progression of HP (or SSLs) to SAC is likely to be associated with overexpression of miRNA-31.
To shed light on the role of miRNA31 on the serrated pathway, Kanthet al[11]conducted a study of 108 colon biopsies with distinct histology types. Different expression was established in 23 miRNAs between NM and serrated lesions. Additionally, six miRNAs showed a different expression pattern between SSLs and HPs, as miRNA-31-5p has been the most significantly modulated.
Noshoet al[71]based on miRNA array analysis, identified that miRNA-31 was the most upregulated inBRAF(V600E) mutation, compared toBRAF-wild type CRCs. Moreover, they performed transfection of the miRNA-31 inhibitor and consequently showed that miRNA-31 might regulateBRAFactivation in CRCs. Therefore, miRNA-31 could be used as a diagnostic biomarker as well as a feasible therapeutic target in the future. Finally, they proved that high miRNA-31 expression was associated with shorter prognosis in patients with CRC.
Higher miRNA-31 expression was associated with cell proliferation and survival in development in CRC, as well as tumor invasion and poor prognosis[72-75]. Kubotaet al[76]pointed out that miRNA-31 could be a potential prognostic biomarker in their study of patients with stage IV of CRC. They also found out a correlation between miRNA-31 overexpression and poor tumor differentiation, as well as advanced disease stages.
Recent studies showed the presence of miRNA-31 in the serum of patients with metastatic CRC, who were treated with anti-EGFR therapy. Igarashiet al[77]found out a correlation between high miRNA-31-5p expression and shorter PFS in CRC patients treated with anti-EGFR therapeutics. Their theory suggested that miRNA-31-5p could be a useful prognostic biomarker for anti-EGFR therapy.
Even though the underlying mechanisms of the role of miRNA-31-5p in CRC remain unknown. It has been postulated that miRNA-31 can directly bind to the 3' untranslated region (3' UTR) of SATB2, which takes part in regulation of transcription and chromatin remodeling. Overexpression of miRNA-31-5p could induce epithelialmesenchymal transition, tumorigenesis, and progression in CRC[78].
Furthermore, another correlation between the expression of miRNA-31 and CRCassociated fibroblast (CAFs) was established, but notin vivoexperimental models. Yanget al[79]elucidated that miRNA-31 inhibits autophagy in CAFs and alters colorectal proliferation and invasion of CRC cells. Thus, more studies must be conducted in this direction because of the lack ofin vivoexperimental models.
Relevance of miRNA-135-B in CRC
In many studies, it has been reported that overexpression of miRNA-135-B has been associated withAPCdysfunction in CRC, leading to the promotion of tumorproliferation, progression, and invasion[63,80]. It was established that miRNA-135-B had been associated with the serrated pathway and colorectal carcinogenesis.
Only few studies indicate that specific miRNA profiles can be used to distinguish neoplastic from benign lesions in colon mucosa[6].A study by Kanthet al[11]was the first that showed the overexpression of specific miRNAs in serrated polyps or serrated carcinoma. In summary, they provided a comprehensive analysis of miRNA gene expression in SSLs, by identifying miRNA-135B, miRNA-378A, miRNA-548, miRNA-9, and miRNA-196B. miRNA-378A-3p was significantly downregulated in SSLs compared to normal colon mucosa. They suggested that these miRNAs are good predictors in SSLs to carcinoma transformation. Additionally, they discovered that miRNA-9 and miRNA-196b were also de-regulated in SSL compared to HP. These miRNAs showed different expression patterns inBRAFmutated-MSI tumors. Interestingly, reduced expression of miRNA-196B has been detected in the plasma of patients with CIMP-positive SSLs or MSI colon cancers[11].
The involvement of miRNA-21 in CRC
MiRNA-21 is one of the most eminent miRNAs involved in the genesis and progression of CRC. Evidence implied that miRNA-21 negatively regulates tumor suppressor phosphatase and tensin homolog (PTEN) gene, which played an essential role in cell proliferation and invasion in CRC[81-84]. An interesting study by Ghareibet al[85]established that miRNA-21 in serum could be feasible, non-invasive biomarker with high sensitivity and specificity (95.8% and 91.7%) for early detection and prognosis in patients with CRC.
In addition, Chenet al[86]report a correlation between tissue and serum miRNA-21 overexpression and poor prognosis in patients with CRC. It is more significant in colon cancers, compared to rectal.
Another interesting study by Yauet al[87]presents the potential role of fecal-based miRNA-21 and miRNA-92a as non-invasive biomarkers for CRC screening. They reported higher expression of miRNA-21 and miRNA-92a in patients with advanced distal CRC compared to the proximal localization, without significant value in the detection of early CRC.
miRNA-21 down-regulates tumor suppressor PDCD4, thus stimulating cancer cell invasion and intravasation. Moreover, the high level of miRNA-21 was associated with metastasis and resistance to chemotherapy of 5-FU in CRC. Thus, it makes miRNA-21 a potential non-invasive biomarker for diagnostic and prognosis for CRC[88].
Recently, several studies have reported the correlation between expression of miRNA-21 and serrated pathway in CRC. A study by Schmitzet al[89]demonstrated different expression of miRNA-21 among NM, HPs, and SSLs. They found overexpression of miRNA-21 in SSLs, whereas normal colon mucosa and HPs exhibited no differences. Opposite to them, Kanthet al[11]proved that there was no statistically significant expression of miRNA-21 in SSLs.
Future investigations are necessary to find out the correlation between expression levels of miRNA-21 and genetic and epigenetic alterations of SSLs.
The role of miRNA-181a-2 in the development of serrated pathway in CRC
miRNA-181 plays a pivotal role in regulation at the post-transcriptional level in many different types of cancer. More specifically, the expression of miRNA-181a and miRNA-181b are strongly associated with the mutation status of the tumor suppressor genep53in colorectal carcinogenesis[90]. The underlying mechanism of how miRNA-181a influences conventional colorectal carcinogenesis could be based on upregulation miRNA-181a through the activation of the Wnt/β-catenin pathway[91].
Little is known about the expression of miRNA-181a in the serrated pathway. A comprehensive analysis of miRNA profile in SACs and MSI-H CRC has been carried out by Kondelovaet al[10]Interesting information about the molecular features of miRNA expression in SACs and MSI-H CRC has been elucidated. Microarray assay showed that 223 miRNAs were differently expressed, as 75 of them were downregulated in SACs compared to MSI-H CRC. On the other hand, 148 miRNAs were upregulated in the same comparison group. Notably, only miRNA-181a-2 showed significant overexpression in MSI-H CRC compared to SACs. It has been established that miRNA-181a-2 has an inverse correlation with nicotinamide phosphoribosyl transferase, which is a transcription factor playing a significant role in organogenesis and stem cell development[92].
In conclusion, their analysis showed that miRNA-181a-2 plays a role in development in different subtypes of CRC from the serrated pathological pathway. Additionally, the up-regulation of miRNA181a-2 was associated with MSI-H status. This study may be a foundation for further researches aiming to elucidate the function of miRNA-181a-2 in CRC[10].
Other significant miRNAs in serrated pathway
Slatteryet al[15]have carried out promising research about different miRNA expression between NM and different types of polyps. They made a comprehensive analysis of miRNA expression among adenomatous polyp (AD), SSLs, and HPs. This study identified 19 differently expressed miRNAs between AD and HP such as let-7i-5p, miRNA-1229-5p, miRNA-1234-5p, miRNA-1249, miRNA-1268B, miRNA-1275, miRNA-194-5p, miRNA-215, miRNA-2392, miRNA-30b-5p, miRNA-331-3p, miRNA-3653, miRNA-3960, miRNA-4281, miRNA-4689, mRNA-4739, miRNA-518a-5p, miRNA-6510-5p and miRNA-939-5p. They concluded that the expression of the abovementioned miRNAs in HP and SSLs are down-regulated and are related to MSI and CIMP. On the other hand, ADs have upregulated miRNA expression and are associated with TP53 andKRAS-mutations. Additionally, their study aimed to identify different miRNA expression and molecular pathways in colorectal carcinogenesis through genomic landscaping of colon polyps[15]. An overview of putative miRNA profile expression in the serrated colorectal pathway is presented in Figure 3.
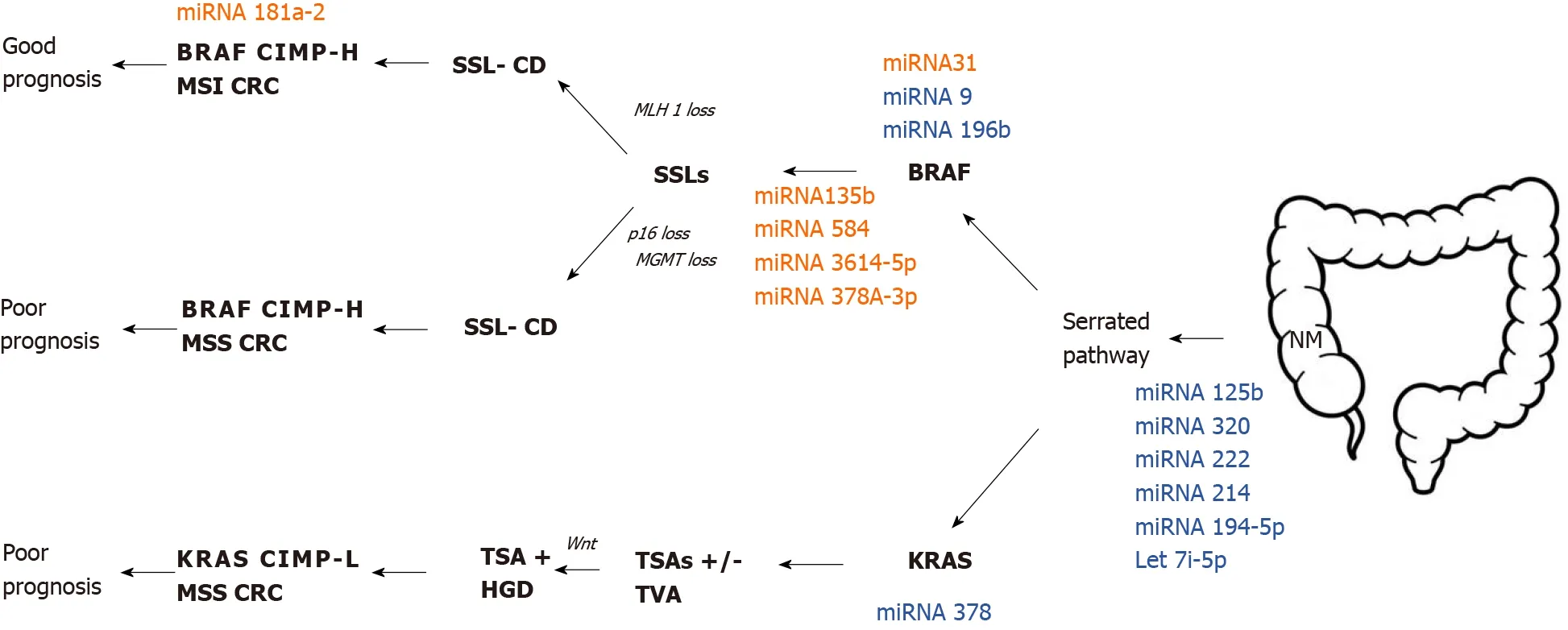
Figure 3 Putative microRNA profile expression in the serrated colorectal pathway. microRNAs in red color showed up-regulation, while the ones in blue color showed down-regulation. miRNA: microRNA; SSL: Sessile serrated lesion; SSL-CD: Sessile serrated lesion with cytological dysplasia; TSA: Traditional serrated adenoma; HGD-H: High-grade dysplasia; TVA: Tubulovillous adenoma; CIMP: CpG island methylator phenotype; CIMP-H: CIMP-high; CIMP-L: CIMP-low; MSI: Microsatellite instability; MSS: Microsatellite stability; NM: Normal mucosa.
HUMAN GUT MICROBIOTA, MUCOSAL IMMUNITY, AND MIRNA IN SERRATED PATHWAY
Human gut microbiota comprises approximately 39 trillion microorganisms that colonize the adult gut system[93]. It plays a significant role in maintaining homeostasis of the intestinal immune system, which represents a natural barrier to pathogen infection[94]but also maintain oral tolerance in the gut. Gut homeostasis can be disturbed by environmental factors such as lifestyle, diets, infections, and antibiotics, leading to dysbiosis. Many recent studies have demonstrated the association between gut dysbiosis and colorectal carcinogenesis[95]. Evidence suggest thatFusobacterium nucleatum(F. nucleatum) has overabundance in gut microbiota in dysbiosis[96]. This finding is in agreement with the fact thatF. nucleatumis involved in mucosal inflammation and contributes to the progression of CRC[97,98]. There are plenty of studies that investigate interactions betweenF. nucleatumand conventional adenoma to carcinoma sequences[99-101]. Itoet al[102]focused onF. nucleatumand serrated carcinoma pathway. In particular, they investigated the putative correlation betweenF. nucleatumand miRNA-31 expression. However, the results of the study did not indicate a significant association between miRNA-31 andF. nucleatum. Nevertheless, Yuet al[103]showed that invasiveF. nucleatummight play a role in developing proximal colon carcinogenesis through the serrated neoplasia process, which may play a less significant role in the traditional adenomas-carcinoma sequence. Bacterial biofilms may not supportF. nucleatuminfiltrate tumor tissues.
Longitudinal studies of immune infiltrate in resected CRC tumors have shown the role of the immune response in the pathophysiology of CRC. miRNAs, as non-coding RNAs, are capable of controlling several post-transcription target genes and performing essential roles in cell proliferation, differentiation, and apoptosis, including the immune cells[104]. In other words, miRNAs are necessary for maintaining the functioning of the immune system. However, abnormal expression of miRNAs is often found in various forms of tumors that contributes to immune deficiencies or immune evasion. Liet al[104]focused on the possible functions of miRNAs in CRC immune response control and the use of specific miRNA targets for CRC therapy. It is assumed that miRNAs possess an immunomodulatory role and can potentially be a part of the anti-cancer target pipeline. However, there may be some drawbacks and threats of using miRNAs as immunotherapeutics.
As discussed above, different miRNA profile variations from the transition of NM to adenoma and CRC identified some miRNA as contributors to those transformations. Moreover, serum miRNAs may be used as markers to track certain changes accompanying carcinogenesis[105]. miRNA profiles obtained in standard colorectal mucosa differ from those in adenomas and CRC. Oncogenes such asc-MetandKRAS, together with the miRNAs could also have pro- or anti-CRC effects, including influencing the immune system. More interestingly, some miRNAs increased their expression in developing CRC, whereas others reduced their expression, such as miRNA-30b[106]. Furthermore, evidence indicates that miRNAs not only participate in colorectal carcinogenesis, but can be used as biomarkers for diagnosing, managing, and follow up the patients.
It is well-known that one of the mechanisms for cancer invasion is to establish complex pathways for disarming the immune system and evading immune surveillance. Nakanishiet al[106]demonstrated that in human serrated tumors, the expression of atypical protein kinases C (PKC) is decreased. Simultaneous inactivation of the encoding genes in the intestinal epithelium of the mouse culminated in random serrated tumorigenesis with a highly reactive and immunosuppressive stroma leading to advanced cancer development. Whereas epithelial PKC deficiency resulted in the death of immunogenic cells and the infiltration of CD8+ T cells that repressed tumor initiation, IFN, and CD8+ T cell responses were impaired by PKC loss, resulting in tumorigenesis[106].
Some tumors may stimulate the immune cells in the tumor stroma to produce a variety of inhibiting cytokines such as transforming growth factor (TGF-β) and IL-10, which suppress the recruitment and activation of antitumor T lymphocytes[107]. Furthermore, IL-6 suppresses the ability of dendritic cells to present antigens by activating the signal transducer and transcription activator 3 (STAT3) and lessens CD4+ T cell-mediated immune response[108]. Thus, an immunotherapy that utilizes monoclonal antibodies that antagonize immunosuppressive cytokines or inactivate immunosuppressive cells may enhance tolerance to cancer and prevent tumor growth[16]. Our team also documented that IL-6 upregulation is crucial for developing both IBD and CRC well before the upregulation of other Th17/Treg associated genes (TGFb1, IL-10, IL-23, and FoxP3 transcription factor) that are critical primarily for the development of CRC[109]. An additional study revealed that intratumoral IL-17-mediated signaling might inhibit immunotherapy responses[110].
In line with this, synergistic therapeutic efficacy was demonstrated by combined therapy with TGF-β receptor inhibitor and anti-PD-L1 checkpoint blockade. A study of human samples confirmed the importance of atypical PKCs during the immunosurveillance defects in human serrated CRC. These results give insight into how this poor-prognosis subtype of CRC to be diagnosed and treated[106].
Since miRNAs modify the differentiation, activation, and distribution of the various immune cells and the intricate cytokine network, miRNAs play an essential role in both innate and adaptive immune responses. miRNAs are closely involved in processes such as control of innate and adaptive immunity activation, regulation of inflammation and cytokine network, trafficking and cytokine crosstalk between the tumor and its microenvironment, miRNAs are promising targets for immunotherapy of different gastroenterological cancers[111]. Thus, miRNAs exert regulatory and protective functions in the digestive system and antitumor defense against gastroenterological cancers development.
In line with this, KRAS-IRF2 (interferon regulatory factor 2) axis also impacts the immune system towards immune suppression[112]. The clinical significance of this observation is the immunotherapy resistance in CRC. However, the biological functions and mechanisms of oncogenicKRASin resistance to immune checkpoint blockade therapy are not fully understood.
Additionally, although various studies have examined the immune environment of CRCs with MSI, only one analysis assessed the immune microenvironment of serrated precursor lesions, including sessile serrated adenoma with dysplasia (SSA-D)[113]. Rauet al[113]studied the density of intraepithelial lymphocytes (IELs) in various serrated polyps and SSAs-D. The investigators observed that the amount of IELs was substantially higher in SSA-D than in SSAs, which displayed significantly higher numbers of IELs relative to HPs and typical adenomas. In their research, Acosta-Gonzalezet al[114]examined the immune properties of the serrated carcinogenesis system and its association with morphological stepwise dysplasia-carcinoma development and MSI status. They confirmed the higher density of IELs in lesions of MSI-H tumors. Additionally, other studies have shown that the total number of frameshift mutations in MSI CRCs correlates with lymphocyte infiltrating tumor density, specifically CD8+ lymphocyte density[115].
Nevertheless, the serrated pathway has two outcomes that differ in their clinical and prognostic characteristics as well as in their methylome profile and histological and molecular characteristics: (1) SSLs; or (2) Sporadic CRC showing MSI-H[42]. The latter subtype of CRC is correlated with deep immune invasion and has a better prognosis than the former[116].
The latest approaches in transcriptomics used to classify human CRC have shown that mesenchymal and/or desmoplastic involvement, together with an immunosuppressive microenvironment, are essential determinants of the worst prognosis of CRC. Importantly, these aggressive CRCs harbor the traits of serrated tumors, suggesting that how aggressive the CRC becomes is determined by initiation by this alternate mechanism. Moreover, molecular markers and profiles of gene expression have indicated that at least two CRC subgroups exist within the serrated pathway: (1) An inflammatory subtype with features of stromal/mesenchymal high immune infiltration (referred to “mesenchymal serrated” CRCs); and (2) MSI (“classical serrated”).BRAFmutation characterized with immune suppression in the tumor environment[117].
However, the tumor stroma's possible activation and the type of immune response associated with the CRC tumor stroma are not yet well understood. SAC may be infiltrated by CD45+ cells that express PD-L1 and decrease CD8+ T cells, which determines that there are multiple immune mechanisms to avoid the immune response[106]. Nevertheless, to create more efficient therapies, understanding the pathogenesis, including the tumor environment on the immunological settings, for both forms of serrated CRC is essential. Although emerging data show that immunotherapy is a promising choice for patients with multiple cancer forms still, there is a substantial clinical gap between the identification of serrated precursor lesions and the effective therapies for treating them.
CONCLUSION
With the growing influence of translational research and molecular pathology, the serrated pathway carcinogenesis became more elucidated based on the association of CIMP and key mutations inBRAF,KRAS,PIK3CA,TP53, andAPC. Furthermore, MSI caused by dysfunction of DNA MMR genes, is considered as another critical pathway in carcinogenesis.
In this review we summarized the most relevant information that have been published in the literature so far about miRNA expression in serrated pathway. Furthermore, we intended to answer the question could miRNA expression tell us more about colorectal serrated pathway carcinogenesis. The answer may come from several studies that have been published related to this issue. The data showed a unique miRNA signature in different types of colonic polyps, as well as in the progression of serrated lesions. Besides, those miRNAs play an important role in serrated carcinogenesis, proliferation, regression, and metastasis. Existing evidence support that miRNAs expression profiling, including miRNA-125b, miRNA-222, miRNA-214, miRNA-335 miRNA-31 miRNA-135-B miRNA-21 miRNA-181a-2,etc., allows us to understand and identify differences between benign and malignant lesions of the colon mucosa, as well as to stratify benign lesions according to their malignant potential.
Moreover, serum miRNAs may be used as markers to track specific changes accompanying serrated carcinogenesis. This assertion is based on the fact that there is a significant difference of miRNA expression between serrated and conventional pathway in colorectal carcinogenesis.
The immunopathology of CRC attracted growing attention since an association between gut dysbiosis and colorectal carcinogenesis was suggested by recent authors. miRNAs are putative regulators of several post-transcription target genes and are thought to play essential role in differentiation and proliferation of immune cells. It is assumed that, different miRNA profile pattern may contribute to alterations in gut immunity and dysbiosis, leading to transition events of NM to adenoma.
The specific miRNA expression in serrated pathway, could be useful tool to find appropriate diagnostic, prognostic and treatment response markers in clinical practice. Thus, in order to understand the real significance of miRNAs in this clinical setting, further studies must be conducted.
 World Journal of Gastroenterology2020年42期
World Journal of Gastroenterology2020年42期
- World Journal of Gastroenterology的其它文章
- Nonalcoholic fatty liver disease in lean subjects: Prognosis, outcomes and management
- Modern surgical strategies for perianal Crohn's disease
- Simultaneous colorectal and parenchymal-sparing liver resection for advanced colorectal carcinoma with synchronous liver metastases: Between conventional and mini-invasive approaches
- Estimation of visceral fat is useful for the diagnosis of significant fibrosis in patients with non-alcoholic fatty liver disease
- Nomograms and risk score models for predicting survival in rectal cancer patients with neoadjuvant therapy
- Attention deficit hyperactivity disorder and gastrointestinal morbidity in a large cohort of young adults
